Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(7):1018-1022. doi:10.7150/ijms.34141 This issue Cite
Research Paper
Torsades de pointes and QT prolongation Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System
1. Pharmacotherapy Division, College of Pharmacy, The University of Texas at Austin, San Antonio, TX, USA
2. Pharmacotherapy Education and Research Center, Long School of Medicine, University of Texas Health-San Antonio, San Antonio, TX, USA
3. Division of Infectious Diseases, Long School of Medicine, University of Texas Health-San Antonio, San Antonio, TX, USA
4. South Texas Veterans Health Care System, San Antonio, TX, USA
5. University Health System, San Antonio, TX, USA
Received 2019-2-15; Accepted 2019-5-17; Published 2019-6-10
Abstract
Introduction: Macrolides, linezolid, imipenem-cilastatin, fluoroquinolones, penicillin combinations, and ceftriaxone are known to be associated with Torsades de pointes/QT prolongation (TdP/QTP). Other antibiotics may also lead to TdP/QTP, but no study has systemically compared TdP/QTP associations for many available antibiotics.
Objectives: The objective of this study was to evaluate the association between TdP/QTP and many available antibiotics using the FDA Adverse Event Report System (FAERS).
Methods: FAERS reports from January 1, 2015 to December 31, 2017 were analyzed. The Medical Dictionary for Regulatory Activities (MedDRA) was used to identify TdP/QTP cases. We calculated the Reporting Odds Ratios (RORs) and corresponding 95% confidence intervals (95%CI) for the association between antibiotics and TdP/QTP. An association was considered to be statistically significant when the lower limit of the 95%CI was greater than 1.0.
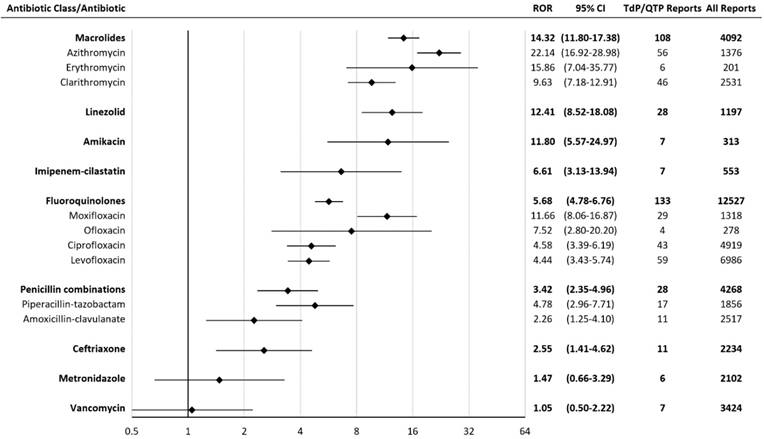
Results: A total of 2,042,801 reports (including 3,960 TdP/QTP reports) were considered, after inclusion criteria were applied. Macrolides had the greatest proportion of TdP/QTP reports. Of the 4,092 reports associated with macrolides, 108 reports (2.6%) were associated with TdP/QTP. Significant TdP/QTP RORs (95%CI) for the antibiotics were (in descending order): macrolides 14.32 (11.80-17.38), linezolid 12.41 (8.52-18.08), amikacin 11.80 (5.57-24.97), imipenem-cilastatin 6.61 (3.13-13.94), fluoroquinolones 5.68 (4.78-6.76), penicillin combinations 3.42 (2.35-4.96), and ceftriaxone 2.55 (1.41-4.62).
Conclusion: This study confirms prior evidence for TdP/QTP associations with macrolides, linezolid, imipenem-cilastatin, fluoroquinolones, penicillin combinations, and ceftriaxone. This study also identifies a new association between amikacin and TdP/QTP.
Keywords: Torsades de pointes, QT prolongation, adverse drug events, antibiotics, antimicrobial stewardship
Introduction
Drug-induced QT interval prolongation (QTP) is able to cause Torsades de pointes (TdP), a potentially fatal ventricular arrhythmia [1]. The risk of TdP/QTP must be considered when selecting antibiotic therapy. In 2010, a study evaluated the risks of TdP with antibiotics using the United States FDA Adverse Event Reporting System (FAERS) and identified macrolides, fluoroquinolones, and linezolid as TdP agents [2]. Macrolides and fluoroquinolones are known to cause QTP via blockade of the rapidly activating delayed rectifier potassium channel (hERG/IKr channel) [3-7]. Linezolid has been associated with TdP [2]; however, a double-blind placebo-controlled four-way crossover study with 40 healthy subjects found that linezolid had no effect on the QT interval itself [8]. An observational cohort study of 1,270 patients indicated that beta-lactamase inhibitors were associated with QTP [9]. Ceftriaxone, when used with lansoprazole, was significantly associated with QTP in a study of FAERS and electronic health records [10]. A case report stated that imipenem-cilastatin and piperacillin-tazobactam caused hypokalemia leading to TdP in a patient [11].
