Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(2):82-89. doi:10.7150/ijms.7.82 This issue Cite
Research Paper
Effects of Losartan on expression of connexins at the early stage of atherosclerosis in rabbits
The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, China
Received 2010-3-5; Accepted 2010-4-30; Published 2010-5-8
Abstract
Aim: to investigate effects of Losartan on expression of connexin 40 and 43 (Cx40 and Cx43), in arteries at the early stage of atherosclerosis in a rabbit model. Methods: A total of 28 male New Zealand white rabbits were divided into following groups: control group, high fat diet group, and Losartan group (10 mg/kg/day). Losartan was administrated in food for two weeks. Iliac arteries were obtained for immunohistochemistry, transmission electron microscopy, Western blot, and reverse transcriptase-polymerase chain reaction (RT-PCR). Results: Transmission electron microscopy revealed abundant gap junctions between neointimal smooth muscle cells (SMCs), which were markedly reduced by treatment. RT-PCR and Western blot assay showed that the mRNA and protein expression of Cx40 and Cx43 were elevated in the neointimal area at the early stage of atherosclerosis. The mRNA and protein expression of Cx43 were significantly down-regulated by losartan treatment but those of Cx40 were not markedly changed. Conclusion: Cx40 and Cx43 in the neointimal SMCs were up-regulated at the early stage of atherosclerosis. Losartan (an angiotensin-converting enzyme inhibitor) could reduce neointima proliferation and down-regulate the elevated protein expression of Cx43, suggesting the rennin-angiotensin system (RAS) plays an important role in the remodeling of gap junction between ventricular myocytes under pathological conditions.
Keywords: vascular proliferation, gap junction, connexin, balloon angioplasty, statin
Introduction
Atherosclerosis is a slow and complicated pathological process. Vascular smooth muscle cell (VSMC) proliferation is one of the important features of atherosclerosis. The gap junction (GJ) and connexin (Cx) between cells play crucial roles in the VSMC disintegration and proliferation (1). At present, Cx40, Cx43 and Cx37 have been identified in the VSMCs (2). A variety of in vitro and in vivo experiments have proved that GJ may be involved in the injury or disease induced activation of vessel wall cells. It is indicated that the expression of Cx43 in the smooth muscle cells was markedly elevated at the early stage of coronary atherosclerosis (3). Recently, evidence showed the expression of Cx40 could also be profoundly increased in the early phase of artery damage.
Angiotensin II (Ang II) is an effective vasoactive peptide, and has been proved to affect the process of atherosclerosis. The regulation process mediated through G-protein-coupled receptors AT1 and AT2 (mostly through AT1 receptor) can also be blocked by specific receptor antagonist (4). Additionally, a study shows that angiotensin-converting enzyme inhibitors (ACEI) can inhibit the Ang II induced proliferation and migration of smooth muscle cells (5).
However, little is known about the relationship between the up-regulation of Cx43 in early atherosclerosis and AngII, whether up-regulation of Cx43 can be antagonized by AT1 antagonists or whether suppressed smooth muscle cell proliferation and migration by AT1 antagonists are mediated through decreasing VSMC seamless connections.
The present study aimed to detect the expression of Cx40 and Cx43 in the artery at early stage of high fat diet induced atherosclerosis and to investigate the effects of AT1 antagonist Losartan on the Cx43 and Cx40 expression in the VSMCs .
Methods
Experimental animal
New Zealand male purebred albino rabbits weighing 2.5-3.3 kg were purchased from the Animal Centre of Medical College of Zhejiang University.
Establishment of the atherosclerosis model
Twenty eight healthy rabbits were randomly divided into three groups: normal control group (Group A, n=7), high fat diet group (Group B, n=7), Losartan group (Group C, n=7). Rabbits in the Group A were fed with commercially available food; and those in the Group B and C were given food containing 1.5% cholesterol (high fat diet) for two weeks. In addition, rabbits in the Group C were treated with Losartan (10mg/kg/d) in the food for eight weeks. These animals were given ad libitum access to water.
