3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(10):2308-2317. doi:10.7150/ijms.112117 This issue Cite
Research Paper
Ellagic acid suppresses the human renal carcinoma cell migration and invasion by targeting the RUNX2/MMP1 expression
1. Division of Nephrology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan.
2. Institute of Medical Sciences, Tzu Chi University, Hualian, Taiwan.
3. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
4. Division of Nephrology, Department of Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
6. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
7. Department of Medicine Research, Buddhist Dalin Tzu Chi Hospital, Chiayi, Taiwan.
8. School of Medicine, Tzu Chi University, Hualian, Taiwan.
*These authors contributed equally
Received 2025-2-13; Accepted 2025-4-11; Published 2025-4-22
Abstract
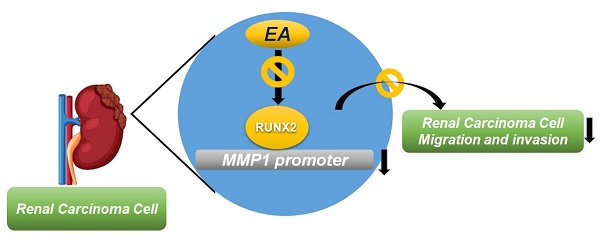
Ellagic acid (EA) exerts anti-carcinogenic activity in various types of cancer. Matrix metalloproteinases (MMPs) are critical mediators in the pathogenesis of renal cell carcinoma (RCC) metastasis. Using in vitro experiments, this study aims to investigate the mechanisms by which EA inhibits RCC migration and invasion. The findings show that EA treatment inhibited RCC cell migration and invasion without reducing cell viability in normal human kidney cells (HK2 cells) and RCC cells (786-O and ACHN). A human proteinase array showed that EA treatment decreased MMP1 mRNA and protein expression levels in 786-O and ACHN cell lines. MMP1 expression is elevated in RCC tissues and correlates with tumor grade, stage, and overall survival in RCC patients. Our molecular docking model indicates a strong interaction between EA and MMP1. The addition of recombinant human MMP1 (Rh-MMP1) to RCC cells increased their migration and invasion; co-treatment with Rh-MMP1 and EA effectively reversed these effects. EA reduced the expression of the transcription factor RUNX2 in both RCC cell lines and knockdown of RUNX2 significantly decreased the migration and invasion abilities of EA-treated 786-O cells. High expression of RUNX2 in RCC patients is associated with higher tumor grade, stage, and poorer survival and correlates positively with MMP1 expression level. These results suggest that EA suppresses RUNX2 targeting of MMP1 expression, thereby conferring anti-invasive properties on RCC cells.
Keywords: ellagic acid, renal cell carcinoma, MMP1, RUNX2, migration, invasion
Introduction
Renal cell carcinoma (RCC) constitutes the largest proportion of all kidney cancers, with a rising incidence over the past decades [1, 2]. The mainstay treatment for advanced RCCs comprises surgical metastasectomy, targeted therapy, and immunotherapy [3, 4]. Despite advancements in anticancer therapeutic strategies, the survival rates for RCC remain low [5, 6].
Matrix metalloproteinases (MMPs) exert proteolytic activity in the metabolism of the extracellular matrix (ECM). MMPs contribute to cancer invasion and metastases via proposed mechanisms that include ECM degradation, promotion of the epithelial-to-mesenchymal transition, aberrant angiogenesis, and induction of inflammatory responses [7, 8]. Both clear cell and papillary RCCs exhibit much higher expression of MMP1 mRNA than non-tumor tissues. In addition, certain subtypes of tissue inhibitors of matrix metalloproteinases (TIMPs) are downregulated in the RCC microenvironment [9]. MMPs have been used as prognostic indicators of advanced RCC. Among patients undergoing immunotherapy for metastatic RCC, higher levels of MMP1 expression were found to correlate with worse progression-free survival [10]. MMP2 and MMP9 overexpression in RCC tumors is reported to be associated with a poorer overall patient prognosis [11]. In RCC cells, the extent of MMP7 expression was found to correlate positively with the degree of angiogenesis, nuclear grade, cancer stage, and patient survival [12]. In summary, various subtypes of MMPs play a critical role in RCC disease progression and related survival.
Ellagic acid (EA), derived from the hydrolysis of ellagitannins found in many plants, is a polyphenol phytochemical involved in a wide variety of physiologic processes [13]. EA eliminates myocardial injury, diminishes the risk of cardiac dysrhythmia, retards the progression of neurodegenerative diseases, and preserves liver function [14-16]. Furthermore, EA protects against carcinogenesis and cancer invasion through the repression of angiogenesis, induction of apoptotic pathways, reversal of the epithelial-to-mesenchymal transition (EMT), stimulation of DNA repair, and downregulation of pro-inflammatory mediators [17, 18]. The molecular mechanisms underlying the anticancer effects of EA have been studied in different types of malignant tumors, including colon cancer, gastric cancer, and hepatocellular carcinoma [19-21]. Our previous studies concluded that several naturally occurring compounds can exert anti-metastatic effects on RCC. Corosolic acid (CA) decreases RCC invasion via the regulation of the extracellular signal-regulated kinase (ERK)-MMP2 signaling pathway. Oxyresveratrol decreases MMP1-mediated RCC invasion and migration through the suppression of ERK and protein kinase Cα phosphorylation [22]. According to the current literature, the antitumor effects of EA on RCC have not been investigated extensively. This study investigates the molecular mechanisms underlying the suppressive effects of EA on RCC cell migration and invasion.
