3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(4):887-896. doi:10.7150/ijms.107159 This issue Cite
Review
Clusterin: structure, function and roles in disease
1. Department of Orthopaedic Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China.
2. Chongqing Municipal Health Commission Key Laboratory of Musculoskeletal Regeneration and Translational Medicine, Chongqing 400016, China.
3. Orthopedic Laboratory of Chongqing Medical University, Chongqing 400016, China.
Received 2024-11-18; Accepted 2025-1-11; Published 2025-1-21
Abstract
Clusterin (CLU) is a glycoprotein that exists in various forms in cells, including nuclear, cytoplasmic, and secreted types. The relative molecular weight of CLU varies significantly due to differences in glycosylation and cleavage. Although CLU is commonly present in mammalian tissues and body fluids, its expression levels differ markedly under physiological and pathological conditions. The existence forms and molecular sizes of CLU in cells vary greatly, contributing to its diverse functions. For example, CLU can participate in the occurrence and development of neurological, fibrotic, and metabolic diseases by regulating cell endocytosis, apoptosis, and other processes. This article will review the structural characteristics, basic functions, and potential regulatory mechanisms of CLU protein in physiological and pathological processes.
Keywords: clusterin, secreted protein, neurological disorders, fibrosis, metabolism disease
1. Introduction
Clusterin (CLU) is a highly glycosylated heterodimeric protein that is widely distributed in human plasma and tissue fluids. Due to the diverse forms of CLU protein in cells, its expression levels vary greatly under physiological and pathological conditions, resulting in different functions in different tissues, organs, and physiological and pathological states. Although there have been several published reviews on this topic, most of them only discussed the function of CLU in specific diseases, such as tumors [1-3], neurodegenerative diseases [3,4], and musculoskeletal diseases [5], without fully exploring the role of CLU in other diseases. Moreover, recent studies have reported that CLU also plays an important role in fibrotic diseases [6,7], metabolic diseases [8], and cardiovascular diseases [9]. Therefore, we conducted this review to summarize the structural characteristics, basic functions, and potential regulatory mechanisms of CLU protein in physiological and pathological processes, providing a theoretical basis for the treatment of CLU protein-related diseases.
2. The structural characteristics and basic functions of CLU protein
CLU, also known as apolipoprotein J, is ubiquitously expressed in various body tissues and fluids [10]. The human CLU gene is located on the short arm of chromosome 8, spanning from region 2 to region 1, covering approximately 16 kb and comprising 11 exons. Under normal conditions, the full-length mRNA encoding CLU initiates translation from the start codon in exon 2, producing a polypeptide chain of 449 amino acids. This nascent polypeptide is guided into the endoplasmic reticulum (ER) by an N-terminal signal peptide, where it undergoes N-glycosylation and other post-translational modifications, resulting in a glycosylated CLU protein with a molecular weight of approximately 60 kDa. Subsequently, CLU is transported to the Golgi apparatus for further processing, including peptide cleavage and additional glycosylation, before being secreted into the extracellular space via secretory vesicles [11]. The secreted CLU protein has a molecular size ranging from 70 to 80 kDa. However, due to varying degrees of glycosylation, SDS-PAGE gel electrophoresis reveals two distinct bands: one corresponding to the intact, uncleaved CLU at approximately 60 kDa, and the other representing the cleaved α and β chains at approximately 40 kDa [12]. CLU is a highly glycosylated protein, with carbohydrates accounting for about 30% of its total mass. The N-linked glycans of CLU primarily consist of a core of three mannose residues and are extensively modified with fucose. These glycans play crucial roles in cellular communication and immune function regulation [13].
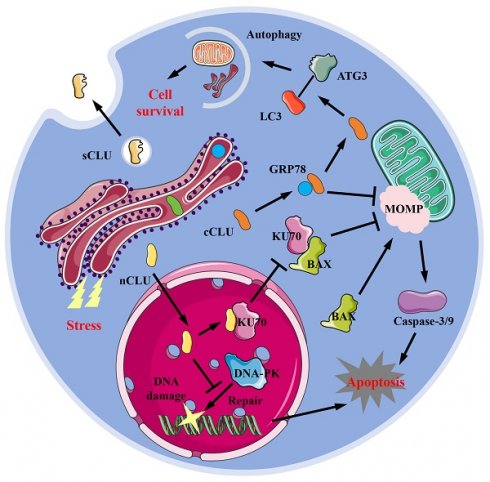
CLU exists in various forms within cells, including the secreted type (sCLU), the cytoplasmic type (cCLU), and the nucleus type (nCLU). sCLU is a highly glycosylated protein, primarily found in blood and cerebrospinal fluid, and is also distributed in other tissues. Under physiological conditions, the concentration of sCLU protein in plasma ranges from 35-105 μg/mL [14], while in cerebrospinal fluid, it is between 1.2-3.6 μg/mL [15]. Due to hydrophobic regions, sCLU typically exists stably in body fluids as dimers or tetramers. sCLU participates in normal physiological processes such as complement regulation [16] and lipid transport [17]. Under pathological conditions, such as Alzheimer's disease (AD) [18-20], fibrosis [6,7], cancer [21,22], and cardiovascular disease [8,23], the local concentration of sCLU protein often increases. Under normal physiological conditions, cells generally produce sCLU protein, but when stimulated by the external environment, some of the produced CLU protein remains within the cell. This form of CLU is known as intracellular CLU (iCLU). The iCLU protein has two physiological structures within the cell: one consists of two chains with lower glycosylation levels than sCLU, known as cCLU; the