Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(2):117-123. doi:10.7150/ijms.13862 This issue Cite
Research Paper
Relationship of Genetic Polymorphisms of Aldosterone Synthase Gene Cytochrome P450 11B2 and Mineralocorticoid Receptors with Coronary Artery Disease in Taiwan
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan;
2. Division of Cardiology, Department of Internal Medicine, Yuan-Sheng Hospital and Changhua Christian Hospital, Yuanlin Branch, Yuanlin, Taiwan;
3. Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan;
4. School of Medicine, Chung Shan Medical University, Taichung, Taiwan;
5. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan;
6. Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung, Taiwan.
Received 2015-9-15; Accepted 2016-1-5; Published 2016-2-1
Abstract
The aldosterone synthase gene, cytochrome P450 11B2 (CYP11B2), and mineralocorticoid receptor (MR) genes have been reported to be associated with coronary artery disease (CAD). In this study, we investigated the association of single nucleotide polymorphisms (SNPs) of CYP11B2 (CYP11B2 T-344C) and MR (MR C3514G and MR C4582A) with CAD in Taiwanese. Six hundred and nine unrelated male and female subjects who received elective coronary angiography were recruited from Chung Shan Medical University Hospital. The enrolled subjects were those who had a positive noninvasive test. CYP11B2 T-344C, MR C3514G and MR C4582A were determined by polymerase chain reaction-restriction fragment length polymorphism. We found that women with CYP11B2 C/C had a higher risk of developing CAD. However, there were no significant differences in the genotype distributions of MR C3514G and MR C4582A between the women with and without CAD. In multivariate analysis, CYP11B2 T-344C was most significantly associated with CAD in Taiwanese women. In conclusions, CYP11B2 C/C was more significantly associated with the development of CAD than diabetes mellitus or hypertension. This implies that CYP11B2 C/C plays a more important role than some conventional risk factors in the development of CAD in Taiwanese women.
Keywords: aldosterone synthase gene, cytochrome P450 11B2, mineralocorticoid receptors, single nucleotide polymorphism, coronary artery disease, Taiwan women.
Introduction
Coronary heart disease is a major cause of mortality and morbidity worldwide affecting millions of people. The causes of coronary heart disease are multifactorial and include conventional and nonconventional factors (1, 2). Male gender, hypertension, smoking, hyperlipidemia, and diabetes mellitus (DM) are conventional risk factors, however, nonconventional risk factors have not yet to be well-defined.
The renin-angiotensin-aldosterone system (RAAS), which affects circulatory homeostasis, regulates the functions of cardiovascular, renal and adrenal glands by regulating blood pressure, fluid and sodium balance (3). RAAS maintains blood pressure through its effect on the kidneys to regulate sodium and water balance, and on peripheral blood vessels to increase systemic vascular resistance (4). Abnormal activity of the RAAS may lead to an array of cardiovascular events such as atherosclerotic coronary artery disease (CAD), plaque rupture and myocardial infarction (3, 5). Local aldosterone synthesis may also play a pathogenic role (6). Renin cleaves angiotensinogen that is synthesized and secreted by the liver to angiotensin I. Circulating angiotensin I is then hydrolyzed to angiotensin II by angiotensin-converting enzyme that is located primarily in the pulmonary and renal endothelium. Angiotensin II initiates a vasoconstrictor response and stimulates aldosterone synthesis by the adrenal glands (7). Aldosterone has been linked to the development of left ventricular cardiac and systemic vascular remodeling, and left heart failure (8, 9). Aldosterone is also known to play an important role in the regulation of blood pressure, cardiac and perivascular fibrosis, increased left ventricular mass and cardiovascular events (10). It is either causative or a disease modifier that facilitates adaptive cardiovascular remodeling (8, 9). Aldosterone acts via binding to the mineralocorticoid receptor (MR) (11).
Aldosterone secretion is regulated largely by the expression level of the final enzyme required for its biosynthesis, aldosterone synthase, which is encoded by the aldosterone synthase gene, cytochrome P450 11B2 (CYP11B2). Aldosterone, or activation of its receptor, MR, has several extra-renal effects that are largely detrimental in the setting of heart disease (12, 13). Because CYP11B2 and its receptor are implicated in the development of cardiovascular diseases and the SNPs were associated with heart disease (16), we hypothesized that CYP11B2 single nucleotide polymorphism (SNP CYP11B2 T-344C) and MR SNPs (MR C3514G and MR C4582A) would be associated with CAD. To the best of our knowledge, few studies have investigated the roles of CYP11B2 T-344C, MR C4582A or MR C3514G in the development of CAD in Taiwan. The aims of this study were to investigate the correlations of CYP11B2 T-344C, MR C4582A and MR C3514G with CAD in Taiwanese.
