3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(4):819-833. doi:10.7150/ijms.103241 This issue Cite
Review
Exploring the Potential of Moringa oleifera in Managing Bone Loss: Insights from Preclinical Studies
1. Department of Biochemistry, Faculty of Medicine, Manipal University College Malaysia, Bukit Baru, 75150, Melaka, Malaysia.
2. Department of Pharmacology, Faculty of Medicine, Manipal University College Malaysia, Bukit Baru, 75150, Melaka, Malaysia.
3. Faculty of Bioeconomics, Food & Health Science, University of Geomatika Malaysia, Setiawangsa, 54200, Kuala Lumpur, Malaysia.
4. Department of Pharmacology, Faculty of Medicine, Universiti Teknologi Mara (UITM), Jalan Hospital, 47000, Sungai Buloh, Selangor, Malaysia.
Received 2024-9-4; Accepted 2025-1-9; Published 2025-1-21
Abstract
Moringa oleifera (MO) is renowned for its remarkable medicinal uses, supported by claims across various cultures and growing scientific evidence. Preclinical experimental evidence indicated that MO may effectively reduce bone loss and promote bone remodelling through its effects on osteoclasts and osteoblasts. In vivo studies demonstrated that MO enhances critical aspects of bone health, such as bone volume, trabecular thickness and overall bone density. Furthermore, MO positively influenced bone biomarkers including alkaline phosphatase and procollagen type 1 N-terminal propeptide, reflecting improved bone formation. Additionally, in vitro and ex vivo studies revealed that MO boosted bone regeneration, stimulated osteoblast activity and reduced inflammation. In terms of mechanisms, MO may modulate signalling pathways related to bone metabolism, such as BMP2, PI3K/Akt/FOXO1, p38α/MAPK14 and RANKL/RANK//OPG pathways. This evidence provides a strong foundation for future clinical research and potential therapeutic applications in managing and preventing bone loss conditions.
Keywords: Moringa oleifera, osteoporosis, bone remodelling, in vitro, in vivo, ex vivo
1. Introduction
Bone remodelling involves the constant regeneration and replacement of bone tissues, which occurs in two separate phases: bone formation and bone resorption. During bone formation, osteoblasts (bone-forming cells) fill the bone cavities with new tissue. While, during resorption, osteoclasts (bone-resorbing cells) disintegrate bone tissue. Under normal and healthy conditions, bone resorption and formation occur in a dynamic and balanced way, with old tissues constantly replaced by new ones [1]. Calcium which is vital for bone health is primarily stored in bone. Calcium phosphate and mineralised collagen serve as structural supporting components for bone tissue. When blood calcium levels drop, various physiological responses contribute to maintaining calcium homeostasis. This includes activation of parathyroid hormone, which causes release of bone calcium, increased intestinal calcium absorption and increased renal calcium reabsorption. Traditionally, serum and urine calcium levels are employed as indications of mineral balance in the body. Meanwhile, excessive urine calcium excretion is linked to bone loss and osteoporosis [2].
Several clinical tests are now available for assessing bone health and evaluating therapy responses. Most of these indicators are also applicable to in vitro and in vivo experimental research. Bone mineral density (BMD), bone microarchitecture and biochemical markers serve as indicators of bone remodelling. In the clinical setting, bone diseases are typically evaluated using dual-energy X-ray absorptiometry (DXA), which measure bone mineral density. A T-score is created to indicate deviation of the measured BMD from the reference values in terms of standard deviation (SD). A T-score of -1 to -2.5 suggests poor bone mass or osteopenia, while a value below -2.5 indicates osteoporosis [3]. Bone microarchitecture can be evaluated using quantitative computed tomography (qCT). Deterioration of bone architecture resulted from bone loss, as demonstrated by decreased trabeculae number, increased inter-trabecular distances, loose connectivity of the trabecular meshwork, reduction of cortical bone thickness and increased porosity [3].
In recent years, cellular components of bone tissue have been widely employed as biomarkers for measuring and monitoring bone turnover and bone loss. Novel markers representing osteoblast or osteoclast metabolic activity can be measured in blood or urine to provide a quantitative estimate of bone remodelling status. Information on bone remodelling status can be used to predict pathological changes or the likelihood of developing certain bone diseases. These biomarkers have been employed mostly to evaluate the efficacy of antiresorptive and anabolic medications used to treat bone diseases and compliance with these medication therapies. They may also be used to enhance fragility fracture risk evaluation [4, 5].
Bone defects can negatively impact a patient's quality of life, and treatments are becoming increasingly expensive. As the population ages, they are more likely to experience bone disorders like pain, fractures, osteoporosis, infections, tumours, rheumatic diseases and oral/maxillofacial pathologies. When bone resorption outpaces bone synthesis, bone tissues are lost, resulting in osteoporosis. Osteoporosis is a prevalent bone disease marked by decreasing bone mass and microstructure alterations. Patients with osteoporosis should regularly calculate their fracture risk as they are prone to fractures. Bone fractures from injury, infection or inflammation can heal through cell proliferation and regeneration [6]. Certain plants contain phytocompounds that can enhance bone repair and prevent bone loss by inhibiting osteoclast cells recruitment, promoting osteoblast growth and lowering inflammation without the negative side effects associated with allopathic treatments [7].
Moringa oleifera (MO), commonly known as the 'drumstick tree' or 'horse radish tree,' is also referred to by various other names, including kelor, marango, mlonge, moonga, mulangay, nebeday, saijhan, sajna and ben oil tree [8]. MO is a unique plant with numerous edible parts, each offering distinct biological activities. Its leaves, fruit pods, seeds, flowers and roots contribute to diverse range of potential health benefits. The leaves of MO are rich in sulphur-containing amino acids, β-carotene, L-ascorbic acid, calcium, potassium, iron and fibre, surpassing the levels found in carrots, oranges, bananas and spinach [9]. MO seed oil primarily contains campesterol, stigmasterol, β-sitosterol, avenasterol and clerosterol, with trace amounts of 24-methylenecholesterol, campestanol, stigmastanol, and 28-isoavenasterol [10]. Meanwhile, MO leaves and pods are rich in ascorbic acid, retinol, riboflavin, nicotinic acid, pyridoxine, α-tocopherol, folic acid, β-carotene, estrogenic substances, β-sitosterol, iron, calcium, phosphorus, copper, protein and essential amino acids such as methionine, cystine, tryptophan and lysine, making them ideal dietary supplements [11]. Furthermore, laboratory studies demonstrated that mice fed with MO have elevated serum levels of calcium, proteins, phosphorus and antioxidants, while showing reduced levels of glucose, triglycerides and cholesterol [12, 13]. MO stands out as an affordable source of nutrition that can potentially alleviate malnutrition. It is especially beneficial for children who lack access to breast milk and exhibit signs of nutrient deficiencies. Intriguingly, MO also provides affordable nutrition and has the potential to combat malnutrition. MO contains lactagogues, which are substances commonly prescribed to breastfeeding mothers to help increase milk supply. These lactagogues, composed of phytosterols, act as precursors to hormones essential for reproductive growth. In addition, MO contains phytosterols such as stigmasterol, sitosterol and campesterol, which support estrogen synthesis. This, in turn, promotes the growth of mammary gland ducts and enhances milk production [14].
Experiments on MO extract revealed its remarkable osteoprotective impact and ability to enhance osteogenesis. Some of MO's therapeutic benefits are directly related to its osteoprotective activities, as addressed further below. MO, which has strong antioxidant and anti-inflammatory properties, can counteract the defects in osteoblastogenesis and osteoclastogenesis-induced by osteoporosis. This review article explores the preclinical experimental evidence regarding MO's efficacy in reducing bone loss.
2. Potential Effects of MO in Treating Bone Loss: In Vivo Studies
This review was based on data obtained from PubMed, Google scholar and EBSCOhost Medline databases from their inception to June 2024. There were 11 published in vivo studies that investigated the potential effects of MO on bones (Table 1). In these studies, MO was extracted either from the leaves, seeds or roots. However, some studies did not indicate the dose/concentration of MO used in their studies. Kusolrat and Kupittayanant [16] conducted a 6-week trial using 0.25 mL/100 g bodyweight/day of MO seed oil. Both ovariectomised (OVX) rats treated with MO from the preventative and recovery investigations showed significantly lower levels of serum alkaline phosphatase (ALP) but higher serum calcium levels compared to OVX control rats. The urine calcium and phosphorus excretion were lower than OVX control rats. The increased bone ALP activity associated with ovariectomy was due to high bone turnover rate, as seen by accelerated bone formation and resorption, which may lead to osteoporosis [15]. These bone marker patterns were also consistent with many prior studies, which found that vegetable oils rich in omega-3 and omega-6 polyunsaturated fatty promoted bone formation [16]. On the other hand, Kusolrat and Kupittayanant reported that OVX rats given MO seed oil have lower total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels, as well as higher high-density lipoprotein cholesterol (HDL-C) level [15]. These hypocholesterolemic and anti-osteoporotic effects in OVX rats were consistent with other studies using dietary soybean, flaxseed and sesame oils [17-19].
Hu et al. [21] found that estradiol (E2) and MO had comparable efficacy in enhancing bone metabolism, microstructure and density in OVX rats. In their study, MO treatment decreased the number of osteoclasts and bone resorption indicators, which was similar to Kusolrat and Kupittayanant's study, where MO treatment enhanced serum calcium levels, potentially by reducing bone resorption. Hu et al. al also reported that MO treatment increased procollagen I n-terminal propeptide (PINP) and decreased bone ALP, demonstrating its osteogenic properties. The results showed that MO intervention increased bone formation, decreased bone resorption and slowed bone turnover. In comparison to the OVX group, the trabecular bone microstructure showed significantly higher levels of bone volume (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N) and connectivity density (Conn.D) in the estradiol (E2) and MO groups. Histopathological study revealed trabecular damage (thinning, fracture, reduced area and wide trabecular spacing) apart from an abundance of fat vacuoles in the OVX group. E2 and MO treatments restored trabecular bone integrity to normal levels while reducing fat vacuoles in the OVX group [20]. The data indicated that removing the ovaries decreased bone mass and destroyed bone microstructure, whereas estradiol and MO therapies may alleviate these effects.