other type is that the formed peptide chains do not undergo cleavage and N-glycosylation modification, and typically function within the nucleus [22], known as nCLU. The common function of cCLU protein is to inhibit cell apoptosis. Research has shown that drugs such as paclitaxel or MG132 treatment can induce stress responses in the endoplasmic reticulum of cells; the GRP78 (glucose regulated protein 78) protein in the endoplasmic reticulum binds to cCLU, stabilizing its conformation and transporting it to the periphery or interior of mitochondria [24]. The cCLU transported to the periphery of mitochondria can inhibit mitochondrial outer membrane perforation (MOMP), thereby preventing the release of cytochrome C and inhibiting cell apoptosis [24,25] (Figure 1). Additionally, cCLU can bind to the LC3 (microtubule-associated protein 1 light chain 3)-ATG3 (autophagy-related protein 3) complex, promoting the esterification of LC3 protein, thereby enhancing cell autophagy ability [26] and resisting cell apoptosis. Conversely, nCLUs, which also belong to the same iCLU, promote cell apoptosis. For instance, nCLU directly binds to Ku70 (X-ray repair cross complementing 6) protein, reducing the complex formed by Ku70 and BAX (BCL2 associated X), causing free BAX to induce intracellular mitochondrial outer membrane perforation, thereby activating downstream apoptotic pathways [27]; nCLU can also inhibit the repair of X-rays induced DNA damage by binding to DNA-PK (DNA dependent kinase complexes), thereby activating downstream apoptosis processes in cancer cells [28] (Figure 1). Therefore, classifying and studying the different existence forms of CLU helps to understand the complex mechanisms of CLU and its role in different diseases, which is of great benefit in elucidating the contradictory effects of CLU shown in previous studies.
3. The role of CLU protein in neurological diseases
3.1 Alzheimer's disease
The CLU protein, primarily expressed and secreted by neuronal cells and astrocytes, is found in cerebrospinal fluid (CSF). Research indicates that the concentration of CLU protein in the CSF of Alzheimer's disease (AD) patients is approximately 40% higher than in normal individuals [29]. Furthermore, the plasma concentration of CLU protein is inversely related to the cognitive ability of AD patients [30] but directly proportional to the incidence and severity of AD [31]. A single nucleotide polymorphism (SNP) in high-risk genes associated with AD has shown a strong correlation between the rs11136000 mutation in the CLU gene and the delayed onset of AD. Immunofluorescence staining of brain tissue from AD patients reveals that CLU protein co-localizes with β-amyloid protein [12,19,29], suggesting an interaction between the two. Additionally, direct injection of recombinant CLU protein into the cerebrospinal fluid of AD model mice, using amyloid precursor protein (APP) mutant mice, can reduce the deposition of amyloid protein in the brain [32]. Research has found that the primary mechanism by which CLU protein alleviates Alzheimer's disease may involve binding to low-density lipoprotein receptor-related protein 2 (LRP2), triggering receptor expressed on myeloid cells 2 (TREM2), or heparan sulfate (HS) on the membrane surface, thereby mediating the clearance of β-amyloid and its polymers in the extracellular matrix [19,33,34] (Figure 2). Moreover, studies have shown that the concentration of CLU protein in the plasma of mice increases significantly after exercise, which can inhibit neuronal apoptosis and hippocampal inflammation, thereby improving cognitive ability in mice [35]. However, in recent years, studies have suggested that CLU protein may promote the progression of AD [36]. Excessive phosphorylation of Tau protein is another typical feature of AD. Research has demonstrated that CLU protein can bind to overly phosphorylated Tau protein, inhibiting Tau protein hydrolysis in lysosomes, leading to Tau aggregation, and causing cell rupture and death [36] (Figure 2). Additionally, after the membrane of dead cells ruptures, CLU-Tau is released and aggregates outside the cell, forming pathological plaques [36] (Figure 2). In the brain tissue of Clu-knockout mice with cerebral ischemia, Han et al. [37] found that the area of hypoxic stress damage was smaller than in control mice, indicating that CLU protein is not beneficial for neuronal survival under stress. The latest study on AD also suggests that CLU is an important cause of pathological plaques [38]. Therefore, the role of CLU in AD cannot be generalized, as there has not been a comprehensive localization and expression analysis of CLU in intracellular studies thus far. Consequently, analyzing its function solely from the perspective of protein expression and disease progression is relatively narrow and incomplete. Although Herring et al. [39] analyzed the expression positions and levels of different RNA types of CLUs in various cells of neural tissue in detail, there remains a lack of comprehensive research in this area.
Forms and potential mechanisms in physiological functions of CLU protein. sCLU, secreted type clusterin; cCLU, cytoplasmic type clusterin; nCLU, nucleus type clusterin; LC3, microtubule-associated protein 1 light chain 3; ATG3, autophagy-related protein 3; GRP78, glucose regulated protein 78; MOMP, mitochondrial outer membrane perforation; KU70, X-ray repair cross complementing 6; BAX, BCL2 associated X; DNA-PK, DNA-dependent protein kinase.