Materials and methods
Subjects
Six hundred and nine unrelated male and female subjects who received elective coronary angiography in Chung Shan Medical University Hospital from April 2007 to March 2009 were recruited. The studied population who received coronary angiography included the subjects who had positive noninvasive test such as the treadmill test, myocardial perfusion scan, or cardiac computed tomography scan. All participants received echocardiographic examinations (Philips Healthcare, SONOS 7500) during their clinic visit. The exclusion criteria included patient refusal, known cerebrovascular attack history, peripheral arterial disease, and incomplete medical chart data. The left ventricular mass (LVM) was calculated using the formula defined by the American Society of Echocardiography: 0.8x {1.04 x [(IVSTD+LVEDD+PWTD)3-(LVEDD)3]}+ 0.6 g, where IVSTD is interventricular septum thickness in diastole, LVEDD is left ventricular end-diastolic dimension, and PWTD is posterior wall thickness in diastole (15). CAD was defined as more than 50% stenosis over any segment of the coronary artery by angiography, a diagnostic gold standard. The collected data included gender, age, co-morbidities such as hypertension and DM, and echocardiographic measurements including LVM, LVEDD and left ventricular end-systolic diameter (LVESD). The study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (CSMUH No: CS07095), and informed consent was obtained from each participant.
Blood sample collection and genomic DNA extraction
Venous blood was drawn from each subject into Vacutainer tubes containing EDTA and stored at 4˚C. Genomic DNA was extracted using QIAamp DNA blood mini kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The DNA was dissolved in TE buffer [10 mM Tris (pH 7.8), 1mM EDTA] and then quantitated by measurements at an optical density of 260 nm. The final preparation was stored at -20˚C and used as templates for polymerase chain reaction.
Selection of CYP11B2 T-344C, MR C3514G and MR C4582A Polymorphisms
We included the CYP11B2 T-344C SNP in the promoter region which was found to affect the production of CYP11B2 in a Chinese population (16). Furthermore, the SNPs MR C3514G and MR C4582A were selected in this study because the gene polymorphism of the SNP has been found to associate with heart disease (14).
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP)
The SNPs CYP11B2 T-344C, MR C3514G, and MR C4582A were determined by PCR-RFLP assay as previously described (14, 17). The primer sequences and restriction enzyme for analysis of the CYP11B2 T-344C, MR C3514G, and MR C4582A gene polymorphisms are described in Table 1. The PCR was performed in a 10 µL volume containing 100 ng DNA template, 1.0 µL of 10× PCR buffer (Invitrogen, Carlsbad, CA), 0.25 U of Taq DNA polymerase (Invitrogen, Carlsbad, CA), 0.2 mM dNTPs (Promega, Madison, WI), and 200 nM of each primer (MDBioInc, Taipei). The Taq DNA polymerase is a relatively low replication fidelity enzyme. To prevent an error occurring, triple experiments were performed in amplification. The PCR cycling conditions were 5 minutes at 94˚C followed by 35 cycles of 1 minute at 94˚C, 1 minute at 60˚C, and 2 minutes at 72˚C, with a final step at 72˚C for 20 minutes to allow for complete extension of all PCR fragments. A 10 µL aliquot of PCR product was subjected to digestion at 37˚C for 4 hours in a 15 µL reaction containing 5 U of restriction enzyme (New England Biolabs, Beverly, MA) and 1.5 µL buffer (New England Biolabs). Digested products were separated on a 3% agarose gel and then staine with ethidium bromide.
Primer sequences and PCR-RFLP conditions for amplification of CYP11B2 and MR SNPs.
| SNP | Sequences | Product | Enzyme |
|---|---|---|---|
| CYP11B2 T-344C | 5'-CAGGAGGAGACCCCATGTGAC-3' 5'-CCTCCACCCTGTTCAGCCC-3' | T/T: 274 bp, 138 bp, 126 bp C/C: 202 bp, 138 bp, 126 bp, 71 bp | HaeIII |
| MR C3514G | 5'-AATCGCTCTCCACTGCTGTA-3 5'-CAATGCCTGGAATAGCTGCT-3' | C/C: 255 bp G/G: 150 bp, 105 bp | BanII |
| MR C4582A | 5'-TTGGGAAAGCCTGCCTCGTT-3' 5'-TCCTGCCATGATCTGTGCGTT-3' | A/A: 286 bp C/C: 286 bp, 194 bp, 92 bp | MspA1I |
Statistical analysis
Chi-square and Fisher's exact tests were used to examine the relationships between clinical characteristics and the genotype frequencies of CYP11B2 T-344C, MR C3514G and MR C4582A with CAD. The Student t test and analysis of variance (ANOVA) with post hoc Scheffe test were used to compare the cardiographic measurements between the subjects with and without CAD as well as among the subjects with different genotypes of the CYP11B2 SNP. Multivariate analysis of the genotype distribution of CYP11B2 T-344C and clinical variables for their relationships with CAD was performed using a logistic regression model after controlling for variable parameters. A significant difference was defined as a P value of less than 0.05. All statistical analyses were performed using SPSS statistical software (version 11.0; SPSS, Inc., Chicago, IL). Odds ratios (ORs) and the 95% confidence intervals (CIs) were estimated using WinPepi software version 10.0 and SPSS.