Materials and Methods
Cell lines and culture condition
The RCC cell lines 786-O (clear cell renal cell carcinoma) and ACHN (papillary renal cell carcinoma) were cultured in RPMI-1640 medium. Human normal proximal renal tubular HK2 cells were cultured in DMEM/F12 medium with 10% FBS, 1% penicillin/ streptomycin, and 1% sodium pyruvate. Cells were passaged after reaching 70-80% confluence.
Cell growth assay
Cell growth rates were assessed using the MTT assay. 786-O, ACHN, and HK2 cells were treated with EA at four concentrations (6.25, 12.5, 25, and 50 μM) for 24 h. After EA treatment, the supernatant medium was removed and ice-cold isopropanol added to dissolve the blue-purple formazan crystals. The OD 570 nm was measured using a spectrophotometer.
Cell migration and invasion assay
The 786-O and ACHN cells were treated with EA (12.5, 25, and 50 μM) for 24 h. The Boyden chamber assay (non-Matrigel coated) was used to assess cell migration. The lower chamber was filled with 10% FBS-containing medium, followed by placement of an 8-µm cellulose nitrate filter. EA-treated 786-O cells (1 × 10⁴) or ACHN cells (2 × 10⁴) were seeded into the upper chamber and incubated for 16 h (786-O cells) or 18 h (ACHN cells) to assess migration. For the cell invasion assay, the lower chamber set up as for the migration assay. Matrigel (0.5 mg/mL) was added to the upper chamber and incubated for 2 h to allow for gel solidification. The cells were incubated for 20 h (786-O) or 24 h (ACHN). The membranes were removed and fixed with 100% methanol for 30 min and then stained with Giemsa's stain (1:20) for 4 h. The migrated cells were observed under a 400× optical microscope, photographed, and quantified for statistical analysis.
RNA extraction and qRT-PCR assay
The RCC cells were washed twice with 1 mL of PBS followed by the addition of 1 mL of Trizol reagent and incubation for 2 mins. Chloroform was added, and the mixture was shaken gently up and down for 3 min. After centrifugation for 15 min, the upper aqueous phase (total RNA) was collected and isopropanol added. After centrifugation for 20 min at 4°C, the RNA pellet was air-dried at room temperature. Nuclease-free water was added to dissolve the RNA pellet, and the RNA concentration was assessed using a spectrophotometer. The reverse transcription assay was performed using the GoScript Reverse Transcription Mix (Promega). DEPC-treated water and total RNA were thoroughly mixed, and the RT reaction was conducted under the following conditions: 25°C for 5 min, 42°C for 60 min, and 70°C for 15 min. PCR assays were performed using nuclease-free water, the forward and reverse primers, GoTaq qPCR Master Mix, and cDNA. The PCR tubes were then placed in the StepOnePlus real-time PCR machine and processed using the built-in SYBR-GREEN system settings. The primers of RUNX2 and MMP1 as list: MMP1: F-5'-CTTGCTCATGCTTTTCGACC-3', R-5'-TCCGGGTAGAAGGGATTTGTG-3'; RUNX2: F-5'-CCGGAATGCCTCTGCTGTTATGA, R-5'-ACTGAGGCGGTCAGAGAACAAACT-3'; GAPDH: F-5'-CATCATCCCTGCCTCTACTG-3', R-5'-GCCTGCTTCACCACCTTC-3'.
Protein extraction and western blot analysis
RCC cells were treated with three different concentrations of EA (12.5, 25, and 50 μM) for 24 h, followed by incubation in NETN protein lysis buffer for 30 minutes. The cells were lysed using ultrasonic homogenization and centrifuged at 13,000 rpm for 30 minutes. The supernatant, containing the total protein fraction, was collected. Proteins were separated via 8-10% SDS-PAGE at 100 V for 1 h and then transferred onto a PVDF membrane in transfer buffer at 100 V for 1 h. The membrane was blocked with 5% nonfat milk for 1 h and then incubated with the MMP1 (SC-21731; dilution 1:1000, Santa Cruz Biotechnology), RUNX2 (#12556, dilution 1:1000, Cell Signaling Technology, Inc) and GAPDH (60004-1-Ig, dilution 1:1000, Proteintech Group, Inc) at 4°C overnight. The secondary antibody was then added and incubated at room temperature for 1 h, followed by three washes with TBST. Protein bands were visualized using a chemiluminescent substrate (ECL) and quantified using the Cytiva ImageQuant 800 system.
Clinical database for human RCC tissues
The relationship between MMP-1 and RUNX2 gene expression in renal cell carcinoma (tumor) and normal kidney tissues (normal) was analyzed using data in the TIMER2.0 database (http://timer.cistrome.org/). Data regarding tumor grade, tumor stage, and overall survival of patients with low or high expression of MMP-1 and RUNX2 were taken from the TISIDB database (http://cis.hku.hk/TISIDB/index.php).
