Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(5):251-259. doi:10.7150/ijms.7.251 This issue Cite
Research Paper
Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain
1. Department of Anesthesiology, Eastern Hepatobiliary Hospital, Second Military Medical University, Shanghai 200438, China
2. Department of Anesthesiology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
3. Institute of Thoracic Cardiac Surgery, Changhai Hospital, PLA, Shanghai 200433, China
Received 2010-7-19; Accepted 2010-8-2; Published 2010-8-2
Abstract
Background: Neuropathic pain is characterized by hyperalgesia, allodynia and spontaneous pain. It often occurs as a result of injury to peripheral nerves, dorsal root ganglions (DRG), spinal cord, or brain. Recent studies have suggested that Toll-like receptor 4 (TLR4) might play a role in neuropathic pain. Methodology/Principal Findings: In this study, we investigated the role of TLR4 in a rat chronic constriction injury (CCI) model and explored the feasibility of treating neuropathic pain by inhibiting TLR4. Our results demonstrated that intrathecal siRNA-mediated suppression of TLR4 attenuated CCI-induced mechanical allodynia and thermal hyperalgesia through inhibiting the activation of NF-κB p65 and production of proinflammatory cytokines (e.g., TNF-α and IL-1β). Conclusions/Significance: These findings suggest that suppression of TLR4 mediated by intrathecally administered siRNA may be a new strategy for the treatment of neuropathic pain.
Keywords: Toll-like receptor 4, neuropathic pain, NF-κB, RNA interference, IL-1β, TNF-α.
Introduction
Neuropathic pain is characterized by hyperalgesia, allodynia and spontaneous pain. It often occurs as a result of injury to peripheral nerves, dorsal root ganglions (DRG), spinal cord, or brain. 7% to 8% of the population suffer from neuropathic pain, and 5% may be severely affected (1-2). Neuropathic pain remains a prevalent and persistent clinical challenge due to unknown pathogenesis. A variety of mechanisms have been proposed for the induction and/or maintenance of neuropathic pain. Recently, investigations have focused on the role of central nervous system (CNS) immune responses after nerve injuries that lead to behavioral hypersensitivity (3-5). A current theory for the etiology of neuropathic pain involves CNS immune activation with cytokine production inducing the expression of final common pain mediators such as TNF-α and IL-1β (6-8).
The Toll-like receptor 4 (TLR4) has recently been implicated in chronic neuropathic pain (9-10). TLR4 is a transmembrane receptor protein containing extracellular domains with leucine-rich repeat and a cytoplasmic signaling domain. The role of TLR4 in innate immune response has been well elucidated. The binding of exogenous (e.g. Lipopolysaccharides, LPS) or endogenous (e.g. members of heat shock protein family and proteoglycans) ligands to TLR4 activates NF-κB and then releases proinflammatory cytokines such as TNF-α, IL-1β and IL-6 (11-13). Previous studies have demonstrated that TLR4 is expressed in microglia of CNS (14-16). Since microglial activation is essential for the release of proinflammatory cytokines (17), it is plausible that TLR4 might be a common mediator through which different pain-inducing signals are linked to the production of proinflammatory factors. Consistent with this notion, N-methyl-D-aspartate (NMDA) receptor-modulated innate immune responses were dependent on TLR4 (18), and mice with TLR4 deficiency demonstrated decreased cytokine production and attenuated neuropathic pain responses upon nerve injury (19).
In this study, we suppressed TLR4 expression using siRNA in a rat CCI model. Knockdown of TLR4 in spinal cord inhibited pain response, and blocked NF-κB activation and production of proinflammatory cytokines (e.g. IL-1β and TNF-α).
Materials and methods
Ethics Statement
All animal experiments were approved by the Administrative Committee of Experimental Animal Care and Use of Second Military Medical University (SYXK(Hu)2007-0003), and conformed to the National Institute of Health guidelines on the ethical use of animals.
Screening siRNA sequence with reporter vector
A scrambled sequence was designed as a mismatch control (MM-siRNA) (5'-GGCGUGUCUCUCUUACGAC-3”). SiRNAs targeting the cDNA sequence of rat TLR4 (GenBank accession NM_019178) were: 5'-CUACCAACAGAGAGGAUAU-3” (siRNA1), 5'-GUCUCAGAUAUCUAGAUCU-3' (siRNA2), 5'-GAGCCGGAAAGUUAUUGUG-3' (siRNA3).