Collection of blood vessels
At the end of the experiment, rabbits were sacrificed. Then the aortas were obtained and washed with cold normal saline. The aortas without evident yellow plaque were cut into pieces and fixed in 10% paraformaldehyde. The blood vessels without obvious yellow speckle were fragmented into 3 pieces: one fixed with 10% neutral buffered formalin, one immersed in 2.5% Glutaral solution, and the third piece stored in liquid nitrogen tank after snap frozen at -80 0C for use.
Plasma cholesterol level
Peripheral blood was collected from the ear vein under local anesthesia immediately before drug treatment/surgical procedures and before sacrificing. Plasma cholesterol levels were measured with an autoanalyzer (Type 7170, Hitachi Corp., Tokyo, Japan).
Pathology and immunochemistry
Iliac arteries were carefully removed and post-fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4) overnight at 4°C. Tissue blocks were rinsed for 1 h with PBS and dehydrated through a series of increasing concentrations of ethanol. After dehydration, these tissues were cleaned with chloroform and xylene and then embedded in paraffin. Tissues were cut into 6 μm sections. The sections were deparaffinized, and endogenous peroxidase was quenched by incubation with 0.3% hydrogen peroxide in methanol for 10 min at room temperature. The sections were either stained with hematoxylin and eosin or incubated with anti-Cx43 monoclonal antibody (Zymed Laboratories Inc, Carlsbad, CA) or anti-Cx40 monoclonal antibody (Alpha Diagnostic International Inc, San Antonio, TX) at a dilution of 1:1000 in blocking buffer (2% bovine serum albumin in PBS) at room temperature overnight. After washed with PBS, the sections were incubated with secondary antibody conjugated to horseradish peroxidase (Histofine simple-stain kit, Nichivei, Japan) for 30 min. The sections were visualized with 3, 3'-diaminobenzidine and hydrogen peroxide, and counterstained with hematoxylin.
Western blot
Protein extracts were prepared in lysis buffer and then repeatedly aspirated (20 times) through a 23-gauge needle followed by centrifugation at 4°C for 30 min at 10,000 g. The supernatant was submitted for protein quantification. Samples (20 μg of total proteins for Cx40 and 4 μg for Cx43) were added with gel loading buffer, and aliquots were loaded onto a 12.5% sodium dodecyl sulfate-polyacrylamide gel. After separation, proteins were transferred to nitrocellulose membranes which were then blocked for 30 min in PBS containing 5% nonfat milk and 0.1% Tween. The membranes were subsequently incubated overnight with either the anti-Cx43 monoclonal antibody (1:1,000, Zymed Laboratories Inc.) or anti-Cx40 monoclonal antibody (1:1,000, Alpha Diagnostic International Inc.) or with anti-actin antibody (1:500, PharMingen, San Jose, CA). After washing in PBS containing 0.1% Tween, membranes were probed for 1 h with the corresponding horseradish peroxidase- conjugated secondary antibodies (anti-rabbit: 1:1,000, anti-mouse: 1:500; Chemicon, Temecula, CA). The time of incubation in ECL detection reagents and exposure to Hyperfilm (Amersham-Pharmacia, Buckinghamshire, UK) were identical for all experimental conditions. The intensity of the bands was determined by laser scanning the films followed by quantitative densitometric analysis using Kodak Digital Science 1D 2.0 Image software.
RT-PCR
Total RNA was extracted using Trizol (Life Technologies, Gaithersburg, MD). Then, 2.5 μg of total RNA were reverse transcribed into cDNA in 50 μl of reaction mixtures using 200 U of recombinant M-MLV reverse transcriptase (Superscript II, GIBCO-BRL) and oligo dT15 as primers. The RT was carried out for 60 min at 42°C followed by an inactivation step at 94°C for 10 min. The RT products were stored at -80°C. The amount of RNA was determined by measuring the absorbance at 260 nm. Oligonucleotide primers were as follows: a) glyceraldehyde phosphated dehydrogenase (GAPDH): forward: 5'-GCGCCTGGTCACCAGGGCTGCTT-3', reverse: 5'-TGCCGAAGTGGTCGTGGATGACCT-3'; b) Cx40-1: forward: 5'-ATGCACACTGTGCGCATGCAGGA-3', reverse: 5'-CAGGTGGTAGAGTTCAGCCAG-3'; c) Cx43-1: forward: 5'-CATCTTCATGCTGGTGGTGT-3', reverse: 5'-TAGTTCGCCCAGTTTTGCTC-3'. Computer-assisted primer selection (Gene Runner, Hastings Software) was conducted to determine the primers for Cx43. The anticipated size of amplification products was 399 bp for Cx40, 283 bp for Cx43, and 465 bp for GAPDH.