Intriguingly, Hu et al. also examined gut flora composition, intestinal permeability and key signalling markers in the mitogen-activated protein kinase (MAPK) pathway to understand MO's osteoprotective mechanisms. Novel gut flora-targeting therapies like probiotics and prebiotics might be efficacious at preventing bone loss [20]. Other studies demonstrated that supplementation with beneficial bacteria such as lactobacilli may offer protection against osteoporosis. Reductions in both the Firmicutes/Bacteroidetes ratio and Lactobacillus abundance are characteristics of the gut microbiota in OVX rats [21, 22]. The study supported previous findings that MO could improve osteoporosis by increasing the Firmicutes/ Bacteroidetes ratio and Lactobacillus abundance. The phylum Fusobacteria, Elusimicrobia and genus Oscillospira were found to have a favourable correlation with BMD. Microorganisms were seen to be more abundant in the normal control group than in the OVX group. Thus, these bacterial taxa may have anti-osteoporotic properties. Subsequently, Hu et al. used Cytoscape v. 3.7.2 to create a target pathway network map in identifying the core pathways and targets of MO in osteoporosis treatment. According to the analysis, MAPK signalling may be the core pathway through which MO increase extracellular signal-regulated kinase (ERK) expression and exerted their anti-osteoporosis effect. Other fascinating findings include Lactobacillus' ability to convert dietary L-histidine into immunomodulatory histamine, which raises cAMP levels and suppresses ERK activation [20]. Macrophages play an important function in bone development. Lactobacillus stimulates the immunological response of bone marrow-derived macrophages by activating MAPKs [23]. In addition, MO contains myricetin, quercetin and kaempferol, which can ameliorate osteoporosis. Other research has shown that myricetin reduces glucocorticoid-induced osteoporosis by modulating ERK signalling, enhancing femoral histology and increasing osteogenic differentiation and matrix mineralisation [24].
Diabetes mellitus is known to be correlated with an increased risk of osteoporosis. Streptozotocin is primarily employed as a chemical to create type 1 diabetes mellitus (T1DM) animal model. Ovariectomised rat is a classic animal model of oestrogen deficiency-induced osteoporosis. Both oestrogen deprivation and T1DM can cause osteoporosis [25]. In an in vivo study by Patel et al. [27], OVX and streptozotocin-induced osteoporosis model in rats was utilised to evaluate MO's preventive effects on bone loss caused by ovariectomy paired with diabetes. Patel et al. employed three separate components: leaves, fruits, and flowers, with findings showing all MO treatments reduced glucose levels in STZ-OVX rats. Among the three components, fruit extract was found to be very effective in lowering the raised glucose levels as well as TRAcP, the osteoclastic bone marker, in STZ-OVX rats. On the other hand, it caused a rise in ALP, the ostoblastic marker, in STZ-OVX rats [26]. The study provided evidence that MO fruit extracts play a major role in the prevention of bone loss in STZ-OVX mice.
Glucocorticoids (GCs) are the leading iatrogenic cause of secondary osteoporosis, with fracture rates increased by up to 75% within the first three months of treatment. GCs increase the production of macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-beta (NF-κB) ligand RANKL and decrease the production of osteoprotegerin (OPG) by osteoblastic cells and osteocytes. These led to increase number and activity of osteoclasts, with direct effects on bone resorption [27]. In the study by Soliman et al., osteoporosis was induced in the jawbone of Albino rats by daily intraperitoneal injection of 200μg/100g body weight of dexamethasone for 30 days. Bone density scan (DEXA) analysis confirmed osteoporosis of the upper jawbone (mandible). However, DEXA analysis showed a significant increase in BMD in the MO leaves-treated group compared to the steroid induced osteoporotic group. Based on Real-time PCR analysis, the MO leaves group also had significantly lower RANKL and higher OPG gene expression levels. Histological evaluation of the MO leaves group revealed significant healing of the mandible microanatomy. Histomorphometric analysis indicated a substantial increase in the percentage bone area in the mandible of the MO leaves group [28].
A similar study from Habib et al. employed a glucocorticoid-induced osteoporosis rat model, in which 100 mg prednisone acetate per kg meal was used as a glucocorticoid source to induce osteoporosis for two weeks. In the study, diets containing dried MO leaves (2.5%), moringa seeds (2.5%) and a mixture of both (2.5%) were fed to rats. Supplementation with MO leaves, seeds, or combinations significantly enhanced blood calcium and phosphorus levels in osteoporotic rats. In osteoporotic rats, serum-free thyroxin (T4) levels increased significantly, while parathormone (PTH) levels decreased significantly. Supplementation with MO (leaves, seeds, or combination) significantly boosted serum T4 levels, while decreasing serum PTH levels. The parathyroid organ produces parathyroid hormone, which regulates bone digestion and calcium homeostasis, among other metabolic hormones. The study discovered that rats with osteoporosis had significantly higher levels of PTH compared to controls. This may stimulate bone resorption to increase serum calcium levels, which could lead to osteoporosis. Following MO supplementation, the rat femoral BMD was significantly higher compared to osteoporotic rats [29]. A similar study conducted by Rawat et al. reported that MO supplementation ameliorated the steroid-induced reductions in BMD of the rat femur, serum calcium and ALP levels [30]. These studies concluded that MO leaves, seeds and their combinations have effective anti-osteoporotic properties.
Long-term glucocorticoid use can result in femoral head necrosis, which causes persistent deformity, fractures and aberrant bone regeneration [31]. In the study from Zhang et al., the chemical structure of purified polysaccharide from the MO plant was analysed using Fourier transform-infrared spectroscopy (FT-IR), methylation and nuclear magnetic resonance spectroscopy (NMR) methods. Osteoporosis was induced in rats by giving 0.2 g/kg injection of dexamethasone every four days. The rats also received daily oral gavages of MO polysaccharide at 6 g/kg (an average of 1.32 g/rat). The structural analysis revealed that MO polysaccharide contains D-galactitol, L-arabinitol and L-rhamnitol, as well as non-reduced L-rhamnopyranosyl, D-galactopyranosyl, L-arabinopyranosyl and D-galactopyranosyl. The study identified that rats given MO polysaccharide have improved bone volume, trabecular thickness, bone density and hexosamine concentration. Meanwhile, micro-CT scan results revealed that MO polysaccharides supplementation could counteract the bone-deleting effects of dexamethasone by maintaining the trabecular bone thickness. MO polysaccharide also increased the expressions of osteocalcin (OCN) and Runt-related transcription factor 2 (Runx2) genes, which could help to preserve bone volume and trabecular thickness parameters. High levels of hydroxyproline (a non-essential amino acid in collagen) and hexosamine (a component of mucopolysaccharide that reduces osteoblastic formation and bone reabsorption) in blood can indicate femoral bone necrosis [32]. MO polysaccharides can enhance collagen and mucopolysaccharide synthesis by increasing the hexosamine-to-hydroxyproline ratio [33]. In the study, it was discovered that ingestion of MO polysaccharides significantly increased the expression level of the COL-1 gene, which is particularly effective at healing damaged tissue. The findings suggest that MO polysaccharides could be used to control femoral head necrosis.
Tooth extraction is the process of removing a tooth from its alveolar bone socket, which can cause an inflammatory response and alveolar bone resorption in the buccolingual and apicocoronal areas of the edentulous ridge [34]. Alveolar bone resorption begins with the binding of RANKL to its receptor, receptor activator of nuclear factor-kappa B (RANK), which is found on preosteoclasts [35]. RANKL/RANK is a critical regulator of osteoclastogenesis. Proinflammatory cytokines including TNF-α, IL-1 and IL-6 can also influence the development of osteoclasts. Osteoprotegerin (OPG) protects bone from excessive resorption by binding to RANKL, preventing it from binding to RANK. RANKL and OPG bonds are the primary factors influencing bone mass and strength [36]. One approach for preventing alveolar bone loss is socket preservation. It is a surgical treatment that preserves the alveolar bone and soft tissues to the greatest extent possible after tooth extraction [37]. Demineralised freeze-dried bovine bone xenograft (DFDBBX) is a biocompatible and osteoinductive replacement material that can be utilised to preserve alveolar bone sockets [38]. The use of DFDBBX and MO combination has received attention as it could lessen the inflammatory response following tooth extraction. Soekobagiono et al., discovered that combining MO leaf extract with DFDBBX significantly reduced the level of RANKL expression in alveolar bone sockets of guinea pigs after tooth extraction [39]. Using immunohistochemistry Djais et al., found that combination of MO and Demineralization Freeze Dried Dentin Matrix (DFDDM) protected tooth extraction sockets against bone loss by increasing OPG and decreasing RANKL expressions [40]. Furthermore, histological and histomorphometric analyses revealed that MO leaf extract significantly increased the surface area of bone and the number of osteoblasts in MO groups compared to control groups, demonstrating its positive effect on bone regeneration in critical-sized defects [41].
Bone healing is a very complex reformative process. Bone tissues commonly heal spontaneously but may be incomplete in complicated circumstances such as large bone defects. Bone healing can be divided into three phases: inflammatory phase, reparative phase and remodelling phase [42]. Al-Azzawi and Al-Ghaban created a femur defect in albino rats to study the potential of MO and marine collagen (MC) in bone healing. Bone microarchitectures during healing was assessed using histological and histomorphometric analysis. Microscopic analysis of serial sections from the intervention location two weeks after treatment with MO extract revealed a considerable increase in osteoblasts compared to the control group. After 2- and 4 weeks, the group treated with MO showed increased osteoblast activity and decreased osteoclast activity, resulting in increased trabecular area and decreased bone marrow area compared to the control group. Intriguingly, after two weeks, the combination of MO and MC groups had more osteoblast cells and larger bone trabecular regions than either group alone. At 4 weeks, the trabecular bone area was significantly larger compared to two weeks, while bone trabeculae seemed thicker with regularly ordered osteocytes [43].
3. Potential Effects of MO in Treating Bone Loss: In Vitro and Ex vivo Studies
There were five in vitro and ex vivo studies which have assessed MO's osteoinductive potential in several cell types, including bone marrow mesenchymal stem cells (BMSCs), human osteosarcoma cell lines (SaOS2 and UMR106) and murine preosteoclast cell lines (RAW 264.7) (Table 2). In vitro studies were conducted to examine MO's effects on cell viability, proliferation and mineralisation, showing that MO can directly promote osteoblast development and differentiation. MO could stimulate cell proliferation, ALP activity, collagen synthesis and calcium deposition of osteoblast cells in a dose-dependent manner. Interestingly, MO leaves, fruits, seeds, flowers and roots have all been shown to possess osteoinductive properties.
Patel et al. investigated the osteoblastogenic potential of MO extracts from various parts (leaves, flowers and fruits) on SaOS2 osteoblast cell line. The MO extracts increased osteoblast proliferation from the dose of 50 μg/mL to 100 μg/mL. The study demonstrated that methanolic extracts of all MO parts promoted osteoblastogenesis. Flowers and fruit extracts were the most effective parts. The flower extract stimulated osteoblast cell proliferation, whereas the fruit extract increased ALP activity, hydroxyproline, calcium and collagen levels. Calcium is the primary component of bone mineral hydroxyapatite, while hydroxyproline is an essential amino acid for collagen production and bone matrix composition Although both extracts promoted bone formation, their mechanisms were slightly different. The flower extract increased osteoblast number, while the fruit extract promoted bone formation activity and mineralisation. [44]. These findings indicated that MO extracts of various parts have positive impact on osteoblastic cell proliferation, activity, bone matrix formation and mineralization.