In this study, we investigated FAERS to analyze the association between TdP/QTP and common antibiotic agents, including macrolides, fluoroquinolones, oxazolidinones, penicillins, carbapenems, cephalosporins, aminoglycosides, metronidazole, and glycopeptide antibiotics.
Methods
Data Source
FAERS is a publicly available database, which is composed of adverse event reports that were submitted to United States Food and Drug Administration (FDA) [12]. FAERS data contain drug information (drug name, active ingredient, route of administration, the drug's reported role in the event) and reaction information. Each report has a primary suspected drug with one or more adverse drug reactions (ADR) and may include other drugs taken by the patient.
Study Design
FAERS data from January 1, 2015 to December 31, 2017 were included in the study. Some reports were submitted to FDA multiple times with updated information. Therefore, duplicate reports were removed by case number, with only the most recently submitted version included in the study. Reports containing drugs which were administered in oral, intramuscular, subcutaneous, intravenous, and parenteral routes were included in the study, while other routes of administration were excluded.
Drug Exposure Definition
Each antibiotic was identified in FAERS by generic and brand names listed in the Drugs@FDA Database [13]. Drugs with a reported role coded as “PS” (Primary Suspect Drug) or “SS” (Secondary Suspect Drug) were evaluated for inclusion [14]. Antibiotics with less than three TdP/QTP reports were excluded from data analysis [15].
Adverse Drug Reaction Definition
FAERS defines ADRs using Preferred Terms (PT) from the Medical Dictionary for Regulatory Activities (MedDRA) [16]. Preferred Terms “Electrocardiogram QT prolonged”, “Long QT syndrome”, and “Torsade de pointes” were used to identify TdP/QTP cases.
Statistical Analysis
A disproportionality analysis was conducted by computing Reporting Odds Ratios (ROR) and corresponding 95% confidence intervals (95%CI) for the association between TdP/QTP and each antibiotic class or individual antibiotic [17]. ROR was calculated as the ratio of the odds of reporting TdP/QTP versus all other ADRs for a given drug, compared with these reporting odds for all other drugs present in FAERS [17]. An association was considered to be statistically significant if the lower limit of 95%CI was above 1.0 [17]. An adjusted ROR was calculated after removing reports of potentially confounding antiarrhythmic drugs from the data analysis. These drugs include amiodarone, azimilide, disopyramide, dofetilide, flecainide, ibutilide, mexiletine, propafenone, propranolol, quinidine, and sotalol. Data analysis was performed using Microsoft Access 2016, Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA), and JMP Pro 13.2.1 (SAS Institute, Cary, NC).
Results
After applying inclusion and exclusion criteria and removing duplicate reports, FAERS contained 2,042,801 reports from January 1, 2015 to December 31, 2017. There were 3,960 TdP/QTP reports from the study period, which were included in the data analysis. Females accounted for 60% of TdP/QTP reports. TdP/QTP patients had a median age (IQR, interquartile range) of 55 (34) years.
Macrolides had the highest TdP/QTP ROR among all antibiotics in the study. Of the 4,092 reports associated with macrolides, 108 reports were associated with TdP/QTP. The RORs for agents significantly associated with TdP/QTP were: macrolides 14.32 (11.80-17.38), linezolid 12.41 (8.52-18.08), amikacin 11.80 (5.57-24.97), imipenem-cilastatin 6.61 (3.13-13.94), fluoroquinolones 5.68 (4.78-6.76), penicillin combinations 3.42 (2.35-4.96), and ceftriaxone 2.55 (1.41-4.62) (Figure 1).