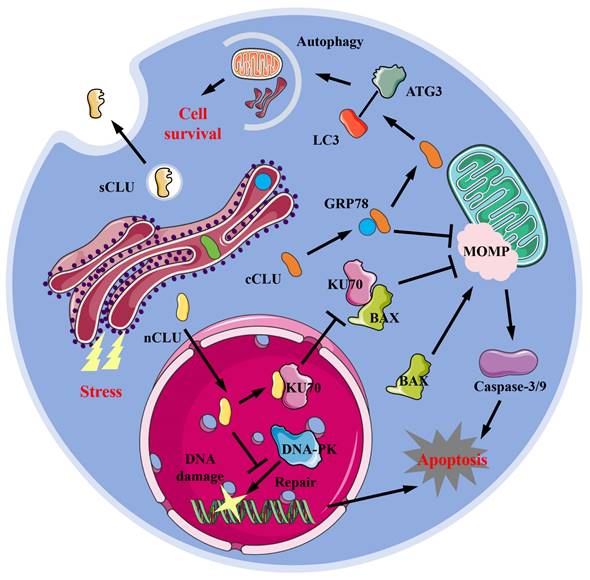
The role of CLU protein in Alzheimer's disease. In neural tissue, sCLU is secreted by neural cells and glial cells. The sCLU can inhibit β-amyloid protein aggregation, promote its transport to microglia and being cleared, and thus reduce its toxicity. Moreover, sCLU can also bind to phosphorylated Tau to exert its toxic effects on nerve cells. sCLU, secreted type clusterin; LRP2, lipoprotein receptor-related protein 2.
3.2 Parkinson's disease
Parkinson's disease (PD) is a highly prevalent degenerative disease of the central nervous system, primarily caused by the formation and accumulation of Lewy bodies in dopamine neurons within the substantia nigra of the midbrain, leading to extensive neuronal death. Research has found that the Clu gene rs11136000 is associated with disease development in PD patients [40]. Analyzing the exosome components of nerve cells in the blood of PD patients, it was discovered that the decrease of CLU content in the blood was closely related to the occurrence of PD [41]. The aggregation of α-synuclein proteins is a crucial step in the formation of Lewy bodies. Immunofluorescence staining results showed that in the brain tissue of PD patients, the CLU protein was mainly distributed at the α-synuclein site of the Lewy body [42]. The main mechanism is that the cCLU protein can interact and bind with the hydrophobic regions on the surface of α-synuclein proteins, weakening their aggregation ability, thereby reducing the formation of Lewy bodies and ultimately decreasing neuronal apoptosis [43]. On the other hand, during the clearance of extracellular α-synuclein proteins and their oligomers by astrocytes, extracellular sCLU would bind to α-synuclein proteins and inhibit the phagocytosis of astrocytes, leading to an increased concentration of extracellular α-synuclein proteins [44], which may, in turn, exacerbate the development of PD.
4. The role of CLU protein in fibrosis related diseases
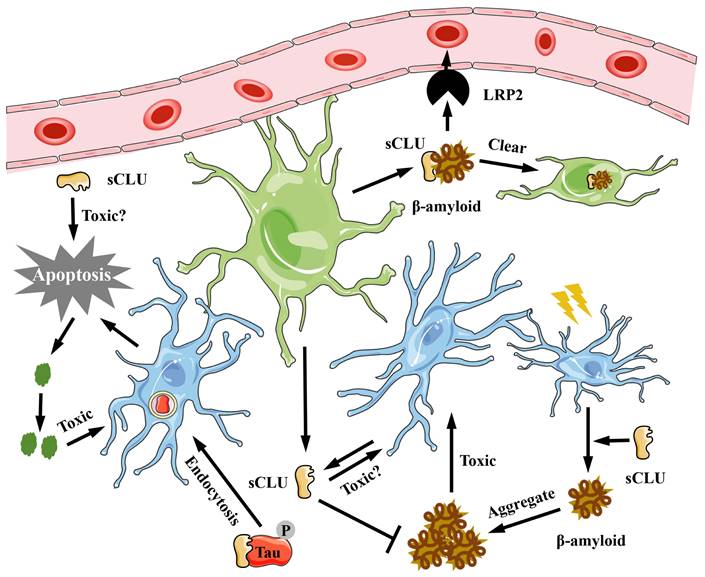
The hallmark of fibrosis is pathological changes resulting from the excessive deposition of extracellular matrix components, such as collagen. In a mouse model of renal fibrosis induced by unilateral ureteral obstruction (UUO), the expression of CLU in the kidney was observed to increase during the progression of renal fibrosis, and a corresponding rise in CLU concentration in urine was detected [45]. Furthermore, the creation of a UUO model in Clu gene knockout mice revealed a more rapid progression of fibrosis [45]. Conversely, in a mouse model of UUO renal fibrosis with Clu overexpression, it was discovered that the overexpression of Clu could suppress the development of renal fibrosis [46]. Studies on pulmonary fibrosis have also demonstrated that the CLU protein can inhibit the progression of pulmonary fibrosis [6], primarily by reducing the transforming growth factor β (TGF-β) expression, preventing the activation of astrocytes and their differentiation into fibroblasts, as well as the expression and deposition of extracellular matrix proteins like collagen (Figure 3). Clinical studies on patients with acute chronic liver failure (ACLF) caused by HBV have indicated that the concentration of CLU protein in the patient's serum can serve as a marker for disease progression in HBV-ACLF. As the disease advances, the concentration of CLU protein in the patient's serum tends to decrease [47]. Research on mouse liver fibrosis found that the expression of CLU protein increased following the onset of liver fibrosis [7]; inhibiting the expression of CLU protein was shown to accelerate the progression of liver fibrosis [48]. The mechanism involved the inhibition of TGF-β-mediated activation of hepatic stellate cells and the production and deposition of a substantial amount of extracellular matrix. Thus, as an extracellular molecular chaperone, CLU not only directly participates in the deposition process of the extracellular matrix but also regulates corresponding cellular physiological processes, playing a crucial role in maintaining extracellular matrix homeostasis.