Effects of MO on bone in in vivo studies. The symbol ↑ denotes an increase, ↓ denotes a decrease, and ↔ denotes no change.
| In vivo Study | Intervention (Dose, Route, and Duration) | Research Findings | Outcome | References |
|---|---|---|---|---|
| Female Wistar rats, 3-month-old, bilateral ovariectomy | MO seed oil (0.25 mL/100g BW/day), oral, 60 days | serum Ca ↑, Serum P ↑, ALP ↓, urine Ca ↓, urine P ↓ | MO seed oil reduced ALP but increased serum calcium, while decreasing urine calcium and phosphorous levels of ovariectomy rats. | Kusolrat & Kupittayanant [15] |
| Female Sprague-Dawley rats, 10-weeks old, bilateral ovariectomy | MO powder (0.1 mg/kg/day), oral, 12 weeks | BMD ↑, Trabecular: Tb Ar ↑, Tb Sp: ↑, Tb Th ↑, Tb N ↔, Cortical: Ct. Th↑, Ct.Ar ↑, Serum Ca ↑, Serum P ↑, ALP ↑, PINP ↑, Urine Ca ↓ , urine P ↓, CTX-1 ↓, DPD/CREA ↓ | MO increased bone mineral density and improved bone metabolism-related indicators and bone microstructure of ovariectomy rats. | Hu et al.,[20] |
| Female Wistar rats, 3-month-old, streptozotocin induced diabetic + ovariectomised (STZ-OVX) | MO leaf, flower and fruit (200mg /100g BW/day), oral, 30 days | ALP ↑, TRAcP↓ | MO protected against diabetic and ovariectomy induced osteoporosis by increasing ALP (bone formation marker) and decreasing TRAcP (bone resorption markers) | Patel et al., [26] |
| Albino rats, 3-month-old, dexamethasone (glucocorticoid)-induced jawbone osteoporotic rats | MO leaves (200mg/kg/day), oral, 30 days | jawbone BMD ↑, RANKL ↓, OPG ↑, RANKL/OPG ↓, bone area percentage ↑ | MO leaf extract reversed steroid induce osteoporosis by reducing RANKL and increasing OPG expressions, increasing bone area and osteoblast numbers. | Soliman et al.,[28] |
| Albino Sprague Dawley rats, 3-month-old, prednisoneacetate(glucocorticoid)-induced osteoporotic rats | MO leaves, seeds or combination of leaves and seeds (2.5%/kg/day), oral, 8 weeks | BMD ↑, serum Ca ↑, Serum P ↑, T4 ↑, PTH ↓ | MO leaves, seeds, and combinations reversed steroid induce osteoporosis by increasing BMD, reducing PTH and increasing serum calcium levels. | Habib et al., [29] |
| Sprague-Dawley rats, 3-month-old, prednisolone (glucocorticoid)-induced osteoporotic rats | MO leaves (200mg/kg/day), oral, 28 days | Bone density ↑, serum Ca ↑, ALP ↑ | MO reverses steroid induced osteoporosis by increasing bone density, serum calcium and ALP levels. | Rawat et al., [30] |
| Sprague-Dawley rats, 3-month-old, dexamethasone (glucocorticoid)-induced osteoporotic rats | MO (roots) polysaccharides (6g/kg/day), oral, 28 days | BMD ↑, BV/TV ↑, Tb Th ↑, hexosamine to hydroxyproline ratio ↑ | MO polysaccharide may treat femoral head necrosis caused by long-term glucocorticoid use by increasing bone histomorphometric parameters and hexosamine to hydroxyproline ratio. | Zhang et al., [32] |
| Male guinea pigs, alveolar bone sockets after tooth extraction | MO leaves extract + demineralized freeze-dried bone bovine xenograft (DFDBBX), 7 and 30 days | RANKL ↓ | MO lowered RANKL expressions of alveolar bone sockets post tooth extraction, which is indicative of bone formation. | Soekobagiono et al., [39] |
| Guinea pigs, alveolar bones after tooth extraction | MO leaves extract + demineralized freeze-dried bone bovine xenograft (DFDBBX), 7 14, and 21 days | RANKL ↓, OPG ↑ | Combining MO with DFDDM preserved tooth extraction sockets by increasing OPG and reducing RANKL expressions. | Djais et al.,[40] |
| Adult male New Zealand rabbits, critical-sized bone defects in the edentulous portions of mandibles. | MO leaves extract | ↑ the surface area of bone, ↑ the number of osteoblasts | MO leaf extract promotes bone repair in critical-sized defects in the edentulous portions of mandible by increasing bone area and osteoblasts number. | Elsadek et al., [41] |
| Albino rats, defect in femora | Dried seeds of MO + marine collagen, 4 weeks | Number of osteoblasts ↑, Number of osteocytes ↑, ALP ↑, TbN ↑. TbAr ↑ | Combination of MO and marine collagen promoted bone healing by increasing bone histomorphometric parameters. | Al-Azzawi and Al-Ghaban [43] |
Abbreviation: BW: body weight; Ca: Calcium; P: Phosphate; ALP: Alkaline phosphatase; BMD: Bone mineral density; Tb.Ar: Trabecular Area; Tb.Th: Trabecular thickness; Tb.N: Trabecular Number; Ct. Th: Cortical Thickness; Ct. Ar: Cortical Area; BV/TV: bone volume/Total volume; PINP: procollagen I n-terminal propeptide; CTX-1: C-terminal telopeptides type I collagen; DPD/CREA: urinary deoxypyridinoline/creatinine ratio; TRAcP: tartrate-resistant acid phosphatase; RANKL: receptor activator of nuclear factor-κB ligand; OPG: osteoproprotegrin; T4: Thyroxine; PTH: Parathyroid hormone
Using a comparable cell line model, Khan et al. discovered that in a cell viability assay, MO leaf extract promoted the growth activity of osteoblast cell line at the concentrations of 25 and 50 μg/mL. However, higher MO concentrations of 100 and 200 μg/mL reduced proliferation activity, probably adversely affected by ROS generation and chromatin condensation. Cell cycle analysis demonstrated that MO extracts at doses of 50 and 100 μg/mL halted cell division at the G2/M phase. The study also found that low doses of MO leaves extract increased the expression of osteogenic genes BMP2 and Runx2, while high doses decreased the expressions [45]. BMP2 is a potent cytokine that promotes mesenchymal cell in vitro differentiation into osteoblasts and in vivo bone formation. BMP2 promotes osteogenesis by activating Smad signalling and regulating the transcription of osteogenic genes such as Runx2, type I collagen, osteocalcin and bone sialoprotein. Runx2 plays a crucial role in early osteogenesis by regulating bone production, late mineralisation and expression of critical bone matrix genes during osteoblast differentiation [46]. The biphasic dose-response of osteoblast-like SaOS-2 cells growth activity towards MO extract suggested potential therapeutic and preventative applications in drug development.
Mesenchymal stem cells (MSCs) are stromal cells that can self-renew and differentiate into many lineages. MSC-based therapy has been proven to significantly increase tissue regeneration in pre-clinical and clinical trials [47]. MSCs can differentiate into a specific set of cell lineages: adipogenesis, osteogenesis and chondrogenesis. It is crucial to search for new mediators that can boost MSCs' osteogenic differentiation capacity [48]. The study by Marupanthorm and Kedpanyapong was the first to show that MO therapy could improve osteogenic differentiation of porcine bone MSCs (BMSCs), as indicated by enhanced ALP expression. Intriguingly, the study had used low concentrations of MO leaves ethanolic extract (100, 200 and 300 ng/ml). The optimal concentration of 100 ng/ml produced the most favourable results on osteogenesis [49]. Following that, Liu et al. investigated the therapeutic effects of MO leaves extracts on rat BMSCs exposed to peroxidative damage by hydrogen peroxide (H2O2). The molecular mechanisms involving the PI3K/Akt/FoxO1 pathway were also investigated. It was found that this pathway was activated in response to peroxidative damage to promote cell survival [50]. FoxO1 is a key regulator of oxidative stress and oxygen-free radical equilibrium during bone formation [51]. The study found that oxidative stress caused by H2O2 reduced FoxO1, while MO leaves extract restored it. On top of that, the study demonstrated that FoxO1 played a crucial role in reducing oxidative damage in BMSCs undergoing osteogenic differentiation. MO leaves extract enhanced the osteogenic differentiation capacity of MSCs, suggesting its potential for bone regeneration and alternative medicine applications.
Osseointegration refers to a time-dependent healing process in which a stable, clinically asymptomatic attachment of artificial materials to bone is achieved and sustained under functional loading [52]. In scientific term, osseointegration is bone ingrowth into a metal implant. The focus is on making the implant surface hydrophilic to improve osseointegration. Hydrophilic implant surfaces can help with bone integration and enhance osseointegration in the early stages of wound healing. Primary stability during installation, biological host bone and direct bone-to-implant contact (BIC) all contribute significantly to implant life and success [53-55]. Histomorphometry at the light microscopic level has been utilised to determine the amount of bone apposition surrounding the implant. For instance, Pachimalla et al. conducted a study to create a hydrophilic gel from MO seed extract applied to the surface of a dental implant grown with human mesenchymal cells to improve bone-implant contact. Transmission electron microscopy (TEM) and histomorphometry analysis revealed that hydrophilic implant surfaces increased BIC and encouraged new bone formation. The addition of bone-proliferating components to these hydrogels would hasten osseointegration and strengthen bone structure [56]. Surface features of implants, including topography, coatings and wettability, influence the behaviour of host osteoblasts and aid in bone formation during osseointegration. In particular, surface topography is essential for the initial adhesion and differentiation of osteoblasts on the implant surface and plays a significant role in long-term bone remodelling [52]. Furthermore, there was no evidence of degeneration, necrosis, fibrosis or inflammation at the new bone-to-implant contact [56].
Periodontitis is a chronic condition characterised by the clinical loss of alveolar bone and degeneration of connective tissue. Previous studies have linked p38α MAPK signalling to osteogenesis and the expression of inflammatory cytokines in periodontitis [57, 58]. Wang et al. have identified phenolic compounds in MO leaves extract using UPLC-ESI-MS/MS. Following that, the anti-periodontitis effects and mechanisms of MO were predicted through network pharmacology and molecular docking. Molecular docking analysis has identified p38α MAPK (MAPK14) pathway and the OPG/RANKL complex as potential targets for the anti-periodontitis effects. The in vitro study using RAW 264.7 pre-osteoclast cell lines demonstrated that MO significantly reduced inflammatory cytokines such as TNF-α, IL-1β, and IL-6, IL-1Ra and IL-10 and lowered OPG/RANKL ratio through MAPK14 inhibition. These MO effects halted the alveolar bone resorption by osteoclasts. In these findings, the anti-periodontitis effect might be attributed to the antioxidant properties of MO leaves extract [59].