Expression of Cx40 and Cx43 was determined by semiquantitative PCR using GAPDH as an internal standard. The samples were subjected to 28 (Cx40) or 30 (Cx43) cycles of amplification as follows: the samples were initially heated to 94°C for 5 min to ensure complete denaturation of DNA (45 sec for subsequent cycles) followed by 45 sec at 57°C (Cx40) or 51°C (Cx43) to anneal primers, and then 1 min at 72°C for extension of annealed primer. The PCR reaction was concluded by a 10-min elongation phase, again at 72°C. The PCR products (10 μl) were visualized on 2% agarose gel. Images were captured using a solid-state black-and-white video camera (Cohu Electronic, Irvine, CA), and the intensity of the bands was determined using Kodak Digital Science 1D 2.0 imaging software.
Statistical Analysis
Data are presented as mean ± SD. Data of multiple groups were analyzed by one-way analysis of variance. Means between two groups were compared with a two-tailed Student t test after an F test for homogeneity of variances. If the data failed to meet the requirements for the parametric t test, a Mann-Whitney U test was performed. A value of P<0 .05 was considered statistically significant.
Results
Changes in the body weight and plasma cholesterol level
The body weight and plasma cholesterol level immediately before experiment and sacrificing were shown in the Table 1. There is no significant difference in the body weight and total cholesterol level at the baseline between different groups (P>0.05); Eight weeks later, plasma cholesterol in the normal group was significantly lower than the other two groups (P<0.01) but no significant difference was observed between the Losartan group and the high fat diet group (P>0.05). The body weight at the end of experiment was did not change significantly compared with that at baseline (P>0.05). Furthermore, there was no significant difference in the body weight among the three groups.
Body weight and plasma cholesterol levels before and after treatment
| Group | n | Plasma cholesterol (mmol/L) | Body weight (kg) | ||
|---|---|---|---|---|---|
| Baseline | 8 weeks | Baseline | 8 weeks | ||
| Normal group | 7 | 1.35±0.18 | 1.37±0.12 | 2.87±0.21 | 3.01±0.24 |
| High fat diet group | 7 | 1.34±0.10 | 1.68±0.13** | 2.81±0.20 | 3.16±0.20 |
| Losartan group | 7 | 1.36±0.18 | 1.61±0.15** | 2.82±0.20 | 3.07±0.16 |
Data were expressed as mean±SD.
**P<0.01 vs Normal group; Losartan group vs high fat diet group P>0.05
Morphologic changes
The internal wall of vessels was slick in the normal group, while lumens were smaller in the high fat diet group than in the normal group. Vessel wall is in the condition of rugosity and the yellow atheromatous plaques were found in the lumens of high fat diet group. However, the vessel wall was expanded to different extents and little yellow atheromatous plaques were observed in the Losartan group.
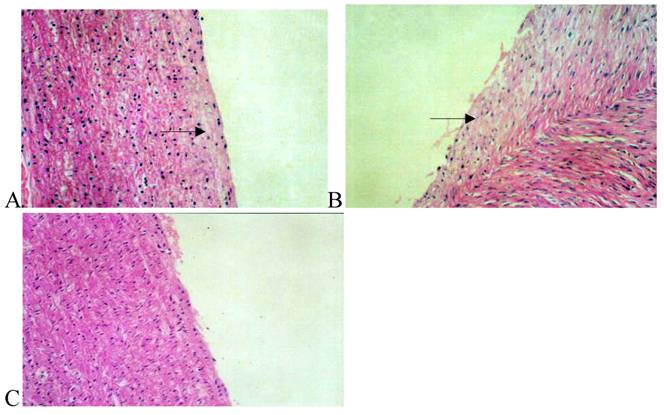
Sections were stained with HE and observed under a light microscope. The vessel wall was round and homogeneous in the thickness. The inner and outer internal elastic laminas were clear and intact in the normal group. The nucleus of endothelial cells was blue and arranged regularly, and the nucleus of smooth muscle cells in the tunica media was shuttle-shaped and no smooth muscle cells appeared to slip into the subcortex.