Prediction of MMP1 binding energy in EA treatment using a molecular docking model
MMP1 structure information was imported into ChemBio3D to generate and optimize three-dimensional models, which were then converted to PDB format. The relevant crystal structures of MMP1 were obtained from the PDB database and used for molecular docking analysis. The binding energy between EA and MMP1 was calculated using AutoDock Vina software. The most likely binding conformation was identified and visualized using PyMOL 1.8.
Statistical analysis
Statistical analysis was performed using SPSS 18.0. Student's t-test was used to determine the significance of differences between the two groups. One-way analysis of variance (ANOVA) was used to analyze data across different groups. Spearman correlation coefficients were used to determine correlations between variables. Statistical significance was set at P < 0.05 or P < 0.01.
Results
Effect of EA treatment on cell growth, migration and invasion of human RCC cells
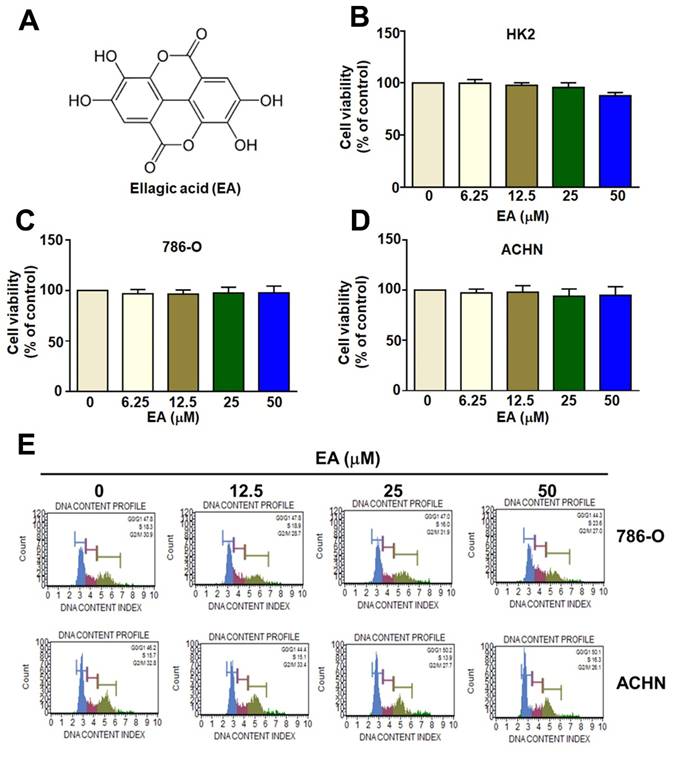
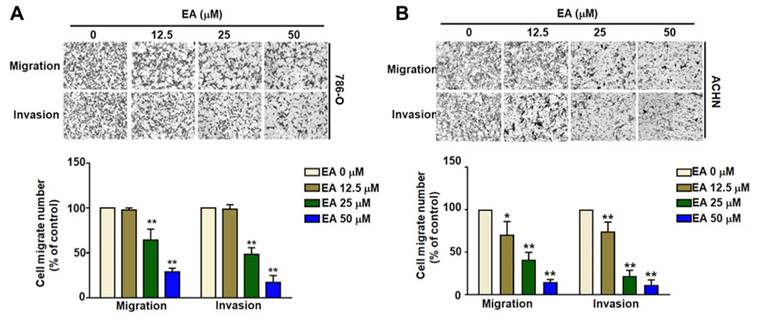
The structure of ellagic acid (EA) is shown in Figure 1A. MTT assays comparing the viability of human HK2, 786-O, and ACHN cell lines showed that EA treatment at concentrations up to 50 µM did not significantly reduce the viability of either normal human renal tubular HK2 cells or RCC cells (Figures 1B-1D). Additionally, EA had no effect on the cell cycle in either of the RCC cell lines (Figures 1E). Thus, EA did not exhibit cytotoxicity in normal kidney cells or kidney cancer cells. Treatment of 786-O and ACHN cells with 25 and 50 µM EA resulted in a significant decrease in cell migration (Figure 2A, 2B). Similar results with invasion in EA-treated with 786-O and ACHN cells. These results showed that EA exerts anti-migration and anti-invasion activity on RCC cells.