All siRNAs were chemically synthesized by United Gene Company (Shanghai, China). The primers amplifying the full length cDNA of rat TLR4 were 5'-CGGGAGCTCTGAATGCTCTCTTGCATCTGGCTGGC-3' (forward) and 5'-CGGGTCGACGCGATACAATTCGACCTGCTG-3' (reverse).
To construct a green fluorescent protein (GFP) tagged TLR4 expressing vector, total RNA was extracted from rat lung tissues using Tri-Reagent (TaKaRa, Japan). RT-PCR was used to obtain the full length TLR4 fragment. After pEGFPC1 vector was linearized by SacⅠ and SalⅠ, the fragment of TLR4 was inserted to construct the reporter vector, pEGFPC1-TLR4. The reporter vector was verified by RT-PCR using primers 5'-CGGGAGCTCTGAATGCTCTCTTGCATCTGGCTGGC-3' and 5'-CGGGTCGACGCGATACAATTCGACCTGCTG-3'.
To construct a green fluorescent protein (GFP) tagged TLR4 expressing vector, total RNA was extracted from rat lung tissues using Tri-Reagent (TaKaRa, Japan). RT-PCR was used to obtain the full length TLR4 fragment. After pEGFPC1 vector was linearized by SacⅠ and SalⅠ, the fragment of TLR4 was inserted to construct the reporter vector, pEGFPC1-TLR4. The reporter vector was verified by RT-PCR using primers 5'-CGGGAGCTCTGAATGCTCTCTTGCATCTGGCTGGC-3' and 5'-CGGGTCGACGCGATACAATTCGACCTGCTG-3'.
To identify the knockdown efficacy of different siRNA oligonucleotides, HEK-293 cells were cotransfected with pEGFPC1-TLR4 and siRNA (iRNA1-3, respectively) with lipofectamine2000 (Invitrogen, USA). EGFP expression was observed under an inverted fluorescence microscope and the fluorescence intensity was quantified by flow cytometry.
To identify the knockdown efficacy of different siRNA oligonucleotides, HEK-293 cells were cotransfected with pEGFPC1-TLR4 and siRNA (iRNA1-3, respectively) using lipofectamine2000 (Invitrogen, USA). An oligonucleotide sequence with no homology to the sequence of TLR4 was used as a mismatch controls. After 48 h, cells were visualized under an inverted fluorescence microscope and the inhibitory effects of different siRNA oligonucleotides were determined by measuring EGFP expression using flow cytometry.
Animals and chronic constriction injury
Male Sprague-Dawley rats (200-250g) were purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences. The chronic constriction injury (CCI) model was established as previously described (20). Briefly, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). The common sciatic nerve was exposed at the mid-thigh level. The nerve was ligated loosely with 4-0 chromic gut thread at 4 sites with an interval of 1 mm, so that the nerve diameter was only slightly reduced. Meanwhile, a sham surgery was performed with the sciatic nerve exposed but not ligated. Upon recovery from anesthesia, animals were housed individually in clear plastic cages with the floor covered by 3-6 cm of sawdust.
Lumbar subarachnoid catheterization
One week prior to CCI, a chronic indwelling catheter was implanted into the subarachnoid space of each rat. Briefly, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). A PE-10 catheter (Becton Dickinson, Sparks, MD, USA) was inserted into the lumbar subarachnoid space between 5th and 6th lumbar vertebrae (L5) and L6 (21). The catheter was chronically implanted and the external portion of the catheter was protected according to Milligan's method (22).
Intrathecal delivery of siRNA
Rats were randomly divided into four groups with 10 rats in each group: a sham group (Sham surgery + Normal saline, NS), a CCI group (CCI + NS), a MM group (CCI + MM siRNA), and a siRNA group (CCI + TLR4-siRNA). 10 μg SiRNA dissolved in 30 μl i-Fect transfection reagent (Neuromics, Edina, MN, USA) was administered intrathecally once daily for 7 days, starting from 1 day before CCI surgery.