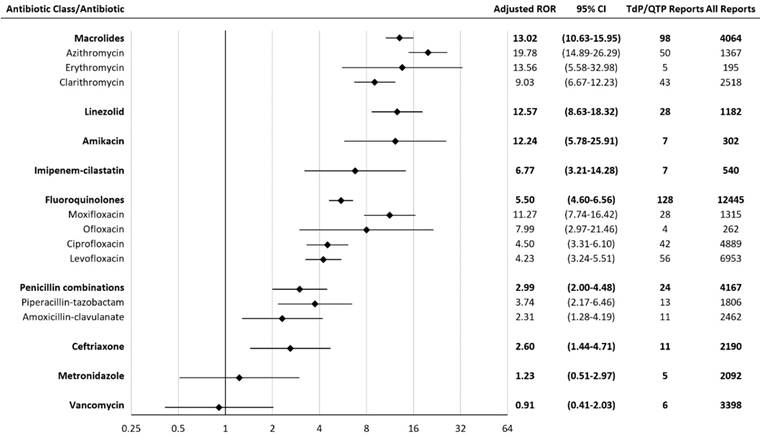
An adjusted ROR was performed to exclude reports among patients who were taking concomitant antiarrhythmic agents. This was done to reduce confounding variables that may also contribute to TdP/QTP. The adjusted RORs for agents significantly associated with TdP/QTP were: macrolides 13.02 (10.63-15.95), linezolid 12.57 (8.63-18.32), amikacin 12.24 (5.78-25.91), imipenem-cilastatin 6.77 (3.21-14.28), fluoroquinolones 5.50 (4.60-6.56), penicillin combinations 2.99 (2.00-4.48), and ceftriaxone 2.60 (1.44-4.71) (Figure 2). The ROR association rank did not differ when adjusted to exclude antiarrhythmic agents.
Amikacin was associated with a total of seven TdP/QTP reports. In these reports, amikacin was the secondary suspect drug of TdP/QTP, while the primary suspect drugs were bedaquiline, clofazimine, linezolid, and ciprofloxacin.
Piperacillin-tazobactam was associated with a total of seventeen TdP/QTP reports. In these reports, three reports had Clostridium difficile colitis and seven reports had electrolyte abnormalities.
Reporting Odds Ratios (RORs) for Torsades de pointes/ QT prolongation with antibiotics. CI = confidence interval; TdP/QTP = Torsades de pointes/QT prolongation.The scale is log-2.
Adjusted Reporting Odds Ratios (RORs) for Torsades de pointes/QT prolongation with antibiotics. CI = confidence interval; TdP/QTP = Torsades de pointes/QT prolongation. The scale is log-2.
Discussion
Our study found a significantly higher ROR for TdP/QTP as compared to all other adverse events for these antibiotics, which were (ROR from highest to lowest) azithromycin, erythromycin, linezolid, amikacin, moxifloxacin, clarithromycin, ofloxacin, imipenem-cilastatin, piperacillin-tazobactam, ciprofloxacin, levofloxacin, ceftriaxone, and amoxicillin-clavulanate. An FAERS study published in 2010 indicated significant TdP associations (from strongest to weakest) with moxifloxacin, levofloxacin, erythromycin, ciprofloxacin, gatifloxacin, clarithromycin, azithromycin, and linezolid [2]. Both studies showed TdP associations with macrolides, fluoroquinolones, and linezolid. The 2010 FAERS study only included TdP in their data analysis while our study included not only TdP but also QTP. Since QTP is a precursor of TdP, including QTP increases sensitivity of signal detection. The 2010 FAERS study included drugs administered through all routes, including topical routes, which may have limited systemic absorption and are less likely to cause TdP. Our study only included drugs administered in oral, subcutaneous, intramuscular, intravenous, and parenteral routes and excluded other routes of administration, such as topical routes. Therefore, TdP/QTP events in our study are more likely to be caused by a drug than those in the 2010 FAERS study [2].
Our study confirmed previously known TdP/QTP associations with macrolides, linezolid, imipenem-cilastatin, fluoroquinolones, penicillin combinations, and ceftriaxone [2,9-11]. Penicillin combinations have a high incidence of diarrhea, and diarrhea may lead to electrolyte abnormalities, which are significant risk factors for TdP/QTP. In our study, out of seventeen piperacillin-tazobactam TdP/QTP reports, three reports had Clostridium difficile colitis and seven reports had electrolyte abnormalities. Amikacin was found to be associated with TdP/QTP in our study, which was not reported in the literature. However, amikacin was the secondary suspect drug in all TdP/QTP reports, while the primary suspect drugs were known to be associated with TdP/QTP. Amikacin might play a role in TdP/QTP but the causal relationship is not warranted.
Limitations
A causal relationship between a drug and an ADR cannot be determined by FAERS. Significant bias may occur because of the spontaneous and voluntary reporting of ADRs. Media attention for a particular ADR might affect the reporting behaviors. The association between a drug and an ADR is confounded by comorbid diseases and concomitant drugs. For example, concomitant QT-prolonging drugs, such as ondansetron, antidepressants, antipsychotics, methadone, arsenic, and azole antifungals, are confounders when studying the associations between TdP/QTP and antibiotics. Diarrhea is also a potential confounder because diarrhea may lead to electrolyte abnormalities, which may cause TdP/QTP. Antibiotics, such as penicillin combinations and fluoroquinolones, cause diarrhea in many patients. The higher TdP/QTP ROR for penicillin combinations might be due to their ability of causing diarrhea. The higher TdP/QTP ROR for fluoroquinolones might be due to a combination of their ability of causing diarrhea and their blockade of hERG/IKr channel.
