3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(4):834-844. doi:10.7150/ijms.104943 This issue Cite
Research Paper
Predictivity of Hepatic Steatosis Index for Gestational Hypertension and Preeclampsia: a Prospective Cohort Study
1. Department of Internal Medicine, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, China.
2. Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, China.
3. Department of Research Management, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, China.
*These authors have contributed equally to this work and share first authorship.
Received 2024-10-10; Accepted 2025-1-9; Published 2025-1-21
Abstract
Context: Previous studies have reported that pregnant women with non-alcoholic fatty liver disease (NAFLD) face an increased risk of gestational hypertension (GH) and preeclampsia (PE). However, no study has assessed the relationship between the Hepatic Steatosis Index (HSI), a biomarker for NAFLD, in early pregnancy and the subsequent risk of GH and PE.
Objective: We aimed to investigate the relationship between HSI in early pregnancy and the risks of GH and PE in Chinese women.
Methods: Based on the China Birth Cohort Study conducted from February 2018 to December 2022, this prospective cohort study collected liver enzyme and body mass index data from pregnant participants during 6-13+6 gestational weeks. The incidences of GH and PE were monitored until delivery.
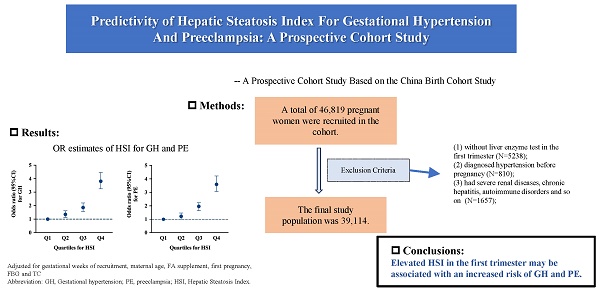
Results: This study included 39,114 pregnant women, and GH and PE incidences were 4.2% and 4.1%, respectively. After multivariable adjustment, the risks of GH (Q2: OR = 1.35, 95% CI = 1.13-1.62; Q3: OR = 1.86, 95% CI = 1.57-2.20; Q4: OR = 3.81, 95% CI = 3.25-4.46) and PE (Q2: OR = 1.22, 95% CI = 1.01-1.47; Q3: OR = 1.96, 95% CI = 1.65-2.32; Q4: OR = 3.60, 95% CI = 3.07-4.22) significantly increased with higher HSI quartiles. Further analysis indicated that compared to women aged 35 years or older, HSI in pregnant women under 35 years had relatively stronger predictive value for GH (OR ≥ 35 = 4.527, 95% CI = 3.762-5.446 vs. OR < 35 = 2.325, 95% CI = 1.729-3.128) and PE (OR ≥ 35 = 4.13, 95% CI = 3.433-4.983 vs. OR < 35 = 2.348, 95% CI = 1.736-3.176).
Conclusion: Elevated HSI may be associated with an increased risk of GH and PE.
Keywords: hepatic steatosis index, non-alcoholic fatty liver disease, gestational hypertension, preeclampsia
1. Introduction
Preeclampsia (PE), a pregnancy-specific disease that manifests after 20 weeks of gestation, is characterized by hypertension and proteinuria. It can lead to various organ dysfunctions, such as liver damage, and cardiac and renal insufficiency, posing significant risks to both mother and fetus [1, 2]. Gestational hypertension (GH) is defined as elevated blood pressure alone, occurring after 20 weeks of gestation without accompanying organic dysfunction [3].
Non-alcoholic fatty liver disease (NAFLD), a leading cause of liver diseases globally [4], is characterized by excessive hepatic fat accumulation and insulin resistance and is defined as the histological presence of steatosis in more than 5% of hepatocytes [5]. Meta-analyses estimate that the prevalence of NAFLD is about 25%, having nearly tripled over the past decade [6, 7].
Established clinical risk factors for PE include hypertension, diabetes, obesity, advanced maternal age (> 35 years), obstructive sleep apnea syndrome, kidney disease, thrombophilia, primiparity, and assisted reproduction [3, 8]. Previous studies have reported that the combined risk of GH and PE is nearly fourfold higher in pregnant women with NAFLD [7, 9]. Thus, the inclusion of NAFLD in predictive risk stratification for PE is gaining recognition [10]. Compared to traditional ultrasound for screening NAFLD, the Hepatic Steatosis Index (HSI) is a more efficient, cost-effective, and equally accurate biomarker derived from body mass index (BMI) and liver enzyme calculations. An HSI value of 36.0 detects NAFLD with a specificity of 93.1% (95% CI, 92.0-94.0) and a positive likelihood ratio of 6.505 (95% CI, 5.628-7.519) [11, 12]. However, data on the relationship between HSI and GH and HSI and PE remain limited.
We collected data from a large prospective cohort study in China to evaluate the predictive value of HSI for GH and PE, aiming to demonstrate the increased risk of GH and PE associated with NAFLD.
2. Materials and Methods
Study population
This study enrolled participants from the China Birth Cohort Study (CBCS) between February 2018 and December 2022 who delivered a live-born singleton at Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Participants were excluded for the following reasons: (1) incomplete liver enzyme tests in the first trimester, (2) a diagnosis of hypertension before pregnancy, (3) severe renal diseases, chronic hepatitis, autoimmune disorders, or related conditions, and (4) missing covariate data. All participants completed baseline questionnaires and clinical laboratory tests at recruitment (6-13+6 gestational weeks) and continued regular follow-ups until delivery [13]. The final study population included 39,114 pregnant women. Figure S1 shows the participant selection process. This study was approved by the Ethics Committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University (approval number: 2018-KY-003-02). All study participants provided written informed consent.
Exposure and covariates
Demographic and obstetric characteristics of study participants, such as maternal age, ethnicity, employment status, educational level, pre-pregnancy height and weight, maternal lifestyle factors (smoking status, alcohol consumption, and folic acid supplementation), gravidity, mode of fertilization, obstetric history, and medical history, were obtained at baseline using a standardized questionnaire. The date of the last menstrual period was confirmed through ultrasound during the first trimester. Pre-pregnancy BMI was calculated as maternal weight (kg)/height squared (m2) and classified into normal (< 24.0 kg/m2), overweight (24.0-27.9 kg/m2), and obese (≥ 28.0 kg/m2) categories, based on established thresholds [14]. Laboratory test results, including fasting blood glucose (FBG), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT), were obtained after approximately 12 hours of fasting during 6-13+6 weeks of gestation and were collected from the medical records. HSI was calculated as (8 × ALT/AST) + BMI + 2 [12].