Evaluation of tactile allodynia and thermal hyperalgesia
The paw withdrawal latency (PWL) to radiant heat and paw withdrawal threshold (PWT) were used to evaluate thermal hyperalgesia and mechanical allodynia respectively as previously described (23-24). To measure PWL, rats were placed in an inverted clear plexiglass cage (23×18×13 cm) on a piece of 3-mm-thick glass plate and allowed to acclimate to their surroundings for 30 minutes before testing. After acclimation, the radiant heat source was positioned under the glass floor directly beneath the hind paw. The radiant heat source consisted of a high-intensity projection lamp bulb (8V, 50W), located 40 mm below the glass floor and projecting through a 5×10-mm aperture in the top of a movable case. A digital timer automatically recorded the duration between the start of stimuli and the paw withdrawal (PWL). Three trials were carried out in each rat with a 5-minute interval. The cut-off was set at 20 seconds to avoid tissue damage.
Mechanical allodynia was assessed with von Frey filaments. Rats were placed on a wire mesh platform, covered with a transparent plastic dome, and allowed to acclimate for 30 minutes before testing. The filament was applied perpendicularly to the plantar surface of the hind paw (ipsilateral to the side of CCI). The paw withdrawal threshold (PWT) was determined by sequentially increasing and decreasing the stimulus intensity (the 'up-and-down' method) (in gram, g), and data were analyzed using the nonparametric method of Dixon (24). Tests were performed 1 day before CCI surgery, and 1, 3, 7, 10 and 14 days after CCI surgery.
Enzyme linked immunosorbent assay (ELISA)
Dorsal spinal cord tissue and cerebrospinal fluid (CSF) samples were prepared as previously described (25). IL-1β and TNF-α in spinal cord tissues and CSF were detected by ELISA (Peprotech, UK).
Spinal cord RNA extraction and real time PCR
Total RNA was extracted from L4-L5 spinal cord tissues. Extracted RNA was pretreated with DNaseⅠ at 37℃ for 30 minutes before reverse transcription reaction was performed using a high capacity cDNA archived kit (TaKaRa, Japan). A Real-Time PCR Detection System (Roche, Switzerland) was used to continuously monitor the intensity of fluorescence, which was directly proportional to the PCR products.
Western blotting
Nuclear extracts were prepared from lumbar spinal cord (L4-L5) tissues as previously described (26). Proteins were separated on an 8% polyacrylamide SDS-PAGE gel and transferred onto a nitrocellulose membrane. The nitrocellulose membrane was blotted with a primary antibody recognizing the p65 subunit of NF-κB (1:100, Santa Cruz, CA, USA), followed by a secondary antibody conjugated with horseradish peroxidase. Protein signals were detected with an ECL system (Amersham Pharmacia Biotech, Uppsala, Sweden). Histone3 (Sigma, St. Louis, MO, USA, 1:500) was used as an internal control.
Statistical analyses
Data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using Student's t-test or multiple ANOVA followed by least-significance difference post-hoc comparison. P<0.05 was considered statistically significant.
Results
Identification of pGEFPC1-TLR4
The TLR4 fragment of 2376 bp was successfully obtained by PT-PCR. Restriction analysis and sequencing results demonstrated that the recombinant pEGFP-TLR4 indeed contained the TLR4 fragment.
Silencing of TLR4 transgene with siRNA in HEK-293 cells
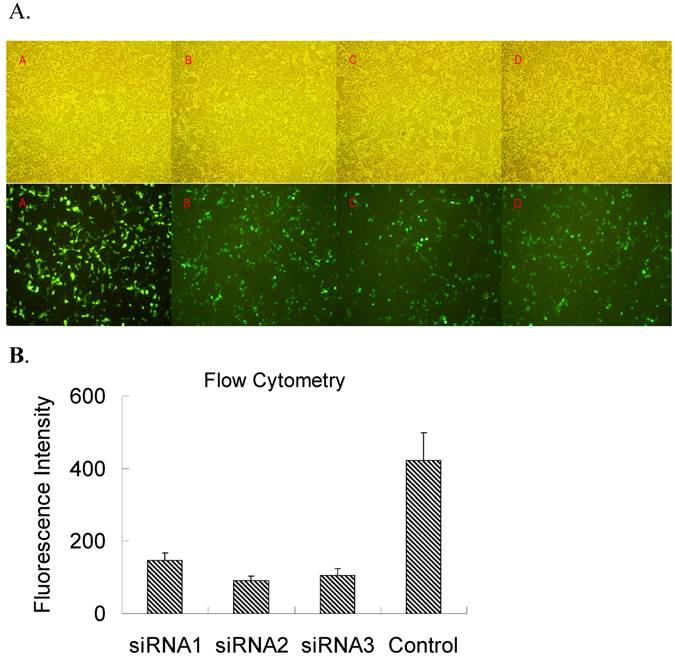
In order to select a siRNA oligonucleotide for an efficient knockdown of TLR4, three siRNA oligonucleotides targeting the rat TLR4 were used to cotransfect pEGFPC1-TLR4 in HEK-293 cells in vitro and then the level of TLR4 transgene expression was evaluated by GFP fluorescence (Figure 1A). Meanwhile, the relative fluorescence intensity was also detected by flow cytometry (Figure 1B). Flow cytometry and fluoresce observation revealed that all 3 siRNAs demonstrated effective inhibition on GFP fluorescence, and TLR4-siRNA2 was the most potent. Therefore, TLR4-siRNA2 was used for further in vivo study.