EA treatment decreased MMP1 protein expression
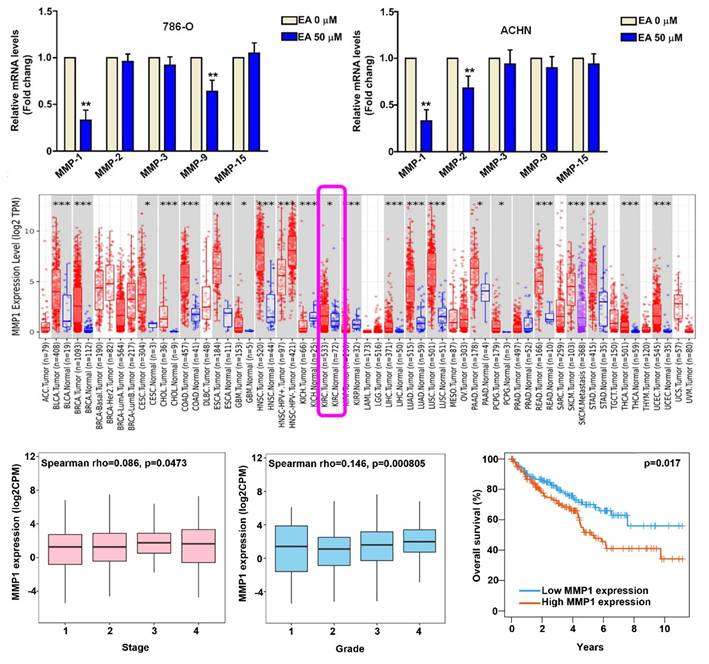
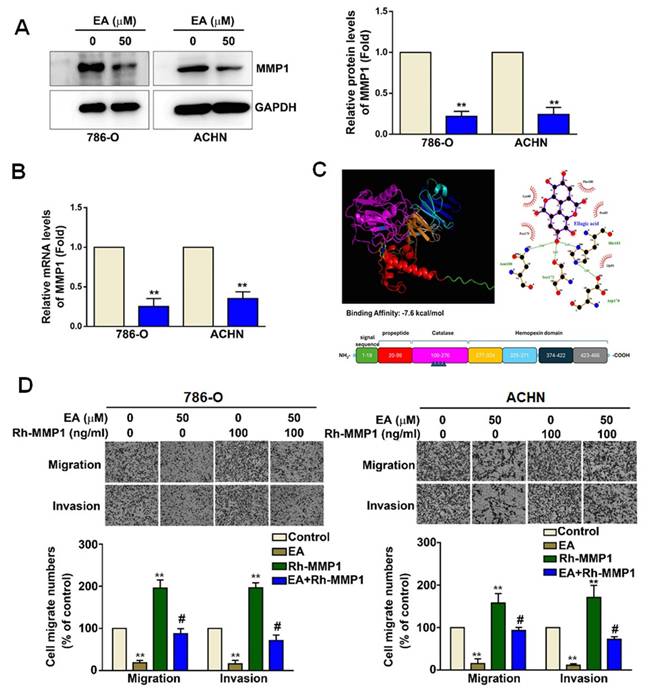
The effect of EA treatment on the mRNA expression of MMPs (MMP1, MMP2, MMP3, MMP9, MMP15) was assessed using the RT-qPCR assay. Based on the results of cell migration and invasion assays, we used 50 µM EA in further experiments. EA treatment of 786-O and ACHN cells resulted in a significant decrease in mRNA levels of MMP1 compared to untreated cells (Figures 3A). To investigate the pathogenic role of MMP1 in RCC tissues, we analyzed data in the TIMER2.0 database. We found that MMP1 expression was significantly higher in RCC tissues than in normal kidney tissues (Figure 3B) and that higher MMP1 expression was associated with a higher tumor stage (P = 0.0473) (Figure 3C) and tumor grade (P = 0.0008) (Figure 3D) and poorer overall 10-year survival (P = 0.017) (Figure 3E). These findings suggest that MMP1 is a key target of EA and may serve as a prognostic factor for RCC.
The inhibitory effect of EA on normal renal cells and RCC cell lines. The molecular structure of EA (EA) is shown in (A). Different concentrations of EA were administered during incubation of HK-2 cells (B), 786-O cells (C), and ACHN cells (D) for up to 24 h. The viability of the three cell lines was assessed using the MTT assay. (E) Flow cytometry was used to determine the cell cycle phase of EA-treated RCC cells.
The role of MMP1 in EA-induced decreases in RCC cell migration and invasion
EA treatment (50 µM) of 786-O and ACHN cells led to a significant decrease in MMP1 protein (Figure 4A) and mRNA expression (Figure 4B). As shown in Figure 4C, molecular docking analysis revealed that the binding energy between EA and MMP1 was significantly lower than -7.3 kJ/mol, indicating that EA has sufficient docking activity to directly interact with MMP1 proteins (Figure 4C). To determine the functional role of MMP1 in the mechanism underlying the effects of EA on RCC cells, we investigated these effects in the presence of overexpressed recombinant human MMP1 (Rh-MMP1). We found that EA treatment of 786-O and ACHN cells significantly decreased the migration and invasion of both lines of RCC cells (Figure 4D). Cells treated with 100 ng/mL Rh-MMP1 alone exhibited greater cell migration and invasion (Figure 4D). Combination treatment of RCC cells with EA and Rh-MMP1 reversed the effect seen with Rh-MMP1 alone, resulting in reduced cell migration and invasion (Figure 4D). These results show that EA inhibits RCC cell migration and invasion by targeting MMP1 expression.
The inhibitory effect of EA on the migration and invasion of human RCC cells. EA treatment inhibited the migration and invasion of the renal cell carcinoma cell lines 786-O (A) and ACHN (B). The histology findings and corresponding histograms of the relative proportions of migrating cells are shown. ** p < 0.01 compared to untreated cells.
