3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(11):1492-1507. doi:10.7150/ijms.87472 This issue Cite
Review
Human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications: applications and research advances
1. Department of Endocrinology, the Second Hospital of Jilin University, Changchun 130041, Jilin, P.R. China.
2. Department of Nephrology, the Second Hospital of Jilin University, Changchun 130041, Jilin, P.R. China.
3. Department of Comparative and Experimental Medicine, Nagoya City University Graduate 24 School of Medical Sciences, Aichi 467-8601, Nagoya, Japan.
4. Institute of Basic Medical Sciences and Laboratory Animal Resource Center, University of Tsukuba, Ibaraki 305-8575, Tsukuba, Japan.
Received 2023-6-25; Accepted 2023-8-22; Published 2023-9-11
Abstract
Diabetes mellitus and its complications pose a major threat to global health and affect the quality of life and life expectancy of patients. Currently, the application of traditional therapeutic drugs for diabetes mellitus has great limitations and can only temporarily control blood glucose but not fundamentally cure it. Mesenchymal stem cells, as pluripotent stromal cells, have multidirectional differentiation potential, high self-renewal, immune regulation, and low immunogenicity, which provide a new idea and possible development direction for diabetes mellitus treatment. Regenerative medicine with mesenchymal stem cells treatment as the core treatment will become another treatment option for diabetes mellitus after traditional treatment. Recently, human umbilical cord mesenchymal stem cells have been widely used in basic and clinical research on diabetes mellitus and its complications because of their abundance, low ethical controversy, low risk of infection, and high proliferation and differentiation ability. This paper reviews the therapeutic role and mechanism of human umbilical cord mesenchymal stem cells in diabetes mellitus and its complications and highlights the challenges faced by the clinical application of human umbilical cord mesenchymal stem cells to provide a more theoretical basis for the application of human umbilical cord mesenchymal stem cells in diabetes mellitus patients.
Keywords: umbilical cord, mesenchymal stem cells, diabetes mellitus, pluripotent, clinical application, regenerative medicine
Introduction
Diabetes mellitus (DM) is the most prevalent metabolic disorder caused by the inability of the pancreas to secrete insulin adequately or the body's inability to use insulin effectively. According to the 9th edition of the International Diabetes Federation (IDF) Atlas of Diabetes, approximately 7.002 million adults aged 20-79 years will have DM worldwide by 2045 (1). Type 1 diabetes mellitus (TIDM) and type 2 diabetes mellitus (T2DM) are the two most common types of DM. Patients with T1DM are primarily treated with insulin replacement therapy to alleviate absolute insulin deficiency, but may be at risk for hypoglycaemia and tumourigenesis. Human islet transplantation is an effective treatment for T1DM, with a combination of impaired hypoglycaemic awareness and severe hypoglycaemic episodes (2). However, islet transplantation may be greatly limited in clinical application due to a shortage of donor islets and immune rejection. Drug therapy is an important treatment modality for patients with T2DM (3), but its side effects (such as diarrhoea, nausea, vomiting, and anaemia) and drug prices remain to be investigated. Meanwhile, persistent hyperglycaemia can cause chronic damage or dysfunction of the eyes, kidneys, heart, blood vessels, and nerves, and intervention in DM and its complications and reduction of mortality are imminent (4, 5).
Mesenchymal stem cells (MSCs) are widely used in various cell therapies because of their many advantages, such as self-renewal capacity, multispectral differentiation ability, tissue damage repair, and lack of co-stimulatory molecules (6). The abundant source of human umbilical cord mesenchymal stem cells (HUC-MSCs), low ethical controversy, low infection risk, high proliferation and differentiation ability, and very low immunogenicity make them uniquely advantageous for DM therapy. Recently, studies related to the treatment of DM with HUC-MSCs have rapidly developed. This review describes the advantages and mechanisms of HUC-MSCs in treating DM and the application and research progress of HUC-MSCs in DM-related complications, providing more options for managing DM and its complications.
Source of HUC-MSCs
Umbilical cord blood (UCB) is a valuable stem cell source. MSCs can be isolated from neonatal umbilical cords by enzymatic digestion and show a positive expression of classical MSC surface markers. The umbilical cord comprises the umbilical artery, umbilical vein, Wharton's jelly (WJ), and external amniotic epithelium surrounding the mucus connective tissue (7). MSCs can be isolated from different umbilical cord parts, including the blood, sub-umbilical vein endothelium, and the WJ. Researchers have successfully isolated and cultured MSCs from the perivascular layer of Wharton collagenous vessels of the human umbilical vein (8). MSCs can also be isolated from non-perivascular areas (sub-amniotic membranes) (9). Platelet-derived growth factor (PDGF) produced by human amniotic cells may induce cell migration from the vascular system to the amnion (10). The human umbilical cord is a rich MSC source.
Advantages of HUC-MSCs
HUC-MSCs have compelling advantages in treating DM, including (1) abundant sources, easy collection, and easy preservation and transportation (11); (2) easy isolation, high purity, and non-tumorigenic (12); (3) high amplification potential (13); (4) functional stability after lyophilisation and recovery (14); (5) no adverse effects of collection on the donor, and ethical issues are circumvented (15); and (6) low probability of infection and transmission of pathogenic microorganisms. In contrast, bone marrow-derived MSCs (BM-MSCs) have a high risk of viral infection and a significant decrease in cell number and proliferation/differentiation capacity with age (16, 17). (7) More primitive and proliferative differentiation capacity. Compared with BM-MSCs, HUC-MSCs have higher pancreatic differentiation potential and proliferative capacity (18). Compared with dental pulp-derived mesenchymal stem cells (PU-MSCs) and adipose tissue-derived mesenchymal stem cells (AD-MSCs), HUC-MSCs have the strongest efficacy in ameliorating glucose and lipid metabolism disorders in T2DM (19). (8) Very low immunogenicity (20). In summary, HUC-MSCs are an ideal source of cells for cell therapy in DM.
Possible mechanisms of HUC-MSCs for DM treatment
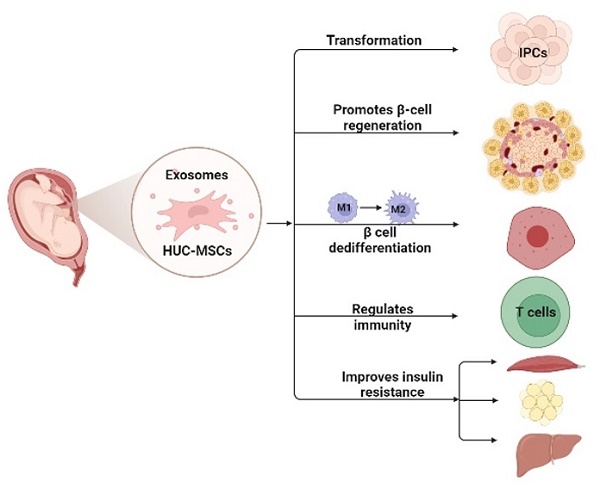
MSCs for DM are cell-based therapeutic approaches that have shown remarkable therapeutic effects in DM because of their self-renewal, differentiation potential, and immunosuppressive properties. Numerous studies have shown that HUC-MSCs are a novel strategy to treat DM, and their possible mechanisms (21) include: 1) homing to the damaged pancreas and acting through local nutrition and secretion of paracrine factors; 2) differentiation into insulin-producing cells (IPCs); 3) reversal of beta-cell (β-cell) dedifferentiation, thereby alleviating β-cell dysfunction and protecting islet β-cells; 4) promotion of islet β-cell regeneration; 5) secretion of anti-inflammatory cytokines and macrophage phenotype regulation, thereby reducing islet β-cell inflammation; and 6) enhancing insulin sensitivity in target tissues and improving insulin resistance (Fig. 1).
Homing effect of HUC-MSCs
One advantage of MSCs for DM mitigation is their ability to home to damaged tissues and then directly proliferate and differentiate to replace damaged cells and repair damaged tissues. Homing is potentially important for recruiting MSCs to the injury and regeneration sites (22). MSCs homing includes both non-systematic and systemic homing. In non-systematic homing, MSCs are locally transplanted into the target tissue and then directed to the injury site via a chemokine gradient. In systemic homing, the molecular mechanisms of MSCs homing include initial tethering by selectins, activation by cytokines, blockade by integrins, exudation or migration using matrix remodelling agents, and extravasation toward chemokine gradients (23). In 2017, HUC-MSCs labelled with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI) were detected in the pancreas of T1DM mice, suggesting that HUC-MSCs may target and migrate to damaged organs to exert therapeutic effects (24). Yin et al. pre-labelled HUC-MSCs with cell membrane-Dil (CM-Dil) to demonstrate their migration in various tissues, thus confirming the implantation of MSCs in the pancreatic islets of T2DM mice. This suggests that homing of HUC-MSCs may be closely related to tissue damage (25). Overall, HUC-MSCs may play a role in treating DM by homing to damaged islets. However, the homing rate of MSCs is low, and MSCs may exert protective effects through other mechanisms.
Paracrine effects of HUC-MSCs
The paracrine properties of MSCs make them a key tissue repair option, and the paracrine effect of MSCs is achieved through the secretion of soluble factors and release of extracellular vehicles (EVs), such as exosomes and microvesicles (26). All the factors secreted by MSCs are called the secretome and comprise various cytokines, chemokines, angiogenic factors, and growth factors. Moreover, up to 80% of the therapeutic effects of MSCs are mediated by paracrine signalling (27). HUC-MSCs secrete soluble molecules such as keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), placental growth factor (PGF), monocyte chemoattractant protein 1 (MCP-1), insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), prostaglandin E2 (PGE2), indoleamine 2,3-deoxygenase (IDO), interleukin-10 (IL-10), interleukin-6 (IL-6), transforming growth factor-β1 (TGF-β1), nitric oxide (NO), human leukocyte antigen-G5 (HLA-G5), tumour necrosis factor-α stimulated gene 6 (TSG-6), and neurotrophic factors (12, 28-30). These factors play a role in promoting tissue regeneration, participating in angiogenesis, promoting ulcer tissue healing, wound healing, modulating immunity, anti-inflammation, anti-apoptosis, and cytoprotection. Recently, there has been intense interest in the synthesis and release of EVs by MSCs via paracrine secretion. Human umbilical cord mesenchymal stem cell-derived exosomes (HucMSC-exs) are nanometer-sized and are capable of rapid diffusion across biological barriers and cell membranes. Numerous studies have shown that HucMSC-exs have anti-inflammatory, anti-apoptotic, tissue repair, neuroprotective, and immunomodulatory properties, suggesting that HucMSC-ex may be a potential DM therapy. HucMSC-ex alleviates T2DM by activating the regenerative capacity of islets (31), improving insulin sensitivity (32), reversing peripheral insulin resistance, and attenuating β-cell destruction (33). Human umbilical cord mesenchymal stem cell-derived small extracellular vesicles (HUC-MSC-sEVs) attenuated structural damage in the pancreas, kidney, and liver of T2DM rats (34). HucMSC-ex protects β-cells from hypoxia-induced apoptosis by carrying miR-21 to attenuate endoplasmic reticulum (ER) stress and inhibit p38 MAPK phosphorylation (35). Exosome-loaded immunomodulatory biomaterials can attenuate the local immune response induced by grafts in DM mice (36). The above studies revealed the potential value of HucMSC-ex and miRs in DM. Overall, HUC-MSCs play a protective role against DM by secreting soluble factors and EVs.