Screening siRNA for an efficient suppression of TLR4 expression in vitro. HEK-293 cells were co-transfected with both pEGFRC1-TLR4 and either one of three independent siRNA oligonucleotides targeting TLR4 (TLR4-siRNA1-3) or a control siRNA (MM-siRNA). Two days after transfection, EGFP fluorescence was observed under microscope (A) or quantified by flow cytometry (B). (A) EGFP fluorescence under an inverted fluorescence microscope (×100) or cell density under an optical microscope (×100). A, control; B, siRNA1; C, siRNA2; D, siRNA3. (B) The quantification of TLR4-EGFP fluorescence intensity upon siRNA knockdown was evaluated by flow cytometry analysis. Immunofluorescence and flow cytometry results revealed that all 3 siRNAs had efficient inhibition on GFP fluorescence, and TLR4-siRNA2 was the most potent.
Effects of TLR4-siRNA on TLR4 and its downstream signaling in CCI rats
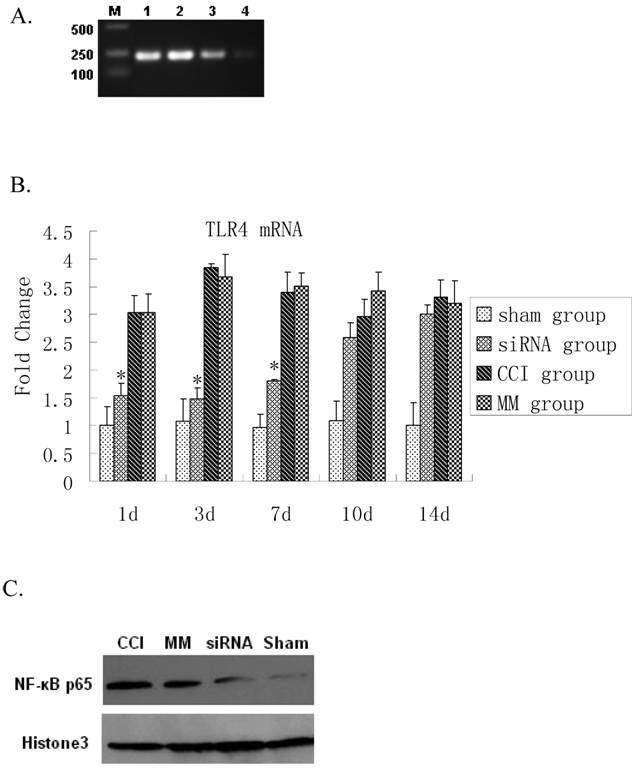
Real time RT-PCR showed a significant up-regulation of TLR4 mRNA expression 1 day after CCI compared to the sham group (P=0.0000). The siRNA-TLR4 decreased TLR4 mRNA expression and continued for 7 days (P=0.0003). However, there was no significant difference in TLR4 mRNA expression between 10-14 days after CCI (Figure 2A, 2B). The TLR4 protein expression in spinal cord tissues was detected by Western blotting. No statistical difference was found between CCI group and MM siRNA group for nuclear TLR4 protein expression (P=0.6062). Interestingly, activation of NF-κB p65 was also blocked by the TLR4-siRNA treatment (P=0.0070) (Figure 2C). Thus, the TLR4 mRNA and protein in spinal cord tissues were decreased by siRNA-TLR4, and inhibitory effects of siRNA on TLR4 expression were confirmed at the mRNA and protein levels.