Intima was increased sharply accompanied by a large amount of foam cells in the high fat diet group. Smooth muscle cells in the tunica media were hyperplasia and arranged in chaos. Parts of smooth muscle cells arranged in length with the tendency of growing to the intima.
The wall of arteries was basically homogeneous in thickness in the Losartan group, only slight fresh endomembrane was seen under the endomembrane, and a small amount of macrophages and inflammatory cells were noted between the smooth muscle cells and the internal elastic lamina was intact (Fig.1).
Immunocytochemistry
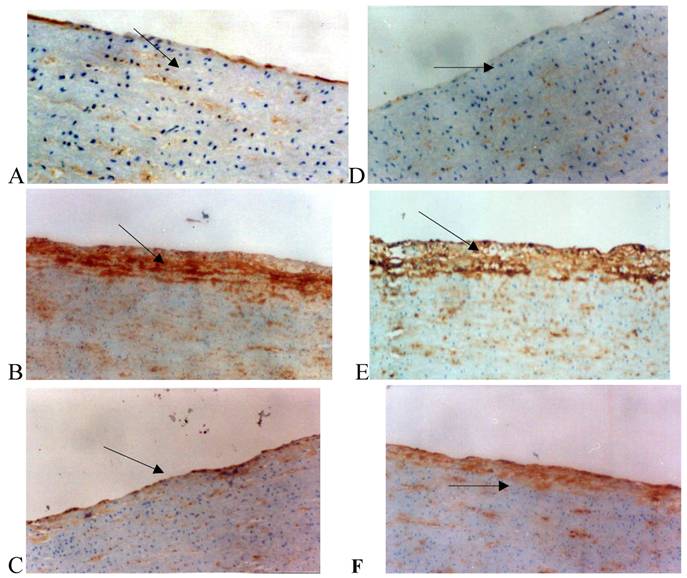
The relatively low expression of Cx43 and Cx40 in VSMCs and endothelial cells was observed in the normal group. However, the expression of Cx43 and Cx40 in the intima and tunica media of the high fat diet group was markedly increased when compared with normal group. In the Losartan group, the expression of Cx40 in the intima did not profoundly change compared with the high fat diet group (Fig. 2).
Transmission electron microscopy
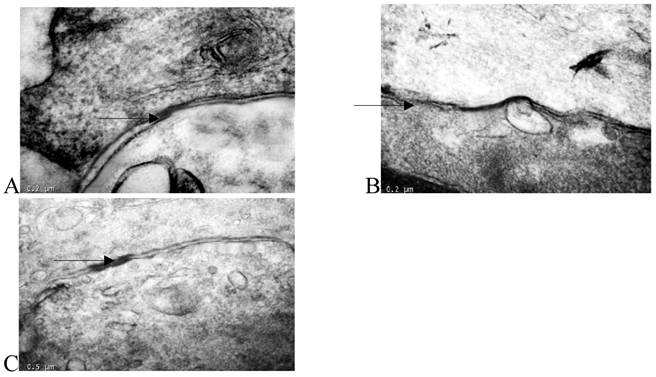
Transmission electron microscopy showed that there were many large-volume gap junction structures between proliferating smooth muscle cells, but few small-volume gap junction structures were observed between normal smooth muscle cells (Fig. 3).