The role of CLU protein in fibrosis related diseases. The sCLU secreted by the lung, kidney and liver can inhibit the activation of fibroblasts and the generation of collagen fibers by suppressing the TGF-β pathway. sCLU, secreted type clusterin; TGF-β, transforming growth factor β.
5. The role of CLU in glucose and lipid metabolism
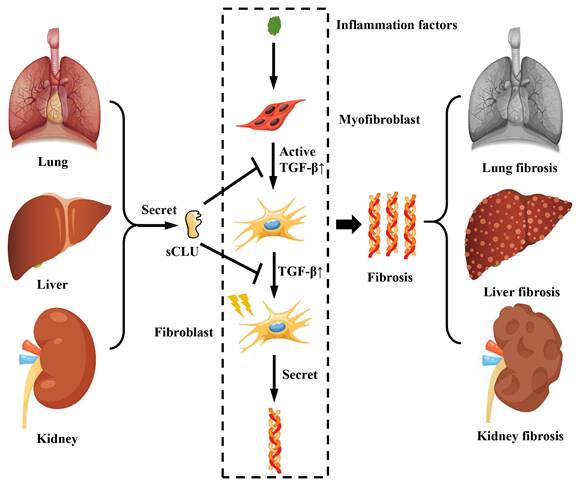
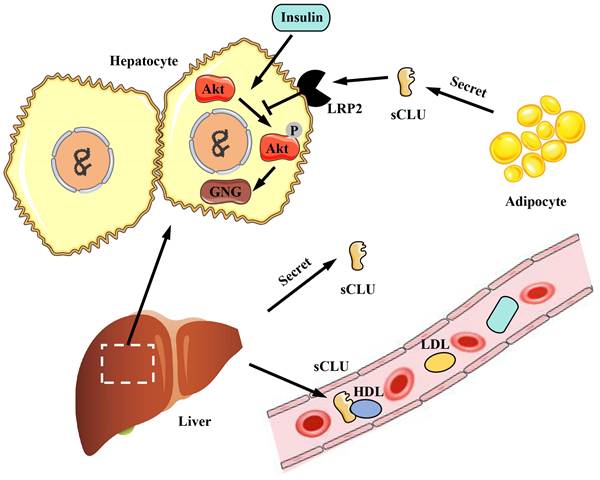
The CLU protein is primarily expressed and secreted into plasma and tissue fluids by tissues and organs such as the liver and heart. CLU plays a significant role in physiological processes, including metabolism and anti-inflammatory effects. Obesity is associated with a high incidence of diabetes, and diabetic patients often exhibit symptoms related to insulin resistance. Interestingly, the CLU protein has been found to regulate insulin resistance [8]. Compared to individuals with normal body weight, obese individuals exhibit increased expression and secretion of the CLU protein in adipocytes [8]. The primary mechanism involves the transport of sCLU to the liver, where it binds to the LRP2 receptor on the surface of liver cells, inhibiting insulin-induced Akt phosphorylation and stimulating downstream gluconeogenesis (GNG) pathways. This results in reduced insulin sensitivity of liver cells and impacts the body's metabolism of glucose and lipids [8] (Figure 4). Additionally, as the liver is the main source of CLU in the body, liver-secreted CLU can also interact with the LRP2 receptor on muscle cell membranes, enhancing the activation of downstream pathways by insulin, improving insulin sensitivity in muscle cells, and increasing glucose uptake by muscle tissue [49] (Figure 5). Beyond metabolic regulation, CLU also influences food intake. The CLU protein can act on the LRP2 receptor in the hypothalamus, triggering Stat3 phosphorylation and suppressing central feeding-related pathways [50], leading to weight loss in mice. In summary, CLU is considered a liver regulatory factor and has garnered increasing interest from researchers, offering new insights into the functional interactions between different organs.
6. The role of CLU protein in cardio-cerebrovascular diseases
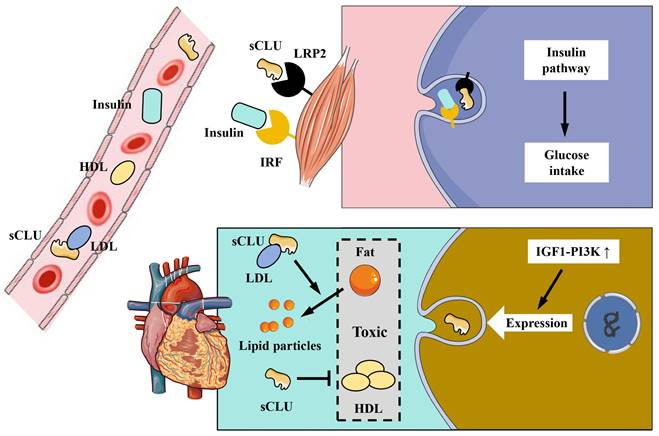
As an apolipoprotein, the CLU protein is an important component of high density lipoprotein (HDL) and plays a crucial role in cardio-cerebrovascular diseases [51]. The primary mechanism of HDL's anti-atherosclerotic effect is that the apolipoproteins within HDL particles activate key enzymes of lipoprotein metabolism, promoting liver cells to clear cholesterol from tissues, thereby slowing down and preventing atherosclerosis. The CLU protein is one of these significant apolipoproteins. It has been shown that the CLU protein in HDL from healthy individuals can reduce heart damage by inhibiting the apoptosis of cardiac endothelial cells; however, HDL particles from patient populations lack CLU protein binding [52], suggesting that CLU is involved in the protective effect of HDL on cardiovascular function. The concentration of CLU protein is also related to cardiac injury. Clinical data analysis has found that sCLU in plasma can serve as a marker for myocardial infarction injury, with its concentration decreasing during early ischemic injury of the heart, while the content of iCLU increases [23]. Protein components such as CLU in HDL can also indicate the therapeutic effect of stroke. Analysis of HDL protein components in the plasma of stroke patients post-treatment revealed a positive correlation between CLU content and recovery progress [41]. Low density lipoprotein (LDL) has the opposite effect of HDL and can promote atherosclerosis. Research has shown that saturated fatty acids can inhibit the binding of CLU protein to LDL, thereby increasing the aggregation of LDL in plasma [53]. It has also been reported that the insulin-like growth factor 1-phosphatidylinositol-3-kinase (IGF1-PI3K) pathway has a protective effect on the heart and enhances cardiac function, and the activation of this pathway can increase CLU expression [54] (Figure 5). In a rat myocardial infarction model experiment, the administration of human CLU protein in the blood was shown to reduce the myocardial infarction area by 75% and lower the mortality rate [55]. All of the above research results indicate that CLU plays a significant role in the development of cardio-cerebrovascular disease.