4. Insight Molecular Mechanism of MO on Bone Remodelling
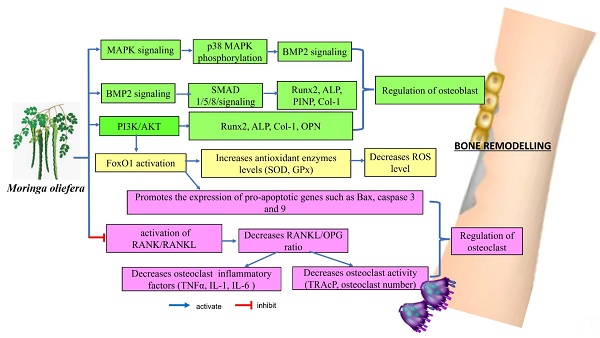
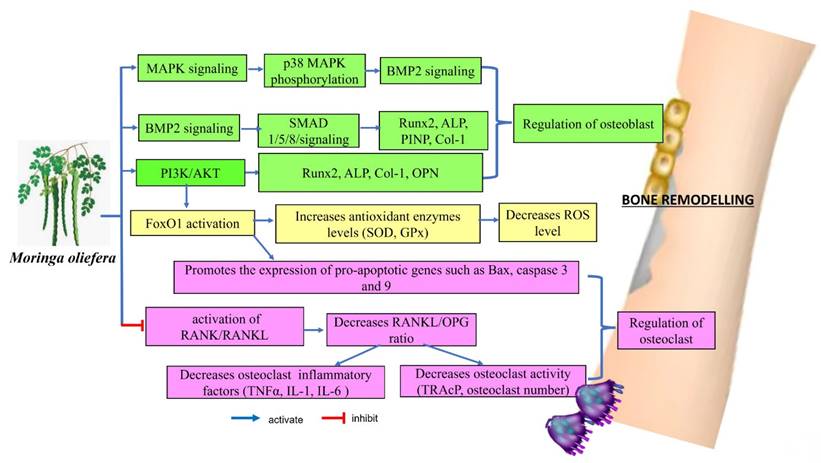
Mitogen-activated protein kinases (MAPKs) are essential dual-specificity protein kinases that participate in signalling pathways which control a variety of biological processes. MAPKs play a distinct role in stress response, cell proliferation, differentiation and survival. Moreover, they serve an important role in converting external signals into appropriate biological responses, which influence processes including inflammation, cell proliferation and death [60]. p38 MAPK is one of the signalling pathways that plays a vital role in regulating the expressions of Runx2 and BMP2, which are essential for osteoblast development and bone formation.
Effects of MO on bone in in vitro/ex vivo studies. The symbol ↑ denotes an increase, ↓ denotes a decrease, and ↔ denotes no change.
| In vitro/Ex vivo Study | Intervention | Research Findings | Outcome | References |
|---|---|---|---|---|
| SaOS2 cell line | 50 μg/mL to 100 μg/mL MO leaves, flowers and fruits | Cell viability ↑, ALP ↑, hydroxyproline content ↑, Ca↑, Col1 ↑ | MO extract from the three plant parts (leaves, flowers and fruits) stimulated osteoblast proliferation, differentiation, and mineralisation. | Patel et al. [44] |
| SaOS2 cell line | 25 μg/mL and 50 μg/mL MO leaves | Cell proliferation ↑, ALP ↑, Runx2 ↑, BMP2 ↑ | Biphasic dose-response effect of MO extract on the development of osteoblast-like SaOS2 cells. | Khan et al. [45] |
| 100 μg/mL and 200 μg/mL MO leaves | Cell proliferation↓, ROS ↑, cell cycle arrest | |||
| BMSC cells, exposed to 100 μmol/L H2O2 and Wortmannin (an inhibitor ofphosphoinositide-3 kinase)) | 10% MO leaves-containing serum | Cell proliferation ↑, ROS ↓, MDA ↓, Runx2 ↑, SOD↑, GSH ↑, PAkt ↑, FoxO1 ↑, Caspase 3 ↓ | MO leaf extract activated the PI3K/Akt/Foxo1 pathway, reducing peroxidative damage and promoting osteogenic induction in rat BMSCs. | Liu et al. [50] |
| UMR106 cell line | MO leaves hydrogel | Cell viability ↑, ALP ↑ | Titanium disc coated with MO hydrogel and seeded with human MSC showed increased proliferation of osteoblast cells. | Pachimalla et al. [56] |
| prototype titanium implants on the human MSC | TEM: Osteoblast proliferation↑, Histomorphometry: Bone implant contact ↑, BV ↑ | |||
| RAW 264.7 cell line | 1000 µg/ml MO leaves | TNF-α, IL-1β, and IL-6, IL-1Ra and IL-10 ↓, OPG ↑, RANKL ↓, OPG/RANKL↑, p38α MAPK ↓ | MO inhibited the expression of inflammatory cytokines and reduced alveolar bone resorption by modulating the expressions of p38α/MAPK14 and OPG/RANKL | Wang et al. [59] |
Abbreviation: ALP: Alkaline phosphatase; Col1-Type 1 Collagen; Runx2: runt-related transcription factor 2; ; BMP2: bone morphogenic protein 2; ROS: Reactive oxygen species; MDA: Malondialdehyde; SOD; Superoxide dismutase; GSH: Glutathione; RANKL: receptor activator of nuclear factor-κB ligand; OPG: osteoprotegerin; FoxO1: Forkhead box O1; PIK/AKT: phosphatidylinositol-3-kinase; TNF-α: tumour necrosis factor-α; IL-1β: interleukin-1β; IL-6: Interleukin-6; IL-1β: Interleukin-1β; IL-1Ra: Interleukin-1Ra; MAPK : mitogen-activated protein kinase; BMSC: bone mesenchymal stem cells; BV: bone volume; TEM: transmission electron microscopy
This pathway affects Runx2 and BMP2 expressions through several mechanisms, including the phosphorylation of transcription factors, modulation of gene promoter activity and interactions with other signalling molecules. By regulating the expression of Runx2 and BMP2, p38 MAPK contributes significantly to bone growth, maintenance and repair [61].
Osteogenesis is the process of new bone formation, where transcription factors are crucial in regulating cell proliferation and differentiation. These transcription factors guide the development of osteoblasts, cells responsible for bone formation, ensuring proper bone growth and maturation [62]. Runt-related transcription factor 2 (Runx2) is a crucial transcription factor governing the differentiation of mesenchymal stem cells into osteoblasts, which subsequently mature into osteocytes. Runx2 serves as a modulator capable of stimulating and inhibiting the differentiation of osteoblasts. For instance, in mice with a homozygous knockout of the Runx2 gene, bone formation was significantly impaired due to insufficient osteoblast differentiation and reduced expression of osteoblastic genes, including alkaline phosphatase (ALP) and osteocalcin (OC), among others [63]. Therefore, it can be said that Runx2 is a key transcription factor and an early indicator of osteoblastogenesis.
As a member of the transforming growth factor-beta (TGF-β) superfamily, Bone morphogenetic proteins (BMPs) are crucial in bone formation and repair. BMP2 is a potent osteoblastogenic factor that stimulates the formation of osteoblasts by activating the Smad signalling pathway. This pathway involves phosphorylation of Smad proteins, complex formation with Smad4, as well as regulation of gene expressions related to osteoblast differentiation and bone formation. Smad1/5/8 proteins formed complex with Smad4 and translocated to the nucleus where they activate Runx2, resulting in increased expression of osteoblastogenic markers [64]. Yoshikawa et al. discovered that BMP-2-induced RANKL mRNA expression was inhibited in primary osteoblasts lacking Smad1, leading to reduced osteoclast formation. In addition to Smad2 and Smad3, which mediate TGF-β signalling, as well as Smad1 and Smad5, which mediate BMP pathways, Smad4 plays a role in osteoclast formation by binding to phosphorylated R-Smads, acting as a Co-Smad in response to TGF-β/BMP stimulation [65]. TGF-β/BMP signalling can be modulated by extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38 MAPK, Ras homolog family member A (RhoA), phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt), with effects depending on the specific cellular context [66].
p38 MAPK was reported to promote osteoclast development, maturation and bone resorption. Previous research on the role of p38 MAPK in osteoclastogenesis primarily involved in vitro studies using RAW264 cell line and primary bone marrow cells [67, 68]. These studies revealed that p38 MAPK acted downstream of RANK following stimulation by RANKL, a key pro-resorptive cytokine essential for osteoclast formation. A complete inhibition of p38 MAPK effectively blocked osteoclastogenesis-induced by RANKL. Additionally, in osteoclast precursors, upon activation by TNF-α, p38 MAPK also operated downstream of the TNF receptor to support early osteoclast differentiation [69].
The differentiation of osteoclasts is primarily regulated RANKL/RANK pathway, where RANKL binds to its receptor RANK on osteoclast precursors, promoting their formation. Conversely, OPG, a soluble decoy receptor, inhibited this process by binding to RANKL and preventing it from interacting with RANK. RANKL also interacted with macrophage colony-stimulating factor (MCSF) to stimulate osteoclast development. These three components worked together to keep bone remodelling in equilibrium [70, 71]. Disruption of this balance may lead to osteoporosis, which can be efficiently treated by blocking the RANKL/RANK pathway [72, 73]. This was confirmed by the fact that OPG knockout mice suffered osteoporosis, demonstrating the relevance of OPG in controlling osteoclast development [74].
TNF-α, IL-1β and IL-6 are inflammatory cytokines that have impact on osteoclast activity, which is responsible for bone resorption. These cytokines influenced osteoclastogenesis (the production of osteoclasts) by interacting with RANKL, the key regulator of bone resorption process [75, 76]. TNF-α promoted bone resorption by activating osteoclasts, and if uncontrolled may lead to bone deterioration. It also suppressed osteoblast function, which reduced bone growth. Elevated TNF-α levels from chronic inflammation or autoimmune diseases can impair bone density, worsen bone loss and increase the risk of osteoporosis [77]. IL-1 is another cytokine which is important in inflammatory bone loss as it influences numerous elements of osteoclast biology, such as differentiation, multinucleation and survival. IL-1β plays a significant role in increasing osteoclast development. It accomplished this by boosting the level of RANKL, which is required for osteoclastogenesis [78]. IL-1β and TNF-α promoted the production of osteoclasts by increasing RANKL expression in stromal cells, leading to differentiation of osteoclast precursors [79, 80]. Furthermore, IL-1 can promote osteoclast development through pathways other than the RANKL/RANK pathway, particularly via the bone marrow-derived macrophages (BMMs) [81]. When coupled with TNF-α, IL-1 activated osteoclasts to resorb bone, resulting in bone loss. Furthermore, IL-1 may impair fracture healing by inhibiting osteoblast migration, which impeded the repair process [82]. IL-6 is another cytokine that has diverse effects on bone metabolism. It is best known as a pro-osteoclastic factor that promoted osteoclast activity. In mouse models with elevated IL-6 levels, an increase in osteoclasts and a decrease in bone trabecular volume could be seen, indicating enhanced bone resorption. IL-6 can induce osteoclast production through RANKL-dependent and independent pathways [83, 84]. It can also directly or indirectly increase osteoclastogenesis by boosting RANKL synthesis by osteoblasts. This dual role highlighted IL-6's ability to promote bone loss via increased osteoclast activity.