Definition of GH and PE
The occurrence of GH and PE was recorded during follow-up and verified by the diagnoses in the medical records after delivery. GH and PE were diagnosed according to the guidelines recommended by the International Society for the Study of Hypertension in Pregnancy (ISSHP) [8]. PE is defined as gestational hypertension accompanied by proteinuria and/or other maternal organ dysfunction at or after 20 weeks of gestation. In contrast, GH is persistent de novo hypertension developing at or after 20 weeks of gestation without features of PE.
Statistical analyses
Participants were classified into three groups: normal, GH, and PE. Baseline characteristics were reported as mean ± standard deviation (SD) or median (interquartile range) for continuous variables. One-way analysis of variance (ANOVA) or Kruskal-Wallis test was used to compare the differences across the normal, GH, and PE groups. Categorical variables were expressed as frequency (%) and compared across groups using the χ2 test. Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for liver enzyme levels (AST, ALT, GGT) and HSI quartiles in relation to GH and PE, adjusting for potential covariates such as gestational age of recruitment, maternal age, pre-pregnancy BMI, folic acid supplementation, primiparity, FBG, and TC. Subgroup analyses were performed by stratifying participants according to maternal age (< 35 years or ≥ 35 years), first-trimester FBG (< 5.6 or ≥ 5.6 mmol/L), and gravidity (> 1 or 1). ORs between subgroups were compared using the z-statistic [15]. Receiver Operating Characteristic Curve (ROC) analysis was used to evaluate the predictive capacity of liver enzyme levels and HSI for GH and PE. Restricted cubic spline (RCS) was applied to develop two non-linear regression models for GH and PE, with four knots positioned at the 5th, 35th, 65th, and 95th percentiles of predictor distributions. Statistical significance was defined as P < 0.05 (two-sided). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
Clinical features of the enrolled pregnant women
Among the 39,114 participants who had an average age of 32.0 years and were at 8 gestational weeks at baseline, 1,668 (4.3%) and 1,588 (4.1%) were diagnosed with GH and PE, respectively. The baseline characteristics of the study participants are shown in Table 1. Participants in the normal group had higher proportions of individuals with higher education levels and natural pregnancies (P<0.001). Furthermore, participants in the GH or PE groups were more likely to have a higher pre-pregnancy BMI or to be experiencing their first pregnancy (P < 0.001). The overall median levels of the liver enzyme indicators—AST, ALT, and GGT—were 14.8 (13.0-17.4) U/L, 12.9 (9.9-18.8) U/L, and 13.0 (11.0-18.0) U/L, respectively. The mean HSI was 31.7 ± 5.2. All serum markers, including liver enzyme indicators, HSI, FBG, and blood lipid indicators (TG, TC, LDL-C, and HDL-C) in the first trimester, showed significant differences among the normal, GH, and PE groups (P < 0.001).
Baseline characteristics of study subjects.
| Variables | Total (N=39114) | Normal (N=35858) | GH (N=1668) | PE (N=1588) | P-value |
|---|---|---|---|---|---|
| Maternal age, year | 32.0±3.8 | 32.0±3.8 | 32.5±4.1 | 32.4±4.2 | <0.001 |
| Maternal employed | 36451 (93.2) | 33437 (93.2) | 1544 (92.6) | 1470 (92.6) | 0.336 |
| Gestational week, weeks | 8.0 (7.0-9.0) | 8.0 (7.0-9.0) | 8.0 (7.0-9.0) | 8.0 (7.0-9.0) | 0.011 |
| Han ethics | 36194 (92.5) | 33189 (92.6) | 1539 (92.3) | 1466 (92.3) | 0.857 |
| Maternal education | <0.001 | ||||
| < College | 2273 (5.8) | 2042 (5.7) | 113 (6.8) | 118 (7.4) | |
| Undergraduate/College | 26963 (68.9) | 24599 (68.6) | 1199 (71.9) | 1165 (73.4) | |
| > Postgraduate or higher | 9878 (25.3) | 9217 (25.7) | 356 (21.3) | 305 (19.2) | |
| Pre-pregnancy BMI, kg/m2 | <0.001 | ||||
| < 24 | 31309 (80.0) | 29361 (81.9) | 995 (59.7) | 953 (60.0) | |
| 24-28 | 6130 (15.7) | 5237 (14.6) | 448 (26.9) | 445 (28.0) | |
| ≥ 28 | 1675 (4.3) | 1260 (3.5) | 225 (13.5) | 190 (12.0) | |
| FA supplement | 34982 (89.4) | 32071 (89.4) | 1490 (89.3) | 1421 (89.5) | 0.989 |
| Alcohol use during the first trimester | 1810 (4.6) | 1670 (4.7) | 71 (4.3) | 69 (4.3) | 0.644 |
| Maternal smoking during the first trimester | 86 (0.2) | 78 (0.2) | 4 (0.2) | 4 (0.3) | 0.945 |
| First pregnancy | 21137 (54.0) | 19251 (53.7) | 938 (56.2) | 948 (59.7) | <0.001 |
| Natural pregnancy | 36724 (93.9) | 33807 (94.3) | 1503 (90.1) | 1414 (89.0) | <0.001 |
| Serum markers during the first trimester | |||||
| AST, U/L | 14.8 (13.0-17.4) | 14.8 (12.9-17.4) | 15.0 (13.0-18.1) | 15.2 (13.2-18.2) | <0.001 |
| ALT, U/L | 12.9 (9.9-18.8) | 12.8 (9.8-18.4) | 15.1 (10.9-23.4) | 15.2 (10.8-23.3) | <0.001 |
| GGT, U/L | 13.0 (11.0-18.0) | 13.0 (11.0-17.0) | 15.0 (12.0-21.0) | 15.0 (12.0-21.0) | <0.001 |
| HSI | 31.7±5.2 | 31.5±5.0 | 34.6±6.0 | 34.5±6.1 | <0.001 |
| FBG, mmol/L | 4.6±0.4 | 4.6±0.4 | 4.7±0.4 | 4.7±0.4 | <0.001 |
| TG, mmol/L | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) | 1.1 (0.8-1.5) | 1.1 (0.9-1.5) | <0.001 |
| TC, mmol/L | 4.2±0.7 | 4.2±0.7 | 4.3±0.7 | 4.4±0.7 | <0.001 |
| LDL-C, mmol/L | 2.2±0.6 | 2.2±0.6 | 2.4±0.6 | 2.4±0.6 | <0.001 |
| HDL-C, mmol/L | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | <0.001 |
Abbreviations: GH, gestational hypertension; PE, preeclampsia; BMI, body mass index; FA, folic acid; FBG, fasting blood glucose; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; HSI, hepatic steatosis index.