MMP1 expression in EA treated-RCC cells and clinical significance of MMP1 in RCC tissues. (A) Effect of 50-µM EA treatment on MMP expression (MMP1, MMP2, MMP3, MMP9, MMP15) in 786-O and ACHN cells. ** p < 0.01 compared to untreated cells. (B) Analysis of Timer2.0 data comparing MMP1 expression between normal tissues and renal cell carcinoma (RCC) cells. (C) Comparison of tumor stage, tumor grade, and 10-year overall survival rates between high and low MMP1 gene expression levels in RCC tumor tissues.
The functional role of MMP1 in EA-treated RCC cells. (A) MMP1 protein expression levels in renal cell carcinoma cell lines with or without EA treatment as assessed by western blot analysis. (B) Relative MMP1 mRNA expression levels as assessed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) assay. (C) Molecular docking of EA and MMP1 protein. (D) EA and recombinant human MMP1 (Rh-MMP1) co-treatment abolished Rh-MMP1-dependent cell migration and invasion in both 786-O and ACHN cells. The histology findings and corresponding histograms of the relative proportions of migrating cells are shown. ** p < 0.01 compared to untreated cells; # p < 0.01 compared to EA-treated cells.
EA inhibit RCC cell migration and invasion through regulation of RUNX2 expression
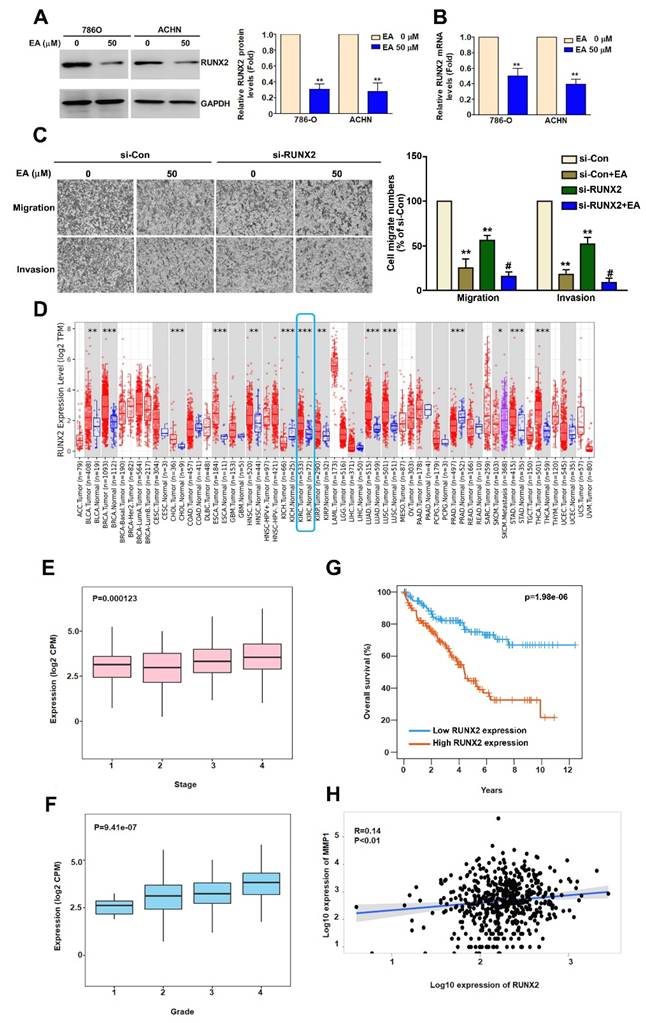
Studies have shown that RUNX2 is involved in tumor cell migration and invasion [23] and that RUNX2 directly targets the MMP1 promoter in TNBC cells [24]. To determine whether EA inhibits RUNX2 expression in RCC cells, we used western blot and RT-qPCR analysis. We found that EA treatment significantly decreased RUNX2 protein and mRNA expression (Figure 5A, 5B). To clarify the role of RUNX2 in EA-treated RCC cells, we found that inhibition of RUNX2 using si-RUNX2 significantly reduced the migration and invasion abilities of EA-treated 786-O cells (Figure 5C). Further analysis of data in the TIMER2.0 database showed higher levels of RUNX2 expression in RCC tissues than in normal kidney tissues (Figure 5D). We also found that RUNX2 expression levels correlated with tumor stage (P = 0.000123) (Figure 5E), tumor grade (P = 9.41e-07) (Figure 5F), and overall survival (P = 1.98e-06) (Figure 5G). The results of Spearman correlation analysis indicate that RUNX2 expression positively correlates with MMP1 expression in human RCC tissues (R = 0.14; P = 0.01) (Figure 5H). These results show that EA inhibited RCC cell migration and invasion by downregulating RUNX2 targeting MMP1 expression, and clinical evidence suggests that the level of RUNX2 expression may be prognostic factor for RCC.