Forkhead box class O (FoxO) proteins are evolutionarily conserved transcription factors that convert environmental signals into gene expression, regulating a variety of cellular processes including proliferation, differentiation, survival, oxidative stress, inflammation, apoptosis and ageing [85]. FoxO proteins protect osteoblast progenitors from oxidative stress, which can damage proteins, lipids and DNA, potentially leading to cell death [86]. Excess reactive oxygen species (ROS) contribute to oxidative stress. When FoxO1 is overexpressed, it enhances the production of these antioxidant enzymes, which helps to neutralise ROS and reduce oxidative stress within cells. Conversely, when FoxO1 is deleted, the protective effect against oxidative stress is lost, leading to increased ROS levels. This heightened oxidative stress can adversely affect the bone marrow, thus increasing osteoclast progenitors, which are precursor cells that develop into bone-resorbing osteoclasts [87]. Thus, the loss of FoxO1 function can disrupt normal bone homeostasis by promoting the proliferation of osteoclast precursors and potentially contributing to bone loss.
Studies have shown that deleting FoxO1, FoxO3 and FoxO4 in mice can elevate oxidative stress and osteoblast apoptosis, as well as a decrease in the number of osteoblasts, bone formation rate and overall bone mass in cancellous and cortical bone regions [87, 88]. This showed that FoxOs played an important role in protecting osteogenesis from oxidative stress. ROS such as H2O2 can activate FoxO targets by binding with β-catenin, a dual function protein, responsible for regulating and coordinating gene transcription and cell to cell adhesion [89].
The PI3K/AKT signalling pathway played a crucial role in regulating various cellular processes such as proliferation, differentiation, metabolism and survival [90]. Findings from Liu et al. indicated that MO leaf extract activates PI3K, facilitating osteogenic differentiation in H2O2-damaged bone marrow stromal cells (BMSCs) [50]. This activation may improve osteogenic differentiation (bone formation) of BMSCs injured by oxidative stress (H2O2). RNA sequencing analysis revealed that the PI3K/Akt pathway is involved in bone regeneration [91]. Another study demonstrated that astragaloside promotes osteogenic differentiation of MC3T3-E1 cells by regulating the PI3K/Akt signaling pathway [92]. Furthermore, an ex vivo study showed that exosomes derived from human-induced pluripotent stem cell-derived mesenchymal stem cells (hiPS-MSC-Exos), when combined with tricalcium phosphate, can repair critical-sized calvarial bone defects by activating the PI3K/Akt signaling pathway [93]. FoxO1 is particularly affected by this pathway through PI3K/AKT-mediated phosphorylation. When AKT activity is inhibited, FoxO1 translocated to the nucleus and promoted the expression of pro-apoptotic genes such as Bax, caspase 3 and 9, leading to increased cell apoptosis [94, 95]. In experimental conditions, dexamethasone treatment inhibited the PI3K/AKT signalling pathway by decreasing the levels of phosphorylated PI3K (p-PI3K) and phosphorylated AKT (p-AKT). This resulted in reduced phosphorylation of FoxO1 (p-FoxO1) and promoted FoxO1's movement into the nucleus. Conversely, the addition of insulin-like growth factor 1 (IGF-1) counteracted the effects of dexamethasone by enhancing p-PI3K, p-AKT and p-FOXO1 levels, while decreasing the total FOXO1 expression and its nuclear translocation. These findings demonstrate that dexamethasone regulated FOXO1 through the PI3K/AKT pathway, specifically by inducing FoxO1's nuclear entry and activating downstream apoptotic signalling pathways [95].
Remarkably, MO may affect several key signalling pathways related to bone metabolism, including the p38α/MAPK14, BMP2, RANKL/RANK/OPG and PI3K/Akt/FoxO1 pathways. These interactions suggested that MO could play a role in enhancing bone formation, regulating bone cell activity, managing inflammation and influencing bone resorption. These actions indicated that MO has potential therapeutic benefits for managing and preventing conditions related to bone loss. The evidence supporting its effects on these signalling pathways provided a promising foundation for future clinical research to explore its effectiveness and safety in bone health applications.
5. Bioactive Compounds of MO with Potential Osteoprotective Effects
Polyphenols are roughly divided into two types: phenolic acids (which have just one phenol ring) and flavonoids (which include many phenol rings). The MO tree contains several essential polyphenols, including phenolic acids and flavonoids, including tannins [96]. The leaves of MO have been reported to have the highest total phenolic content, ranging from 2000 to 12,200 mg GAE/100 g. The most prevalent flavonoids found in the MO tree are kaempferol glycosides (including glucosides, malonyl glucosides, and rutinosides), as well as quercetin. Myricetin, epicatechin, and rutin are all present in small amounts [97].
Proposed molecular mechanism of Moringa oleifera on bone remodelling.
Kaempferol (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) is a naturally occurring flavonoid that enhances the nutritional value of many fruits and vegetables. It is also found in various botanical plants that have long been used in traditional medicine, such as Ginkgo biloba and propolis [98]. Kaempferol and its derivatives are known for their antioxidant, anti-inflammatory, and potential therapeutic qualities, making them valuable for both nutritional and medical applications. Preclinical investigations have shown that kaempferol has significant bone-protecting effects. In the review by Wong et al., kaempferol supplementation has demonstrated bone-sparing effects in various models, including newborn rats, glucocorticoid-induced, ovariectomy-induced osteoporotic models, and bone fracture models. These bone-protective effects are achieved through multiple mechanisms: kaempferol inhibits adipogenesis, inflammation, oxidative stress, osteoclastic autophagy, and osteoblastic apoptosis, while promoting osteoblastic autophagy. The anti-osteoporotic effects of kaempferol are mediated by the regulation of key signaling pathways, including the estrogen receptor, bone morphogenetic protein-2 (BMP-2), nuclear factor-kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin (mTOR) pathways [99].
Quercetin is a broad group of naturally occurring flavonoid compounds found in plant-based diets. It is also a member of a class of plant-derived, nonsteroidal chemicals known as phytoestrogens [100]. Quercetin has been found to improve bone metabolism by modulating a variety of cellular processes. It suppresses RANKL-mediated osteoclastogenesis, hence preventing excessive bone resorption. Furthermore, quercetin inhibits osteoblast apoptosis, oxidative stress, and the inflammatory response, all of which contribute to bone loss. On the other hand, quercetin stimulates osteogenesis, angiogenesis, and antioxidant expression, all of which are required for bone growth and repair. Furthermore, it promotes adipocyte and osteoclast apoptosis, which improves bone health by reducing fat cell growth and suppressing excessive bone resorption [101, 102].
Carotenoids are a broad collection of fat-soluble pigments that give different fruits, vegetables, fungi, bacteria, and algae their red, orange, and yellow colour. Carotenoids, including β-carotene, are precursors to vitamin A, making these pigments essential for human nutrition. Fresh Moringa (Moringa oleifera) leaves contain high levels of β-carotene, with quantities ranging from 6.6 to 17.4 mg per 100 g. This is significantly higher than the β-carotene level of other typical sources such as carrots, pumpkins, and apricots. Dried Moringa leaves contain significantly more β-carotene, with concentrations ranging from 23.31 to 39.6 mg per 100 g dry matter [103]. This makes Moringa leaves a good source of this essential nutrient, especially when dried. Kulczyński et al. conducted a narrative review and found that carotenoids may improve bone health. Increased carotene intake and blood levels are consistently linked to improvements in critical bone health markers such as bone mineral density (BMD), alkaline phosphatase (ALP), C-terminal telopeptide (CTx), tartrate-resistant acid phosphatase (TRAP), and RANKL. Furthermore, carotenoids have been related to a lower risk of fracture, implying that they contribute to bone strength and integrity. These findings underscore carotenoids' potential involvement in improving overall bone health [104].
6. Safety Studies of MO
Safety studies on herbal medicines typically involved acute and subacute toxicity testing in laboratory animals. The acute toxicity study of methanol extract of MO revealed a high safety profile, as methanol extract of MO did not cause noticeable toxicity or mortality at the dose of 2000 mg/kg in any of the animals throughout the observation period. Haematological and biochemical parameters remained stable compared to the control group. These findings confirmed the safety of methanol extract of MO and supported its use as herbal medicine [105]. However, a study by Oyagbemi found that rats administered methanol extract at doses of 200 and 400 mg/kg for 8 weeks exhibited significant increments (p < 0.05) in serum ALT, AST, BUN and creatinine levels, indicating potential hepatic and kidney damage [106]. Meanwhile, other research found genotoxicity at supra-supplementation levels of 3000 mg/kg body weight, with intake remaining safe at levels of 1000 mg/kg or less [107].
The aqueous extract of MO leaves has also been found to adversely affect conception and pregnancy outcomes in rats. A dose of 58 mg/kg body weight of cold aqueous extract or 50 mg/kg body weight of hot aqueous extract administered before mating resulted in 100% and 83.3% infertility, respectively. The 58 mg/kg body weight dose was noted to be equivalent to 250 mg of the dry plant product. When rats were given these doses after mating, an 80% abortion rate was reported with the cold aqueous extract and a 50% abortion rate with the hot aqueous extract. In comparison, there were no abortions in the control group, which only received distilled water [108]. In another study, Ajuogu et al. investigated the effects of adding 0 to 15 g/kg MO leaf powder in the diet on reproductive hormones and semen quality of rabbits after 12 weeks. In female rabbits, follicle-stimulating hormone (FSH) levels were lower with increasing MO powder doses compared to the control group. Conversely, male rabbits (bucks) displayed higher FSH levels with 15 g/kg MO powder compared to the control. Additionally, semen quality and sperm count improved with higher doses of MO powder. The study also revealed that while MO positively influenced buck fertility, it caused infertility in female rabbits [109].