Associations of liver enzyme levels in the first trimester with risk of GH and PE.
| Variables | GH | PE | ||
|---|---|---|---|---|
| Model 1 | Model 2* | Model 1 | Model 2* | |
| AST, U/L | ||||
| Q1 (<13.00) | 1.000 | 1.000 | 1.000 | 1.000 |
| Q2 (13.00-14.79) | 0.990 (0.858-1.142) | 1.025 (0.888-1.185) | 1.017 (0.876-1.181) | 1.041 (0.895-1.211) |
| Q3 (14.80-17.39) | 0.964 (0.836-1.111) | 1.002 (0.868-1.157) | 1.060 (0.915-1.227) | 1.090 (0.940-1.264) |
| Q4 (≥17.40) | 1.206 (1.053-1.381) | 1.131 (0.985-1.298) | 1.356 (1.179-1.559) | 1.256 (1.090-1.447) |
| ALT, U/L | ||||
| Q1 (<9.90) | 1.000 | 1.000 | 1.000 | 1.000 |
| Q2 (9.90-12.89) | 1.186 (1.013-1.39) | 1.104 (0.941-1.294) | 1.078 (0.916-1.268) | 1.009 (0.856-1.188) |
| Q3 (12.90-18.79) | 1.441 (1.238-1.677) | 1.248 (1.070-1.455) | 1.443 (1.239-1.682) | 1.255 (1.075-1.464) |
| Q4 (≥18.80) | 2.187 (1.897-2.522) | 1.610 (1.390-1.865) | 2.090 (1.809-2.414) | 1.538 (1.325-1.785) |
| GGT, U/L | ||||
| Q1 (<11.00) | 1.000 | 1.000 | 1.000 | 1.000 |
| Q2 (11.00-12.99) | 1.100 (0.914-1.324) | 1.047 (0.869-1.261) | 1.331 (1.103-1.605) | 1.274 (1.055-1.537) |
| Q3 (13.00-17.99) | 1.633 (1.394-1.914) | 1.410 (1.201-1.655) | 1.690 (1.430-1.997) | 1.467 (1.239-1.737) |
| Q4 (≥18.00) | 2.713 (2.323-3.168) | 1.847 (1.570-2.172) | 2.890 (2.456-3.400) | 1.978 (1.669-2.344) |
| HSI | ||||
| Q1 (<28.06) | 1.000 | 1.000 | 1.000 | 1.000 |
| Q2 (28.06-30.66) | 1.377 (1.151-1.647) | 1.354 (1.131-1.621) | 1.226 (1.020-1.475) | 1.220 (1.014-1.468) |
| Q3 (30.67-34.29) | 1.926 (1.627-2.281) | 1.858 (1.567-2.203) | 1.999 (1.688-2.367) | 1.957 (1.651-2.321) |
| Q4 (≥34.30) | 4.088 (3.503-4.772) | 3.808 (3.254-4.457) | 3.833 (3.278-4.482) | 3.602 (3.072-4.224) |
* Model 1 was crude model; Model 2 adjusted for gestational weeks of recruitment, maternal age, maternal BMI before pregnancy (except for HSI), FA supplement, first pregnancy, FBG and TC.
Abbreviations: GH, gestational hypertension; PE, preeclampsia; OR, odds ratio; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; HSI, hepatic steatosis index; Q1, quartile 1; Q2, quartile 2; Q3, quartile 3; Q4, quartile 4; BMI, body mass index; FA, folic acid; FBG, fasting blood glucose; TC, total cholesterol.
Associations of liver enzyme levels in the first trimester with risk of GH and PE
The associations between AST, ALT, GGT, HSI, and the risks of GH and PE are shown in Table 2. After adjusting for confounding variables, the risk of GH was significantly higher among participants in the third and fourth quartiles of ALT and GGT (ALT Q3: OR = 1.248, 95% CI: 1.070-1.455, Q4: OR = 1.610, 95% CI: 1.390-1.865; GGT Q3: OR = 1.410, 95% CI: 1.201-1.655, Q4: OR = 1.847, 95% CI: 1.570-2.172) compared with those in the first quartile. Moreover, the risk of GH increased significantly with higher HSI quartiles compared with the first quartile (Q2: OR = 1.354, 95% CI: 1.131-1.621; Q3: OR = 1.858, 95% CI: 1.567-2.203; Q4: OR = 3.808, 95% CI: 3.254-4.457). Similarly, PE risk increased with higher liver enzyme quartiles and HSI. In fully adjusted models, the risk of PE was significantly higher among participants in the highest quartile of AST (OR = 1.256, 95% CI: 1.090-1.447) and the third and fourth quartiles of ALT (Q3: OR = 1.255, 95% CI: 1.075-1.464, Q4: OR = 1.538, 95% CI: 1.325-1.785). Furthermore, the risk of PE increased significantly with higher quartiles of GGT and HSI (GGT Q2: OR = 1.274, 95% CI: 1.055-1.537; Q3: OR = 1.467, 95% CI: 1.239-1.737; Q4: OR = 1.978, 95% CI: 1.669-2.344; HSI Q2: OR = 1.220, 95% CI: 1.014-1.468; Q3: OR = 1.957, 95% CI: 1.651-2.321; Q4: OR = 3.602, 95% CI: 3.072-4.224).