This figure illustrates the possible mechanism of HUC-MSCs for the treatment of DM. HUC-MSCs exert beneficial effects on diabetes by differentiating into IPCs, promoting islet β-cell regeneration, reversing β-cell dedifferentiation, reducing islet β-cell inflammation, and improving insulin resistance. IPCs insulin-producing cells.
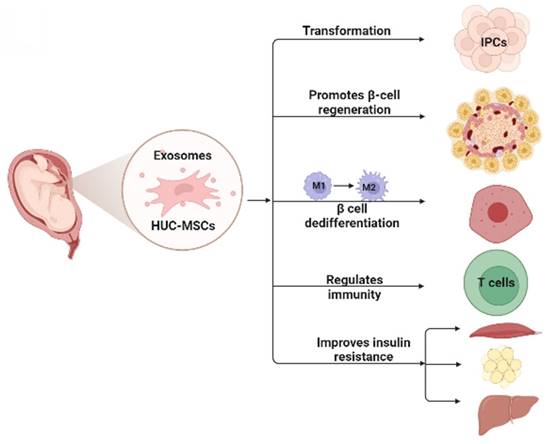
Differentiation of HUC-MSCs into IPCs
HUC-MSCs ameliorate hyperglycaemia and weight loss in DM rats by differentiating them into IPCs (37-39). HUC-MSCs induced to differentiate into IPCs (40, 41) express pancreatic β-cell differentiation-related genes (e.g. nestin, pancreatic duodenal homeobox-1 (PDX-1), neurogenin3 (NGN3), paired box 6 (PAX6), paired box 4 (PAX4), nk2 homeobox 2 (NKX2.2), nk6 homeobox 1 (NKX6.1), glucose transporter 2 (GLUT-2), and insulin (INS) genes) (42, 43), and promote the secretion of serum C-peptide and INS in DM rats (44, 45). Additionally, HUC-MSCs promote the survival, function, and number of islet-like cell clusters (46). Co-culture of HUC-MSCs with T1DM rat pancreatic cells promotes survival, proliferation, and induced differentiation of HUC-MSCs into IPCs (47). Furthermore, the differentiation of IPCs is a very complex process, and the initial stage of nestin preselection, appropriate induction reagents (48), and extracellular matrix (49) are necessary for the in vitro culture of IPCs from HUC-MSCs. PDX-1 (50, 51), inhibition of Notch signalling (52) and laminin 411 (53) effectively regulate the differentiation of MSCs into IPCs. Under hypoxic conditions, UCB-MSCs also efficiently differentiate into IPCs (54, 55). Additionally, factors that effectively promote the efficacy of IPC action include Port-A catheter transplantation (56), suspension culture (57), and addition of the histone deacetylase (HDAC) inhibitor TMP269 (58). Overall, HUC-MSCs can replace damaged islet β cells by inducing differentiation into IPCs, which are ideal seed cells to treat DM.
HUC-MSCs can effectively improve islet β-cell function
β-Cell dedifferentiation is thought to be an important contributor to β-cell dysfunction in T2DM (59). Pro-inflammatory cytokines can lead to β-cell dysfunction and de-differentiation. MSCs reduce endogenous interleukin-1b (IL-1b) production in T2DM islets by secreting IL-1Ra, thereby reducing islet injury and reversing β-cell dedifferentiation (60). Additionally, it has been shown that the interleukin-1 receptor antagonist (IL-1Ra) can also regulate the phenotypic transition of macrophages (61). In db/db mice, early infusion of HUC-MSCs reduced β-cell dedifferentiation markers, such as aldehyde dehydrogenase 1 family member A3+ (ALDH1A3+), and increased the proportion of Ins+ β cells and Pdx1+/Ins+ cells (62). This suggests that MSCs transplantation may be a therapeutic strategy for protecting and restoring β-cell function in patients with T2DM. Additionally, the potential mechanisms for the therapeutic effects of MSCs on DM may involve islet regeneration, including direct differentiation into functionally competent β-cells. Pax4, in concert with Pdx1, Ngn3, and MAF bZIP transcription factor A (MafA), can induce the differentiation of HUC-MSCs into pancreatic β-like cells (pβLCs) functional pancreatic β cells (63). MSCs participate in the repair process by secreting various cytokines and growth factors with paracrine and autocrine activities, which may contribute to endogenous β-cell regeneration and islet structural recovery (21). Wei et al. found that HUC-MSCs protect islets from hypoxia-induced dysfunction (64) and secrete IGF-1 to exert a trophic effect on islets (65). Bao et al. found that HUC-MSCs overexpressing tissue inhibitors of matrix metalloproteinase (TIMP)-1 induced weight loss and hypoglycaemia and improved islet function and survival in T1DM mice (66). Lu et al. found that HUC-MSC transplantation is safe and effective in T1DM patients and may better protect residual β-cells (67). Hu et al. found that the combination of HUC-MSCs and selegiline was effective in improving hyperglycaemia, promoting islet β-cell regeneration, and inhibiting islet alpha cell (α-cell) production in T2DM rats (68). Although the exact mechanism needs to be further explored, this study may provide a new therapeutic approach for DM.
Interaction of HUC-MSCs with various immune cells and cytokines
HUC-MSCs and macrophage polarization
DM is characterised by mild chronic inflammation, which is often accompanied by inflammatory cell infiltration in islets. Macrophage infiltration of islets and autoimmune destruction of β-cells are important features of the chronic inflammatory process in T1DM. Macrophages may be a major contributor to the development of chronic inflammation and insulin resistance in patients with T2DM (3). UC-MSC transplantation induces an increase in M2 macrophages in pancreatic islets, adipose tissue, liver, and skeletal muscle. HUC-MSCs produce anti-inflammatory mediators and growth factors that suppress inflammation and improve insulin sensitivity and β-cell regeneration (25). HUC-MSCs reduce insulin resistance by secreting IL-6 (69) and IL-10 (70) to promote M2 macrophage polarization. MCP-1 secreted by HUC-MSCs synergistically regulates macrophage polarisation with IL-6 (71). Additionally, low-dose decitabine may prolong the antidiabetic effects of MSCs and promote sustainable β-cell recovery by polarising macrophages to the M2 phenotype (72). Overall, HUC-MSCs can reduce islet β-cell inflammation by polarising macrophages to the M2 anti-inflammatory phenotype, thereby alleviating islet dysfunction in patients with DM.
HUC-MSCs and other immune cells
MSCs not only act on innate immune cells but also interact with other immune cells, thus regulating multiple effector functions (73). MSCs regulate antigen presentation by dendritic cells (DCs), cytotoxicity of natural killer (NK) cells and neutrophil activation. MSCs induce peripheral tolerance in T cells and exert effective tissue protection through the release of anti-inflammatory, anti-apoptotic, and trophic molecules (74). Li et al. found that regulatory T cells (Treg)/T helper cell 17 (Th17) and Treg/T helper cell 1 (Th1) cell ratios increased significantly after 4 weeks of transplantation of HUC-MSCs, while the Th17/Th1 cell ratio remained unchanged (75), suggesting that HUC-MSCs ameliorate immune disorders in T2DM by repairing Treg cells. HUC-MSCs can reduce blood glucose, increase C-peptide levels, and Treg production in T2DM patients (76), suggesting that HUC-MSCs with powerful immunomodulatory ability are safe and effective in T2DM patients, and microencapsulated HUC-MSCs reduce effector Th1 cells and repair the Treg/Th17 ratio (77), suggesting that HUC-MSCs may treat T1DM by modulating immunity. In addition, HUC-MSCs have shown efficacy in other autoimmune diseases, such as T1DM combined with Sjogren syndrome (SS) (78, 79). Overall, MSCs may represent a new strategy to treat immune-mediated diseases.
HUC-MSCs improve insulin resistance
Insulin resistance (IR) is one of the most common and important pathological features of T2DM. MSCs can exert immunomodulatory and anti-inflammatory effects through paracrine effects, thereby increasing insulin sensitivity and improving insulin resistance in T2DM rats (80). Umbilical cord mesenchymal stem cell-conditioned medium (UC-MSC-CM) may improve IR in C2C12 cells by improving glucose transporter 4 (GLUT4) translocation, insulin signalling pathways, and mitochondrial content and function (81). HUC-MSCs also improve IR by modulating the balance between PTEN-mediated PI3K/Akt and ERK/MAPK signalling pathways (82). UC-MSCs infusion and fasting-mimicking diet (FMD) synergistically modulate the systemic inflammatory microenvironment and improve hyperglycaemia and lipid metabolism disorders in T2DM mice (83). Glucagon-like peptide-1 (GLP-1) gene modification of HUC-MSCs improves fasting glucose, IR, and β-cell function in T2DM mice (84). HUC-MSCs combined with liraglutide can downregulate the TLR4/NF-kB inflammatory pathway and oxidative stress while improving glucose metabolism and inhibiting islet β-cell apoptosis in an ASK1/JNK/BAX pathway-dependent manner in T2DM rats (85, 86). In conclusion, HUC-MSCs act as an effective treatment for T2DM by improving IR, thereby providing a potential avenue for developing novel clinical T2DM therapies.
HUC-MSCs and other types of diabetes
Recently, with advances in regenerative medicine research, HUC-MSCs may provide a new treatment option for other types of DM. Hu et al. found that HUC-MSCs therapy could restore the function of residual islet β cells in patients with new-onset T1DM over a longer period. This suggests that implantation of HUC-MSCs is expected to be an effective strategy for treating new-onset T1DM (87). Yang et al. found that HUC-MSCs reduced inflammatory responses and attenuated pancreatic injury in rats with severe acute pancreatitis (SAP) (88). Kong et al. found that HUC-MSCs ameliorated chronic pancreatitis in rats via the AKT-mTOR-S6K1 signalling pathway, which provides a basis for the clinical application of HUC-MSCs in treating pancreatitis (89). In 2019, HucMSC-ex delivered exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression, suggesting a therapeutic role of HUC-MSCs in pancreatic exocrine diseases (90). Additionally, transplantation of HUC-MSCs can effectively alleviate weight loss symptoms, reduce blood glucose levels, and improve offspring survival in gestational diabetes mellitus (GDM) patients (91). However, it has been shown that GDM adversely affects the proliferative capacity and viability of HUC-MSCs (92). Therefore, to address this situation, it is crucial to identify conditions that improve the survival of HUC-MSCs, reduce apoptosis, and promote proliferation.