Conclusions
This study confirms prior evidence for significant TdP/QTP associations with macrolides, linezolid, imipenem-cilastatin, fluoroquinolones, penicillin combinations, and ceftriaxone. This study also discovers a new association between amikacin and TdP/QTP. Results obtained from FAERS should be interpreted with caution in the context of data limitations. Antibiotic stewardship is needed to prevent TdP/QTP and to improve health outcomes.
Abbreviations
ADR: adverse drug reaction; FDA: Food and Drug Administration; FAERS: FDA Adverse Event Reporting System; CI: confidence interval; IQR: interquartile range; MedDRA: Medical Dictionary for Regulatory Activities; QTP: QT Prolongation; ROR: Reporting Odds Ratio; TdP: Torsades de pointes; PT: Preferred Term.
Acknowledgements
No funding was sought for this research study. Dr. Frei was supported, in part, by a NIH Clinical and Translational Science Award (National Center for Advancing Translational Sciences, UL1 TR001120, UL1 TR002645, and TL1 TR002647) while the study was being conducted. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the authors' affiliated institutions.
Authors' contributions
Study concept and design: Teng, Walter, and Frei. Statistical analysis: Teng. Interpretation of data: Teng and Frei. Drafting of the manuscript: Teng and Gaspar. Critical revision of the manuscript for important intellectual content: All authors. Study supervision: Frei.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358:169-76
2. Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf. 2010;33:303-14
3. Abo-Salem E, Fowler JC, Attari M, Cox CD, Perez-Verdia A, Panikkath R, Nugent K. Antibiotic-induced cardiac arrhythmias. Cardiovasc Ther. 2014;32:19-25
4. Antzelevitch C, Sun ZQ, Zhang ZQ, Yan GX. Cellular and ionic mechanisms underlying erythromycin-induced long QT intervals and torsade de pointes. J Am Coll Cardiol. 1996;28:1836-48
5. Anderson ME, Mazur A, Yang T, Roden DM. Potassium current antagonist properties and proarrhythmic consequences of quinolone antibiotics. J Pharmacol Exp Ther. 2001;296:806-10
6. Volberg WA, Koci BJ, Su W, Lin J, Zhou J. Blockade of human cardiac potassium channel human ether-a-go-go-related gene (HERG) by macrolide antibiotics. J Pharmacol Exp Ther. 2002;302:320-7
7. Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406:341-3
8. Damle B, Labadie RR, Cuozzo C, Alvey C, Choo HW, Riley S, Kirby D. Lack of an effect of standard and supratherapeutic doses of linezolid on QTc interval prolongation. Antimicrob Agents Chemother. 2011;55:4302-7 (PMC3165302)
9. Keller GA, Alvarez PA, Ponte ML, Belloso WH, Bagnes C, Sparanochia C, Gonzalez CD, Villa Etchegoyen MC, Diez RA, Di Girolamo G. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11:86-98
10. Lorberbaum T, Sampson KJ, Woosley RL, Kass RS, Tatonetti NP. An integrative data science pipeline to identify novel drug interactions that prolong the QT interval. Drug Saf. 2016;39:433-41 (PMC4835515)
11. Kumar V, Khosla S, Stancu M. Torsade de pointes Induced by hypokalemia from imipenem and piperacillin. Case Rep Cardiol. 2017;2017:4565182. (PMC5468583)
12. Food and Drug Administration. FDA Adverse Event Reporting System (FAERS). Available from http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed July 24. 2018
13. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed July 24. 2018
14. McConeghy KW, Soriano MM, Danziger LH. A quantitative analysis of FDA adverse event reports with oral bisphosphonates and Clostridium difficile. Pharmacotherapy. 2016;36:1095-101
15. Evans SJW, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483-6
16. MedDRA MSSO. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 21.0. Available from http://www.meddra.org/sites/default/files/guidance/file/smq_intguide_21_0_english.pdf. Accessed July 24. 2018
17. Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427-36
Author contact
![]() Corresponding author: Christopher R. Frei, PharmD, FCCP, BCPS, Director, Pharmacotherapy Education and Research Center, Long School of Medicine, University of Texas Health-San Antonio, 7703 Floyd Curl Dr., MSC-6220, San Antonio, TX 78229; Email: freicedu
Corresponding author: Christopher R. Frei, PharmD, FCCP, BCPS, Director, Pharmacotherapy Education and Research Center, Long School of Medicine, University of Texas Health-San Antonio, 7703 Floyd Curl Dr., MSC-6220, San Antonio, TX 78229; Email: freicedu

 Global reach, higher impact
Global reach, higher impact