Results
The clinical characteristics of the enrolled individuals are shown in Table 2. Of the 609 subjects, 423 individuals were male and 186 female, and 417 had CAD and 192 did not. There were no significant differences in age, LVM, LVEDD and LVESD between the two groups. The patients with DM and hypertension had a higher risk of developing CAD [P<0.001; OR: 1.96, 95% CI: 1.35-2.85; and P=0.007; OR: 2.01, 95% CI: 1.33-3.03, respectively] (Table 2).
Relationships between clinical variables and coronary artery disease (CAD)
| Variables | Negative CAD (N=192) | Positive CAD (N=417) | Odds ratio and 95% confidence interval | P value |
|---|---|---|---|---|
| Race | Taiwanese | Taiwanese | ||
| Residence | Mid-Taiwan | Mid-Taiwan | ||
| Gender | <0.001a | |||
| male | 114 | 309 | Reference | |
| female | 78 | 108 | 0.51 (0.35-0.75) | |
| Age (years) | 66.9±11.6 | 65.9±11.5 | 0.314 | |
| Diabetes mellitus | <0.001a | |||
| negative | 129 | 213 | Reference | |
| positive | 63 | 204 | 1.96 (1.35-2.85) | |
| Hypertension | 0.007a | |||
| negative | 59 | 86 | Reference | |
| positive | 113 | 331 | 2.01 (1.33-3.03) | |
| Left ventricular mass (g) | 193.48±35.73 | 197.61±39.10 | 0.275 | |
| left ventricular end- diastolic diameter (mm) | 50.11±5.34 | 49.87±5.58 | 0.664 | |
| left ventricular end- systolic diameter (mm) | 34.95±6.10 | 35.31±6.39 | 0.568 |
Statistical analysis: Chi-square or independent Student t tests
aP<0.05.
SD: standard deviation.
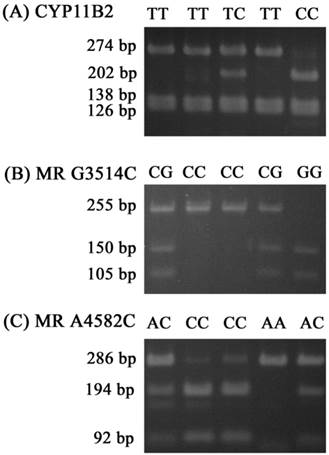
For the CYP11B2 gene polymorphism, the wild homozygous alleles (T/T) yielded 274-, 138- and 126-base pair (bp) products, the heterozygous alleles (T/C) yielded 274-, 202-, 138-, 126-and 71-bp products, while the mutant homozygous alleles (C/C) yielded 202-, 138-, 126- and 71-bp products. For MR C3514G, the wild homozygous alleles (C/C) yielded a 255-bp product, the heterozygous alleles (C/G) yielded 255-, 150-and 105-bp products, while the mutant homozygous alleles (G/G) yielded 150- and 105-bpproducts. For MR C4582A, the wild homozygous alleles (C/C) yielded 194- and 92-bp products, the heterozygous alleles (C/A) yielded 286-, 194- and 92-bp products, while the mutant homozygous alleles (A/A) yielded a 286-bp product (Fig. 1).
The minor allele frequencies of CYP11B2 T-344C, MR C3514G and MR C4582A of the subjects without CAD were all >5% (28.4%, 20.1% and 15.6%, respectively). In these subjects, the genotype frequency of CYP11B2 (P=0.279, χ2 value: 4.12) met Hardy-Weinberg equilibrium. The frequencies of MR G3514C (P>0.05, χ2 value: 0.018) and MR C4582A (P=0.851, χ2 value: 0.59) were also in Hardy-Weinberg equilibrium.
Polymerase chain reaction-restriction fragment length polymorphisms of CYP11B2 T-344C, MR G3514C, and MR A4582C genes. (A) PCR products of CYP11B2 T-344C gene polymorphisms were subjected to enzymatic digestion by incubation with Hae III, for 4 hours at 37°C and then submitted to electrophoresis in 3% agarose gels. The wild homozygous alleles (T/T) yielded 274-, 138- and 126-base pair (bp) products, the heterozygous alleles (T/C) yielded 274-, 202-, 138-, 126- and 71-bp products, while the mutant homozygous alleles (C/C) yielded 202-, 138-, 126- and 71-bp products. (B) PCR products of the MR G3514C gene polymorphism were subjected to enzymatic digestion by incubation with Ban II. The wild homozygous alleles wild (C/C) yielded a 255-bp product, the heterozygous alleles (C/G) yielded 255-, 150- and 105-bp products, while the mutant homozygous alleles (G/G) yielded 150- and 105-bp products. (C) PCR products of the MR A4582C gene polymorphism were subjected to enzymatic digestion by incubation with MspA1I. The wild homozygous alleles (C/C) yielded 194- and 92-bp products, the heterozygous alleles (C/A) yielded 286-, 194- and 92-bp products, while the mutant homozygous alleles (A/A) yielded a 286-bp product.