In conclusion, MSC therapy presents a novel approach for treating DM, displaying substantial efficacy in both basic and clinical trials. HUC-MSCs exhibit the capacity to migrate towards damaged pancreatic islets, facilitated by homing and paracrine effects, thus assuming a reparative role. Additionally, they induce differentiation into IPCs, replacing impaired islet β-cells and enabling the secretion of C peptide and insulin. Furthermore, they counteract β-cell de-differentiation, thereby safeguarding pancreatic β-cells, and facilitate regeneration of islet β-cells along with structural revitalization, consequently enhancing islet β-cell functionality. Their impact extends to immune cells, encompassing macrophages, DCs, NK cells, neutrophils, and T cells, thereby exerting immunomodulatory and anti-inflammatory properties. This therapeutic modality also ameliorates insulin resistance by targeting insulin-responsive organs. In summary, the collective mechanisms through which HUC-MSCs operate synergistically culminate in an amelioration of diabetic symptoms (Table. 1). Nevertheless, the homing rate of MSCs remains limited, prompting the need for further investigations to enhance their homing rate, bolster their survival rate post-transplantation, and optimize overall efficacy and safety. Notably, contemporary research endeavors have augmented the efficacy of diabetes mellitus treatment via preemptive treatments of MSCs, including hypoxic pre-conditioning. While current studies yield promising clinical outcomes, the full spectrum of optimal efficacy warrants deeper exploration.
HUMSCs and complications of T2DM
Diabetic nephropathy
Diabetic nephropathy (DN) is one of the most serious complications of DM and a major cause of end-stage chronic kidney disease. HUC-MSCs act mainly by promoting paracrine mechanisms, such as mitogenic, anti-fibrotic, anti-inflammatory, antioxidant, anti-apoptotic, cytoprotective and immunomodulatory. HUC-MSC transplantation is expected to be an effective therapeutic approach for preventing and treating DN. An et al. found that HUC-MSCs lowered blood glucose, improved renal function and renal histopathological changes in DN nonhuman primates (93). Fang et al. found that IGF-1 secreted by HUC-MSCs promoted renal tubular cell proliferation and reduced apoptosis, thus exerting a protective effect on the kidneys (94). Additionally, HUC-MSCs inhibited the levels of inflammatory factors IL-6, IL-1β, tumour necrosis factor-alpha (TNF-α), TGF-β, MCP-1, and nuclear factor-κB (NF-κB) and downregulated the expression of fibronectin alpha-smooth muscle actin (α-SMA) and collagen IV, suggesting that HUC-MSCs benefit podocytes under high glucose (HG) by suppressing inflammation and fibrosis while delaying the progression of DN (95-97). Nie et al. found that HUC-MSCs decreased malondialdehyde levels and 4-hydroxynonenal (4-HNE) protein expression and increased the antioxidant enzymes catalase (CAT) and glutathione peroxidase (GPX) (98). HUC-MSCs attenuated the expression of TGF-β1, α-SMA, collagen I, and heat shock protein 47 (HSP47) mRNA and increased the expression of E-cadherin and bone morphogenetic protein 7 (BMP-7) mRNA, suggesting that HUC-MSCs can prevent renal injury in DN rats via paracrine humoral factors (99, 100). Notably, HUC-MSCs improved renal function in mice, mainly due to immunomodulatory effects rather than direct implantation and trans-differentiation into renal cells (101). Overall, HUC-MSCs can improve DN through the above-mentioned mechanisms, and may be a promising DN therapeutic strategy.
The possible modes of action of MSCs in the treatment of diabetes are discussed in the table.
| Mode of action of HUC-MSCs | |||
|---|---|---|---|
| Mode of action | Mechanism | References | |
| Homing effects | Systemic homing | Initial tethering by selectins Activation by cytokines Blockade by integrins Exudation or migration using matrix remodelling agents Extravasation toward chemokine gradients | (22, 23) |
| Non-systematic homing | Directed to the injury site via a chemokine gradient. | ||
| Paracrine effects | Secrete soluble molecules (KGF, HGF, VEGF, FGF, PGF, MCP-1, IGF-1, EGF, PGE2, IDO, IL-10, IL-6, TGF-β1, NO, HLA-G5, TSG-6, and neurotrophic factors) | Promoting tissue regeneration and angiogenesis Promoting ulcer tissue healing and wound healing Modulating immunity, anti-inflammation, anti-apoptosis, and cytoprotection. | (12, 28-30) |
| Release of EVs, such as exosomes and microvesicles | Activating the regenerative capacity of islets Improving insulin sensitivity Reversing peripheral insulin resistance Attenuating β-cell destruction | (31-33) | |
| Differentiation into IPCs | Induced to differentiate into IPCs | Replace some damaged islet β-cells to secrete C peptide and INS | (40, 45) |
| Improve islet β-cell function | Protection of pancreatic islet beta cells | Secreting IL-1Ra to reduce islet injury and reverse β-cell dedifferentiation | (60, 62) |
| Promoting the regeneration of pancreatic islet beta cells | Induced to differentiate into pβLCs functional pancreatic β cells | (63) | |
| Immunomodulatory effects | Macrophage | Suppress inflammation and improve insulin sensitivity by secreting IL-6 and IL-10 to promote M2 macrophage polarization. | (25, 69-71) |
| DCs, NK cells, neutrophil, and T cells | Regulate antigen presentation by DCs Regulate cytotoxicity of NK Regulate neutrophil activation Induce peripheral tolerance in T cells | (74) | |
| Improve insulin resistance | Liver, fat and skeletal muscle | Improve IR in C2C12 cells and improve lipid metabolism disorders in T2DM mice | (81, 83) |
HUC-MSCs, Human umbilical cord mesenchymal stem cells; KGF, Keratinocyte growth factor; HGF, Hepatocyte growth factor; VEGF, Vascular endothelial growth factor; FGF, Fibroblast growth factor; PGF, Placental growth factor; MCP-1, Monocyte chemoattractant protein 1; IGF-1, Insulin-like growth factor 1; EGF, Epidermal growth factor; PGE2, Prostaglandin E2; IDO, Indoleamine2,3-deoxygenase; IL-10, Interleukin-10; IL-6, Interleukin-6; TGF-β1, Transforming growth factor-β1; NO, Nitric oxide; HLA-G5, Human leukocyte antigen-G5; TSG-6, Tumor necrosis factor α stimulated gene 6; EVs, Extracellular vehicles; β-cell, Beta cell; IPCs, Insulin-producing cells; INS, Insulin; IL-1Ra, Interleukin-1 Receptor antagonist; PβLCs, Pancreatic β-like cells; DCs, Dendritic cells; NK cells, Natural killer cell; IR, Insulin resistance; T2DM, Type 2 diabetes mellitus.
Diabetic retinopathy
Diabetic retinopathy (DR) is a common cause of visual impairment and blindness in working-age individuals. Microangiopathy and inflammatory responses are key components of DR. Recently, MSCs have received increasing attention for their tissue damage repair therapy, anti-inflammatory effects, and pro-angiogenic effects, and they offer potential options for the treatment of DR. HUC-MSCs play an anti-inflammatory role and inhibit retinal neuronal apoptosis by upregulating the expression of adiponectin (APN) and neurotrophin-4 (NT-4) and downregulating the expression of myocardial infarction-associated transcript (MIAT), IL-1β, IL-6, and high-sensitivity C-reactive protein (hs-CRP) (102, 103). HUC-MSCs increase the number of surviving retinal ganglion cells (RGCs) and improve neuroprotection through a BDNF-dependent mechanism, suggesting that HUC-MSCs may slow DR progression through paracrine humoral factors (104, 105). Additionally, numerous studies have shown that HucMSC-ex have anti-inflammatory, anti-apoptotic, tissue repair, neuroprotective, and immunomodulatory properties. Moreover, EVs are nanometer-sized and can diffuse rapidly through the retina (106). Fu et al. found that HucMSC-ex effectively prevented early retinal vascular damage and retinal thickening, and alleviated DM-induced structural damage to the retina (107). Li et al. found that HucMSC-derived exosomes shuffled microRNA-17-3p ameliorated the inflammatory response and oxidative damage in DR mice by targeting STAT1, providing new insights into novel targeted therapies for DR (108). In 2021, HucMSC-derived exosomes shuffled microRNA-18b exerted anti-apoptotic and anti-inflammatory effects in DR rats by mediating the MAP3K1/NF-κB axis, suggesting that miR-18b is critical for HucMSC-ex treatment of DR (109). Zhang et al. found that HucMSC-ex overexpressing miR-126 was able to reduce hyperglycemia-induced retinal inflammation by targeting and regulating high mobility group box 1 (HMGB1) (110). Overall, these studies have laid a solid foundation for HUC-MSCs in DR treatment.
Diabetic central nervous system complications
DM is a risk factor for acute stroke and can lead to a higher risk of ischaemic stroke and a worse prognosis (111-113). Cerebral haemorrhage, neurological deficits, and white matter (WM) damage can be severe after stroke in DM mice (114). Inflammatory and immune responses play important roles in ischaemic stroke prognosis, and human umbilical cord blood cells (HUCBCs) are widely accepted to repair the central nervous system (115). Stem cell-rich HUCBCs can survive, migrate, differentiate, and restore neurological function in the ischaemic brain microenvironment of stroke rats (116). Lin et al. found that CD34-immunosorted human umbilical cord blood haematopoietic stem cells (HUCB34) after hypoxic preconditioning promoted neuronal progenitor cell (NPCs) homing to the ischaemic brain and enhanced neuronal synapse regeneration (117). Chen et al. found that HUCBCs promote vascular and WM remodelling by upregulating miR-126 expression while promoting M2 macrophage polarisation and inducing neural repair by decreasing vascular cell adhesion molecule-1 (VCAM-1) and MCP-1 expression (118). HUCBCs increase the density of oligodendrocyte progenitors and oligodendrocytes, increase angiopoietin 1 (Ang-1), expression, and decrease the expression of ischaemic border zone (IBZ) RAGE, matrix metalloproteinase 9 (MMP-9), and toll-like receptor 4 (TLR4), suggesting that HUCBCs have a therapeutic effect on nerve repair in DM rats with stroke (119, 120). Therefore, MSCs therapy may be a promising therapeutic option for diabetic patients with central nervous system complications.