Western blot assay
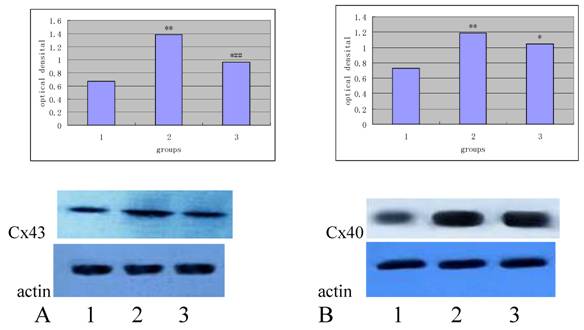
To optimize the protocol, different amounts of total proteins were tested for Western blot analysis. The amount of the protein was increased sequentially from 0.5 μg, 1.0 μg, 1.5 μg, 2.0 μg, 3.0 μg, 4.0 μg, 5.0 μg, 6.0 μg, 8.0 μg, 10.0 μg, 15.0 μg, 17.5 μg, 20.0 μg, 25.0 μg and 30.0 μg. The optimal concentration was 4 μg for Cx43 and 20 μg for Cx40, respectively. The relative optical density of the Cx43 and Cx40 of different groups was shown in Table 2.
The relative optical density of the Cx43 and Cx40 of different groups
| Normal group | High fat diet group | Losartan group | |
|---|---|---|---|
| Cx43 (n=7) | 0.63±0.10 | 1.39±0.08** | 0.96±0.09*## |
| Cx40 (n=7) | 0.71±0.13 | 1.17±0.12** | 1.07±0.14 |
**P<0.01 vs normal group, ##P<0.01 vs high fat diet group.
As shown in Table 2, the expression of Cx43 and Cx40 in the high-fat diet group is significantly increased when compared with the normal group (P<0.01), and the expression of Cx43 in the Losartan group was higher than that in the normal group (P<0.01), but significantly lower than that in the high-fat diet group (P<0.05). There was no significant difference in the expression of Cx40 between losartan group and high-fat diet group (P>0.05).
Figure 4 and 5 independently showed the protein expression of Cx43 and Cx40 in the iliac arteries of different groups.
Hematoxylin/eosin staining of the iliac arteries in the normal group (panel A), high fat diet group (panel B), and high fat diet plus losartan group (panel C). In panel A, the elastic lamina was clear and intact; endothelia and smooth muscle cells (arrow) arranged regularly. In panel B, the elastic lamina was incomplete and neointima was exposed. Numerous smooth muscle cells near the internal elastic lamina arranged irregularly and extended to the intima (arrow). After treatment with losartan as shown in panel C, less neointima was markedly exposed.
Immunohistochemistry for connexin 40 (Cx40) and connexin 43 (Cx43) in the rabbit iliac arteries. Cx40 expression was shown in panel A, B, and C and Cx43 expression in panel D, E, and F. Panel A: Cx40 staining (brown spots, arrow) between the smooth muscle cells in the tunica media of iliac arteries from the normal group. Panel B: Cx40 staining was distributed in both the tunica media and neointima with more prominent staining in the latter. Panel C: After losartan treatment, Cx40 staining was markedly reduced in the neointima of arteries. The distribution of Cx43 was similar to that of Cx40.
Thin-section electron micrographs indicated gap junctions between smooth muscle cells in the rabbit iliac arteries of the normal group (panel A), high fat diet group (panel B), and high fat diet plus losartan group (panel C). In panel B, neointimal smooth muscle cells showed abundant and large gap junction when compared with normal group and losartan treated group. Bar=0.2 μm for panels A, B, C and D, × 65,000.
Protein expression of Cx43 (panel A) and Cx40 (panel B) in the rabbit iliac arteries of different groups. Top panels independently represented the expression of Cx43 and Cx40 from different groups and bottom panels are Actin. 1) normal control (n =7); 2) high fat diet group (n =7); 3) high fat diet + losartan (n = 7). *P<0.05, **P<0.01 vs normal group, ##P<0.01 vs high fat diet group.
mRNA expression of Cx40 (panel A) and Cx43 (panel B) in the rabbit iliac arteries of different groups by RT-PCR. Top panels represented mRNA expression of Cx40 and Cx43 from each of the groups and bottom panels are Actin. 1) normal control (n =7); 2) high fat diet group (n =7); 3) high fat diet group + losartan (n =7). *P <0.05, **P<0.01 vs normal group, ##P<0.01 vs high fat diet group.