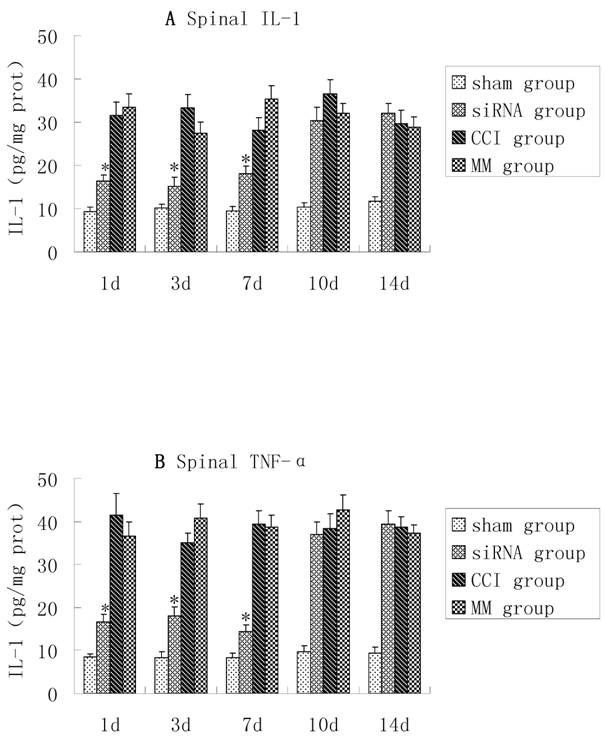
TNF-α and IL-1β were up-regulated in the dorsal spinal cord tissues of CCI rats, and there were no significant differences in TNF-α and IL-1β between the CCI group and MM group (P>0.05). However, compared with the MM group, the production of TNF-α and IL-1β in spinal cord tissues was significantly lower in the CCI group during the course of TLR4-siRNA treatment, indicating that intrathecal administration of TLR4-siRNA significantly attenuated TLR4 induction in the CCI rats (Figure 3).
Inhibition of TLR4 signaling upon TLR4-siRNA in CCI rats. Either saline or 10μg of selected siRNA was administered intrathecally once daily for 7 days as described in Materials and Methods. Tissue biopsy was performed from lumbar L4-L5 spinal cord tissues at indicated time points as described. A RT-PCR analyses of TLR4 mRNA expression in rat lumbar spinal cord tissues in four groups one day after CCI. Maker, DL200; Lane 1, CCI group; Lane 2, MM group; Lane 3, siRNA-TLR4 group; Lane 4, sham group. B Real-time quantitative RT-PCR analyses of TLR4 mRNA expression in rat lumbar spinal cord tissues in four groups (* P<0.05 VS MM group), sham group (Sham surgery + NS), CCI group (CCI + NS), MM group (CCI + MM siRNA), siRNA group (CCI + TLR4-siRNA). C Western blotting showed the levels of NF-κB P65 protein in spinal cord of rat tissues in four groups. Interestingly, the expression of κB p65 protein in the TLR4-siRNA treatment group was significantly lower than the MM group (P=0.0070).
CCI-induced pro-inflammatory cytokines in lumbosacral spinal cord tissues were inhibited upon TLR4-siRNA administration. Student t-test was performed and IL-1 (A) and TNF-α (B) production in the spinal cord tissues in the siRNA group was significantly lower compared with the MM group (* P<0.05 VS MM group). Sham group (Sham surgery + NS), CCI group (CCI + NS), MM group (CCI + MM siRNA), siRNA group (CCI + TLR4-siRNA).
Suppression of TLR4 attenuates neuropathic pain in CCI rats
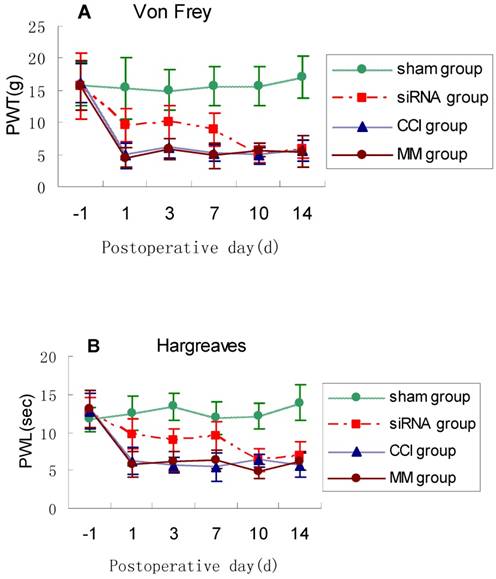
To examine the impact of TLR4-siRNA treatment on pain response in vivo, modulation of pain perception in the Bennett model of neuropathic pain was investigated. PWT and PWL were used to measure mechanical allodynia and thermal hyperalgesia, respectively. The PWL and PWT were significantly shorter in the CCI rats compared with sham controls. Mechanical allodynia and thermal hyperalgesia induced by CCI was attenuated by intrathecal administration with TLR4-siRNA (p<0.05, Fig. 4), but not mismatched siRNA.