The results of subgroup analyses are shown in Table 3. The relationship between ALT, GGT, and HSI with the risk of GH was stronger among pregnant women under 35 years of age. Similarly, the risk of PE associated with HSI was higher in pregnant women younger than 35 years. There was no significant difference in the association between the top quartiles of AST, ALT, GGT, and HSI and GH or PE across subgroups stratified by baseline FBG levels and gravidity (All P > 0.05).
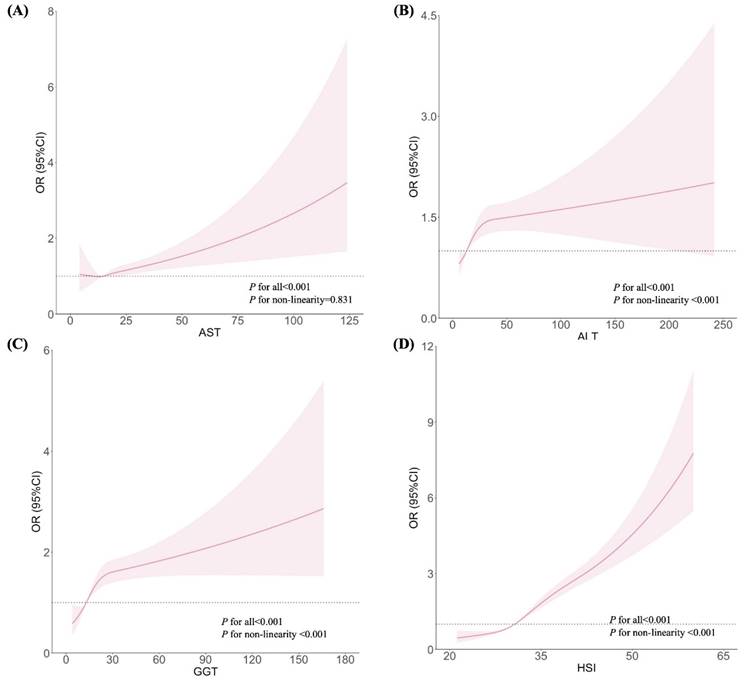
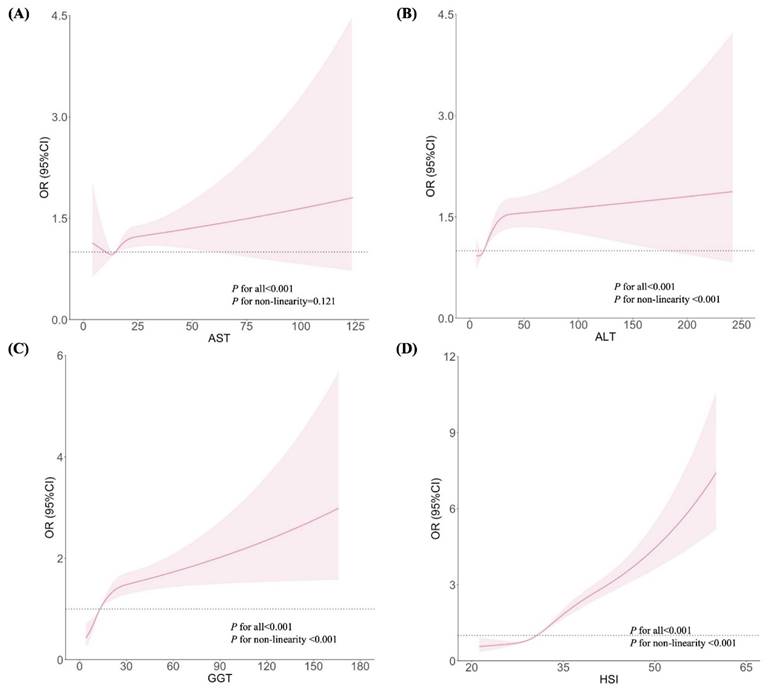
Figures 1 and 2 show the dose-response relationships between AST, ALT, GGT, and HSI with the risks of GH and PE, respectively. After adjustment for confounders, non-linear relationships were demonstrated between ALT, GGT, and HSI and the risk of GH (Figures 1B, 1C, and 1D; all P for non-linearity < 0.001). Similar non-linear associations were observed between ALT, GGT, HSI and the risk of PE (Figures 2B, 2C, and 2D; all P for non-linearity < 0.001). Maternal AST levels showed a linear association with the risks of GH (Figure 1A; P < 0.001, P for non-linearity = 0.831) and PE (Figure 2A; P < 0.001, P for non-linearity = 0.121).
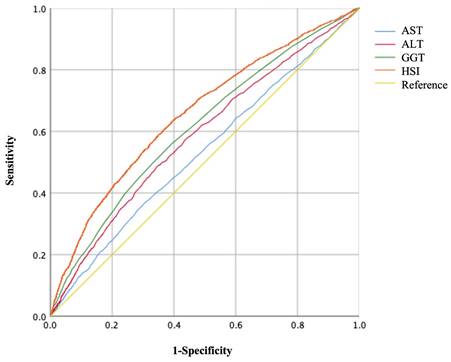
The areas under the ROC curves (AUC) to predict the risks of GH and PE using AST, ALT, GGT, and HSI are shown in Figures 3, 4, and Table 5. Maternal HSI in the first trimester showed the best predictive performance for GH and PE, yielding AUCs of 0.663 (95% CI: 0.649-0.676) and 0.657 (95% CI: 0.643-0.671), respectively, which were significantly higher than those for AST, ALT, and GGT (all P < 0.001).