EA inhibits RUNX2 expression, which is associated with the migration and invasion of human RCC cells, and highlights the clinical significance of RUNX2. (A, B) Effect of EA treatment (50 µM) on RUNX2 mRNA and protein expression in RCC cell lines. (C) The inhibitory of migration and invasion in siRNA-RUNX2 (si-RUNX2) combined with EA in 786-O cells. ** p < 0.01 compared to untreated cells; # p < 0.05 compared to EA-treated cells. (D) Comparison of RUNX2 expression levels between normal and renal cell carcinoma tissues using Timer2.0 data. Comparison of (E) tumor stage, (F) tumor grade, and (G) 12-year overall survival rates between high and low RUNX2 gene expression levels in RCC tumor tissues. (H) Correlation between MMP1 and RUNX2 gene expression levels in RCC tumor tissues.
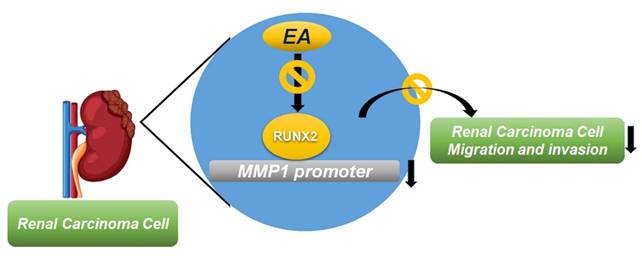
Summary of EA effects on RUNX2 regulation of MMP1 activity, which further suppresses the migration and invasion of RCC cells.
Discussion
The results of this study show that EA treatment of RCC cells inhibits their invasion and migration capabilities, likely by mediating MMP1 expression levels. Our analysis of clinical data in the TCGA database shows that higher MMP1 and RUNX2 levels in tumor tissues are associated with higher tumor grade and stage and poorer overall outcomes of RCC patients. We found that EA, a naturally occurring compound, can retard metastatic behaviors in human RCCs by suppressing the expression of RUNX2, which targets MMP1 expression.
MMPs, when activated by growth factors and inflammatory cytokines, exert proteolytic activity that contributes to ECM degradation and remodeling [25, 26]. Reorientation of collagen fibers in the ECM is associated with increased cancer invasion and even can predict worse survival in cancer patients [27]. MMP1 (interstitial collagenase-1) is upregulated in many types of metastatic cancers, and an inverse relationship has been observed between MMP1 transcription levels and clinical outcomes [28, 29]. Previous evidence and the results of the MMP1 is linked to RCC risk at the genetic level. One study indicated an association between an MMP1 genetic variant and predisposition to kidney cancers [30]. However, the correlation between polymorphisms in the MMP1 promoter region and risk of developing RCCs yielded conflicting results [31, 32]. In human clear cell RCCs, higher grades of tumors were found to have lower collagen fiber content and higher collagenolytic activity of MMP1 [33]. One of the main results of our study shows that EA significantly decreased MMP1 expression in RCC cells; EA treatment of these cells also affected the expression of MMP2 and MMP9. The anti-invasive effects of EA on different malignant tumor types, exerted by mediating MMP expression, have been studied formerly. Colon carcinogenesis induced by 1,2-dimethyl hydrazine (DMH) was associated with augmented expression of MMP2 and MMP9, and EA co-treatment with DMH significantly reduced the protein expression of MMP2 and MMP9 [34]. In cultured human gastric cancer cells, EA treatment hindered the acidic microenvironment-induced upregulation of MMP7 and MMP9 mRNAs as well as tumor migration and invasion [35]. The same study also reported that cyclooxygenase activity and the degree of EMT, both of which were increased under acidic conditions and correlated with tumor invasiveness, were inhibited by EA [35]. Patients with late-stage RCCs have a low chance of survival despite standard anticancer therapies. Clinical trials of MMP1 inhibitors and drugs targeting other types of MMPs show that these are promising agents against metastatic cancers [36, 37]. EA decreases the expression of MMP1 in RCC tumors, thereby inhibiting tumor invasion. Therefore, EA can be regarded as a potentially effective compound for treating metastatic RCCs; subsequent clinical studies are needed to evaluate the safety and efficacy of EA.
RUNX2 is a nuclear transcription factor involved in regulating osteoblast differentiation and chondrocyte maturation [38]. Several studies have implicated RUNX2 in the progression of various malignant tumors. For example, high RUNX2 expression plays a significant oncogenic role in hepatocellular carcinoma [39], renal cell carcinoma [40], highly invasive breast cancer [41], and glioma [42]. Guo et al. suggested that RUNX2 promotes gastric cancer tumorigenesis through YAP1 [23]. Additionally, studies on triple-negative breast cancer have confirmed that RUNX2 increases TGF-β-mediated regulation of CD44+/CD24- breast cancer stem cells, leading to increased cancer stemness, EMT, and apoptosis resistance, as well as conferring resistance to epirubicin [43]. Both in vitro and in vivo experiments have demonstrated that RUNX2 directly regulates MMP1 transcriptional activity, promoting TNBC tumorigenesis and increasing chemoresistance [24]. In pancreatic cancer, RUNX2 has been shown to regulate the transcriptional activity of the extracellular matrix proteins SPARC and MMP1, thereby influencing the tumor microenvironment [44]. In chondrosarcoma, IL-1β has been found to regulate p38, which in turn promotes RUNX2-mediated MMP-13 transcription and translation, playing a crucial role in tumor progression [45]. Based on these findings, our study confirms that EA inhibits MMP1 expression by downregulating RUNX2, thereby suppressing the migration and invasion abilities of renal cancer cells. This study had a few limitations. We will investigate whether RUNX2 directly regulates MMP1 transcriptional activity and whether the in vivo metastasis mouse assay supports the anti-metastatic effect of EA observed in vitro remain to be further suggested in future studies.