Diabetic autonomic neuropathy
Diabetic autonomic neuropathy (DAN) is a serious and common complication of DM that has significant adverse effects on patient survival and quality of life (121). Diabetic cystopathy (DC) is considered a manifestation of diabetic neuropathy, and its pathogenesis may be related to long-term hyperglycaemia, bladder wall remodelling induced polyuria, and oxidative stress leading to smooth muscle cell and neuronal damage (122). Wu et al. found that HUC-MSCs overexpressing nerve growth factor (NGF) could secrete neurotrophic factors and cytokines in the rat spinal cord and could also differentiate into NeuN neurones and glial fibrillary acidic protein (GFAP)-positive astrocytes to effectively prevent bladder hypertrophy and remodelling, thereby reversing the progression of DC and restoring bladder function (123). Shin et al. found that HUC-MSC transplantation improved urinary function in DM rats, which provides a rationale for HUC-MSC treatment of DM-related detrusor underactivity (DUA) (124). Wu et al. found that HUC-MSC transplantation may improve diabetic erectile dysfunction in rats by increasing the production of paracrine growth factors (VEGF), endothelial nitric oxide synthase (eNOS), IGF1, and basic fibroblast growth factor (bFGF) (125). In conclusion, transplantation of HUC-MSCs may be a new potential therapeutic option for DAN.
Diabetic foot disease
Diabetic foot ulcers (DFU) are full-length lesions that occur in the skin of the foot of patients with DM, accompanied by infection and tissue destruction caused by neuropathy and/or peripheral artery disease (PAD) (126). DFU has a high disability and mortality rate, which severely affects the quality of life of patients, shortens life expectancy, and imposes a heavy socioeconomic burden (127, 128). In recent years, HUC-MSCs have achieved good therapeutic effects in the treatment of DFU. Zhao et al. found that HUC-MSCs specifically homed to ulcerated tissue and promoted epithelialisation of ulcerated tissue, possibly by stimulating the release of cytokeratin 19 from keratin-forming cells and promoting extracellular matrix formation (129). Shi et al. found that HUC-MSCs promoted wound healing in DFU rats by transdifferentiating, regulating inflammation, and providing growth factors that promote angiogenesis, cell proliferation, and collagen deposition (130). Xia et al. found that HUC-MSCs prevented or cured foot ulcers in DFU rats by reversing the neuronal structure and function by upregulating NGF and promoting significant angiogenesis in the femoral nerve-innervated gastrocnemius muscle (131). HUC-MSCs induce angiogenesis (132, 133), promote tissue repair and regeneration (134), and reduce muscle damage and apoptosis in the ischaemic hind limbs of DM mice (135). Transplantation of HUC-MSCs significantly improved skin temperature, ankle-arm pressure index, transcutaneous partial pressure of oxygen, and claudication distance in patients with postoperative diabetic foot disease. This is accompanied by a significant increase in neovascularization and complete or gradual ulcer healing (136). In conclusion, transplantation of HUC-MSCs may be a potential strategy for clinical application in DFU, although its long-term effects remain to be elucidated.
Impaired wound healing in DM
Impaired wound healing is a common DM complication. DM is associated with persistent inflammation and a defective tissue repair response. Impaired angiogenesis is an important factor in delaying chronic diabetic wound healing. Poorly healing wounds in DM mice exhibit a persistent inflammatory response, a deficiency of M2 macrophages (137, 138), a prolonged accumulation of pro-inflammatory M1 macrophages, elevated levels of pro-inflammatory cytokines and proteases, and reduced levels of various growth factors (139-141). HUC-MSCs can self-renew, multi-directionally differentiate, and secrete multiple cytokines and growth factors, and their mechanisms to improve diabetic wound healing mainly include 1) promoting diabetic wound healing by differentiating into keratin-forming cells (142); 2) secreting molecules related to wound healing in a paracrine manner (VEGF, PDGF, KGF, TGF-β1, SMA, scavenger receptor class B type1 (SR-B1), and platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31)) to promote angiogenesis (143-146); 3) regulating the activity, function, and proliferative capacity of vascular endothelial cells by reducing oxidative stress and inflammatory response, thereby promoting angiogenesis (147, 148); 4) inducing functional recovery of vascular endothelial cells by modulating macrophage phenotype (149); and 5) stimulating diabetic fibroblast activity and promoting cell proliferation, collagen synthesis, and glycosaminoglycan levels, thereby playing a role in skin wound healing play a role (150). Moreover, HUC-MSCs may be more effective than fibroblasts in stimulating diabetic wound healing (151, 152). Additionally, HUC-MSCs accelerate wound healing in diabetic rats by increasing epidermal and dermal thickness and density, accelerating epithelial and collagen regeneration, and increasing angiogenesis (153). Han et al. found that the Wnt signalling pathway activation promoted the proliferation and differentiation of HUC-MSCs, thereby facilitating the healing of diabetic skin wounds (154). Yue et al. found that c-Jun overexpression promotes the proliferation and migration of HUC-MSCs in vitro and accelerates diabetic wound closure, re-epithelialization, and angiogenesis in vivo (155). The development of new technologies has extensively improved the therapeutic efficacy of HUC-MSCs. HUC-MSCs can improve skin wound healing in diabetic mice by combining with Pluronic F127 hydrogel (156), tissue-engineered scaffolds (157, 158), or Cas9-AAV6 engineering modification (159). Overall, HUC-MSC transplantation may have a therapeutic effect on impaired diabetic wound healing; however, its specific therapeutic modalities and safety need to be further explored.
HUC-MSCs infusion is safe and effective for COVID-19 with diabetes
Currently, coronavirus disease 2019 (COVID-19) is a serious global public health problem and is significantly associated with an increased risk of developing DM (160). At the same time, patients with DM are at a high risk of developing severe COVID-19 infections, have a complex disease process, and have significantly higher mortality rates (161, 162). Severe COVID-19 is thought to result from the hyper-inflammatory state and overactive immune response caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as well as cytokine storm and immune thrombosis. The SARS-CoV-2 spike glycoprotein binds to angiotensin-converting enzyme 2 (ACE2), and the serine protease transmembrane protease serine 2 (TMPRSS2) initiates S proteins that can facilitate viral entry into cells, viral replication, and cell-to-cell transmission (163). The activity of ACE2 is increased in DM mice (164, 165) and significantly increased in patients with DM treated with angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) (166), suggesting that patients with DM may be at increased COVID-19 risk.
MSCs can achieve immunomodulation by secreting various cytokines through paracrine pathways or by interacting directly with the immune cells (167). ACE2 and TMPRSS2 were expressed at low levels in HUC-MSCs, suggesting that HUC-MSCs may have the ability to "evade" viral infection and thus exert immunomodulatory effects (168). HUC-MSCs reduced the levels of inflammatory molecules associated with the COVID-19 "cytokine storm", including interferon-γ (IFNγ), IL1β, IL-6, and TNFα, and regulated upon activation of normal T cell expressed and secreted factor (RANTES). Additionally, no serious adverse events related to HUC-MSC infusion have been observed (169, 170). HUC-MSCs improved respiratory distress and reduced inflammatory biomarkers in patients with critically ill COVID-19-induced automated resources directory service (ARDS) (171). Tao et al. found that UC-MSCs significantly increased pulmonary static compliance, maintained a stable partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio, and improved renal function in critically ill COVID-19 patients, suggesting that UC-MSCs transplantation may have a positive therapeutic effect in critically ill COVID-19 patients (172). In conclusion, HUC-MSC therapy may be a potential treatment option for DM combined with COVID-19.
Opportunities and challenges
HUC-MSCs have shown impressive results in treating DM and its complications, but most studies are still in the preclinical stage. Improving the survival and efficacy of HUC-MSCs after transplantation in a challenging metabolic environment may be an interesting topic in the future, as elevated palmitate levels in the sera of obese and T2DM patients lead to a shift from an immunosuppressive to an immunostimulatory state in MSCs, suggesting that the metabolic disease environment alters the immunomodulatory efficacy of healthy donor MSCs (173). Boland et al. found that culturing HUC-MSCs in xeno-free conditions attenuated palmitate-induced impairment of the immunomodulatory function of HUC-MSCs (174). Additionally, the mode of MSCs administration affects therapeutic efficacy, with intravenous delivery methods being more effective than intraperitoneal grafts (175). Local delivery causes MSCs to cluster into "spheroids", thereby altering gene expression and phenotype. In 2020, researchers found that budesonide could act synergistically with prostaglandin E2 (PGE2) produced by spheroid MSCs to inhibit T cell proliferation at the PGE2 receptors EP2 and EP4 (176). Moreover, IPCs may be immunogenic and trigger immune responses after transplantation into the host owing to changes in the immune microenvironment and immune cell infiltration, thus reducing cell survival and further differentiation (177). However, encapsulation of IPCs with alginate has been shown to avoid graft rejection, which greatly improves the efficacy of allogeneic or xenogeneic MSCs in the treatment of DM (178).
Stem cell banking is the most important life resource for human beings, which can provide high-quality seed cell resources for stem cell therapy. By establishing a standardized production process of MSCs, it can improve stem cell preparation quality and promote the sustainable development of stem cell clinical applications. Actively promoting the clinical translation of stem cell therapy and improving the survival rate and efficacy of HUC-MSCs after transplantation will become the top priority of stem cell technology research nowadays. With the development of technology, the field of stem cell research has become a frontier hotspot. The implantation of the bioartificial pancreas (179), the labelling of nanoparticles (NP) (180), and the co-microencapsulation of HUC-MSCs/human pancreatic islet-derived progenitor cells (hIDC) (181) may provide new tools for cellular therapy of DM. Carboxylic acid-functionalized single-walled carbon nanotubes (f-SWCNT-COOH) (182) and some cytokines (183) can increase the viability and ex vivo expansion of hematopoietic stem cell (HSC) and/or hematopoietic stem progenitor cell (HSPC). These studies provide new perspectives for developing DM cell transplantation therapies based on HUC-MSCs. We believe that the effectiveness of HUC-MSC therapy will be greatly improved by applying advanced technologies such as gene modification, nanotechnology, magnetic targeting technology, and tissue engineering technology. We believe that soon, HUC-MSCs may provide a better solution for the clinical treatment of DM and its complications, and thus can bring new hope to a greater extent in DM patients worldwide.