Adjusted odds ratios for GH with the highest quantile of liver enzyme levels, stratified by baseline characteristics.
| Subgroups | Number of subjects | AST | ALT | GGT | HSI | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | ||
| Maternal age, years | |||||||||
| < 35 | 28365 | 1.212 (1.029-1.427) | 0.105 | 1.914 (1.603-2.284) | <0.001 | 2.054 (1.686-2.502) | 0.039 | 4.502 (3.744-5.414) | <0.001 |
| ≥ 35 | 9161 | 0.942 (0.729-1.218) | 1.054 (0.808-1.376) | 1.427 (1.071-1.900) | 2.351 (1.748-3.161) | ||||
| FBG in the first trimester, mmol/L | |||||||||
| < 5.6 | 37189 | 1.115 (0.971-1.281) | 0.142 | 1.616 (1.394-1.873) | 0.467 | 1.864 (1.584-2.194) | 0.164 | 3.877 (3.312-4.538) | 0.380 |
| ≥ 5.6 | 337 | 3.652 (0.758-17.590) | 0.972 (0.246-3.862) | 0.559 (0.101-3.089) | 1.889 (0.397-8.996) | ||||
| Gravidity | |||||||||
| > 1 | 17337 | 1.133 (0.924-1.389) | 0.991 | 1.542 (1.239-1.918) | 0.654 | 1.752 (1.386-2.215) | 0.584 | 3.721 (2.893-4.786) | 0.825 |
| 1 | 20189 | 1.124 (0.930-1.357) | 1.651 (1.352-2.015) | 1.920 (1.532-2.407) | 3.858 (3.152-4.721) | ||||
*Adjusted for gestational weeks of recruitment, maternal age (excepted for subgroup analyses stratified by maternal age), maternal BMI before pregnancy (excepted for HSI), FA supplement, first pregnancy (excepted for subgroup analyses stratified by gravidity), FBG (excepted for subgroup analyses stratified by FBG) and TC.
**P-value is from the test for the difference between the two OR derived from subgroup analysis using z statistic.
Abbreviations: GH, gestational hypertension; OR, odds ratio; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; HSI, hepatic steatosis index; FBG, fasting blood glucose.
Associations between liver enzyme indicators and GH, plotted with restricted cubic splines. Odds ratios and 95% CI were estimated using logistic regression model adjusted for the maternal age, pre-pregnancy BMI (excepted for HSI), FA supplement, first pregnancy, FBG, TC and gestational week.
Associations between liver enzyme indicators and PE, plotted with restricted cubic splines. Odds ratios and 95% CI were estimated using logistic regression model adjusted for the maternal age, pre-pregnancy BMI (excepted for HSI), FA supplement, first pregnancy, FBG, TC and gestational week.
Receiver operating characteristic (ROC) curves of liver enzyme indicators to predict GH.
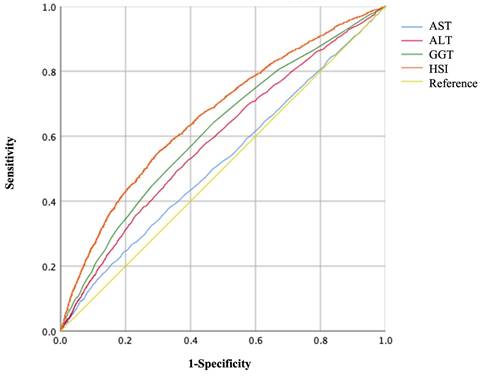
Receiver operating characteristic (ROC) curves of liver enzyme indicators to predict PE.
Adjusted odds ratios for PE with the highest quantile of liver enzyme levels, stratified by baseline characteristics.
| Subgroups | Number of subjects | AST | ALT | GGT | HSI | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | OR* (95%CI) | P-value** | ||
| Maternal age, years | |||||||||
| < 35 | 28326 | 1.293 (1.092-1.532) | 0.480 | 1.591 (1.333-1.898) | 0.418 | 2.101 (1.712-2.579) | 0.276 | 4.138 (3.435-4.985) | 0.002 |
| ≥ 35 | 9120 | 1.157 (0.892-1.500) | 1.387 (1.051-1.831) | 1.707 (1.260-2.313) | 2.341 (1.730-3.166) | ||||
| FBG in the first trimester, mmol/L | |||||||||
| < 5.6 | 37102 | 1.246 (1.080-1.437) | 0.623 | 1.542 (1.328-1.792) | 0.886 | 1.960 (1.652-2.325) | 0.555 | 3.653 (3.115-4.284) | 0.717 |
| ≥ 5.6 | 344 | 1.674 (0.477-5.872) | 1.385 (0.360-5.332) | 3.719 (0.452-30.599) | 5.295 (0.676-41.451) | ||||
| Gravidity | |||||||||
| > 1 | 17247 | 1.370 (1.104-1.699) | 0.304 | 1.592 (1.270-1.995) | 0.720 | 2.197 (1.687-2.861) | 0.306 | 3.831 (2.935-5.002) | 0.552 |
| 1 | 20199 | 1.178 (0.975-1.422) | 1.505 (1.233-1.836) | 1.830 (1.465-2.287) | 3.455 (2.830-4.219) | ||||
*Adjusted for gestational weeks of recruitment, maternal age (excepted for subgroup analyses stratified by maternal age), maternal BMI before pregnancy (excepted for HSI), FA supplement, first pregnancy (excepted for subgroup analyses stratified by gravidity), FBG (excepted for subgroup analyses stratified by FBG) and TC.
**P-value is from the test for the difference between the two OR derived from subgroup analysis using z statistic.
Abbreviations: PE, preeclampsia; OR, odds ratio; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; HSI, hepatic steatosis index; FBG, fasting blood glucose.
Area under curves (AUCs) to predict GH and PE by different liver enzyme indicators.
| Indicators | GH | PE | ||
|---|---|---|---|---|
| AUC (95% CI) | P-value** | AUC (95% CI) | P-value** | |
| AST | 0.523 (0.508-0.537) * | <0.001 | 0.532 (0.517-0.547) * | <0.001 |
| ALT | 0.587 (0.573-0.601) * | <0.001 | 0.585 (0.570-0.600) * | <0.001 |
| GGT | 0.615 (0.601-0.629) * | <0.001 | 0.612 (0.597-0.626) * | <0.001 |
| HSI | 0.663 (0.649-0.676) * | - | 0.657 (0.643-0.671) * | - |
* P-value <0.05 for AUC of each indicator for GH and PE.
** P-value for comparison between AUC of HSI and other indices for GH and PE.
Abbreviations: AUC, area under curve; GH, gestational hypertension; PE, preeclampsia; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transferase; HSI, hepatic steatosis index.