There were no significant differences in the genotype distributions of CYP 11B2 T-344C, MR C3514G and MR C4582A SNPs between the subjects with and without CAD (Table 3). When stratified by the gender, these findings remained insignificant in the male subgroup (Table 4). The female subjects with CYP11B2 C/C had a higher risk of developing CAD, however this risk was not found in the women who had only one mutant allele C (heterozygous T/C) (Table 5). There were no significant differences in the genotype distributions of MR C3514G and MR C4582A SNPs between the women with and without CAD (Table 5). In addition, we also found that women with DM had a tendency to develop CAD (P=0.042; OR: 1.85, 95% CI: 0.98-3.53; Table 5). The women with hypertension had a higher risk of developing CAD (P=0.016; OR: 2.44, 95% CI: 1.10-5.48) (Table 6). In multivariate analysis we found that the CYP11B2 T-344C SNP and hypertension were significantly associated with the development of CAD in the female subjects (P<0.001 OR: ∞, 95% CI: >1.23-∞ and P=0.021, OR: 2.51, 95% CI: 1.14-5.56, respectively; Table 6).
We next investigated the association of the CYP11B2 T-344C SNP with cardiographic measurements, and found that the women with CYP11B2 C/C had a significantly higher LMV compared to those with T/T (237.90±54.16 vs.189.45 ±38.30 g, P=0.022) and those with T/T or T/C (237.90±54.16 vs.192.02±40.10 g, P=0.005; Table 7). Of the women with CAD, those with CYP11B2 C/C had a significantly higher LMV compared to those with T/T (237.90±54.16 vs.188.83 ±41.85 g, P=0.027) and those with T/T or T/C (237.90±54.16 vs.187.73±39.90 g, P=0.005). The women having CAD with the mutant homozygous CC also exhibited a significantly greater LVEDD compared to those with T/T or T/C (52.48±2.60 vs.47.93±4.84 mm, P=0.026). Regardless of the presence of CAD, CYP11B2 C/C seemed to exacerbate the left ventricle function in the female subjects. However LVM and LVEDD were not associated with the development of CAD in the women (Women with CAD vs. those without CAD: for LVM, 191.40±42.76 vs. 192.88±40.50 g, P=0.835; for LVEDD, 48.26±4.85 vs. 49.16±5.10 mm, P=0.285; for LVESD, 33.81±5.34 vs. 33.67±4.41 mm, P=0.866). This implies that CYP11B2 C/C but not LMV or LVEDD predispose Taiwanese women to CAD.
Genotype distributions of single nucleotide polymorphisms of aldosterone synthase gene, cytochrome P450 11B2 (CYP11B2), CYP11B2 T-344C and mineralocorticoid receptor (MR C3514G and MR C4582A) in subjects with or without coronary artery disease (CAD)
| Variables | Negative CAD (N=192) | Positive CAD (N=417) | Odds ratio and 95% confidence interval | P value |
|---|---|---|---|---|
| CYP11B2 T-344C | ||||
| T/Ta | 93 | 222 | Reference | 0.090 |
| T/C | 89 | 159 | 0.75 (0.52-1.08) | |
| C/C | 10 | 36 | 1.51 (0.70-3.55) | |
| T/Ta | 93 | 99 | Reference | 0.271 |
| T/C and C/C | 222 | 195 | 0.83 (0.58-1.18) | |
| T/T and T/Ca | 182 | 381 | Reference | 0.137 |
| C/C | 10 | 36 | 1.72 (0.81-3.97) | |
| MR C3514G | ||||
| C/Ca | 123 | 267 | Reference | 0.888 |
| C/G | 61 | 129 | 0.97 (0.66-1.44) | |
| G/G | 8 | 21 | 1.21 (0.50-3.25) | |
| C/Ca | 123 | 267 | Reference | 0.994 |
| C/G and G/G | 69 | 150 | 1.00 (0.69-1.46) | |
| C/C and C/Ga | 184 | 396 | Reference | 0.640 |
| G/G | 8 | 21 | 0.98 (0.41-2.62) | |
| MR C4582A | ||||
| C/Ca | 138 | 270 | Reference | 0.116 |
| C/A | 48 | 138 | 1.47 (0.98-2.22) | |
| A/A | 6 | 9 | 0.77 (0.24-2.68) | |
| C/Ca | 138 | 270 | Reference | 0.082 |
| C/A and A/A | 54 | 147 | 1.39 (0.94-2.06) | |
| C/C and C/Aa | 186 | 408 | Reference | 0.574 |
| A/A | 6 | 9 | 0.68 (0.21-2.37) |
Statistical analysis: Chi-square or Fisher's exact tests
aUsed as references for comparison to evaluate the odds ratio of other genotypes.