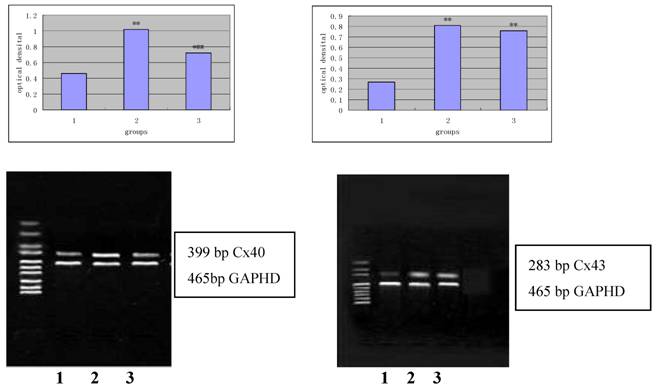
mRNA expression of Cx43 and Cx40 by RT-PCR
As shown in Figure 5, after 2% agarose gel electrophoresis, the bands were visualized and length of amplified products of Cx43, Cx40 and GAPDH was about 283 bp, 399bp and 465bp, respectively, which were within the anticipated size.
The relative optical density of the amplified products of Cx43 and Cx40 from different groups is shown in Table 3.
As shown in Table 3, the mRNA expression of Cx43 and Cx40 in the high-fat diet group was significantly increased when compared with the normal group (P <0.01). The mRNA expression of Cx43 in the Losartan group was higher than in the normal group (P<0.05), but significantly lower than in the high-fat diet group (P<0.01). No significant difference in the mRNA expression of Cx40 was observed between the Losartan group, losartan group and high-fat diet group (P>0.05).
The relative optical density of the amplified products of Cx43 and Cx40 from different groups
| Normal group | High fat diet group | Losartan group | |
|---|---|---|---|
| Cx43 (n=7) | 0.46±0.12 | 1.02±0.13** | 0.71±0.14 ## |
| Cx40 (n=7) | 0.28±0.14 | 0.82±0.12** | 0.74±0.12** |
**P<0.01 vs normal group, ##P<0.01 vs high fat diet group.
Discussion
This study shows that many large gap junction structures exist between the proliferating vascular smooth muscle cells in the early atherosclerosis of rabbits with elevated expression of Cx43 and Cx40 mRNAs and proteins. Losartan, an AT1 antagonist, may not reduce the level of blood lipids induced by high-fat diet, but it may inhibit the expression of Cx43 mRNA and protein, suppress proliferation of vascular smooth muscle cells. These results are consistent with a hypothesis that the anti-atherosclerosis role of AT1 receptor inhibitors may be independent on lipid level, but may be partly dependent on the inhibition of gap junctional communication and thus the proliferation of vascular smooth muscle cells.
Intima of artery has mutual communication between cells and between cells and their environment. These cells in the vessel walls are involved in the development of atherosclerosis (6). In the normal vessel walls, GJ plays a very important role in regulating seamless connection (7).
The transition and hyperplasia of VSMCs (from contraction transformation to the secretary) is a key step in the development of atherosclerosis (4). During this process, VSMC originally came from vascular media must be released from the riveting of gap junctional connection. Cx43 was up-regulated when the smooth muscle cells changed from contraction type to the conversion type in vitro (8).
Cx43 is required for the formation of the atherosclerotic plaque. Previous study demonstrated that rats lack of Cx43 expression showed 50% lower rate of attack with atherosclerotic plaque compared with normal rats (9). Our study demonstrates an association of the formation of atherosclerosis and the expression and function of gap junction. White blood cells (WBC) can be induced by chemokines through gap junction formed between WBC and endothelium and resulted in the formation of atherosclerosis (9). Javid et al. also found that in the early stage of atherosclerosis, the number of Cx43 gap junction plaques increased and the diameter of gap junction became smaller in during endometrial thickening. During the progression of the disease, the diameter of gap junction plaques increased and the number of gap junction plaques decreased (10). Ang II, a strong vasoconstrictor substance, stimulates mitosis that results in the proliferation of VSMCs and fibroblasts and collagen deposition. Ang II is also involved in the initiation and development of atherosclerosis (11). One study found that the activity of ACE, the number of Ang II, and angiotensin receptor 1 were significantly increased in coronary atherosclerosis plaques (12). Ang II targets to vascular endothelial cells, monocytes, macrophages, and smooth muscle cells, promotes angiogenesis and lipid metabolism, and participates in the pathological process (11). Another study showed that Ang II up-regulated the expression of Cx43 in WB rat liver cells, which resulted in changes of cell metabolism conditions and second messengers (13). Cx43 expression can be induced by treating neonatal rat ventricular muscle cells with Ang II for 24 hours; the reaction can be inhibited by AT1 receptor antagonist Losartan, suggesting that Ang II functions through the AT1 (14).