TLR4-siRNA treatment relieved neuropathic pain. Rats were administered with TLR4-siRNA one day before CCI, and then pain response was monitored during and after siRNA treatment. Student t-test was performed and significant differences were observed on 1, 3, 7d between the siRNA group and MM group (* P<0.05 VS MM group). Mechanical allodynia (A) and thermal hyperalgesia (B) was relieved upon TLR4-siRNA treatment in CCI rats. Sham group (Sham surgery + NS), CCI group (CCI + NS), MM group (CCI + MM siRNA), siRNA group (CCI + TLR4-siRNA).
Discussion
Increasing evidences indicate that TLR4 is implicated in neuropathic pain. In this study, siRNA of TLR4 were used to investigate whether blockage of TLR4 mRNA could be used for pain treatment and whether the downstream factors such as NF-κB or proinflammatory factors could be decreased after knockdown of TLR4. Our results demonstrated that siRNA-mediated suppression of TLR4 attenuated CCI-induced mechanical allodynia and thermal hyperalgesia. The NF-κB expression and the production of TNF-α and IL-1β were inhibited after siRNA-TLR4 injection.
TLR4 antagonists and antisense oligonucleotides reduce neuropathic pain in animal models (19, 27). However, systemic administration of a TLR4 inhibitor may result in non-specific toxicity as well as systemic side effects. For example, TLR4 knockout mice are prone to infection (28). Local administration of TLR4 antisense oligonucleotide has demonstrated some hypotheses. siRNA is a more promising and advantageous strategy. Several studies have indicated that RNAi is more potent than antisense oligonucleotides even in cases where site selection was optimized for antisense effectiveness (29). One of the potential advantages of RNAi technologies is the ability to design precisely targeted therapeutics for almost any gene, regardless of the function of the gene product, whether that function is clearly defined, and in the absence of protein structure information (30). SiRNA targeting P2X3, δ-opioid receptor and NMDA receptor has been explored as potential means to manage pain (31-33). RNAi primarily acts within the cytoplasmic compartment, which is easier to access using nonviral methods than the nucleus, but ensuring efficient uptake and long-term stability in vivo is still likely to be difficult (34-35). In this study, the anti-nociception effect disappeared after discontinuation of siRNA-TLR4, suggesting that vectors or viral methods as lentivirus, adenovirus are needed to achieve long-term stable expression of siRNA.
The activation of TLR4 triggers two major downstream signaling cascades, the NF-kB and mitogen-activated protein kinase (MAPK) transduction cascades (36). The NF-kB cascade leads to release pro-inflammatory cytokines (IL-6, IL-1β, TNF-α). The MAPK kinases activate extracellular signal-regulated kinase (ERK), p38 MAPK, and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) (37). MAPKs are important for intracellular signal transduction and play critical roles in regulating neural plasticity and inflammatory responses (38-39). Accumulating evidence shows that all three MAPK pathways contribute to pain sensitization after inflammatory and nerve injury via distinct molecular and cellular mechanisms (40-42). The present study showed that NF-kB and pro-inflammatory cytokines (IL-1β, TNF-α) could be down-regulated by siRNA-TLR4 in the CCI rats. Whether MAPK kinases are down-regulated by siRNA of TLR4 in CCI rats needs to be further studied.
Meanwhile, we also found that the CCI-induced mechanical allodynia and thermal hyperalgesia and the production of TNF-α and IL-1β were simultaneously inhibited after siRNA-TLR4 injection. Sun T et al (43) also observed that the changes of mechanical and thermal pain thresholds and spinal TNF-α and IL-1β mRNA expression were isochronous, and effective suppression on CCI-induced up-regulation of TNF-β mRNA and IL-1α mRNA expression might be conducive to reduce mechanical allodynia and thermal hyperalgesia in neuropathic pain rats. These findings were coincident with the study of Jancálek R and so on (44-46). Thus, we believed that TNF-α and IL-1β might mediate the CCI-induced allodynia. But the critical experiment of inhibiting NF-kB or TNF-α or IL-1β during the CCI to demonstrate there has the same effect as TLR4 inhibition on CCI-induced allodynia should be verified in future.