Relationships of genotype distribution of single nucleotide polymorphisms of cytochrome P450 11B2 (CYP11B2 T-344C) and mineralocorticoid receptor (MR C3514G and C4582A) with coronary artery disease (CAD) in Taiwanese men (N=423).
| Variables | Negative CAD (N=114) | Positive CAD (N=309) | Odds ratio (OR) and 95% confidence interval | P valuea |
|---|---|---|---|---|
| CYP11B2 T-344C | ||||
| T/Tb | 50 | 162 | Reference | 0.285a |
| T/C | 54 | 122 | 0.70 (0.44-1.10) | |
| C/C | 10 | 25 | 0.77 (0.35-1.72) | |
| T/Tb | 50 | 162 | Reference | 0.118 |
| T/C and C/C | 64 | 147 | 0.71 (0.46-1.09) | |
| MR C3514G | ||||
| C/Cb | 77 | 197 | Reference | 0.658 |
| C/G | 31 | 98 | 1.24 (0.76-2.00) | |
| G/G | 6 | 14 | 0.91 (0.34-2.46) | |
| C/Cb | 77 | 197 | Reference | 0.469 |
| C/G and G/G | 37 | 112 | 1.18 (0.75-1.87) | |
| MR C4582A | ||||
| C/Cb | 80 | 201 | Reference | 0.456 |
| C/A | 30 | 100 | 1.33 (0.82-2.15) | |
| A/A | 4 | 8 | 0.80 (0.23-2.72) | |
| C/Cb | 80 | 201 | Reference | 0.322 |
| C/A and A/A | 34 | 108 | 1.26 (0.80-2.01) |
Statistical analysis: Chi-square or Fisher's exact tests
aP<0.05.
bUsed as references for comparison to evaluate the odds ratio of other genotypes.
Relationships of genotype distribution of single nucleotide polymorphisms of cytochrome P450 11B2 (CYP11B2 T-344C) and mineralocorticoid receptor (MR C3514G and C4582A) with coronary artery disease (CAD) in Taiwanese women (N=186)
| Variables | Negative CAD (N=78) | Positive CAD (N=108) | Odds ratio (OR) and 95% confidence interval | P valuea |
|---|---|---|---|---|
| CYP11B2 T-344C | ||||
| T/Tb | 43 | 60 | Reference | 0.010a |
| T/C | 35 | 37 | 0.76 (0.40-1.45) | |
| C/C | 0 | 11 | ∞ (1.67-∞) | |
| T/Tb | 43 | 60 | Reference | 0.954 |
| T/C and C/C | 35 | 48 | 0.98 (0.53-1.84) | |
| T/T and T/Cb | 78 | 97 | Reference | 0.003a |
| C/C | 0 | 11 | ∞ (1.93-∞) | |
| MR C3514G | ||||
| C/Cb | 46 | 70 | Reference | 0.223 |
| C/G | 30 | 31 | 0.68 (0.35-1.33) | |
| G/G | 2 | 7 | 2.30 (0.41-23.51) | |
| C/Cb | 46 | 70 | Reference | 0.417 |
| C/G and G/G | 32 | 38 | 0.78 (0.41-1.49) | |
| C/C and C/Gb | 76 | 101 | Reference | 0.308 |
| G/G | 2 | 7 | 2.63 (0.48-26.56) | |
| MR C4582A | ||||
| C/Cb | 58 | 69 | Reference | 0.158 |
| C/A | 18 | 38 | 1.77 (0.88-3.66) | |
| A/A | 2 | 1 | 0.42 (0.01-8.32) | |
| C/Cb | 58 | 69 | Reference | 0.130 |
| C/A and A/A | 20 | 39 | 1.64 (0.83-3.30) | |
| C/C and C/Ab | 76 | 107 | Reference | 0.573 |
| A/A | 2 | 1 | 0.36 (0.01-6.97) |
Statistical analysis: Chi-square or Fisher's exact tests
aP<0.05.
bUsed as references for comparison to evaluate the odds ratio of other genotypes.
Univariate and multivariate analyses of genotype distributions of single nucleotide polymorphisms of cytochrome P450 11B2 (CYP11B2 T-344C) and clinical variables for coronary artery disease (CAD) in Taiwanese women
| Univariate analysis | Negative CAD (N=78) | Positive CAD (N=108) | OR and 95% CI | P valuea |
|---|---|---|---|---|
| CYP11B2 T-344C | 0.003a | |||
| T/T and T/Cb | 78 | 97 | Reference | |
| C/C | 0 | 11 | ∞ (1.93-∞) | |
| Diabetes mellitus | 0.042a | |||
| negativeb | 50 | 53 | Reference | |
| positive | 28 | 55 | 1.85 (0.98-3.53) | |
| Hypertension | 0.016a | |||
| negativeb | 22 | 15 | Reference | |
| positive | 56 | 93 | 2.44 (1.10-5.48) | |
| Multivariate analysis | P valuea | |||
| CYP11B2 T-344C | <0.001a | |||
| T/T and T/Cb | 78 | 97 | Reference | |
| C/C | 0 | 11 | ∞ (>1.23-∞) | |
| Diabetes mellitus | 0.097 | |||
| negativeb | 50 | 53 | Reference | |
| positive | 28 | 55 | 1.69 (0.91-3.16) | |
| Hypertension | 0.021a | |||
| negativeb | 22 | 15 | Reference | |
| positive | 56 | 93 | 2.51 (1.14-5.56) |
Statistical analysis: univariate analysis using the chi-square or Fisher's exact tests; multivariate analysis using a logistic regression model after controlling for CYP11B2, diabetes mellitus and hypertension.
aP<0.05.
bUsed as references.