4. Discussion
The results indicated that the proportion of subjects with higher education levels and natural pregnancies was greater in the normal group (P < 0.001). Furthermore, participants in the GH or PE groups were more likely to have higher pre-pregnancy BMI or be experiencing their first pregnancy (P < 0.001). All serum markers, including liver enzyme indicators, HSI, FBG, and blood lipid indicators (TG, TC, LDL-C, and HDL-C) in the first trimester, showed significant differences among the normal, GH, and PE groups (P < 0.001). In our cohort study, the incidence of GH and PE gradually increased with higher HSI. After model calibration, women in the highest quartile of HSI had a threefold increase in the odds of GH compared to those in the lowest quartile. The risk of PE significantly increased with higher HSI quartiles, with a threefold increase in the odds of PE in the highest quartile. Pregnant women under the age of 35 years also exhibited a stronger relationship between HSI and GH and between HSI and PE compared to women over 35 years.
In our study population, the rates of developing GH and PE were 4.3% and 4.1% among pregnant women who delivered a live-born singleton, similar to previous reports [2, 16]. Our findings align with prior research indicating that blood glucose levels, primiparity, assisted reproduction, pre-pregnancy BMI, and advanced maternal age all positively correlate with the risks of GH and PE [3, 8]. Additionally, we found that pregnant women in the GH and PE groups had higher levels of TC, TG, LDL-C, and HDL-C than the normal group. Since atherogenic dyslipidemia is a known proximate cause of endothelial dysfunction, this association may help explain our findings [17].
Based on the results of the present study, after adjusting for confounders, non-linear relationships were demonstrated between ALT, GGT, HSI and the risk of GH (all P for non-linearity < 0.001). Similar non-linear associations were found between ALT, GGT, HSI, and the risk of PE (all P for non-linearity < 0.001). Maternal AST levels were linearly associated with the risk of both GH (P < 0.001) and PE (P < 0.001). Several studies support our results. A Korean study involving 2,322 women revealed that those with elevated ALT levels in the first trimester had three times the odds of developing PE (OR = 3.24, 95% CI: 1.06-8.41) [18]. A prospective cohort study from China involving 5,685 pregnant women found that, after adjusting for potential covariates, high-normal levels of ALT and GGT were associated with an increased risk of GH (ORALT = 1.21, 95% CI: 1.05-1.38; ORGGT = 1.23, 95% CI: 1.09-1.39) and PE (ORALT = 1.15, 95% CI: 1.03-1.28; ORGGT = 1.28, 95% CI: 1.16-1.41), respectively [19]. Moreover, a prospective cohort study of 1,041 women in South China indicated that elevated GGT levels increased the risk of PE and GH in early pregnancy by 2.6 times (OR = 2.61, 95% CI: 1.05-6.83) [20]. In all three studies, pregnant women with known chronic viral hepatitis B, hepatitis C, or other liver diseases were excluded. The authors also imposed that unknown elevated liver enzyme levels may indicate pre-existing NAFLD [18] [19].
However, previous studies have reported no correlation between AST levels and the risk of PE. In our study, AST was positively correlated with an increased risk of PE, showing a 1.2-fold higher risk in the highest quartile of HSI compared to the lowest among all pregnant participants. Similar conclusions were reported by Elad Mei-Dan et al. and A Bülez et al. [21, 22]. It is well known that AST plays a role in amino acid metabolism and the tricarboxylic acid cycle [23]. We suggest that AST may affect the epithelial-mesenchymal transition [24] by inhibiting the Phospho-Akt signaling pathway [25] in patients with PE.
HSI is a non-invasive biomarker used to diagnose NAFLD and has demonstrated good performance in detecting the presence of hepatic steatosis in both clinical and epidemiological studies [26], [12, 27-30]. Our study proposes that HSI may serve as a risk factor for GH and PE. Several studies have similarly supported our conclusions. Monika Sarkar et al. demonstrated that, after controlling for confounding factors, pregnant women with NAFLD exhibited an elevated risk of PE, including HELLP syndrome (Hemolysis, Elevated Liver enzymes, Low platelets) and eclampsia (OR = 3.1, 95% CI = 2.6-3.8), when compared to those without chronic liver disease [7]. Hydar et al. conducted a meta-analysis of 13,641 participants and found that pregnant women with NAFLD had a significantly higher likelihood of GH (OR = 1.83; 95% CI = 1.03-3.26; P = 0.041; n = 2) and PE (OR = 2.43; 95% CI = 1.46-4.04; P = 0.001; n = 3). A meta-analysis reported that the incidence of the composite outcome of PE, eclampsia, or HELLP syndrome in pregnancies with NAFLD was almost four times higher than in the control group (OR = 3.91, 95% CI = 2.71-5.64, n = 4) [9]. A retrospective study in China involving 14,708 pregnant women revealed that, after adjusting for potential confounding factors, NAFLD significantly elevated the risk of GH (OR = 3.054, 95% CI = 2.191-4.257) and PE/eclampsia (OR = 3.994, 95% CI = 2.591-6.005) [31].
Our study demonstrated that, compared to pregnant women aged 35 years or older, HSI exhibited stronger predictive power in those under the age of 35 years for GH (OR≥35 = 4.527, 95% CI = 3.762-5.446 vs. OR<35 = 2.325, 95% CI = 1.729-3.128) and PE (OR≥35 = 4.13, 95% CI = 3.433-4.983 vs. OR<35 = 2.348, 95% CI = 1.736-3.176). We suggest that younger patients might be more susceptible to the effects of NAFLD. For example, Walker RW et al. reported that the same variant in the PNPLA3 gene played different roles depending on the age of NAFLD onset, suggesting that age may play a crucial role in the development of NAFLD [32]. This finding may explain the varying effects of age on GH and PE.
NAFLD could lead to hepatic and peripheral insulin resistance and exacerbate liver inflammation [33]. NAFLD frequently coexists with obesity, diabetes, dyslipidemia, hypertension, and other metabolic disorders [34] and is increasingly recognized as a key feature and liver-specific manifestation of metabolic syndrome [35].