Overall, our study demonstrated that the inhibitory effect of EA on renal cancer cell migration and invasion is mediated through the inhibition of the RUNX2/MMP1 axis. These findings provide novel and important reference data for elucidating the molecular mechanism underlying the inhibitory effect of EA on renal cancer cell migration and invasion, while also contributing to the development of clinical therapeutic strategies and new therapeutic targets for human RCC.
Acknowledgements
StepOnePlus PCR was performed in the Instrument Center of Chung Shan Medical University, which is supported by National Science Council, Ministry of Education and Chung Shan Medical University. The authors would like to thank Convergence CT for assistance with English editing during development of the manuscript.
Funding
This research was funded by Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan (TCMF-CP 113-03) and Chung Shan Medical University Hospital (CSH-2021-C-007).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S. et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol. 2022;82:399-410
2. Saad AM, Gad MM, Al-Husseini MJ, Ruhban IA, Sonbol MB, Ho TH. Trends in Renal-Cell Carcinoma Incidence and Mortality in the United States in the Last 2 Decades: A SEER-Based Study. Clin Genitourin Cancer. 2019;17:46-57.e5
3. Powles T, Albiges L, Bex A, Comperat E, Grünwald V, Kanesvaran R. et al. Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:692-706
4. Rose TL, Kim WY. Renal Cell Carcinoma: A Review. JAMA. 2024;332:1001-10
5. Das P, Booth A, Donaldson R, Berfeld N, Nordstrom B, Carroll R. et al. Patient Characteristics, Treatment Patterns, and Outcomes for Patients With Renal Cell Carcinoma in England: A Retrospective Cohort Study. Clin Genitourin Cancer. 2024;22:102081
6. Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A. et al. Epidemiology of Renal Cell Carcinoma. World J Oncol. 2020;11:79-87
7. Mustafa S, Koran S, AlOmair L. Insights Into the Role of Matrix Metalloproteinases in Cancer and its Various Therapeutic Aspects: A Review. Front Mol Biosci. 2022;9:896099
8. Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415-33
9. Hagemann T, Gunawan B, Schulz M, Füzesi L, Binder C. mRNA expression of matrix metalloproteases and their inhibitors differs in subtypes of renal cell carcinomas. Eur J Cancer. 2001;37:1839-46
10. Nagasaka H, Kishida T, Kouro T, Igarashi Y, Takebe S, Yamamoto S. et al. MMP1, IL-1β, sTNFR-1, and IL-6 are prognostic factors for patients with unresectable or metastatic renal cell carcinoma treated with immune checkpoint inhibitors. Int J Clin Oncol. 2024;29:832-9
11. Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7:3113-9
12. Miyata Y, Iwata T, Ohba K, Kanda S, Nishikido M, Kanetake H. Expression of matrix metalloproteinase-7 on cancer cells and tissue endothelial cells in renal cell carcinoma: prognostic implications and clinical significance for invasion and metastasis. Clin Cancer Res. 2006;12:6998-7003
13. Vattem DA, Shetty K. Biological functionality of ellagic acid: a review. J Food Biochem. 2005;29:234-66
14. Chen P, Chen F, Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8:1465
15. Gupta A, Singh AK, Kumar R, Jamieson S, Pandey AK, Bishayee A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv Nutr. 2021;12:1211-38
16. Kannan MM, Quine SD. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism. 2013;62:52-61
17. Lu G, Wang X, Cheng M, Wang S, Ma K. The multifaceted mechanisms of ellagic acid in the treatment of tumors: State-of-the-art. Biomed Pharmacother. 2023;165:115132
18. Zhang HM, Zhao L, Li H, Xu H, Chen WW, Tao L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol Med. 2014;11:92-100
19. Liu Z, Huang H, Yu Y, Li L, Shi X, Wang F. Exploring the mechanism of ellagic acid against gastric cancer based on bioinformatics analysis and network pharmacology. J Cell Mol Med. 2023;27:3878-96
20. Ni X, Shang FS, Wang TF, Wu DJ, Chen DG, Zhuang B. Ellagic acid induces apoptosis and autophagy in colon cancer through the AMPK/mTOR pathway. Tissue Cell. 2023;81:102032
21. Qiu S, Zhong C, Zhao B, Li G, Wang J, Jehan S. et al. Transcriptome analysis of signaling pathways targeted by Ellagic acid in hepatocellular carcinoma cells. Biochim Biophys Acta Gen Subj. 2021;1865:129911
22. Wu TK, Hsieh YH, Hung TW, Lin YC, Lin CL, Liu YJ. et al. The Anti-Metastatic Action of Oxyresveratrol via Suppression of Phosphoryl-ERK/-PKCα-Mediated Sp1/MMP1 Signaling in Human Renal Carcinoma Cells. Environ Toxicol. 2024;39:5264-73