In summary, results from our study confirmed the role of TLR4 pathway in CCI induced neuropathic pain. In addition, we have also demonstrated that suppression of TLR4 with intrathecal siRNA delivery could alleviate pain responses in a rat CCI model, suggesting that siRNA targeting TLR4 could be of practical value in clinical situation.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380-7
2. Torrance N, Smith B.H, Bennett M.I, Lee A.J. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006:7281-9
3. Guo L.H, Schluesener H.J. The innate immunity of the central nervous system in chronic pain: the role of Toll-like receptors. Cell. Mol. Life. Sci. 2007;64:1128-36
4. Zeilhofer H.U. Loss of glycinergic and GABAergic inhibition in chronic pain--contributions of inflammation and microglia. Int. Immunopharmacol. 2008;8:182-7
5. Marchand F, Perretti M, McMahon S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005;6:521-32
6. Sommer C, Galbraith J.A, Heckman H.M, Myers R.R. Pathology of experimental compression neuropathy producing hyperesthesia. J. Neuropathol. Exp. Neurol. 1993;52:223-33
7. Sorkin L.S, Doom C.M. Epineural application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J. Peripheral. Nervous. Syst. 2000;5:96-100
8. Guneli E, Kazikdas K.C, Kolatan E. Ghrelin may attenuate proinflammatory cytokine-mediated neuropathic pain. Med. Hypotheses. 2007;69:356-60
9. Tanga F.Y, Raghavendra V, DeLeo J.A. Quantitative real-time RT- PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochemistry International. 2004;45:397-407
10. Cao L, Tanga F.Y, Deleo J.A. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896-903
11. Vabulas R.M, Ahmad-Nejad P, Ghose S, Kirschning C.J, Issels R.D, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin V 1 receptor signal pathway. J. Biol. Chem. 2002;277:15107-12
12. Tsan M.F, Gao B. Cytokine function of heat shock proteins. Am. J. Physiol. 2004;286:C739-C744
13. Kitaoka Y, Munemasa Y, Nakazawa T, Ueno S. NMDA-induced interleukin-1beta expression is mediated by nuclear factor-kappa B p65 in the retina. Brain. Res. 2007;1142:247-55
14. Eklind S, Mallard C, Leverin A.L, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H. Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur. J. Neurosci. 2001;13:1101-6
15. Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett P.L, Jensen F.E, .PRosenberg A, Volpe J.J, Vartanian T. The Toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002;22:2478-86
16. Blanco A.M, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front. Biosci. 2007;12:2616-30
17. Detloff M.R, Fisher L.C, McGaughy V, Longbrake E.E, Popovich P.G, Basso D.M. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337-47
18. Glezer I, Zekki H, Scavone C, Rivest S. Modulation of the innate immune response by NMDA receptors has neuropathological consequences. J. Neurosci. 2003;23:11094-103
19. Tanga F.Y, Nutile-McMenemy N, DeLeo J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad.Sci. 2005;19102:5856-61
20. Bennett G.J, Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87-107
21. Storkson R.V, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J. Neurosci Methods. 1996;65:167-72
22. Milligan E.D, Hinde J.L, Mehmert K.K, Maier S.F, Watkins L.R. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J. Neurosci Methods. 1999;90:81-6
23. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77-9
24. Chaplan S.R, Bach F.W, Pogrel J.W, Chung J.M, Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55-63
25. Milligan E.D, O'Connor K.A, Nguyen K.T, Armstrong C.B, Twining C, Gaykema R.P, Holguin A, Martin D, Maier S.F, Watkins L.R. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21:2808-19
26. An G, Lin T.N, Liu J.S, Xue J.J, He Y.Y, Hsu C.Y. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann. Neurol. 1993;33:457-64
27. Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56:1312-9