The mechanism by which NAFLD affects GH and PE during pregnancy may involve pregnancy-induced insulin resistance, a normal adaptation to ensure adequate carbohydrate supply for fetal growth [36]. NAFLD during pregnancy intensifies this state of insulin resistance [37]. Vascular insulin resistance promotes vascular contraction by impairing the phosphatidylinositol 3-kinase pathway and subsequently reducing endothelial nitric oxide (NO) production, thereby promoting the onset of PE [38-40]. Furthermore, NAFLD induces a systemic inflammatory response, demonstrated by elevated inflammatory factors, including Interleukin 6 (IL-6) Tumour necrosis factor α(TNF-α), and C-C motif ligand 2(CCL2) [41, 42]. This inflammation leads to endothelial dysfunction [43], contributing to the development of PE [44].
While invasive liver biopsy is the gold standard for diagnosing NAFLD [45], its large-scale use is challenging. Magnetic resonance imaging is time-consuming and expensive [46], whereas ultrasound exhibits low sensitivity and depends on operator skill [47]. Consequently, HSI represents a suitable biomarker for NAFLD screening.
This study has several strengths. First, we found that elevated HSI in early pregnancy predicts an increased risk for GH and PE. To our knowledge, this is the first large-scale cohort study linking pregnancy HSI with the risk of GH and PE, providing new evidence of the impact of NAFLD on these conditions. This discovery bears direct significance for pregnancy counseling for women with NAFLD. Second, we found that HSI predicts GH and PE more strongly in pregnant women under 35. Given the potential role of genetic factors in the onset age of NAFLD, our findings offer new clinical evidence to guide future research on genetic influences in NAFLD.
Our study had some limitations. First, this single-center observational study is subject to potential selection and information biases despite the large sample size. Second, similar to most studies, we could not perform tissue biopsies to confirm the presence of NAFLD in these participants [5]. Third, this study included only pregnant women with live singletons, suggesting that further research should consider including cases of pregnancy loss.
5. Conclusion
In conclusion, our study showed that elevated HSI in the first trimester was significantly associated with an increased risk of GH and PE in a large sample of Chinese pregnant women. Notably, higher levels of AST in early pregnancy were also positively correlated with an increased risk of PE. Moreover, our results suggest that HSI in pregnant women under 35 had a stronger predictive value for GH and PE. These findings illustrate the importance of NAFLD as a potential risk factor for GH and PE and highlight the need for in-depth research into perinatal primary prevention measures for women with NAFLD.
Abbreviations
PE: pre-eclampsia; GH: gestational hypertension; NAFLD: non-alcoholic fatty liver disease; HSI: hepatic steatosis index; BMI: body mass index; CBCS: China birth cohort study; FBG: fasting blood glucose; TG: triglyceride; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: γ-glutamyl transferase; ISSHP: International Society for the Study of Hypertension in Pregnancy; SD: standard deviation; ANOVA: one-way analysis of variance; ROC: receiver operating characteristic; AUC: area under curve; OR: odds ratio; CI: confidence interval; NO: nitric oxide;.; IL-6: Interleukin 6; TNF-α: Tumour necrosis factor α.; CCL2: C-C motif ligand 2.
Supplementary Material
Supplementary figure 1: Flow chart.
Acknowledgements
We thank all pregnant women participated in CBCS and the medical staff for collecting clinical information.
Funding
This work was supported by the National Key Research and Development Program of China (2016YFC1000101), Leading Talents in the Construction Project of High Level Public Health Technical Talents in Beijing (2022-1-003), Beijing Municipal Administration of Hospitals Incubating Program (PX2022058), and Beijing Obstetrics and Gynecology Hospital, Capital Medical University (FCYYJC202404).
Author contributions
Chenghong Yin, Ruixia Liu, Wentao Yue, Lin Zhang and Shen Gao designed the study and interpreted the results. Lin Zhang and Shen Gao conducted analyses and drafted the manuscript. Shaofei Su and Enjie Zhang provided data curation. Jianhui Liu and Shuanghua Xie contributed to clinical interpretation of data. Yue Zhang contributed to quality control of study. Chenghong Yin, Ruixia Liu and Wentao Yue revision and supervision the manuscript. All authors participated in the CBCS establishment and agreed to be accountable for all aspects of the work and approved the final manuscript.
Data availability
Anonymized data are available upon reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2020;76:1690-702
2. Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV. et al. Pre-eclampsia. Nature reviews Disease primers. 2023;9:8
3. Gestational Hypertension and Preeclampsia. ACOG Practice Bulletin, Number 222. Obstetrics and gynecology. 2020;135:e237-e60
4. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology. 2018;15:11-20
5. Byrne CD, Targher G. NAFLD: a multisystem disease. Journal of hepatology. 2015;62:S47-64
6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64:73-84
7. Sarkar M, Grab J, Dodge JL, Gunderson EP, Rubin J, Irani RA. et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. Journal of hepatology. 2020;73:516-22
8. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S. et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy hypertension. 2018;13:291-310
9. El Jamaly H, Eslick GD, Weltman M. Systematic review with meta-analysis: Non-alcoholic fatty liver disease and the association with pregnancy outcomes. Clinical and molecular hepatology. 2022;28:52-66
10. Kwon JY. Non-alcoholic fatty liver disease in pregnancy, paving the way for adverse pregnancy outcome risk assessment. Clinical and molecular hepatology. 2022;28:50-1
11. Wang C, Cai Z, Deng X, Li H, Zhao Z, Guo C. et al. Association of Hepatic Steatosis Index and Fatty Liver Index with Carotid Atherosclerosis in Type 2 Diabetes. International journal of medical sciences. 2021;18:3280-9
12. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010;42:503-8
13. Yue W, Zhang E, Liu R, Zhang Y, Wang C, Gao S. et al. The China birth cohort study (CBCS). European journal of epidemiology. 2022;37:295-304
14. Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomedical and environmental sciences: BES. 2004;17(Suppl):1-36
15. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219