Relationships of genotype distributions of single nucleotide polymorphisms of cytochrome P450 11B2 (CYP11B2 T-344C) with cardiographic measurements in Taiwanese women (N=186).
| Variables | LVM (g) | P valuea | LVEDD (mm) | P valuea | LVESD (mm) | P valuea |
|---|---|---|---|---|---|---|
| CYP11B2 T-344C | ||||||
| T/T | 189.45±38.30 | 0.022a | 48.54±5.16 | 0.170 | 33.72±5.23 | 0.403 |
| T/C | 190.85±42.92 | 0.030a | 48.39±4.73 | 0.158 | 33.50±4.67 | 0.359 |
| C/Cb | 237.90±54.16 | 52.48±2.60 | 36.55±2.79 | |||
| T/T and T/C | 190.02±40.10 | 0.005a | 48.48±4.97 | 0.052 | 33.63±4.99 | 0.158 |
| C/Cb | 237.90±54.16 | 52.48±2.60 | 36.55±2.79 |
Statistical analysis: analysis of variance (ANOVA) with post hoc Scheffe test.
LVM: left ventricular mass; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; SD: standard deviation.
aP<0.05.
bGenotype C/C was compared with other genotypes.
Discussion
This study showed that patients with DM and hypertension had a higher risk of developing CAD. This risk was still present in the female subgroup after stratification by gender. Hypertension and DM, which are conventional risk factors, occurred more frequently in the subjects with CAD. In the Framingham Heart Study, high-normal blood pressure (defined as a systolic blood pressure of 130-139 mmHg, diastolic blood pressure of 85-89 mmHg, or both) increased the risk of cardiovascular disease by 2-fold compared with healthy individuals (18). Patients with DM have been reported to be 2 to 8 times more likely to experience future cardiovascular events than age- and ethnically-matched individuals without DM (19). However, multivariate analysis in the current study showed that hypertension but not DM was significantly associated with the development of CAD in Taiwanese women.
We conducted this study to define the relationship of a nonconventional risk factor, genetic polymorphism, with CAD in Taiwanese. We found no significant differences in the genotype distributions of CYP11B2 T-344C, MR C3514G and MR C4582A SNPs between the subjects with and without CAD. When stratified by gender, the findings remained insignificant in the male subgroup. However, the women with CYP11B2 C/C had a higher risk of developing CAD, although this risk was not found in the women who had only one mutant allele C. There were no significant differences in the genotype distributions of MR C3514G and MR C4582A SNPs between the women with and without CAD. A common single nucleotide polymorphism, T to C transition for position -344, occurs within the promoter region of CYP11B2 (20). In an in vitro study, the C allele was found to bind steroidogenic transcription factor 1 four times more than the T allele (21), and it has also been linked to increased aldosterone production (22, 23). The CYP11B2 promoter polymorphism has been linked to hypertension (24), and the -344C allele in particular to the risk of acute myocardial infarction (25). In a study of angiotensin II receptor blockers, the CC genotype was found to significantly predict a positive response to antihypertensive treatment (26). However, an association of the -344 genotype with aldosterone levels has been inconsistent, with several studies reporting an association between the -344T allele and higher levels (15, 27). Moreover, a meta-analysis suggested that the -344T>C polymorphism in the CYP11B2 gene might be associated with susceptibility to CAD in Caucasians and Asians (28). However without stratification by the gender, Mishra et al. reported that CYP11B2 was not associated with either CAD or left ventricular dysfunction in an Indian population (29).
Even when stratified by gender, the patients with the MR C3514G and MR C4582A SNPs were still not associated with CAD in our study. This may be due to not specific enough binding of MR with its ligands. MR can bind cortisol and aldosterone with nearly equal affinity (30). Hudson et al. demonstrated the structure of the human MR DNA binding domain in complex with a canonical DNA response element. The overall structure is similar to the glucocorticoid receptor DNA binding domain, however small changes in the mode of DNA binding and lever arm conformation may explain the differential effects on gene regulation by the mineralocorticoid and glucocorticoid receptors (31). Glucocorticoids activate MR in most tissues at basal levels and glucocorticoid receptors at stress levels (32). Inactivation of cortisol and corticosterone by 11β-hydroxysteroid dehydrogenase allows aldosterone to activate MR within aldosterone target cells and limits the activation of glucocorticoid receptors. Genetic polymorphisms of the MR gene could potentially affect both cortisol- and aldosterone-mediated MR effects in the brain and kidneys, respectively (33), which may then complicate the role of MRs in CAD. In addition, Sia et al. revealed no significant differences in the genetic distribution of MR between normotensive and hypertensive patients, nor were there differences in the echocardiographic measurements (34).