28. Jordan J.M, Woods M.E, Olano J, Walker D.H. The absence of Toll-like receptor 4 signaling in C3H/HeJ mice predisposes them to overwhelming rickettsial infection and decreased protective Th1 responses. Infect Immun. 2008;76:3717-24
29. Achenbach T, Brunner B, Heermeier K. Oligonucleotide-based knockdown technologies: antisense versus RNA interference. Chembiochem. 2003;4:928-35
30. Uprichard SL. The therapeutic potential of RNA interference. FEBS Letters. 2005;579:5996-6007
31. Dorn G, Patel S, Wotherspoon G, Hemmings-Mieszczak M, Barclay J, Natt F.J, Martin P, Bevan S, Fox A, Ganju P, Wishart W, Hall J. siRNA relieves chronic neuropathic pain. Nucleic. Acids. Res. 2004;32:e49
32. Tan P.H, Yang L.C, Shih H.C, Lan K.C, Cheng J.T. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene. Ther. 2005;12:59-66
33. Luo M.C, Zhang D.Q, Ma S.W, Huang Y.Y, Shuster S.J, Porreca F, Lai J. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol. Pain. 2005;1:29-36
34. Knight SW, Bass BL. A role for the RNase III enzyme DCR - 1 in RNA interference and germ line development in Caenorhabditis elegans. Science. 2001;293:2269- 71
35. Elbashir S, Harborth J, Weber K. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199-213
36. Denkers E.Y, Butcher B.A, Del Rio L, Kim L. Manipulation of mitogen-activated protein kinase/nuclear factor-kappaB-signaling cascades during intracellular Toxoplasma gondii infection. Immunol Rev. 2004;201:191-205
37. Maulik D, Ashraf Q.M, Mishra O.P, Delivoria-Papadopoulos M. Activation of p38 mitogen-activated protein kinase (p38 MAPK), extracellular signal-regulated kinase (ERK) and c-jun N-terminal kinase (JNK) during hypoxia in cerebral cortical nuclei of guinea pig fetus at term: role of nitric oxide. Neurosci Lett. 2008;439:94-9
38. Ji R.R, Gereau R.W, Malcangio M, Strichartz G.R. MAP kinase and pain. Brain Res. Rev. 2009;60:135-48
39. Gao Y.J, Ji R.R. Activation of JNK pathway in persistent pain. Neurosci. Lett. 2008;437:180-3
40. Crown E.D, Gwak Y.S, Ye Z, Johnson K.M, Hulsebosch C.E. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008;213:257-67
41. Gu Y.W, Su D.S, Tian J, Wang X.R. Attenuating phosphorylation of p38 MAPK in the activated microglia: a new mechanism for intrathecal lidocaine reversing tactile allodynia following chronic constriction injury in rats. Neurosci. Lett. 2008;431:129-34
42. Li M.M, Yu Y.Q, Fu H, Xie F, Xu L.X, Chen J. Extracellular signal-regulated kinases mediate melittin-induced hypersensitivity of spinal neurons to chemical and thermal but not mechanical stimuli. Brain. Res. Bull. 2008;77:227-32
43. Liu S, Yang J, Wang L, Jiang M, Qiu Q, Ma Z, Liu L, Li C, Ren C, Zhou J, Li W. Tibia Tumors -Induced Cancer Pain Involves Spinal p38 Mitogen-Activated Protein Kinase Activation via TLR4-Dependent Mechanisms. Brain Res. 2010 [Epub ahead of print]
44. Sun T, Cui CB, Luo JG, Zhang L, Fu ZJ, Song WG. Effect of electroacupuncture on the expression of spinal glial fibrillary acidic protein, tumor necrosis factor-alpha and interleukin-1beta in chronic neuropathic pain rats. Zhen Ci Yan Jiu. 2010;35(1):12-6
45. Sahbaie P, Shi X, Guo TZ, Qiao Y, Yeomans DC, Kingery WS, Clark JD. Role of substance P signaling in enhanced nociceptive sensitization and local cytokine production after incision. Pain. 2009;145(3):341-9
46. Jancálek R, Dubový P, Svízenská I, Klusáková I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J Neuroinflammation. 2010;10:7-11
Author contact
![]() Corresponding author: Wei-feng Yu, Department of Anesthesiology, Eastern Hepatobiliary Hospital, Second Military Medical University. Address: No. 225, Changhai Road, Shanghai 200438, P.R. China. E-mail: ywf808com,Tel: 86-21-65564166.
Corresponding author: Wei-feng Yu, Department of Anesthesiology, Eastern Hepatobiliary Hospital, Second Military Medical University. Address: No. 225, Changhai Road, Shanghai 200438, P.R. China. E-mail: ywf808com,Tel: 86-21-65564166.

 Global reach, higher impact
Global reach, higher impact