The role of CLU protein in glucose and lipid metabolism in liver. The sCLU protein produced by adipocytes can enter hepatocytes through LRP2 and inhibit Akt phosphorylation, thus promoting gluconeogenesis and reducing insulin sensitivity of hepatocytes. Besides, the sCLU secreted by the liver has the similar effect, and can also be transported to the circulatory system to function. sCLU, secreted type clusterin; LRP2, lipoprotein receptor-related protein 2; Akt, protein kinase B; GNG, gluconeogenesis; HDL, high density lipoprotein; LDL, low density lipoprotein.
The role of CLU protein in muscle and the cardiovascular system. In muscle system, insulin and sCLU can produce synergistic effects to promote the activation of the insulin pathway and enhance the glucose uptake. In cardiovascular system: sCLU can inhibit the aggregation of LDL and reduce its cardiovascular toxicity; HDL and sCLU can promote fat decomposition and then attenuate the cardiovascular damage of fat; activation of the IGF1-PI3K pathway in cardiac cells can promote the generation of sCLU and play a role in protecting cardiomyocytes from the toxicity of fat and LDH. sCLU, secreted type clusterin; LRP2, lipoprotein receptor-related protein 2; IRF, insulin receptor family; HDL, high density lipoprotein; LDL, low density lipoprotein; IGF1, insulin-like growth factor 1; PI3K, phosphatidylinositol 3-kinase.
7. The role of CLU in tumor regulation
Various isoforms of CLU have been reported to have varying effects on cancer cells. Under the stimulation of paclitaxel, the expression of cCLU in prostate cancer cells increased, and cCLU was transported from GRP78 to mitochondria. This process could stabilize the outer membrane of mitochondria and reduce the release of cytochrome C, thereby inhibiting cell apoptosis [24]. In osteosarcoma cells, cCLU inhibited the dissociation of Ku70 and BAK complexes and the translocation of BAX to the mitochondrial membrane, stabilizing the outer mitochondrial membrane and preventing MOMP from occurring [56]. cCLU can also regulate pathways related to autophagy. In experiments using human prostate cancer cells as models, it was found that cCLU can bind to the LC3-ATG3 complex, promote the lipidation of LC3 protein, and thereby enhance cell autophagy and the ability of cancer cells to resist external environmental stimuli [26]. Research has reported that cells with high expression of N-cadherin also had higher levels of cCLU, which was beneficial for the survival of cancer cells [21]. In oral cancer cells, overexpression of CLU was beneficial for the activation of the AMPK/Akt/mTOR-guided cell autophagy pathway and improved the survival rate of oral cancer cells [57]. However, nCLU exhibited different regulatory functions. Under ionizing radiation, the nCLU produced by cells can bind to Ku70, leading to an increase in intracellular BAK content [27]; it can also inhibit the repair of DNA by DNA-PK in damaged cells, thereby enhancing the occurrence of downstream cell apoptosis [22]. The TAK1-NF-κB pathway was over-activated in small cell lung cancer patients with Clu deletion; in vitro treatment with a TAK1-NF-κB inhibitor can inhibit the generation and accelerate the death of cancer cells [58]. CLU also showed the function of inhibiting the aforementioned pathway, which provided more options for diversified treatment of cancer [58].
8. The role of CLU in musculoskeletal disorders
Currently, limited research has been conducted on the role of CLU in musculoskeletal diseases. Studies have shown that the expression levels of CLU in the cartilage of osteoarthritis (OA) patients [59], as well as in the serum and synovial fluid of hip OA and knee OA patients [60], were significantly higher compared to those in the normal control population. A recent study further demonstrated that CLU plays a crucial cytoprotective role in OA. Specifically, the knockdown of CLU significantly inhibited chondrocyte proliferation and promoted the expression of markers associated with inflammation and oxidative stress [61]. In bone development studies, it was observed that CLU gene expression decreased during the differentiation process of mouse bone marrow mesenchymal stem cells (mBMSCs). sCLU exhibited a dose-dependent inhibitory effect on mBMSC differentiation into osteoblasts and an induction of adipogenic differentiation [62]. Additionally, overexpression of CLU was noted in atrophic and degenerated fibers of osteoporotic muscles. Silencing CLU via siRNA restored myoblast proliferation and differentiation capacity, thereby identifying CLU as a marker of muscle degeneration [63]. Moreover, increased expression of CLU protein was observed in osteocytes and osteoblasts of osteoporosis (OP) patients, while sCLU levels were reduced [64]. sCLU not only hindered BMSC differentiation into osteoblasts by inhibiting the ERK1/2 signaling pathway [62] but also suppressed osteoclastogenesis by reducing the proliferation of M-CSF-dependent osteoclast precursor cells [65]. In conclusion, CLU plays a significant role in bone metabolism under various pathological conditions and warrants further in-depth investigation.