MO is commercially accessible in capsule and powder forms, which is marketed for a variety of applications. Several research have investigated the clinical safety and therapeutic potential of various MO components in human health. Notably, Agrawal and Mehta studied the utilisation of MO seed kernels to treat bronchial asthma. Their clinical experiment indicated that MO seed extract was safe to deliver to patients, with no side effects reported [110]. Stohs and Hartman conducted a review of five human studies on the effects of whole leaf powders of M. oliefera when taken orally. According to the studies, these powders appear to have anti-hyperglycemic, anti-dyslipidemic, and antioxidant properties in humans, without causing severe side effects. These human trials, which used whole leaf powder at doses of up to 50 g and 8 g per day for 40 days, revealed no side effects [111]. In a randomised controlled trial by Taweerutchana et al., 25% of the 16 patients who received MO experienced diarrhoea, which resolved on its own within a few days. There were no cases of hypoglycemia, and no significant changes in renal or liver function tests were observed from baseline to end of study [112]. Meanwhile, in a clinical trial of 26 pregnant women who ate yoghurt with 4.3 g of dried, ground MO leaves daily, all births were uneventful except for three premature deliveries [113]. Furthermore, six randomised controlled trials of MO as a galactagogue in nursing mothers found no adverse effects [114]. The variable methods of administration and dosing may have contributed to the varied findings. Currently, most commercially available MO products are not controlled by bodies such as the Food and Drug Administration (FDA) and therefore their exact contents are not known. The systematic review by Popoola et al. (covering studies from 1990 to 2019) presents solid evidence of MO's pharmacological multifunctional therapeutic agent with neuroprotection, cardioprotection, anti-inflammatory, and antidiabetic properties. These findings lend credence to the safety profile of MO preparations in people, showing its potential as a treatment for a variety of disorders with minimal toxicity [115]. However, more study, including human clinical trials, is required to establish the efficacy, appropriate doses, and long-term safety of MO. There have been no human clinical trials examining the use of MO as a therapy for osteoporosis. This limitation makes it difficult to draw firm conclusions about its efficacy and safety in humans for this specific ailment. However, preclinical evidence (i.e., studies in animals or in vitro) indicating osteogenic (bone-forming) actions and anti-osteoporosis qualities could serve as a foundation for future study, including clinical trials.
Cytochrome P-450 (CYP) enzymes are a large family of enzymes involved in the metabolism of a wide range of substances, including drugs [116]. In vitro studies of MO and cytochrome P-450 (CYP) enzymes revealed that MO may have the ability to suppress some CYP isoenzymes, notably CYP3A4, CYP1A2 and CYP2D6 [117, 118]. Thus, co-administration of MO with pharmaceuticals that are substrates for certain CYP enzymes may cause substantial drug interactions, affecting treatment efficacy and safety. However, in a trial of HIV-infected patients, the pharmacokinetics of nevirapine, which is a medicine metabolised by CYP3A4, remained unchanged when combined with 1.85 grams of MO powder daily for 14 days [119]. The absence of significant interaction with nevirapine in the study suggested no clinically relevant impact on the metabolism of nevirapine.
While these studies provide valuable information, it is crucial to consider that interactions can be drug-specific and context-dependent. Additional studies may be needed to explore interactions with other drugs metabolised by CYP3A4 to confirm the findings in broader populations. Caution is always recommended when combining herbal supplements with prescription medications. Variability in individual responses and differences in product formulations warrant careful consideration and monitoring.
To put MO promising preclinical effects into practice, well-designed human clinical trials are required. These studies would have to assess dose-effect relationships within human-adjusted ranges to verify that the dosages utilised are both effective and safe. Furthermore, the trials must demonstrate that there are no safety risks associated with the use of MO preparations in humans, especially given individual diversity in sensitivity to the plant's bioactive components.
7. Conclusion and Perspective
Research on the impact of MO on bone health is on the rise. The objective of this article was to examine the preclinical studies on MO with emphasis on its osteogenic and osteoinductive abilities in preventing or treating bone loss. Preclinical research revealed the potential benefits of MO as functional food, notably for bone health. These results provide the framework for human clinical trials to investigate further into MO's role in reducing osteoporosis risk and enhancing general bone health. If MO is proven to be effective, it can be incorporated into treatment regimens for osteoporosis.
MO is being explored as a potential treatment for osteoporosis owing to its dual role as both an anabolic and antiresorptive agent. It enhanced bone repair by increasing the number of osteoblasts, which are crucial for new bone formation. MO could be highly beneficial in repairing bone defects and supporting osteointegration of dental implants. Its ability to enhance bone regeneration, stimulate osteoblast activity and reduce inflammation makes it a promising candidate for improving bone healing and implant outcomes.
In terms of mechanisms, MO directly modulated the expressions of Runx2 and BMP2 to regulate osteoblast differentiation, while decreasing the RANKL/OPG ratio. It also activated the PI3K/Akt/Foxo1 pathway, which reduced oxidative damage and promoted osteogenic induction. Additionally, MO lowered bone resorption by affecting the expressions of p38α/MAPK14 and RANKL/OPG ratio. These dual effects on osteoblasts and osteoclasts are beneficial for maintaining balance in bone remodelling, which is crucial for managing osteoporosis. Furthermore, MO enhanced the growth, proliferation and differentiation of mesenchymal stem cells into osteoblasts, offering potential advantages for stem cell applications and scaffold-based approaches to improve bone restoration.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cook CV, Lighty AM, Smith BJ, Ford Versypt AN. A review of mathematical modeling of bone remodeling from a systems biology perspective. Frontiers in Systems Biology. 2024;4:1368555
2. Taxel P, Kenny A. Differential diagnosis and secondary causes of osteoporosis. Clinical cornerstone. 2000;2:11-9
3. Roux J-P, Duboeuf F, Sornay-Rendu E, Rinaudo L, Ulivieri FM, Wegrzyn J. et al. The relationship between bone strain index, bone mass, microarchitecture and mechanical behavior in human vertebrae: an ex vivo study. Osteoporosis International. 2024;35:1069-75
4. Inderjeeth CA, Inderjeeth DC. The use of anabolic agents in the treatment of osteoporosis: a clinical update. Current Opinion in Endocrinology, Diabetes and Obesity. 2024;31:157-163
5. Ferrari S, Pepe J. Anabolic Agents in the Treatment of Postmenopausal Osteoporosis. Bone Metabolism, Parathyroid Glands, and Calciotropic Hormones: Springer. 2024 p. 1-19
6. Sharma A, Sharma L, Goyal R. Molecular signaling pathways and essential metabolic elements in bone remodeling: An implication of therapeutic targets for bone diseases. Current Drug Targets. 2021;22:77-104
7. Hanga-Farcaș A, Miere F, Filip GA, Clichici S, Fritea L, Vicaș LG. et al. Phytochemical Compounds Involved in the Bone Regeneration Process and Their Innovative Administration: A Systematic Review. Plants. 2023;12:2055
8. Arshad S, Sajjad B, Aftab A, Yousaf Z, Alotaibi MO. Moringa. Essentials of Medicinal and Aromatic Crops: Springer. 2023 p. 1063-89
9. Su B, Chen X. Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Frontiers in veterinary science. 2020;7:53
10. González-Romero J, Guerra-Hernández EJ, Rodríguez-Pérez C. Bioactive compounds from Moringa oleifera as promising protectors of in vivo inflammation and oxidative stress processes. Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress: Elsevier. 2022 p. 379-99
11. Singh U, Dwivedi C, Kulsum U, Verma N, Saraf S, Pradhan DK. Phytochemistry and medicinal uses of moringa oleifera: an overview. Journal of Drug Delivery and Therapeutics. 2017;7:104-16
12. Bais S, Singh GS, Sharma R. Antiobesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Advances in Biology. 2014;2014:162914
13. Salami AT, Okonkwo CE, Attah FA, Olagoke OC. Bioactive Moringa olifera seed extracts attenuates cholesterol gall stones in hyperglycaemic Swiss mice. Comparative Clinical Pathology. 2021;30:207-16
14. Nurachma E, Lushinta L, Puspitaningsih R, Sholikah I. The Effect of Moringa Pudding on Increasing Breast Milk for Postpartum Mothers. Asian Journal of Engineering, Social and Health. 2024;3:1100-9
15. Kusolrat P, Kupittayanant S. Effects of Moringa oleifera Lam. seed oil on lipid profiles and bone biomarkers in ovariectomized rats. Progress in Applied Science and Technology. 2018;8:173-83
16. Bjørklund G, Dadar M, Doşa MD, Chirumbolo S, Pen JJ. Insights into the effects of dietary omega-6/omega-3 polyunsaturated fatty acid (PUFA) ratio on oxidative metabolic pathways of oncological bone disease and global health. Current Medicinal Chemistry. 2021;28:1672-82
17. Hassan HA, Abdel-Wahhab MA. Effect of soybean oil on atherogenic metabolic risks associated with estrogen deficiency in ovariectomized rats: dietary soybean oil modulate atherogenic risks in overiectomized rats. Journal of physiology and biochemistry. 2012;68:247-53
18. Al-Madhagy S, Ashmawy NS, Mamdouh A, Eldahshan OA, Farag MA. A comprehensive review of the health benefits of flaxseed oil in relation to its chemical composition and comparison with other omega-3-rich oils. European journal of medical research. 2023;28:240