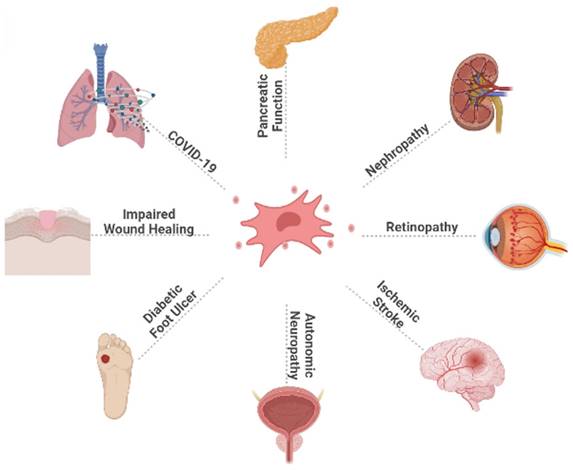
This figure illustrates the broad effect of HUC-MSCs on DM and its complications, as well as the therapeutic effect of HUC-MSCs on diabetic patients infected with COVID-19. COVID-19: coronavirus disease 2019.
Conclusions
The DM epidemic and its complications pose a major threat to global health, accompanied by high morbidity and mortality. Currently, there are many ways to treat DM, such as traditional oral hypoglycaemic therapy and insulin injections, but they can only temporarily control blood glucose levels and cannot cure diabetes, and have insufficient control over diabetic complications, in addition to long-term use of hypoglycaemic drugs or insulin injections, which significantly reduces patient compliance. Recently, regenerative medicine with MSCs treatment as the core has provided new ideas and possible development directions for DM treatment. The characteristics of HUC-MSCs, such as abundant source, less ethical controversy, lower risk of infection, higher proliferation and differentiation ability, and very low immunogenicity, make them stand out among MSCs of different tissue sources. We mainly describe the application of HUC-MSCs in DM and its complications. HUC-MSCs transplantation is expected to be an efficient and ideal treatment for DM and its complications, and its application area will gradually expand (Fig. 2). Currently, HUC-MSCs therapy is still in the exploration stage, and further research is needed to improve the homing rate, survival rate, efficacy, and safety of MSCs after transplantation. With the gradual maturation of technology and theory, the fundamental treatment of diabetes will usher in a greater breakthrough. We believe that HUC-MSCs transplantation can provide more options for the management of DM and its complications and bring longer-term benefits to patients.
Abbreviations
DM: diabetes mellitus
IDF: international diabetes federation
T1DM: type 1 diabetes mellitus
T2DM: type 2 diabetes mellitus
MSCs: mesenchymal stem cells
HUC-MSCs: human umbilical cord mesenchymal stem cells
UCB: umbilical cord blood
WJ: wharton's jelly
PDGF: platelet derived growth factor
BM-MSCs: bone marrow-derived mesenchymal stem cells
PU-MSCs: pulp-derived mesenchymal stem cells
AD-MSCs: adipose tissue-derived mesenchymal stem cells
β-cell: beta cell
DiI: 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate
CM-Dil: cell membrane-dil
EVs: extracellular vesicles
KGF: keratinocyte growth factor
HGF: hepatocyte growth factor
VEGF: vascular endothelial growth factor
FGF: fibroblast growth factor
PGF: placental growth factor
MCP-1: monocyte chemoattractant protein 1
IGF-1: insulin-like growth factor 1
EGF: epidermal growth factor
PGE2: prostaglandin E2
IDO: indoleamine2,3-deoxygenase
IL-10: interleukin-10
IL-6: interleukin-6
TGF-β1: transforming growth factor-β1
NO: nitric oxide
HLA-G5: human leukocyte antigen-G5
TSG-6: tumor necrosis factor α stimulated gene 6
HucMSC-ex: human umbilical cord mesenchymal stem cell-derived exosome
HUC-MSCs-sEVs: human umbilical cord mesenchymal stem cell-derived small extracellular vesicle
ER: endoplasmic reticulum
PDX-1: pancreatic duodenal homeobox-1
NGN3: neurogenin3
PAX6: paired box 6
PAX4: paired box 4
NKX2.2: nk2 homeobox 2
NKX6.1: nk6 homeobox 1
GLUT-2: glucose transporter 2
INS: insulin
HDAC: histone deacetylase
IL-1b: interleukin-1b
IL-1Ra: interleukin-1 receptor antagonist
MafA: MAF bZIP transcription factor A
PβLCs: pancreatic β-like cells
TIMP: tissue inhibitors of matrix metalloproteinase
α-cell: alpha cell
DCs: dendritic cells
NK: cytotoxicity of natural killer cell
Treg: regulatory T cells
Th17: t helper cell 17
Th1: t helper cell 1
SS: sjogren syndrome
UC-MSC-CM: umbilical cord-mesenchymal stem cell-conditioned medium
GLUT4: glucose transporter 4
FMD: fasting-mimicking diet
GLP-1: glucagon-like peptide-1
SAP: severe acute pancreatitis
GDM: gestational diabetes mellitus
DN: diabetic nephropathy
TNF-α: tumor necrosis factorTh17- alpha
NF-κB: nuclear factor-κB
α-SMA: alpha-smooth muscle actin
HG: high glucose
HNE: 4-hydroxynonenal
CAT: catalase
GPX: glutathione peroxidase
HSP47: heat shock protein 47
BMP-7: bone morphogenetic protein 7
DR: diabetic retinopathy
APN: adiponectin
NT-4: neurotrophin-4
MIAT: myocardial infarction-associated transcript
hs-CRP: high-sensitivity C-Reactive Protein
RGCs: retinal ganglion cells
HMGB1: high mobility group box 1
WM: white matter
HUCBCs: human umbilical cord blood cells
HUCB34: CD34-immunosorted human umbilical cord blood hematopoietic stem cells
NPCs: neuronal progenitor cells
VCAM-1: vascular cell adhesion molecule-1
Ang-1: angiopoietin 1
IBZ: ischemic border zone
MMP-9: matrix metalloproteinase 9
TLR4: toll-like receptor 4
DAN: diabetic autonomic neuropathy
DC: diabetic cystopathy
NGF: nerve growth factor
GFAP: glial fibrillary acidic protein
DUA: detrusor underactivity
eNOS: endothelial nitric oxide synthase
bFGF: basic fibroblast growth factor
DFU: diabetic foot ulcer
PAD: peripheral artery disease
SR-B1: scavenger receptor class B type1
PECAM-1/CD31: platelet endothelial cell adhesion molecule-1
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
ACE2: angiotensin converting enzyme 2
TMPRSS2: transmembrane protease, serine 2
ACEI: angiotensin converting enzyme inhibitor
ARB: angiotensin receptor blocker
IL-2R: interleukin-2R
IL-8: interleukin-8
IFNg: interferon-g
IFNγ: interferon-γ
RANTES: regulated upon activation normal T cell expressed and secreted factor
ARDS: automated resources directory service
PaO2: partial pressure of oxygen
FiO2: fraction of inspiration oxygen
PGE2: prostaglandin E2
NP: nanoparticles
hIDC: human pancreatic islet-derived progenitor cells
f-SWCNT-COOH: carboxylic acid functionalized single walled carbon nanotubes
HSC: hematopoietic stem cell
HSPC: hematopoietic stem progenitor cell
Acknowledgements
Figures for this work were created in BioRender.com and are gratefully acknowledged.
Funding
The financial support for this work from the National Natural Science Foundation of China (Grant No. 81471028) and Jilin Provincial Department of science and technology (Grant No. 3D5223990429) is gratefully acknowledged.