A report on the Framingham study suggests that variance in aldosterone levels is primarily due to non-genetic factors (35). However, we examined the genetic polymorphism of CYP11B2, the gene responsible for aldosterone synthase, in subjects who received coronary catheterization in Taiwan, and found that the C/C allele occurred more frequently in females who had CAD, and that it was associated with higher LVM and LVEDD. In contrast, no C/C alleles were detected in the women who did not have CAD. These results suggest that a genetic variation in aldosterone production may lead to a different prognosis. Bress et al., Takai et al. and Pojoga et al. found that the CYP11B2 -344C/C genotype was over-represented among individuals with extreme elevation of aldosterone in patients with dilated cardiomyopathy or cardiovascular diseases (36, 37). The association of the CYP11B2 -344CC genotype with high serum aldosterone levels may explain the reported association between this SNP and greater LVM and decreased event-free survival among African Americans with heart failure (36, 38). In the current study, CYP11B2 C/C predisposed Taiwanese women to CAD. Regardless of the presence of CAD, CYP11B2 C/C exacerbated left ventricle function including LVM and LVEDD in the Taiwanese women; however, LVM and LVEDD were not associated with the development of CAD in these women. In multivariate analysis, CYP11B2 C/C exhibited a more significant association with and a higher risk of developing CAD than DM or hypertension. This implies that the genetic factor CYP11B2 C/C plays a more important role than some conventional risk factors and functional parameters for the development of CAD in Taiwanese women. Nevertheless, previous studies on the CYP11B2 T-344C polymorphism have shown a significant (21, 39) or lack of association with hypertension and other cardiovascular parameters (40). Moreover, Jia et al. suggested that the -344C allele may be associated with a decreased risk of idiopathic hyperaldosteronism (41). Further studies are warranted to elucidate the role of CYP11B2 C/C in the development of CAD.
One of the limitations of our study is the low sample size. Furthermore, the level of CYP11B2 gene of CAD patients versus non-CAD control to see how SNP CYP11B2 T-344C, in particular, that carrying homozygotic CC mutation, affect CYP11B2 in atherosclerosis is worth for further investigation, which will be included in our future work.
In conclusion, in the present study, we used the candidate gene approach to determine whether the genetic variants of CYP11B2 T-344C, MR C3514G and MR C4582A are important effectors in CAD patients. We found no significant differences in the genotype distributions of CYP11B2 T-344C, MR C3514G and MR C4582A SNPs between subjects with and without CAD. When stratified by gender in multivariate analysis, CYP11B2 T-344C exhibited a strong association with the development of CAD in Taiwanese women.
Acknowledgements
This study was supported by research grants from Chung Shan Medical University Hospital, (CSH-2013-C-025); no interest conflict.
Competing Interests
None declared.
References
1. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078-84
2. Greenland P, Alpert JS, Beller GA. et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103
3. Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121-8
4. Beuschlein F. Regulation of aldosterone secretion: from physiology to disease. Eur J Endocrinol. 2013;168:R85-93
5. Brasier AR, Recinos A 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257-66
6. Duprez D, De Buyzere M, Rietzschel ER, Clement DL. Aldosterone and vascular damage. Curr Hypertens Rep. 2000;2:327-34
7. Santos RA, Ferreira AJ, Simoes ESAC. Recent advances in the angiotensin- converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519-27
8. Vasan RS, Evans JC, Larson MG. et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33-41
9. Lieb W, Xanthakis V, Sullivan LM. et al. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085-92
10. Jansen PM, Danser AH, Imholz BP, van den Meiracker AH. Aldosterone-receptor antagonism in hypertension. J Hypertens. 2009;27:680-91
11. Dorrance AM. Interfering with mineralocorticoid receptor activation: the past, present, and future. F1000Prime Rep. 2014;6:61
12. Shen JZ, Young MJ. Corticosteroids, heart failure, and hypertension: a role for immune cells? Endocrinology. 2012;153:5692-700
13. Pitt B, Remme W, Zannad F. et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-21
14. Poch E, González D, Giner V. et al. Molecular basis of salt sensitivity in human hypertension. Evaluation of renin-angiotensin-aldosterone system gene polymorphisms. Hypertension. 2001;38:1204-9
15. Park SH, Shub C, Nobrega TP, Bailey KR, Seward JB. Two-dimensional echocardiographic calculation of left ventricular mass as recommended by the American Society of Echocardiography: correlation with autopsy and M-mode echocardiography. J Am Soc Echocardiogr. 1996;9:119-28