16. Wang X, Zhang E, Tian Z, Zhao R, Huang K, Gao S. et al. The association between dyslipidaemia in the first trimester and adverse pregnancy outcomes in pregnant women with subclinical hypothyroidism: a cohort study. Lipids in health and disease. 2024;23:13
17. Poornima IG, Indaram M, Ross JD, Agarwala A, Wild RA. Hyperlipidemia and risk for preclampsia. Journal of clinical lipidology. 2022;16:253-60
18. Lee SM, Park JS, Han YJ, Kim W, Bang SH, Kim BJ. et al. Elevated Alanine Aminotransferase in Early Pregnancy and Subsequent Development of Gestational Diabetes and Preeclampsia. Journal of Korean medical science. 2020;35:e198
19. Zhang Y, Sheng C, Wang D, Chen X, Jiang Y, Dou Y. et al. High-normal liver enzyme levels in early pregnancy predispose the risk of gestational hypertension and preeclampsia: A prospective cohort study. Frontiers in cardiovascular medicine. 2022;9:963957
20. Chen Y, Ou W, Lin D, Lin M, Huang X, Ni S. et al. Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated With the Development of Gestational Hypertension and Preeclampsia. Frontiers in cardiovascular medicine. 2021;8:756140
21. Bülez A, Hansu K, Çağan ES, Şahin AR, Dokumacı H. Artificial Intelligence in Early Diagnosis of Preeclampsia. Nigerian journal of clinical practice. 2024;27:383-8
22. Mei-Dan E, Wiznitzer A, Sergienko R, Hallak M, Sheiner E. Prediction of preeclampsia: liver function tests during the first 20 gestational weeks. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26:250-3
23. Rink C, Gnyawali S, Stewart R, Teplitsky S, Harris H, Roy S. et al. Glutamate oxaloacetate transaminase enables anaplerotic refilling of TCA cycle intermediates in stroke-affected brain. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2017;31:1709-18
24. Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell adhesion & migration. 2015;9:317-24
25. Shrimali NM, Agarwal S, Kaur S, Bhattacharya S, Bhattacharyya S, Prchal JT. et al. α-Ketoglutarate Inhibits Thrombosis and Inflammation by Prolyl Hydroxylase-2 Mediated Inactivation of Phospho-Akt. EBioMedicine. 2021;73:103672
26. Preveden T, Veres B, Ruzic M, Pete M, Bogic S, Kovacevic N. et al. Triglyceride-Glucose Index and Hepatic Steatosis Index for the assessment of liver steatosis in HCV patients. Minerva gastroenterology. 2023;69:254-60
27. Sviklāne L, Olmane E, Dzērve Z, Kupčs K, Pīrāgs V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. Journal of gastroenterology and hepatology. 2018;33:270-6
28. Lee J, Ha J, Jo K, Lim DJ, Lee JM, Chang SA. et al. Male-specific association between subclinical hypothyroidism and the risk of non-alcoholic fatty liver disease estimated by hepatic steatosis index: Korea National Health and Nutrition Examination Survey 2013 to 2015. Scientific reports. 2018;8:15145
29. Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. Journal of hepatology. 2013;58:1007-19
30. Nakano M, Kawaguchi M, Kawaguchi T. Almost identical values of various non-invasive indexes for hepatic fibrosis and steatosis between NAFLD and MASLD in Asia. Journal of hepatology. 2024;80:e155-e7
31. Qian Y, Zhang Y, Fan X, Yan H, Li X, Fan Y. et al. Nonalcoholic Fatty Liver Disease and Adverse Pregnancy Outcomes in Women With Normal Prepregnant Weight. The Journal of clinical endocrinology and metabolism. 2023;108:463-71
32. Walker RW, Belbin GM, Sorokin EP, Van Vleck T, Wojcik GL, Moscati A. et al. A common variant in PNPLA3 is associated with age at diagnosis of NAFLD in patients from a multi-ethnic biobank. Journal of hepatology. 2020;72:1070-81
33. Byrne CD. Ectopic fat, insulin resistance and non-alcoholic fatty liver disease. The Proceedings of the Nutrition Society. 2013;72:412-9
34. Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H. et al. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Frontiers in endocrinology. 2022;13:1087260
35. Bugianesi E, Gastaldelli A, Vanni E, Gambino R, Cassader M, Baldi S. et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634-42
36. Sivan E, Homko CJ, Chen X, Reece EA, Boden G. Effect of insulin on fat metabolism during and after normal pregnancy. Diabetes. 1999;48:834-8
37. Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ. et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62:238-48
38. Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ. et al. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;204:327.e1-6
39. Roberts JM, Gammill H. Insulin resistance in preeclampsia. Hypertension (Dallas, Tex: 1979). 2006;47:341-2
40. Wu P, Green M, Myers JE. Hypertensive disorders of pregnancy. BMJ (Clinical research ed). 2023;381:e071653
41. Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH. et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism. 2008;7:496-507
42. Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I. et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. Journal of hepatology. 2006;44:1167-74
43. Muniyappa R, Sowers JR. Role of insulin resistance in endothelial dysfunction. Reviews in endocrine & metabolic disorders. 2013;14:5-12
44. Guan X, Fu Y, Liu Y, Cui M, Zhang C, Zhang Q. et al. The role of inflammatory biomarkers in the development and progression of pre-eclampsia: a systematic review and meta-analysis. Frontiers in immunology. 2023;14:1156039
45. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. The New England journal of medicine. 2014;371:1131-41
46. Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L. et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2
47. Leoni S, Tovoli F, Napoli L, Serio I, Ferri S, Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World journal of gastroenterology. 2018;24:3361-73
Author contact
![]() Corresponding authors: Chenghong Yin, MD, PhD, Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 52275422 (yinchhedu.cn). Ruixia Liu, PhD, Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 52277607 (liuruixiaedu.cn). Wentao Yue, PhD, Department of Research Management, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 85985110 (yuewtedu.cn).
Corresponding authors: Chenghong Yin, MD, PhD, Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 52275422 (yinchhedu.cn). Ruixia Liu, PhD, Department of Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 52277607 (liuruixiaedu.cn). Wentao Yue, PhD, Department of Research Management, Beijing Obstetrics and Gynecology Hospital, Capital Medical University. Beijing Maternal and Child Health Care Hospital. No.251, Yaojiayuan Rd, Beijing 100026, China. Tel.: +86 010 85985110 (yuewtedu.cn).

 Global reach, higher impact
Global reach, higher impact