Author contributions
Luyao Li contributed to the conceptualization and wrote the manuscript.Jicui Li contributed to reviewing the draft and making necessary modifcations, and Haifei Guan participated in drawing the figures. Chuan Zhang,Hisashi Oishi and Satoru Takahashi designed the study. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes research and clinical practice. 2019;157:107843
2. Ridler C. Diabetes: Islet transplantation for T1DM. Nature reviews Endocrinology. 2016;12(7):373
3. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. International journal of medical sciences. 2014;11(11):1185-200
4. Papatheodorou K, Papanas N, Banach M, Papazoglou D, Edmonds M. Complications of Diabetes 2016. Journal of diabetes research. 2016;2016:6989453
5. Ali MK, Pearson-Stuttard J, Selvin E, Gregg EW. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia. 2022;65(1):3-13
6. DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators of inflammation. 2010;2010:865601
7. Davies JE, Walker JT, Keating A. Concise Review: Wharton's Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem cells translational medicine. 2017;6(7):1620-30
8. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem cells (Dayton, Ohio). 2003;21(1):105-10
9. Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem cells and development. 2010;19(4):491-502
10. Grzywocz Z, Pius-Sadowska E, Klos P, Gryzik M, Wasilewska D, Aleksandrowicz B. et al. Growth factors and their receptors derived from human amniotic cells in vitro. Folia histochemica et cytobiologica. 2014;52(3):163-70
11. Harris DT. Umbilical cord tissue mesenchymal stem cells: characterization and clinical applications. Current stem cell research & therapy. 2013;8(5):394-9
12. Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W. et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem cell research & therapy. 2020;11(1):519
13. Simões IN, Boura JS, dos Santos F, Andrade PZ, Cardoso CM, Gimble JM. et al. Human mesenchymal stem cells from the umbilical cord matrix: successful isolation and ex vivo expansion using serum-/xeno-free culture media. Biotechnology journal. 2013;8(4):448-58
14. Choudhery MS, Badowski M, Muise A, Harris DT. Utility of cryopreserved umbilical cord tissue for regenerative medicine. Current stem cell research & therapy. 2013;8(5):370-80
15. Li J, Xu SQ, Zhao YM, Yu S, Ge LH, Xu BH. Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Molecular medicine reports. 2018;18(6):4969-77
16. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Experimental hematology. 2000;28(8):875-84
17. Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mechanisms of ageing and development. 2001;122(7):713-34
18. Wu LF, Wang NN, Liu YS, Wei X. Differentiation of Wharton's jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue engineering Part A. 2009;15(10):2865-73
19. Ma Y, Wang L, Yang S, Liu D, Zeng Y, Lin L. et al. The tissue origin of human mesenchymal stem cells dictates their therapeutic efficacy on glucose and lipid metabolic disorders in type II diabetic mice. Stem cell research & therapy. 2021;12(1):385
20. Wang H, Qiu X, Ni P, Qiu X, Lin X, Wu W. et al. Immunological characteristics of human umbilical cord mesenchymal stem cells and the therapeutic effects of their transplantion on hyperglycemia in diabetic rats. International journal of molecular medicine. 2014;33(2):263-70
21. Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetology & metabolic syndrome. 2017;9:36
22. Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. Stem cells (Dayton, Ohio). 2020;38(10):1241-53
23. Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-38
24. Maldonado M, Huang T, Yang L, Xu L, Ma L. Human umbilical cord Wharton jelly cells promote extra-pancreatic insulin formation and repair of renal damage in STZ-induced diabetic mice. Cell communication and signaling: CCS. 2017;15(1):43
25. Yin Y, Hao H, Cheng Y, Gao J, Liu J, Xie Z. et al. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. International immunopharmacology. 2018;60:235-45
26. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem cell research & therapy. 2018;9(1):63
27. Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem cells and development. 2017;26(9):617-31
28. Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A. et al. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes & metabolism. 2009;35(2):85-93
29. Stiner R, Alexander M, Liu G, Liao W, Liu Y, Yu J. et al. Transplantation of stem cells from umbilical cord blood as therapy for type I diabetes. Cell and tissue research. 2019;378(2):155-62
30. Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16(5):579-85
31. Sharma R, Kumari M, Mishra S, Chaudhary DK, Kumar A, Avni B. et al. Exosomes Secreted by Umbilical Cord Blood-Derived Mesenchymal Stem Cell Attenuate Diabetes in Mice. Journal of diabetes research. 2021;2021:9534574
32. Chen MT, Zhao YT, Zhou LY, Li M, Zhang Q, Han Q. et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Enhance Insulin Sensitivity in Insulin Resistant Human Adipocytes. Current medical science. 2021;41(1):87-93
33. Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B. et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS nano. 2018;12(8):7613-28
34. Yap SK, Tan KL, Abd Rahaman NY, Saulol Hamid NF, Ooi J, Tor YS. et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Ameliorated Insulin Resistance in Type 2 Diabetes Mellitus Rats. Pharmaceutics. 2022 14(3)
35. Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M. et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem cell research & therapy. 2020;11(1):97
36. Mohammadi MR, Rodriguez SM, Luong JC, Li S, Cao R, Alshetaiwi H. et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Communications biology. 2021;4(1):685
37. Moshrefi M, Yari N, Nabipour F, Bazrafshani MR, Nematollahi-mahani SN. Transplantation of differentiated umbilical cord mesenchymal cells under kidney capsule for control of type I diabetes in rat. Tissue & cell. 2015;47(4):395-405
38. Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP. et al. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell transplantation. 2011;20(3):455-66
39. Montanucci P, Pescara T, Alunno A, Bistoni O, Basta G, Calafiore R. Remission of hyperglycemia in spontaneously diabetic NOD mice upon transplant of microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells (hUCMS). Xenotransplantation. 2019;26(2):e12476
40. Prabakar KR, Domínguez-Bendala J, Molano RD, Pileggi A, Villate S, Ricordi C. et al. Generation of glucose-responsive, insulin-producing cells from human umbilical cord blood-derived mesenchymal stem cells. Cell transplantation. 2012;21(6):1321-39
41. Su X, Fang S, Zhang D, Zhang Q, He Y, Lu X. et al. Quantitative Raman spectral changes of the differentiation of mesenchymal stem cells into islet-like cells by biochemical component analysis and multiple peak fitting. Journal of biomedical optics. 2015;20(12):125002
42. Phuc PV, Nhung TH, Loan DT, Chung DC, Ngoc PK. Differentiating of banked human umbilical cord blood-derived mesenchymal stem cells into insulin-secreting cells. In vitro cellular & developmental biology Animal. 2011;47(1):54-63
43. Wang HW, Lin LM, He HY, You F, Li WZ, Huang TH. et al. Human umbilical cord mesenchymal stem cells derived from Wharton's jelly differentiate into insulin-producing cells in vitro. Chinese medical journal. 2011;124(10):1534-9
44. Hu YH, Wu DQ, Gao F, Li GD, Yao L, Zhang XC. A secretory function of human insulin-producing cells in vivo. Hepatobiliary & pancreatic diseases international: HBPD INT. 2009;8(3):255-60
45. Yu YB, Bian JM, Gu DH. Transplantation of insulin-producing cells to treat diabetic rats after 90% pancreatectomy. World journal of gastroenterology. 2015;21(21):6582-90
46. Chao KC, Chao KF, Chen CF, Liu SH. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters. Cell transplantation. 2008;17(6):657-64
47. Wang G, Li Y, Wang Y, Dong Y, Wang FS, Ding Y. et al. Roles of the co-culture of human umbilical cord Wharton's jelly-derived mesenchymal stem cells with rat pancreatic cells in the treatment of rats with diabetes mellitus. Experimental and therapeutic medicine. 2014;8(5):1389-96
48. Seyedi F, Farsinejad A, Moshrefi M, Nematollahi-Mahani SN. In vitro evaluation of different protocols for the induction of mesenchymal stem cells to insulin-producing cells. In vitro cellular & developmental biology Animal. 2015;51(8):866-78
49. Gao F, Wu DQ, Hu YH, Jin GX. Extracellular matrix gel is necessary for in vitro cultivation of insulin producing cells from human umbilical cord blood derived mesenchymal stem cells. Chinese medical journal. 2008;121(9):811-8
50. He D, Wang J, Gao Y, Zhang Y. Differentiation of PDX1 gene-modified human umbilical cord mesenchymal stem cells into insulin-producing cells in vitro. International journal of molecular medicine. 2011;28(6):1019-24
51. Wang XL, Hu P, Guo XR, Yan D, Yuan Y, Yan SR. et al. Reprogramming human umbilical cord mesenchymal stromal cells to islet-like cells with the use of in vitro-synthesized pancreatic-duodenal homebox 1 messenger RNA. Cytotherapy. 2014;16(11):1519-27
52. Hu YH, Wu DQ, Gao F, Li GD, Zhang XC. Notch signaling: a novel regulating differentiation mechanism of human umbilical cord blood-derived mesenchymal stem cells into insulin-producing cells in vitro. Chinese medical journal. 2010;123(5):606-14
53. Qu H, Liu X, Ni Y, Jiang Y, Feng X, Xiao J. et al. Laminin 411 acts as a potent inducer of umbilical cord mesenchymal stem cell differentiation into insulin-producing cells. Journal of translational medicine. 2014;12:135
54. Sun B, Meng XH, Liu R, Yan S, Xiao ZD. Mechanism study for hypoxia induced differentiation of insulin-producing cells from umbilical cord blood-derived mesenchymal stem cells. Biochemical and biophysical research communications. 2015;466(3):444-9
55. Chandravanshi B, Bhonde R. Small molecules exert anti-apoptotic effect and reduce oxidative stress augmenting insulin secretion in stem cells engineered islets against hypoxia. European journal of pharmacology. 2016;791:424-32
56. Tsai PJ, Wang HS, Shyr YM, Weng ZC, Tai LC, Shyu JF. et al. Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats. Journal of biomedical science. 2012;19(1):47
57. Seyedi F, Farsinejad A, Nematollahi-Mahani SA, Eslaminejad T, Nematollahi-Mahani SN. Suspension Culture Alters Insulin Secretion in Induced Human Umbilical Cord Matrix-Derived Mesenchymal Cells. Cell journal. 2016;18(1):52-61
58. Belame Shivakumar S, Bharti D, Baregundi Subbarao R, Park JM, Son YB, Ullah I. et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton's jelly mesenchymal stem cells using small molecules. Journal of cellular physiology. 2019;234(4):3933-47
59. Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M. et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2016;101(3):1044-54
60. Wang L, Liu T, Liang R, Wang G, Liu Y, Zou J. et al. Mesenchymal stem cells ameliorate β cell dysfunction of human type 2 diabetic islets by reversing β cell dedifferentiation. EBioMedicine. 2020;51:102615
61. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K. et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11002-7
62. Li B, Cheng Y, Yin Y, Xue J, Yu S, Gao J. et al. Reversion of early- and late-stage β-cell dedifferentiation by human umbilical cord-derived mesenchymal stem cells in type 2 diabetic mice. Cytotherapy. 2021;23(6):510-20
63. Zhang T, Wang H, Wang T, Wei C, Jiang H, Jiang S. et al. Pax4 synergistically acts with Pdx1, Ngn3 and MafA to induce HuMSCs to differentiate into functional pancreatic β-cells. Experimental and therapeutic medicine. 2019;18(4):2592-8