19. Boulbaroud S, El-Hessni A, Azzaoui F, Mesfioui A. Sesame seed oil and flaxseed oil affect plasma lipid levels and biomarkers of bone metabolism in ovariectomized Wistar rats. Biology and Medicine. 2012;4:102
20. Hu X-H, Yang X-Y, Lian J, Chen Y, Zheng C-Y, Tao S-Y. et al. Moringa oleifera leaf attenuate osteoporosis in ovariectomized rats by modulating gut microbiota composition and MAPK signaling pathway. Biomedicine & Pharmacotherapy. 2023;161:114434
21. Wu X, Kim MJ, Yang HJ, Park S. Chitosan alleviated menopausal symptoms and modulated the gut microbiota in estrogen-deficient rats. European Journal of Nutrition. 2021;60:1907-19
22. Chen Q, Wang B, Wang S, Qian X, Li X, Zhao J. et al. Modulation of the gut microbiota structure with probiotics and isoflavone alleviates metabolic disorder in ovariectomized mice. Nutrients. 2021;13:1793
23. Wang B, Wu Y, Liu R, Xu H, Mei X, Shang Q. et al. Lactobacillus rhamnosus GG promotes M1 polarization in murine bone marrow-derived macrophages by activating TLR2/MyD88/MAPK signaling pathway. Animal Science Journal. 2020;91:e13439
24. Fan S, Gao X, Chen P, Li X. Myricetin ameliorates glucocorticoid-induced osteoporosis through the ERK signaling pathway. Life sciences. 2018;207:205-11
25. Bortolin RH, da Graça Azevedo Abreu BJ, Abbott Galvão Ururahy M, Costa de Souza KS, Bezerra JF, Bezerra Loureiro M. et al. Protection against T1DM-induced bone loss by zinc supplementation: biomechanical, histomorphometric, and molecular analyses in STZ-induced diabetic rats. PloS one. 2015;10:e0125349
26. Patel C, Ayaz RM, Parikh P. Studies on the osteoprotective and antidiabetic activities of Moringa Oleifera plant extract. IOSR Journal of Pharmacy and Biological Sciences. 2015;5:19-22
27. Hsu CH, Hsu CL, Langley A, Wojcik C, Iraganje E, Grygiel-Górniak B. Glucocorticoid-induced osteoporosis—from molecular mechanism to clinical practice. Drugs & Therapy Perspectives. 2024;40:315-329
28. Soliman T, Ali Z, Zayed M, Sabry D, AbuBakr N. The anti-osteoporotic effect of Moringa oleifera leaves extract on glucocorticoids-induced jawbone osteoporosis in Albino rats. Brazilian Dental Science. 2021 24
29. Habib M, Al-Moalem MH. Effect of Moringa Leaves and Seeds on Osteoporosis in Rats. Journal of Food and Dairy Sciences. 2018;3:129-35
30. Rawat P, Ahmad A, Pant D, Verma M, Bisht P. Evaluation of anti-osteoporotic potential of Moringa olifera Leaves in rats. Journal Of Veterinary Pharmacology And Toxicology. 2020;19:25-8
31. Xia C, Xu H, Fang L, Chen J, Yuan W, Fu D. et al. β-catenin inhibition disrupts the homeostasis of osteogenic/adipogenic differentiation leading to the development of glucocorticoid-induced osteonecrosis of the femoral head. Elife. 2024;12:RP92469
32. Zhang B, Lu C, Xu Z, Guo H, Zhang G, Hao Y. The effect of Moringa oleifera polysaccharides on the regulation of glucocorticoid-induced femoral head necrosis: In vitro and in vivo. Arabian Journal of Chemistry. 2022;15:103410
33. Yan M, Jiang X, Wang G, Wang A, Wang X, Wang X. et al. Preparation of self-assembled collagen fibrillar gel from tilapia skin and its formation in presence of acidic polysaccharides. Carbohydrate polymers. 2020;233:115831
34. Nemcovsky CE, del Fabbro M, Beitlitum I, Taschieri S. Treatment Alternatives Following Extraction of Teeth with Periodontal-Endodontic Lesions. Endodontic-Periodontal Lesions: Evidence-Based Multidisciplinary Clinical Management. 2019:141-94
35. Usui M, Onizuka S, Sato T, Kokabu S, Ariyoshi W, Nakashima K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Japanese Dental Science Review. 2021;57:201-8
36. Cheng K, Gao S, Mei Y, Zhou D, Song C, Guo D. et al. The bone nonunion microenvironment: A place where osteogenesis struggles with osteoclastic capacity. Heliyon. 2024;10:e31314
37. Khan J, Bandi S, Gangineni S, Kummari S, Pradeep DG, Hinduja T. Evaluation of Alveolar Ridge Dimensions by Socket Preservation Therapy Using a Bone Graft and Platelet-Rich Fibrin: A Randomized Controlled Trial. Cureus. 2024;16:e60388
38. Azhar IS, Kresnoadi U, Rahayu RP. Potency of Garcinia mangostana L peel extract combined with demineralized freeze-dried bovine bone xenograft on IL-1 β expression, osteoblasts, and osteoclasts in alveolar bone. Dent J (Majalah Kedokt Gigi). 2017;50:166-70
39. Soekobagiono S, Alfiandy A, Dahlan A. RANKL expressions in preservation of surgical tooh extraction treated with Moringa (Moringa oleifera) leaf extract and demineralized freeze-dried bovine bone xenograft. Dent J (Majalah Kedokteran Gigi). 2017;50:149-53
40. Djais AI, Oktawati S, Thahir H, Hatta M, Sukmana BI, Dewi N. et al. Effect of the Combination of Demineralization Freeze Dried Dentin Matrix (DFDDM) and Moringa Oleifera Lam on Nuclear Factor Kapa B as a Marker of Bone. Systematic Reviews in Pharmacy. 2020 11
41. Elsadek NA, Aboukhadr MA, Kamel FR, Mostafa HM, El-Kimary GI. Moringa oleifera leaf extract promotes the healing of critical sized bone defects in the mandibles of rabbits. BDJ open. 2024;10:22
42. Duda GN, Geissler S, Checa S, Tsitsilonis S, Petersen A, Schmidt-Bleek K. The decisive early phase of bone regeneration. Nature Reviews Rheumatology. 2023;19:78-95
43. Al-Azzawi AS, Al-Ghaban NM. Histomorphometric evaluation of the effect of local application of Moringa oliefera/Marine collagen on bone healing in rats. J Res Med Dent Sci. 2021;9:225
44. Patel C, Rangrez A, Parikh P. The anti-osteoporotic effect of Moringa oliefera on osteoblastic cells: SaOS 2. IOSR J Pharm Biol Sci. 2013;5:10-7
45. Khan MI, Siddiqui S, Barkat MA, Alhodieb FS, Ashfaq F, Barkat HA. et al. Moringa oleifera leaf extract induces osteogenic-like differentiation of human osteosarcoma SaOS2 cells. Journal of Traditional and Complementary Medicine. 2022;12:608-18
46. Arya PN, Saranya I, Selvamurugan N. RUNX2 regulation in osteoblast differentiation: a possible therapeutic function of the lncRNA and miRNA-mediated network. Differentiation. 2024;140:100803
47. Zhang X, Kuang Q, Xu J, Lin Q, Chi H, Yu D. MSC-Based Cell Therapy in Neurological Diseases: A Concise Review of the Literature in Pre-Clinical and Clinical Research. Biomolecules. 2024;14:538
48. Al-Sharabi N, Mohamed-Ahmed S, Shanbhag S, Kampleitner C, Elnour R, Yamada S. et al. Osteogenic human MSC-derived extracellular vesicles regulate MSC activity and osteogenic differentiation and promote bone regeneration in a rat calvarial defect model. Stem cell research & therapy. 2024;15:33
49. Marupanthorn K, Kedpanyapong W. The Effects of MoringaOleifera Lam. Leaves Extract on Osteogenic Differentiation of Porcine Bone Marrow Derived Mesenchymal Stem Cells. 4th International Conference on Advances in Agricultural. 2016 p. 1-4
50. Liu M, Ding H, Wang H, Wang M, Wu X, Gan L. et al. Moringa oleifera leaf extracts protect BMSC osteogenic induction following peroxidative damage by activating the PI3K/Akt/Foxo1 pathway. Journal of Orthopaedic Surgery and Research. 2021;16:1-14
51. Feng Y, Tang X. FoxO1 as the critical target of puerarin to inhibit osteoclastogenesis and bone resorption. Journal of Pharmacy and Pharmacology. 2024;76:813-823
52. Palermo A. Improvement of Osseointegration Through Autologous Growth Factors. Saving Dental Implants. 2024:522-33
53. Shu T, Wang X, Li M, Ma S, Cao J, Sun G. et al. Nanoscaled Titanium Oxide Layer Provokes Quick Osseointegration on 3D-Printed Dental Implants: A Domino Effect Induced by Hydrophilic Surface. ACS nano. 2023;18:783-97
54. Chen C-L, Hung W-C, Tseng C-C, Shen Y-K, Cho Y-C, Lan W-C. et al. An innovative bioactive surface with potential hemocompatibility performance for enhancing osseointegration at early-stage implantation. Ceramics International. 2023;49:33748-54
55. Silva IRd, Barreto ATdS, Seixas RS, Paes PNG, Lunz JdN, Thiré RMdSM. et al. Novel strategy for surface modification of titanium implants towards the improvement of osseointegration property and antibiotic local delivery. Materials. 2023;16:2755
56. Pachimalla PR, Mishra SK, Chowdhary R. Evaluation of hydrophilic gel made from Acemannan and Moringa oleifera in enhancing osseointegration of dental implants. A preliminary study in rabbits. Journal of Oral Biology and Craniofacial Research. 2020;10:13-9
57. Kirkwood KL. Targeting MAPK/MKP signaling as a therapeutic axis in periodontal disease. Emerging Therapies in Periodontics. 2020:55-71
58. Đorđević IO, Kukolj T, Obradović H, Trivanović D, Petrović A, Mojsilović S. et al. Interleukin-17 modulates uPA and MMP2 expression in human periodontal ligament mesenchymal stem cells: involvement of the ERK1/2 MAPK pathway. Archives of Biological Sciences. 2022;74:15-24
59. Wang F, Long S, Zhang J. Moringa oleifera Lam. leaf extract safely inhibits periodontitis by regulating the expression of p38α/MAPK14-OPG/RANKL. Archives of Oral Biology. 2021;132:105280
60. Kassouf T, Sumara G. Impact of conventional and atypical MAPKs on the development of metabolic diseases. Biomolecules. 2020;10:1256
61. Brennan CM, Emerson Jr CP, Owens J, Christoforou N. p38 MAPKs—roles in skeletal muscle physiology, disease mechanisms, and as potential therapeutic targets. JCI insight. 2021;6:e149915
62. Chan WCW, Tan Z, To MKT, Chan D. Regulation and role of transcription factors in osteogenesis. International journal of molecular sciences. 2021;22:5445
63. Qin X, Jiang Q, Komori H, Sakane C, Fukuyama R, Matsuo Y. et al. Runt-related transcription factor-2 (Runx2) is required for bone matrix protein gene expression in committed osteoblasts in mice. Journal of Bone and Mineral Research. 2020;36:2081-95
64. Jann J, Gascon S, Roux S, Faucheux N. Influence of the TGF-β superfamily on osteoclasts/osteoblasts balance in physiological and pathological bone conditions. International journal of molecular sciences. 2020;21:7597
65. Yoshikawa Y, Yoshizawa T, Domae E, Hieda Y, Takeyama A, Hirota S. et al. RNA interference-mediated knockdown of Smad1 inhibits receptor activator of nuclear factor κB ligand expression induced by BMP-2 in primary osteoblasts. Archives of Oral Biology. 2015;60:1319-26
66. Zhang YE. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harbor perspectives in biology. 2017;9:a022129
67. Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). Journal of Biological Chemistry. 2000;275:31155-61
68. Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S. et al. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU. 1. Journal of Biological Chemistry. 2004;279:45969-79
69. Yu X, Wu Q, Ren Z, Chen B, Wang D, Yuan T. et al. Kaempferol attenuates wear particle-induced inflammatory osteolysis via JNK and p38-MAPK signaling pathways. Journal of Ethnopharmacology. 2024;318:117019
70. Hansen MS, Madsen K, Price M, Søe K, Omata Y, Zaiss MM. et al. Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity. Bone Research. 2024;12:5
71. Rattajak P, Aroonkesorn A, Smythe C, Wititsuwannakul R, Pitakpornpreecha T. Pleurotus sajor-caju (Fr.) Singer β-1, 3-Glucanoligosaccharide (Ps-GOS) Suppresses RANKL-Induced Osteoclast Differentiation and Function in Pre-Osteoclastic RAW 264.7 Cells by Inhibiting the RANK/NFκB/cFOS/NFATc1 Signalling Pathway. Molecules. 2024;29:2113
72. Jin Y, Zhou B-H, Zhao J, Ommati MM, Wang S, Wang H-W. Fluoride-induced osteoporosis via interfering with the RANKL/RANK/OPG pathway in ovariectomized rats: Oophorectomy shifted skeletal fluorosis from osteosclerosis to osteoporosis. Environmental Pollution. 2023;336:122407
73. Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. BioMed research international. 2020;2020:6910312
74. Zhang M, Chen M, Li Y, Rao M, Wang D, Wang Z. et al. Delayed denervation-induced muscle atrophy in Opg knockout mice. Frontiers in Physiology. 2023;14:1127474
75. de Oliveira G, de Andrade Rodrigues L, da Silva AAS, Gouvea LC, Silva RCL, Sasso-Cerri E. et al. Reduction of osteoclast formation and survival following suppression of cytokines by diacerein in periodontitis. Biomedicine & Pharmacotherapy. 2024;177:117086
76. Umur E, Bulut SB, Yiğit P, Bayrak E, Arkan Y, Arslan F. et al. Exploring the Role of Hormones and Cytokines in Osteoporosis Development. Biomedicines. 2024;12:1830
77. Yao Q, He L, Bao C, Yan X, Ao J. The role of TNF-α in Osteoporosis, Bone Repair and Inflammatory Bone Diseases: A Review. Tissue and Cell. 2024;89:102422
78. Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, Di Benedetto P. et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediators of inflammation. 2015;2015:782382
79. Lee B, Kim T-H, Jun J-B, Yoo D-H, Woo J-H, Choi SJ. et al. Direct inhibition of human RANK+ osteoclast precursors identifies a homeostatic function of IL-1β. The Journal of Immunology. 2010;185:5926-34
80. Kitaura H, Kimura K, Ishida M, Kohara H, Yoshimatsu M, Takano-Yamamoto T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Journal of Immunology Research. 2013;2013:181849
81. Hu K, Shang Z, Yang X, Zhang Y, Cao L. Macrophage polarization and the regulation of bone immunity in bone homeostasis. Journal of Inflammation Research. 2023;16:3563-80
82. Kikyo N. Circadian regulation of macrophages and osteoclasts in rheumatoid arthritis. International journal of molecular sciences. 2023;24:12307
83. Feng W, Liu B, Liu D, Hasegawa T, Wang W, Han X. et al. Long-term administration of high-fat diet corrects abnormal bone remodeling in the tibiae of interleukin-6-deficient mice. Journal of Histochemistry & Cytochemistry. 2016;64:42-53
84. Kuroyanagi G, Kamiya N, Yamaguchi R, Kim HK. Interleukin-6 receptor blockade improves bone healing following ischemic osteonecrosis in adolescent mice. Osteoarthritis and Cartilage Open. 2023;5:100386
85. Tia N, Singh AK, Pandey P, Azad CS, Chaudhary P, Gambhir IS. Role of Forkhead Box O (FOXO) transcription factor in aging and diseases. Gene. 2018;648:97-105
86. Ambrogini E, Almeida M, Martin-Millan M, Paik J-H, DePinho RA, Han L. et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell metabolism. 2010;11:136-46
87. Iyer S, Han L, Ambrogini E, Yavropoulou M, Fowlkes J, Manolagas SC. et al. Deletion of FoxO1, 3, and 4 in osteoblast progenitors attenuates the loss of cancellous bone mass in a mouse model of type 1 diabetes. Journal of Bone and Mineral Research. 2017;32:60-9
88. Lu Y, Alharbi M, Zhang C, O'Connor JP, Graves DT. Deletion of FOXO1 in chondrocytes rescues the effect of diabetes on mechanical strength in fracture healing. Bone. 2019;123:159-67
89. Yao H, Yao Z, Zhang S, Zhang W, Zhou W. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Molecular medicine reports. 2018;17:6681-90
90. Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050-60
91. Teng Z, Zhu Y, Hao Q, Yu X, Teng Y, Yue Q. et al. Long non-coding RNA taurine upregulated gene 1 is downregulated in osteoporosis and influences the osteogenic differentiation of bone marrow mesenchymal stem cells. PeerJ. 2021;9:e11251
92. Jing WB, Ji H, Jiang R, Wang J. Astragaloside positively regulated osteogenic differentiation of pre-osteoblast MC3T3-E1 through PI3K/Akt signaling pathway. Journal of orthopaedic surgery and research. 2021;16:1-10
93. Zhang J, Liu X, Li H, Chen C, Hu B, Niu X. et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem cell research & therapy. 2016;7:1-14
94. Ma Y, Wang H. PI3K/Akt/FoxO: a novel participant in signal transduction in bone cells under mechanical stimulation. Cell biology international. 2012;36:923-6
95. Sun F, Zhou JL, Wei SX, Jiang ZW, Peng H. Glucocorticoids induce osteonecrosis of the femoral head in rats via PI3K/AKT/FOXO1 signaling pathway. PeerJ. 2022;10:e13319
96. Hassan MA, Xu T, Tian Y, Zhong Y, Ali FAZ, Yang X. et al. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine. 2021;93:153771
97. Ma Z, Ahmad J, Zhang H, Khan I, Muhammad S. Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South African Journal of Botany. 2020;129:40-6
98. Shahbaz M, Imran M, Momal U, Naeem H, Alsagaby SA, Al Abdulmonem W. et al. Potential effect of kaempferol against various malignancies: recent advances and perspectives. Food and Agricultural Immunology. 2023;34:2265690
99. Wong SK, Chin K-Y, Ima-Nirwana S. The osteoprotective effects of kaempferol: the evidence from in vivo and in vitro studies. Drug design, development and therapy. 2019;13:3497-514
100. Tomczyk-Warunek A, Winiarska-Mieczan A, Blicharski T, Blicharski R, Kowal F, Pano IT. et al. Consumption of phytoestrogens affects bone health by regulating estrogen metabolism. The Journal of Nutrition. 2024;154:2611-2627
101. Feng Y, Dang X, Zheng P, Liu Y, Liu D, Che Z. et al. Quercetin in Osteoporosis Treatment: A Comprehensive Review of Its Mechanisms and Therapeutic Potential. Current Osteoporosis Reports. 2024;22:353-365
102. Wong SK, Chin K-Y, Ima-Nirwana S. Quercetin as an agent for protecting the bone: a review of the current evidence. International journal of molecular sciences. 2020;21:6448
103. Manavalan N, Boopathi NM, Raveendran M. Medicinal and Therapeutic Properties of Moringa. The Moringa Genome. 2021:31-9
104. Kulczyński B, Sidor A, Brzozowska A, Gramza-Michałowska A. The role of carotenoids in bone health-a narrative review. Nutrition. 2023;119:112306
105. Marref SE, Melakhessou MA, Becheker I, Mekersi N, Marref C. Acute Oral Toxicity, Anti-Inflammatory and Anti-pyretic Activities of Methanol Extract of Moringa oleifera LAM in Albino Rats. African Journal of Biological Sciences. 2024;6:2432-2441
106. Oyagbemi AA, Omobowale TO, Azeez IO, Abiola JO, Adedokun RA, Nottidge HO. Toxicological evaluations of methanolic extract of Moringa oleifera leaves in liver and kidney of male Wistar rats. Journal of basic and clinical physiology and pharmacology. 2013;24:307-12
107. Asare GA, Gyan B, Bugyei K, Adjei S, Mahama R, Addo P. et al. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. Journal of ethnopharmacology. 2012;139:265-72
108. Attah A, Moody J, Sonibare M, Salahdeen H, Akindele O, Nnamani P. et al. Aqueous extract of Moringa oleifera leaf used in Nigerian ethnomedicine alters conception and some pregnancy outcomes in Wistar rat. South African Journal of Botany. 2020;129:255-62
109. Ajuogu PK, Mgbere OO, Bila DS, McFarlane JR. Hormonal changes, semen quality and variance in reproductive activity outcomes of post pubertal rabbits fed Moringa oleifera Lam. leaf powder. Journal of ethnopharmacology. 2019;233:80-6
110. Agrawal B, Mehta A. Antiasthmatic activity of Moringa oleifera Lam: A clinical study. Indian Journal of pharmacology. 2008;40:28-31
111. Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytotherapy Research. 2015;29:796-804
112. Taweerutchana R, Lumlerdkij N, Vannasaeng S, Akarasereenont P, Sriwijitkamol A. Effect of Moringa oleifera leaf capsules on glycemic control in therapy-naive type 2 diabetes patients: a randomized placebo controlled study. Evidence-Based Complementary and Alternative Medicine. 2017;2017:6581390
113. Bisanz JE, Enos MK, PrayGod G, Seney S, Macklaim JM, Chilton S. et al. Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of Moringa-supplemented probiotic yogurt. Applied and environmental microbiology. 2015;81:4965-75
114. Raguindin PFN, Dans LF, King JF. Moringa oleifera as a galactagogue. Breastfeed Med. 2014;9:323-4
115. Popoola JO, Aworunse OS, Oyesola OL, Akinnola OO, Obembe OO. A systematic review of pharmacological activities and safety of Moringa oleifera. Journal of Herbmed Pharmacology. 2020;9:174-90
116. Esteves F, Rueff J, Kranendonk M. The central role of cytochrome P450 in xenobiotic metabolism—a brief review on a fascinating enzyme family. Journal of xenobiotics. 2021;11:94-114
117. Showande SJ, Fakeye TO, Kajula M, Hokkanen J, Tolonen A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs—Implication for herb-drug interactions. Food science & nutrition. 2019;7:44-55
118. Fantoukh OI, Albadry MA, Parveen A, Hawwal MF, Majrashi T, Ali Z. et al. Isolation, synthesis, and drug interaction potential of secondary metabolites derived from the leaves of miracle tree (Moringa oleifera) against CYP3A4 and CYP2D6 isozymes. Phytomedicine. 2019;60:153010
119. Monera-Penduka TG, Maponga CC, Wolfe AR, Wiesner L, Morse GD, Nhachi CF. Effect of Moringa oleifera Lam. leaf powder on the pharmacokinetics of nevirapine in HIV-infected adults: a one sequence cross-over study. AIDS research and therapy. 2017;14:1-7
Author contact
![]() Corresponding author: anazrunedu.my; 03-61267008.
Corresponding author: anazrunedu.my; 03-61267008.

 Global reach, higher impact
Global reach, higher impact