9. Summary and prospects
Overall, CLU, a protein with potential protective effects on physiological functions, exhibits complex expression patterns and functions across various pathological conditions. Summarizing the expression patterns and signaling pathways of CLU under physiological and pathological conditions can aid in understanding and applying CLU. CLU may serve as a serological marker for neurological diseases, cardio-cerebrovascular diseases, and fibrosis-related diseases, enabling the prediction of disease progression. As an extracellular chaperone protein, CLU's most critical role is to participate in the clearance of extracellular matrix components such as amyloid and collagen, thereby alleviating the occurrence and development of diseases. Although the conclusion that the extracellular CLU protein alleviates neurological disorders like AD remains controversial, a deeper understanding of the mechanism by which CLU functions as a molecular chaperone in extracellular matrix clearance can assist in establishing future prevention and treatment strategies for AD. Additionally, the iCLU protein plays a significant role in the processes of apoptosis and autophagy, potentially alleviating myocardial infarction, diabetes, and other diseases. Consequently, the iCLU and its molecular regulatory mechanisms could serve as potential drug targets for treating these diseases in the future. The function of the CLU protein extends beyond the organs that express it; it is also a protein that interacts with the circulatory system. The CLU protein secreted by the liver can act on muscle tissue and brain neurons, playing a vital regulatory role between organs. The role and mechanism of CLU in tumors are not yet fully understood, and the effects of CLU vary depending on the different forms present in various tumors. Therefore, accurately understanding the impact of CLU on cellular physiology in different environments will help to advance research on tumors and develop new treatment approaches.
Abbreviations
CLU: clusterin; ER: endoplasmic reticulum; sCLU: secreted type of clusterin; cCLU: cytoplasmic type of clusterin; nCLU: nucleus type of clusterin; iCLU: intracellular clusterin; AD: Alzheimer's disease; GRP78: glucose regulated protein 78; MOMP: mitochondrial outer membrane perforation; LC3: microtubule-associated protein 1 light chain 3; ATG3: autophagy-related protein 3; Ku70: X-ray repair cross complementing 6; BAX: BCL2 associated X; DNA-PK: DNA dependent kinase complexes; CSF: cerebrospinal fluid; SNP: single nucleotide polymorphism; APP: amyloid precursor protein; LRP2: lipoprotein receptor-related protein 2; TREM2: triggering receptor expressed on myeloid cells 2; HS: heparan sulfate; PD: Parkinson's disease; UUO: unilateral ureteral obstruction; TGF-β: transforming growth factor β; ACLF: acute chronic liver failure; GNG: gluconeogenesis; Akt: protein kinase B; HDL: high density lipoprotein; LDL: low density lipoprotein; IGF1: insulin-like growth factor 1; PI3K: phosphatidylinositol-3-kinase; IRF: insulin receptor family; OA: osteoarthritis; mBMSCs: mouse bone marrow mesenchymal stem cells; OP: osteoporosis.
Funding
This work was supported by the China Postdoctoral Science Foundation (2024M763910) and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0972).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Martín-García D, García-Aranda M, Redondo M. Therapeutic Potential of Clusterin Inhibition in Human Cancer. Cells. 2024;13(8):665
2. Zhang Y, Lv X, Chen L. et al. The role and function of CLU in cancer biology and therapy. Clin Exp Med. 2023;23(5):1375-1391
3. Sultana P, Novotny J. Clusterin: a double-edged sword in cancer and neurological disorders. EXCLI J. 2024;23:912-936
4. Palihati N, Tang Y, Yin Y. et al. Clusterin is a Potential Therapeutic Target in Alzheimer's Disease. Mol Neurobiol. 2024;61(7):3836-3850
5. Zhang K, Liu K, Yu D. et al. The therapeutic and prognostic role of clusterin in diverse musculoskeletal diseases: a mini review. Physiol Res. 2022;71(6):739-747
6. Peix L, Evans IC, Pearce DR. et al. Diverse functions of clusterin promote and protect against the development of pulmonary fibrosis. Sci Rep. 2018;8(1):1906
7. Seo HY, Lee SH, Lee JH. et al. Clusterin Attenuates Hepatic Fibrosis by Inhibiting Hepatic Stellate Cell Activation and Downregulating the Smad3 Signaling Pathway. Cells. 2019;8(11):1442
8. Bradley D, Blaszczak A, Yin Z. et al. Clusterin Impairs Hepatic Insulin Sensitivity and Adipocyte Clusterin Associates With Cardiometabolic Risk. Diabetes Care. 2019;42(3):466-475
9. Bass-Stringer S, Tai CMK, McMullen JR. IGF1-PI3K-induced physiological cardiac hypertrophy: Implications for new heart failure therapies, biomarkers, and predicting cardiotoxicity. J Sport Health Sci. 2021;10(6):637-647