64. Wei L, Zhang L, Yang L, Wang X, Zhao C, Zhao D. Protective Effect of Mesenchymal Stem Cells on Isolated Islets Survival and Against Hypoxia Associated With the HIF-1α/PFKFB3 Pathway. Cell transplantation. 2022;31:9636897211073127
65. Zhou Y, Hu Q, Chen F, Zhang J, Guo J, Wang H. et al. Human umbilical cord matrix-derived stem cells exert trophic effects on β-cell survival in diabetic rats and isolated islets. Disease models & mechanisms. 2015;8(12):1625-33
66. Bao Y, Zhao Z, Gao H. Effect of hTIMP-1 overexpression in human umbilical cord mesenchymal stem cells on the repair of pancreatic islets in type-1 diabetic mice. Cell biology international. 2021;45(5):1038-49
67. Lu J, Shen SM, Ling Q, Wang B, Li LR, Zhang W. et al. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem cell research & therapy. 2021;12(1):340
68. Hu J, Wang F, Sun R, Wang Z, Yu X, Wang L. et al. Effect of combined therapy of human Wharton's jelly-derived mesenchymal stem cells from umbilical cord with sitagliptin in type 2 diabetic rats. Endocrine. 2014;45(2):279-87
69. Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y. et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem cells (Dayton, Ohio). 2016;34(3):627-39
70. Xue J, Gao J, Gu Y, Wang A, Yu S, Li B. et al. Human umbilical cord-derived mesenchymal stem cells alleviate insulin resistance in diet-induced obese mice via an interaction with splenocytes. Stem cell research & therapy. 2022;13(1):109
71. Yin Y, Hao H, Cheng Y, Zang L, Liu J, Gao J. et al. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell death & disease. 2018;9(7):760
72. Xue J, Cheng Y, Hao H, Gao J, Yin Y, Yu S. et al. Low-Dose Decitabine Assists Human Umbilical Cord-Derived Mesenchymal Stem Cells in Protecting β Cells via the Modulation of the Macrophage Phenotype in Type 2 Diabetic Mice. Stem cells international. 2020;2020:4689798
73. Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell proliferation. 2020;53(1):e12712
74. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature reviews Immunology. 2008;8(9):726-36
75. Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, Li H. et al. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Current pharmaceutical design. 2013;19(27):4893-9
76. Kong D, Zhuang X, Wang D, Qu H, Jiang Y, Li X. et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clinical laboratory. 2014;60(12):1969-76
77. Montanucci P, Alunno A, Basta G, Bistoni O, Pescara T, Caterbi S. et al. Restoration of t cell substes of patients with type 1 diabetes mellitus by microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells: An in vitro study. Clinical immunology (Orlando, Fla). 2016;163:34-41
78. Qi J, Tang X, Li W, Chen W, Yao G, Sun L. Mesenchymal stem cells inhibited the differentiation of MDSCs via COX2/PGE2 in experimental sialadenitis. Stem cell research & therapy. 2020;11(1):325
79. Liu Y, Li C, Wang S, Guo J, Guo J, Fu J. et al. Human umbilical cord mesenchymal stem cells confer potent immunosuppressive effects in Sjögren's syndrome by inducing regulatory T cells. Modern rheumatology. 2021;31(1):186-96
80. Sun X, Hao H, Han Q, Song X, Liu J, Dong L. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem cell research & therapy. 2017;8(1):241
81. Kim KS, Choi YK, Kim MJ, Hwang JW, Min K, Jung SY. et al. Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell. Diabetes & metabolism journal. 2021;45(2):260-9
82. Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem cell research & therapy. 2020;11(1):401
83. Zhao N, Gao YF, Bao L, Lei J, An HX, Pu FX. et al. Glycemic control by umbilical cord-derived mesenchymal stem cells promotes effects of fasting-mimicking diet on type 2 diabetic mice. Stem cell research & therapy. 2021;12(1):395
84. Chang Y, Dong M, Wang Y, Yu H, Sun C, Jiang X. et al. GLP-1 Gene-Modified Human Umbilical Cord Mesenchymal Stem Cell Line Improves Blood Glucose Level in Type 2 Diabetic Mice. Stem cells international. 2019;2019:4961865
85. Xu X, Wang W, Lin L, Chen P. Liraglutide in combination with human umbilical cord mesenchymal stem cell could improve liver lesions by modulating TLR4/NF-kB inflammatory pathway and oxidative stress in T2DM/NAFLD rats. Tissue & cell. 2020;66:101382
86. Wang W, Wu RD, Chen P, Xu XJ, Shi XZ, Huang LH. et al. Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes. Diabetes/metabolism research and reviews. 2020;36(2):e3212
87. Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H. et al. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocrine journal. 2013;60(3):347-57
88. Yang B, Bai B, Liu CX, Wang SQ, Jiang X, Zhu CL. et al. Effect of umbilical cord mesenchymal stem cells on treatment of severe acute pancreatitis in rats. Cytotherapy. 2013;15(2):154-62
89. Kong L, Xu X, Zhang H, Zhou Y, Huang H, Chen B. et al. Human umbilical cord-derived mesenchymal stem cells improve chronic pancreatitis in rats via the AKT-mTOR-S6K1 signaling pathway. Bioengineered. 2021;12(1):1986-96
90. Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y. et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer letters. 2019;442:351-61
91. Wu D, Zou S, Chen H, Li X, Xu Y, Zuo Q. et al. Transplantation routes affect the efficacy of human umbilical cord mesenchymal stem cells in a rat GDM model. Clinica chimica acta; international journal of clinical chemistry. 2017;475:137-46
92. Wajid N, Naseem R, Anwar SS, Awan SJ, Ali M, Javed S. et al. The effect of gestational diabetes on proliferation capacity and viability of human umbilical cord-derived stromal cells. Cell and tissue banking. 2015;16(3):389-97
93. An X, Liao G, Chen Y, Luo A, Liu J, Yuan Y. et al. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem cell research & therapy. 2019;10(1):363
94. Fang TC, Pang CY, Chiu SC, Ding DC, Tsai RK. Renoprotective effect of human umbilical cord-derived mesenchymal stem cells in immunodeficient mice suffering from acute kidney injury. PloS one. 2012;7(9):e46504
95. Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C. et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem cell research & therapy. 2020;11(1):336
96. Xian Y, Lin Y, Cao C, Li L, Wang J, Niu J. et al. Protective effect of umbilical cord mesenchymal stem cells combined with resveratrol against renal podocyte damage in NOD mice. Diabetes research and clinical practice. 2019;156:107755
97. Wang Y, Liu J, Zhang Q, Wang W, Liu Q, Liu S. et al. Human umbilical cord mesenchymal stem cells attenuate podocyte injury under high glucose via TLR2 and TLR4 signaling. Diabetes research and clinical practice. 2021;173:108702
98. Nie P, Bai X, Lou Y, Zhu Y, Jiang S, Zhang L. et al. Human umbilical cord mesenchymal stem cells reduce oxidative damage and apoptosis in diabetic nephropathy by activating Nrf2. Stem cell research & therapy. 2021;12(1):450
99. Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes research and clinical practice. 2012;98(3):465-73
100. Park JH, Park J, Hwang SH, Han H, Ha H. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury. Transplantation proceedings. 2012;44(4):1123-6
101. Chang JW, Hung SP, Wu HH, Wu WM, Yang AH, Tsai HL. et al. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell transplantation. 2011;20(2):245-57
102. Yu C, Yang K, Meng X, Cao B, Wang F. Downregulation of Long Noncoding RNA MIAT in the Retina of Diabetic Rats with Tail-vein Injection of Human Umbilical-cord Mesenchymal Stem Cells. International journal of medical sciences. 2020;17(5):591-8
103. Chen SN, Xu ZG, Ma YX, Chen S, He GH, Han M. et al. Protective effect of LIF-huMSCs on the retina of diabetic model rats. International journal of ophthalmology. 2021;14(10):1508-17
104. Gao X, He GH, Zhang XT, Chen S. Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway. International journal of ophthalmology. 2021;14(11):1683-9
105. Zhang W, Wang Y, Kong J, Dong M, Duan H, Chen S. Therapeutic efficacy of neural stem cells originating from umbilical cord-derived mesenchymal stem cells in diabetic retinopathy. Scientific reports. 2017;7(1):408
106. Yu B, Li XR, Zhang XM. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World journal of stem cells. 2020;12(3):178-87
107. Fu Y, Gao X, He GH, Chen S, Gu ZH, Zhang YL. et al. Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging. International journal of ophthalmology. 2021;14(12):1828-33
108. Li W, Jin LY, Cui YB, Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. International immunopharmacology. 2021;90:107010
109. Xu Z, Tian N, Li S, Li K, Guo H, Zhang H. et al. Extracellular vesicles secreted from mesenchymal stem cells exert anti-apoptotic and anti-inflammatory effects via transmitting microRNA-18b in rats with diabetic retinopathy. International immunopharmacology. 2021;101(Pt B):108234
110. Zhang W, Wang Y, Kong Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investigative ophthalmology & visual science. 2019;60(1):294-303
111. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39(10):2749-55
112. Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Burvill PW, Anderson CS. et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29(12):2491-500
113. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210-5
114. Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42(2):445-52
115. Newman MB, Davis CD, Kuzmin-Nichols N, Sanberg PR. Human umbilical cord blood (HUCB) cells for central nervous system repair. Neurotoxicity research. 2003;5(5):355-68
116. Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE. et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32(11):2682-8
117. Lin CH, Lee HT, Lee SD, Lee W, Cho CW, Lin SZ. et al. Role of HIF-1α-activated Epac1 on HSC-mediated neuroplasticity in stroke model. Neurobiology of disease. 2013;58:76-91
118. Chen J, Ning R, Zacharek A, Cui C, Cui X, Yan T. et al. MiR-126 Contributes to Human Umbilical Cord Blood Cell-Induced Neurorestorative Effects After Stroke in Type-2 Diabetic Mice. Stem cells (Dayton, Ohio). 2016;34(1):102-13
119. Yan T, Venkat P, Ye X, Chopp M, Zacharek A, Ning R. et al. HUCBCs increase angiopoietin 1 and induce neurorestorative effects after stroke in T1DM rats. CNS neuroscience & therapeutics. 2014;20(10):935-44
120. Yan T, Venkat P, Chopp M, Zacharek A, Ning R, Cui Y. et al. Neurorestorative Therapy of Stroke in Type 2 Diabetes Mellitus Rats Treated With Human Umbilical Cord Blood Cells. Stroke. 2015;46(9):2599-606
121. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes care. 2003;26(5):1553-79
122. Yuan Z, Tang Z, He C, Tang W. Diabetic cystopathy: A review. Journal of diabetes. 2015;7(4):442-7
123. WenBo W, Fei Z, YiHeng D, Wei W, TingMang Y, WenHao Z. et al. Human Umbilical Cord Mesenchymal Stem Cells Overexpressing Nerve Growth Factor Ameliorate Diabetic Cystopathy in Rats. Neurochemical research. 2017;42(12):3537-47
124. Shin JH, Ryu CM, Ju H, Yu HY, Song S, Hong KS. et al. Therapeutic Efficacy of Human Embryonic Stem Cell-Derived Multipotent Stem/Stromal Cells in Diabetic Detrusor Underactivity: A Preclinical Study. Journal of clinical medicine. 2020 9(9)
125. Wu JH, Wang DY, Sheng L, Qian WQ, Xia SJ, Jiang Q. Human umbilical cord Wharton's jelly-derived mesenchymal stem cell transplantation could improve diabetic intracavernosal pressure. Asian journal of andrology. 2022;24(2):171-5
126. Subrata SA, Phuphaibul R. Diabetic foot ulcer care: a concept analysis of the term integrated into nursing practice. Scandinavian journal of caring sciences. 2019;33(2):298-310
127. American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes care. 2014;37(Suppl 1):S14-80