16. Barbato A, Russo P, Siani A. et al. Aldosterone synthase gene (CYP11B2) C-344T polymorphism, plasma aldosterone, renin activity and blood pressure in a multi-ethnic population. J Hypertens. 2004;22:1895-1901
17. Ludwig M, Bolkenius U, Wickert L, Bidlingmaier F. Common polymorphisms in genes encoding the human mineralocorticoid receptor and the human amiloride-sensitive sodium channel. J Steroid Biochem Mol Biol. 1998;64:227-30
18. Vasan RS, Larson MG, Leip EP. et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291-7
19. Howard BV, Rodriguez BL, Bennett PH. et al. Prevention Conference VI: Diabetes and Cardiovascular disease: Writing Group I: epidemiology. Circulation. 2002;105:e132-7
20. White PC, Hautanen A, Kupari M. Aldosterone synthase (CYP11B2) polymorphisms and cardiovascular function. J Steroid Biochem Mol Biol. 1999;69:409-12
21. White PC, Slutsker L. Haplotype analysis of CYP11B2. Endocr Res. 1995;21:437-42
22. Brand E, Chatelain N, Mulatero P. et al. Structural analysis and evaluation of the aldosterone synthase gene in hypertension. Hypertension. 1998;32:198-204
23. White PC, Rainey WE. Editorial: polymorphisms in CYP11B genes and 11-hydroxylase activity. J Clin Endocrinol Metab. 2005;90:1252-5
24. Connell JM, Fraser R, MacKenzie SM. et al. The impact of polymorphisms in the gene encoding aldosterone synthase (CYP11B2) on steroid synthesis and blood pressure regulation. Mol Cell Endocrinol. 2004;217:243-7
25. Hautanen A, Toivanen P, Manttari M. et al. Joint effects of an aldosterone synthase (CYP11B2) gene polymorphism and classic risk factors on risk of myocardial infarction. Circulation. 1999;100:2213-18
26. Ortlepp JR, Hanrath P, Mevissen V, Kiel G, Borggrefe M, Hoffmann R. Variants of the CYP11B2 gene predict response to therapy with candesartan. Eur J Pharmacol. 2002;445:151-2
27. Paillard F, Chansel D, Brand E. et al. Genotype-phenotype relationships for the renin-angiotensin- aldosterone system in a normal population. Hypertension. 1999;34:423-9
28. Liu Y, Liu HL, Han W, Yu SJ, Zhang J. Association between the CYP11B2 gene -344T>C polymorphism and coronary artery disease: a meta-analysis. Genet Mol Res. 2015;14:3121-8
29. Mishra A, Srivastava A, Mittal T, Garg N, Mittal B. Impact of renin-angiotensin-aldosterone system gene polymorphisms on left ventricular dysfunction in coronary artery disease patients. Dis Markers. 2012;32:33-41
30. Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505-11
31. Hudson WH, Youn C, Ortlund EA. Crystal structure of the mineralocorticoid receptor DNA binding domain in complex with DNA. PLoS One. 2014;9:e107000
32. Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4:965-94
33. Zennaro MC, Lombes M. Mineralocorticoid resistance. Trends Endocrinol Metab. 2004;15:264-70
34. Sia SK, Chiou HL, Chen SC, Tsai CF, Yang SF, Ueng KC. Distribution and phenotypic expression of mineralocorticoid receptor and CYP11B2 T-344C polymorphisms in a Taiwanese hypertensive population. Mol Biol Rep. 2013;40:3705-11
35. Kathiresan S, Larson MG, Benjamin EJ. et al. Clinical and genetic correlates of serum aldosterone in the community: the Framingham Heart Study. Am J Hypertens. 2005;18:657-65
36. Bress A, Han J, Patel SR. et al. Association of aldosterone synthase polymorphism (CYP11B2 -344T>C) and genetic ancestry with atrial fibrillation and serum aldosterone in African Americans with heart failure. PLoS One. 2013;8:e71268
37. Takai E, Akita H, Kanazawa K. et al. Association between aldosterone synthase (CYP11B2) gene polymorphism and left ventricular volume in patients with dilated cardiomyopathy. Heart. 2002;88:649-50
38. McNamara DM, Tam SW, Sabolinski ML. et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol. 2006;48:1277-82
39. Matsubara M, Sato T, Nishimura T. et al. CYP11B2 polymorphisms and home blood pressure in a population-based cohort in Japanese: the Ohasama study. Hypertens Res. 2004;27:1-6
40. Schunkert H, Hengstenberg C, Holmer SR. et al. Lack of association between a polymorphism of the aldosterone synthase gene and left ventricular structure. Circulation. 1999;99:2255-60
41. Jia M, Zhang H, Song X. et al. Association of CYP11B2 polymorphisms with susceptibility to primary aldosteronism: a meta-analysis. Endocr J. 2013;60:861-70
Author contact
![]() Corresponding author: Po-Hui Wang, M.D., Ph.D. Institute of Medicine, Chung Shan Medical University, Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital,110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 886-4-24738493 E-mail: wang082160com.tw.
Corresponding author: Po-Hui Wang, M.D., Ph.D. Institute of Medicine, Chung Shan Medical University, Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital,110, Section 1, Chien-Kuo North Road, Taichung, 40201, Taiwan. Tel.: 886-4-24739595 ext. 21721; Fax: 886-4-24738493 E-mail: wang082160com.tw.

 Global reach, higher impact
Global reach, higher impact