10. Rodríguez-Rivera C, Garcia MM, Molina-Álvarez M. et al. Clusterin: Always protecting. Synthesis, function and potential issues. Biomed Pharmacother. 2021;134:111174
11. O'Sullivan J, Whyte L, Drake J. et al. Alterations in the post-translational modification and intracellular trafficking of clusterin in MCF-7 cells during apoptosis. Cell Death Differ. 2003;10(8):914-27
12. Satapathy S, Wilson MR. The Dual Roles of Clusterin in Extracellular and Intracellular Proteostasis. Trends Biochem Sci. 2021;46(8):652-660
13. Xin M, Xu Y, You S. et al. Precision Structural Interpretation of Site-Specific N-Glycans in Seminal Plasma. J Proteome Res. 2022;21(7):1664-1674
14. Murphy BF, Kirszbaum L, Walker ID. et al. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988;81(6):1858-64
15. Polihronis M, Paizis K, Carter G. et al. Elevation of human cerebrospinal fluid clusterin concentration is associated with acute neuropathology. J Neurol Sci. 1993;115(2):230-3
16. Menny A, Lukassen MV, Couves EC. et al. Structural basis of soluble membrane attack complex packaging for clearance. Nat Commun. 2021;12(1):6086
17. Wilson MR, Satapathy S, Jeong S. et al. Clusterin, other extracellular chaperones, and eye disease. Prog Retin Eye Res. 2022;89:101032
18. Moezzi SM, Mozafari N, Fazel-Hoseini SM. et al. Apolipoprotein J in Alzheimer's Disease: Shedding Light on Its Role with Cell Signaling Pathway Perspective and Possible Therapeutic Approaches. ACS Chem Neurosci. 2020;11(24):4060-4072
19. Wojtas AM, Kang SS, Olley BM. et al. Loss of clusterin shifts amyloid deposition to the cerebrovasculature via disruption of perivascular drainage pathways. Proc Natl Acad Sci U S A. 2017;114(33):E6962-E6971
20. Chen F, Swartzlander DB, Ghosh A. et al. Clusterin secreted from astrocyte promotes excitatory synaptic transmission and ameliorates Alzheimer's disease neuropathology. Mol Neurodegener. 2021;16(1):5
21. Osuka S, Zhu D, Zhang Z. et al. N-cadherin upregulation mediates adaptive radioresistance in glioblastoma. J Clin Invest. 2021;131(6):e136098
22. Praharaj PP, Patra S, Panigrahi DP. et al. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188500
23. Cubedo J, Padró T, Vilahur G. et al. Glycosylated apolipoprotein J in cardiac ischaemia: molecular processing and circulating levels in patients with acute ischaemic events. Eur Heart J. 2022;43(2):153-163
24. Li N, Zoubeidi A, Beraldi E. et al. GRP78 regulates clusterin stability, retrotranslocation and mitochondrial localization under ER stress in prostate cancer. Oncogene. 2013;32(15):1933-42
25. Gao S, Li H, Cai Y. et al. Mitochondrial binding of α-enolase stabilizes mitochondrial membrane: its role in doxorubicin-induced cardiomyocyte apoptosis. Arch Biochem Biophys. 2014;542:46-55
26. Zhang F, Kumano M, Beraldi E. et al. Clusterin facilitates stress-induced lipidation of LC3 and autophagosome biogenesis to enhance cancer cell survival. Nat Commun. 2014;5:5775
27. Trougakos IP, Gonos ES. Regulation of clusterin/apolipoprotein J, a functional homologue to the small heat shock proteins, by oxidative stress in ageing and age-related diseases. Free Radic Res. 2006;40(12):1324-34
28. Leskov KS, Criswell T, Antonio S. et al. When X-ray-inducible proteins meet DNA double strand break repair. Semin Radiat Oncol. 2001;11(4):352-72
29. Howlett DR, Hortobágyi T, Francis PT. Clusterin associates specifically with Aβ40 in Alzheimer's disease brain tissue. Brain Pathol. 2013;23(6):623-32
30. Jones N. Alzheimer disease: plasma clusterin predicts degree of pathogenesis in AD. Nat Rev Neurol. 2010;6(9):469
31. Schrijvers EM, Koudstaal PJ, Hofman A. et al. Plasma clusterin and the risk of Alzheimer disease. JAMA. 2011;305(13):1322-6
32. de Retana SF, Marazuela P, Solé M. et al. Peripheral administration of human recombinant ApoJ/clusterin modulates brain beta-amyloid levels in APP23 mice. Alzheimers Res Ther. 2019;11(1):42
33. Yeh FL, Wang Y, Tom I. et al. TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron. 2016;91(2):328-40
34. Itakura E, Chiba M, Murata T. et al. Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J Cell Biol. 2020;219(3):e201911126
35. De Miguel Z, Khoury N, Betley MJ. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600(7889):494-499
36. Yuste-Checa P, Trinkaus VA, Riera-Tur I. et al. The extracellular chaperone Clusterin enhances Tau aggregate seeding in a cellular model. Nat Commun. 2021;12(1):4863
37. Han BH, DeMattos RB, Dugan LL. et al. Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 2001;7(3):338-43
38. Liu X, Che R, Liang W. et al. Clusterin transduces Alzheimer-risk signals to amyloidogenesis. Signal Transduct Target Ther. 2022;7(1):325
39. Herring SK, Moon HJ, Rawal P. et al. Brain clusterin protein isoforms and mitochondrial localization. Elife. 2019;8:e48255
40. Sampedro F, Marín-Lahoz J, Martínez-Horta S. et al. CLU rs11136000 promotes early cognitive decline in Parkinson's disease. Mov Disord. 2020;35(3):508-513