128. Santema KTB, Stoekenbroek RM, Koelemay MJW, Reekers JA, van Dortmont LMC, Oomen A. et al. Hyperbaric Oxygen Therapy in the Treatment of Ischemic Lower- Extremity Ulcers in Patients With Diabetes: Results of the DAMO(2)CLES Multicenter Randomized Clinical Trial. Diabetes care. 2018;41(1):112-9
129. Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q. et al. Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International journal of biological sciences. 2013;10(1):80-9
130. Shi R, Lian W, Jin Y, Cao C, Han S, Yang X. et al. Role and effect of vein-transplanted human umbilical cord mesenchymal stem cells in the repair of diabetic foot ulcers in rats. Acta biochimica et biophysica Sinica. 2020;52(6):620-30
131. Xia N, Xu JM, Zhao N, Zhao QS, Li M, Cheng ZF. Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neuroscience letters. 2015;597:84-9
132. Luo Y, Liang F, Wan X, Liu S, Fu L, Mo J. et al. Hyaluronic Acid Facilitates Angiogenesis of Endothelial Colony Forming Cell Combining With Mesenchymal Stem Cell via CD44/ MicroRNA-139-5p Pathway. Frontiers in bioengineering and biotechnology. 2022;10:794037
133. Zhao L, Guo Z, Chen K, Yang W, Wan X, Zeng P. et al. Combined Transplantation of Mesenchymal Stem Cells and Endothelial Colony-Forming Cells Accelerates Refractory Diabetic Foot Ulcer Healing. Stem cells international. 2020;2020:8863649
134. Hashemi SS, Mohammadi AA, Kabiri H, Hashempoor MR, Mahmoodi M, Amini M. et al. The healing effect of Wharton's jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. Journal of cosmetic dermatology. 2019;18(6):1961-7
135. Shen WC, Liang CJ, Wu VC, Wang SH, Young GH, Lai IR. et al. Endothelial progenitor cells derived from Wharton's jelly of the umbilical cord reduces ischemia-induced hind limb injury in diabetic mice by inducing HIF-1α/IL-8 expression. Stem cells and development. 2013;22(9):1408-18
136. Qin HL, Zhu XH, Zhang B, Zhou L, Wang WY. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2016;124(8):497-503
137. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W. et al. Differential roles of macrophages in diverse phases of skin repair. Journal of immunology (Baltimore, Md: 1950). 2010;184(7):3964-77
138. Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H. et al. Suppressed recruitment of alternatively activated macrophages reduces TGF-β1 and impairs wound healing in streptozotocin-induced diabetic mice. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2015;70:317-25
139. Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2006;23(6):594-608
140. Goren I, Kämpfer H, Podda M, Pfeilschifter J, Frank S. Leptin and wound inflammation in diabetic ob/ob mice: differential regulation of neutrophil and macrophage influx and a potential role for the scab as a sink for inflammatory cells and mediators. Diabetes. 2003;52(11):2821-32
141. Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579-87
142. Fong CY, Tam K, Cheyyatraivendran S, Gan SU, Gauthaman K, Armugam A. et al. Human Wharton's jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. Journal of cellular biochemistry. 2014;115(2):290-302
143. Shrestha C, Zhao L, Chen K, He H, Mo Z. Enhanced healing of diabetic wounds by subcutaneous administration of human umbilical cord derived stem cells and their conditioned media. International journal of endocrinology. 2013;2013:592454
144. Palma MB, Luzzani C, Andrini LB, Riccillo F, Buero G, Pelinski P. et al. Wound Healing by Allogeneic Transplantation of Specific Subpopulation From Human Umbilical Cord Mesenchymal Stem Cells. Cell transplantation. 2021;30:963689721993774
145. Huang J, Wu S, Wu M, Zeng Q, Wang X, Wang H. Efficacy of the therapy of 5-aminolevulinic acid photodynamic therapy combined with human umbilical cord mesenchymal stem cells on methicillin-resistant Staphylococcus aureus-infected wound in a diabetic mouse model. Photodiagnosis and photodynamic therapy. 2021;36:102480
146. Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Materials science & engineering C, Materials for biological applications. 2021;120:111671
147. Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y. et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Frontiers in bioengineering and biotechnology. 2022;10:829868
148. Wei Q, Wang Y, Ma K, Li Q, Li B, Hu W. et al. Extracellular Vesicles from Human Umbilical Cord Mesenchymal Stem Cells Facilitate Diabetic Wound Healing Through MiR-17-5p-mediated Enhancement of Angiogenesis. Stem cell reviews and reports. 2022;18(3):1025-40
149. Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem cell research & therapy. 2020;11(1):39
150. Jung JA, Yoon YD, Lee HW, Kang SR, Han SK. Comparison of human umbilical cord blood-derived mesenchymal stem cells with healthy fibroblasts on wound-healing activity of diabetic fibroblasts. International wound journal. 2018;15(1):133-9
151. You HJ, Namgoong S, Han SK, Jeong SH, Dhong ES, Kim WK. Wound-healing potential of human umbilical cord blood-derived mesenchymal stromal cells in vitro-a pilot study. Cytotherapy. 2015;17(11):1506-13
152. Moon KC, Lee JS, Han SK, Lee HW, Dhong ES. Effects of human umbilical cord blood-derived mesenchymal stromal cells and dermal fibroblasts on diabetic wound healing. Cytotherapy. 2017;19(7):821-8
153. Nilforoushzadeh MA, Raoofi A, Afzali H, Gholami O, Zare S, Nasiry D. et al. Promotion of cutaneous diabetic wound healing by subcutaneous administration of Wharton's jelly mesenchymal stem cells derived from umbilical cord. Archives of dermatological research. 2023Mar;315(2):147-159
154. Han Y, Sun T, Han Y, Lin L, Liu C, Liu J. et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. European journal of medical research. 2019;24(1):10
155. Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z. c-Jun Overexpression Accelerates Wound Healing in Diabetic Rats by Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem cells international. 2020;2020:7430968
156. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. International journal of nanomedicine. 2020;15:5911-26
157. Milan PB, Lotfibakhshaiesh N, Joghataie MT, Ai J, Pazouki A, Kaplan DL. et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta biomaterialia. 2016;45:234-46
158. Montanucci P, di Pasquali C, Ferri I, Pescara T, Pennoni I, Siccu P. et al. Human Umbilical Cord Wharton Jelly-Derived Adult Mesenchymal Stem Cells, in Biohybrid Scaffolds, for Experimental Skin Regeneration. Stem cells international. 2017;2017:1472642
159. Srifa W, Kosaric N, Amorin A, Jadi O, Park Y, Mantri S. et al. Cas9-AAV6-engineered human mesenchymal stromal cells improved cutaneous wound healing in diabetic mice. Nature communications. 2020;11(1):2470
160. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. The lancet Diabetes & endocrinology. 2022;10(5):311-21
161. Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H. et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The lancet Diabetes & endocrinology. 2020;8(10):813-22
162. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2020;127:104354
163. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80.e8
164. Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE. et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132-9
165. Roca-Ho H, Riera M, Palau V, Pascual J, Soler MJ. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. International journal of molecular sciences. 2017 18(3)
166. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory medicine. 2020;8(4):e21
167. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell stem cell. 2013;13(4):392-402
168. Hernandez JJ, Beaty DE, Fruhwirth LL, Lopes Chaves AP, Riordan NH. Dodging COVID-19 infection: low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. Journal of translational medicine. 2021;19(1):149
169. Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D. et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem cells translational medicine. 2021;10(5):660-73
170. Khanh VC, Fukushige M, Chang YH, Hoang NN, Yamashita T, Obata-Yasuoka M. et al. Wharton's Jelly Mesenchymal Stem Cell-Derived Extracellular Vesicles Reduce SARS-CoV2-Induced Inflammatory Cytokines Under High Glucose and Uremic Toxin Conditions. Stem cells and development. 2021;30(15):758-72
171. Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE. et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem cell research & therapy. 2021;12(1):91
172. Tao J, Nie Y, Wu H, Cheng L, Qiu Y, Fu J. et al. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. Journal of infection in developing countries. 2020;14(10):1138-45
173. Ma W, Wu JH, Wang Q, Lemaitre RN, Mukamal KJ, Djoussé L. et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. The American journal of clinical nutrition. 2015;101(1):153-63
174. Boland LK, Burand AJ, Boyt DT, Dobroski H, Di L, Liszewski JN. et al. Nature vs. Nurture: Defining the Effects of Mesenchymal Stromal Cell Isolation and Culture Conditions on Resiliency to Palmitate Challenge. Frontiers in immunology. 2019;10:1080
175. El-Hossary N, Hassanein H, El-Ghareeb AW, Issa H. Intravenous vs intraperitoneal transplantation of umbilical cord mesenchymal stem cells from Wharton's jelly in the treatment of streptozotocin-induced diabetic rats. Diabetes research and clinical practice. 2016;121:102-11
176. Burand AJ Jr, Di L, Boland LK, Boyt DT, Schrodt MV, Santillan DA. et al. Aggregation of Human Mesenchymal Stromal Cells Eliminates Their Ability to Suppress Human T Cells. Frontiers in immunology. 2020;11:143
177. Yang XF, Chen T, Ren LW, Yang L, Qi H, Li FR. Immunogenicity of insulin-producing cells derived from human umbilical cord mesenchymal stem cells. Experimental and therapeutic medicine. 2017;13(4):1456-64
178. Ngoc PK, Phuc PV, Nhung TH, Thuy DT, Nguyet NT. Improving the efficacy of type 1 diabetes therapy by transplantation of immunoisolated insulin-producing cells. Human cell. 2011;24(2):86-95
179. Teotia RS, Kadam S, Singh AK, Verma SK, Bahulekar A, Kanetkar S. et al. Islet encapsulated implantable composite hollow fiber membrane based device: A bioartificial pancreas. Materials science & engineering C, Materials for biological applications. 2017;77:857-66
180. Li X, Wei Z, Wu L, Lv H, Zhang Y, Li J. et al. Efficacy of Fe(3)O(4)@polydopamine nanoparticle-labeled human umbilical cord Wharton's jelly-derived mesenchymal stem cells in the treatment of streptozotocin-induced diabetes in rats. Biomaterials science. 2020;8(19):5362-75
181. Montanucci P, Pescara T, Greco A, Leonardi G, Marini L, Basta G. et al. Co-microencapsulation of human umbilical cord-derived mesenchymal stem and pancreatic islet-derived insulin producing cells in experimental type 1 diabetes. Diabetes/metabolism research and reviews. 2021;37(2):e3372
182. Bari S, Chu PP, Lim A, Fan X, Gay FP, Bunte RM. et al. Protective role of functionalized single walled carbon nanotubes enhance ex vivo expansion of hematopoietic stem and progenitor cells in human umbilical cord blood. Nanomedicine: nanotechnology, biology, and medicine. 2013;9(8):1304-16
183. Fan X, Gay FP, Lim FW, Ang JM, Chu PP, Bari S. et al. Low-dose insulin-like growth factor binding proteins 1 and 2 and angiopoietin-like protein 3 coordinately stimulate ex vivo expansion of human umbilical cord blood hematopoietic stem cells as assayed in NOD/SCID gamma null mice. Stem cell research & therapy. 2014;5(3):71
Author contact
![]() Corresponding author: Department of Endocrinology, the Second Hospital of Jilin University, Changchun 130041, Jilin, P.R. China; E-mail: wangs93com.
Corresponding author: Department of Endocrinology, the Second Hospital of Jilin University, Changchun 130041, Jilin, P.R. China; E-mail: wangs93com.

 Global reach, higher impact
Global reach, higher impact