The study also confirmed that Cx40, another important protein formed gap junction, is expressed in the abdominal aortic smooth muscle cells in rabbits and up-regulated in the early proliferation of atherosclerosis. The study showed that Cx43 and Cx40 protein can not form a heterogeneous gap junction. It is also not known about the role they play in cell growth and differentiation (15).
This study showed that Cx40 mRNA expression can not be changed by Losartan. Polontchouk et al. observed that, after the neonatal rat heart cells were treated with Ang II after 24 h, expression of Cx43, but not Cx40, and GJ communication increased (16). These results suggest that the expression of Cx40 mRNA induced by high-fat diet is independent of the role of Ang II.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Chadjichristos CE, Derouette JP, Kwak BR. Connexins in atherosclerosis. Adv Cardiol. 2006;42:255-67
2. Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22(2):225-30
3. Cai W, Ruan LM, Wang YN, Chen JZ. Effects of angiotensin II on connexin 43 of VSMCs in arteriosclerosis. J Zhejiang Univ Sci B. 2006;7(8):648-53
4. Balakumar P, Kaur T, Singh M. Potential target sites to modulate vascular endothelial dysfunction: current perspectives and future directions. Toxicology. 2008;245(1-2):49-64
5. Li J, Hirose N, Kawamura M, Arai Y. Antiatherogenic effect of angiotensin converting enzyme inhibitor and angiotensin II receptor antagonist in the cholesterol-fed rabbits. Atherosclerosis. 1999;143:315-26
6. Gouni-Berthold I, Sachinidis A. Possible non-classic intracellular and molecular mechanisms of LDL cholesterol action contributing to the development and progression of atherosclerosis. Curr Vasc Pharmacol. 2004;2(4):363-70
7. Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79(4):631-46
8. Yeh HI, Lupu F, Dupont E, Severs NJ. Upregulation of connexin43 gap junctions between smooth muscle cells after balloon catheter injury in the rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17(11):3174-84
9. Rama A, Matsushita T, Charolidi N, Rothery S, Dupont E, Severs NJ. Up-regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF-beta-treated human aortic smooth muscle cells. Eur J Cell Biol. 2006;85(5):375-86
10. Javid PJ, Watts SW, Webb RC. Inhibition of nitric oxide-induced vasodilation by gap junction inhibitors: a potential role for a cGMP-independent nitric oxide pathway. J Vasc Res. 1996;33(5):395-404
11. Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond). 2007;112(8):417-28
12. Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD. et al. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4(1):67-78
13. Bokkala S, Reis HM, Rubin E, Joseph SK. Effect of angiotensin II and ethanol on the expression of connexin 43 in WB rat liver epithelial cells. Biochem J. 2001;357:769-77
14. Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE. Effects of angiotensin II on expression of gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Mol Cell Cardiol. 1998;32:800-7
15. Polontchouk L, Ebelt B, Jackels M, Dhein S. Chronic effects of endothelin-1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB J. 2002;16:87-9
16. White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995;6(4):459-70
Author contact
![]() Corresponding author: Wei Cai, The First Affiliated Hospital, College of Medicine, Zhejiang University, #79 Qingchun Road, Hangzhou, Zhejiang 310003, China. Tel: +8613606508046; Fax: +8657185286912; Email: doc1998net
Corresponding author: Wei Cai, The First Affiliated Hospital, College of Medicine, Zhejiang University, #79 Qingchun Road, Hangzhou, Zhejiang 310003, China. Tel: +8613606508046; Fax: +8657185286912; Email: doc1998net

 Global reach, higher impact
Global reach, higher impact