41. Jiang C, Hopfner F, Katsikoudi A. et al. Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2020;91(7):720-729
42. Sasaki K, Doh-ura K, Wakisaka Y. et al. Clusterin/apolipoprotein J is associated with cortical Lewy bodies: immunohistochemical study in cases with alpha-synucleinopathies. Acta Neuropathol. 2002;104(3):225-30
43. Whiten DR, Cox D, Horrocks MH. et al. Single-Molecule Characterization of the Interactions between Extracellular Chaperones and Toxic α-Synuclein Oligomers. Cell Rep. 2018;23(12):3492-3500
44. Filippini A, Mutti V, Faustini G. et al. Extracellular clusterin limits the uptake of α-synuclein fibrils by murine and human astrocytes. Glia. 2021;69(3):681-696
45. Yuan Y, Zhang F, Wu J. et al. Urinary candidate biomarker discovery in a rat unilateral ureteral obstruction model. Sci Rep. 2015;5:9314
46. Jung GS, Kim MK, Jung YA. et al. Clusterin attenuates the development of renal fibrosis. J Am Soc Nephrol. 2012;23(1):73-85
47. Liu H, Li Y, Gao F. et al. Serum Clusterin: A Potential Marker for Assessing the Clinical Severity and Short-Term Prognosis of Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Dis Markers. 2020;2020:8814841
48. Trautwein C, Friedman SL, Schuppan D. et al. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62(1 Suppl):S15-24
49. Vorselen D, Wang Y, de Jesus MM. et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell-target interactions. Nat Commun. 2020;11(1):20
50. Gil SY, Youn BS, Byun K. et al. Clusterin and LRP2 are critical components of the hypothalamic feeding regulatory pathway. Nat Commun. 2013;4:1862
51. Plubell DL, Fenton AM, Rosario S. et al. High-Density Lipoprotein Carries Markers That Track With Recovery From Stroke. Circ Res. 2020;127(10):1274-1287
52. Riwanto M, Rohrer L, Roschitzki B. et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127(8):891-904
53. Ruuth M, Lahelma M, Luukkonen PK. et al. Overfeeding Saturated Fat Increases LDL (Low-Density Lipoprotein) Aggregation Susceptibility While Overfeeding Unsaturated Fat Decreases Proteoglycan-Binding of Lipoproteins. Arterioscler Thromb Vasc Biol. 2021;41(11):2823-2836
54. Pereira RM, Mekary RA, da Cruz Rodrigues KC. et al. Protective molecular mechanisms of clusterin against apoptosis in cardiomyocytes. Heart Fail Rev. 2018;23(1):123-129
55. Sposito AC, de Lima-Junior JC, Moura FA. et al. Reciprocal Multifaceted Interaction Between HDL (High-Density Lipoprotein) and Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2019;39(8):1550-1564
56. Trougakos IP, Lourda M, Antonelou MH. et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-Bax protein complex. Clin Cancer Res. 2009;15(1):48-59
57. Naik PP, Mukhopadhyay S, Praharaj PP. et al. Secretory clusterin promotes oral cancer cell survival via inhibiting apoptosis by activation of autophagy in AMPK/mTOR/ULK1 dependent pathway. Life Sci. 2021;264:118722
58. Chen Z, Fan Z, Dou X. et al. Inactivation of tumor suppressor gene Clusterin leads to hyperactivation of TAK1-NF-κB signaling axis in lung cancer cells and denotes a therapeutic opportunity. Theranostics. 2020;10(25):11520-11534
59. Connor JR, Kumar S, Sathe G. et al. Clusterin expression in adult human normal and osteoarthritic articular cartilage. Osteoarthritis Cartilage. 2001;9(8):727-37
60. Fandridis E, Apergis G, Korres DS. et al. Increased expression levels of apolipoprotein J/clusterin during primary osteoarthritis. In Vivo. 2011;25(5):745-9
61. Tarquini C, Pucci S, Scioli MG. et al. Clusterin exerts a cytoprotective and antioxidant effect in human osteoarthritic cartilage. Aging (Albany NY). 2020;12(11):10129-10146
62. Abdallah BM, Alzahrani AM, Kassem M. Secreted Clusterin protein inhibits osteoblast differentiation of bone marrow mesenchymal stem cells by suppressing ERK1/2 signaling pathway. Bone. 2018;110:221-229
63. Pucci S, Greggi C, Polidoro C. et al. Clusterin silencing restores myoblasts viability and down modulates the inflammatory process in osteoporotic disease. J Transl Med. 2019;17(1):118
64. Visconti VV, Greggi C, Cariati I. et al. Deregulated Clusterin as a Marker of Bone Fragility: New Insights into the Pathophysiology of Osteoporosis. Genes (Basel). 2022;13(4):652
65. Choi B, Kang SS, Kang SW. et al. Secretory clusterin inhibits osteoclastogenesis by attenuating M-CSF-dependent osteoclast precursor cell proliferation. Biochem Biophys Res Commun. 2014;450(1):105-9
Author contact
![]() Corresponding author: Wei Shui, Postal Address: No.1 YouYi Road, Yu Zhong District, 400016, Chongqing, China. Telephone: 86-13618220790; Email: sjuiweicom.
Corresponding author: Wei Shui, Postal Address: No.1 YouYi Road, Yu Zhong District, 400016, Chongqing, China. Telephone: 86-13618220790; Email: sjuiweicom.

 Global reach, higher impact
Global reach, higher impact