23. Guo Z, Zhou K, Wang Q, Huang Y, Ji J, Peng Y. et al. The transcription factor RUNX2 fuels YAP1 signaling and gastric cancer tumorigenesis. Cancer Sci. 2021;112:3533-44
24. Si W, Xu X, Wan L, Lv F, Wei W, Xu X. et al. RUNX2 facilitates aggressiveness and chemoresistance of triple negative breast cancer cells via activating MMP1. Front Oncol. 2022;12:996080
25. Alexander CM, Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989;1:974-82
26. Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol. 2019;137:57-83
27. Popova NV, Jücker M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers (Basel). 2022;14:238
28. Ala-aho R, Kähäri VM. Collagenases in cancer. Biochimie. 2005;87:273-86
29. Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823-30
30. Ricketts C, Zeegers MP, Lubinski J, Maher ER. Analysis of germline variants in CDH1, IGFBP3, MMP1, MMP3, STK15 and VEGF in familial and sporadic renal cell carcinoma. PLoS One. 2009;4:e6037
31. Hirata H, Naito K, Yoshihiro S, Matsuyama H, Suehiro Y, Hinoda Y. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter is associated with conventional renal cell carcinoma. Int J Cancer. 2003;106:372-4
32. Piccoli MF, Figueira M, Andreoni C, Marumo JT, Schor N, Bellini MH. Lack of association between matrix metalloproteinase-1 (MMP-1) promoter polymorphism and risk of renal cell carcinoma. Int Braz J Urol. 2007;33:622-9
33. Młynarczyk G, Kudelski J, Darewicz B, Bruczko-Goralewska M, Romanowicz L. Suppressed Expression but Not Activity of Collagenases MMP-1 and MMP-13 in Human Renal Carcinoma. Pathobiology. 2019;86:201-7
34. Umesalma S, Nagendraprabhu P, Sudhandiran G. Antiproliferative and apoptotic-inducing potential of ellagic acid against 1,2-dimethyl hydrazine-induced colon tumorigenesis in Wistar rats. Mol Cell Biochem. 2014;388:157-72
35. Lim SC, Hwang H, Han SI. Ellagic Acid Inhibits Extracellular Acidity-Induced Invasiveness and Expression of COX1, COX2, Snail, Twist 1, and c-myc in Gastric Carcinoma Cells. Nutrients. 2019;11:3023
36. Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix Metalloproteinases Inhibitors in Cancer Treatment: An Updated Review (2013-2023). Molecules. 2023;28:5567
37. Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther. 2018;17:1147-55
38. Chen PC, Liu JF, Fong YC, Huang YL, Chao CC, Tang CH. CCN3 Facilitates Runx2 and Osterix Expression by Inhibiting miR-608 through PI3K/Akt Signaling in Osteoblasts. Int J Mol Sci. 2019;20:3300
39. Cao Z, Sun B, Zhao X, Zhang Y, Gu Q, Liang X. et al. The Expression and Functional Significance of Runx2 in Hepatocellular Carcinoma: Its Role in Vasculogenic Mimicry and Epithelial-Mesenchymal Transition. Int J Mol Sci. 2017;18:500
40. Zhang X, Ren Z, Liu B, Wei S. RUNX2 Mediates Renal Cell Carcinoma Invasion through Calpain2. Biol Pharm Bull. 2022;45:1653-9
41. Zhang F, Cho WC. Therapeutic potential of RUNX1 and RUNX2 in bone metastasis of breast cancer. Expert Opin Ther Targets. 2023;27:413-7
42. Yamada D, Fujikawa K, Kawabe K, Furuta T, Nakada M, Takarada T. RUNX2 Promotes Malignant Progression in Glioma. Neurochem Res. 2018;43:2047-54
43. Lv F, Si W, Xu X, He X, Wang Y, Li Y. et al. RUNX2 prompts triple negative breast cancer drug resistance through TGF-beta pathway regulating breast cancer stem cells. Neoplasia. 2024;48:100967
44. Kayed H, Jiang X, Keleg S, Jesnowski R, Giese T, Berger MR. et al. Regulation and functional role of the Runt-related transcription factor-2 in pancreatic cancer. Br J Cancer. 2007;97:1106-15
45. Pei Y, Harvey A, Yu XP, Chandrasekhar S, Thirunavukkarasu K. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: potential role of Runx2 in mediating p38 effects. Osteoarthritis Cartilage. 2006;14:749-58
Author contact
![]() Corresponding author: Dr. Jen-Pi Tsai, PhD, Division of Nephrology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan. Institute of Medical Sciences, Tzu Chi University, Hualian, Taiwan. School of Medicine, Tzu Chi University, Hualian, Taiwan. Email: dm315797com.tw, Tel.: +886-5-2648000 ext 5246.
Corresponding author: Dr. Jen-Pi Tsai, PhD, Division of Nephrology, Department of Internal Medicine, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan. Institute of Medical Sciences, Tzu Chi University, Hualian, Taiwan. School of Medicine, Tzu Chi University, Hualian, Taiwan. Email: dm315797com.tw, Tel.: +886-5-2648000 ext 5246.

 Global reach, higher impact
Global reach, higher impact