3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(4):754-763. doi:10.7150/ijms.103639 This issue Cite
Research Paper
Long sleep duration and good sleep quality reduced incident peptic ulcer disease in a large Taiwanese population follow-up study
1. Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
2. Teaching and Research Center, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
3. Department of Psychiatry, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
4. Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
5. Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
6. School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan.
7. Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
8. Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
9. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Received 2024-9-13; Accepted 2025-1-11; Published 2025-1-21
Abstract
Poor sleep has been associated with diseases including cardiovascular, obesity and mental disorders. However, there is limited information on the correlation between sleep duration and quality with peptic ulcer disease (PUD). This study aimed to investigate the impact of sleep duration and quality on the incidence of PUD in a large Taiwanese population follow-up study. The study participants were recruited from the Taiwan Biobank. Sleep duration, sleep quality, and the presence of PUD were assessed using self-reported questionnaires. The participants were categorized into three groups based on sleep duration: < 7 hours/day, 7 hours/day, and > 7 hours/day. Sleep quality was divided into five levels: very poor, poor, normal, good and very good. The association between sleep duration and quality with incident PUD was analyzed using multiple logistic regression after controlling for confounders. We collected data from 22,561 participants (excluding those with pre-existing PUD, missing basic information, or lacking sleep data). Over an average follow-up period of 43 months, multivariable analysis showed that sleep duration > 7 hours/day (vs. < 7 hours/day; hazard ratio [HR], 0.771; 95% confidence interval [CI], 0.668 to 0.890; p < 0.001) was significantly associated with incident PUD. Further, sleep duration (per 1 hour/day; HR, 0.933; 95% CI, 0.889 to 0.979; p = 0.005) was also significantly associated with incident PUD. Those with poor sleep quality (vs. very poor quality; HR, 0.649; 95% CI, 0.491 to 0.858; p = 0.002), normal sleep quality (vs. very poor quality; HR, 0.611; 95% CI, 0.469 to 0.795; p < 0.001), good sleep quality (vs. very poor quality; HR, 0.507; 95% CI, 0.382 to 0.671; p < 0.001), and very good sleep quality (vs. very poor quality; HR, 0.493; 95% CI, 0.367 to 0.662; p < 0.001) were significantly associated with incident PUD. We found that longer sleep duration and better sleep quality were independent protective factors for PUD. Future research should explore the underlying mechanisms and verify whether improving sleep can directly reduce the incidence of PUD.
Keywords: sleep duration, sleep quality, peptic ulcer disease, Taiwan biobank
Introduction
Both sleep duration and quality play crucial roles in maintaining overall health and well-being [1]. Regarding sleep duration, the American Academy of Sleep Medicine and the Sleep Research Society recommend 7 hours or more per night for adults to promote optimal health [2-4]. Sleep quality is determined by the frequency of awakenings during the night, along with the percentage, duration, and type of sleep stages [5, 6]. According to the National Sleep Foundation, shorter sleep latencies, fewer awakenings, reduced wake after sleep onset, and higher sleep efficiency are indicators of good sleep quality [7]. A short duration of sleep has been associated with various adverse health outcomes, including obesity, hypertension, type 2 diabetes, metabolic syndrome and cardiovascular diseases [8-12]. In contrast, a prolonged sleep duration has been associated with increased risks of cerebrovascular events, coronary artery heart disease, and diabetes [13-15]. A U-shaped association between overall mortality and sleep duration has been suggested, with the lowest risk at 7 hours of sleep; however, the results of previous studies have been conflicting [2, 3]. Poor sleep quality has been reported to lead to neuropsychological problems, including anxiety, depression, and cognitive impairment [1, 16].
Peptic ulcer disease (PUD) is a common gastrointestinal disease involving mucosal break in the stomach or proximal duodenum [17]. Helicobacter pylori (H. pylori) infection and the use of nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common etiologies of PUD [17-19]. The global incidence of PUD has been decreasing over the past decades (from 63.84 in 1990 to 44.26 per 100,000 population in 2019), possibly due to H. pylori eradication therapy, effective gastric acid-suppressing medications, and awareness of the adverse effects of NSAIDs [20-22]. The hospitalization rate of PUD varies among countries, with the highest incidence in Eastern Europe and Eastern Asia [20]. Despite the decreasing trends in incidence and mortality rates, PUD and associated complications of bleeding and perforation remain a major burden on healthcare systems worldwide, particularly as hospitalized patients tend to be older, with multiple comorbidities, or taking anticoagulants and NSAIDs [23, 24].
Sleep disturbance has also been linked to gastrointestinal diseases [25], and previous studies have reported associations between poor sleep quality with PUD recurrence and rebleeding [26-28]. A Korean study reported that women who slept less than 7 hours a night had nearly twice the risk of having PUD compared to those who slept more than 9 hours a night [29]. However, the cross-sectional retrospective design of the study limited the validity of the association. Due to the limited data and lack of large retrospective cohort studies addressing this issue, the present study aimed to investigate the associations between sleep duration and quality with the incidence of PUD using longitudinal data from 22,561 participants in the Taiwan Biobank (TWB).
Materials and Methods
Data source and study population
This study was based on data from the TWB, a nationwide prospective community-based research database. Participants aged 30 to 70 years without cancer have been enrolled in the TWB from over 30 recruitment centers in Taiwan since 2008. The methods and details regarding the establishment of the TWB have been described previously. Each participant underwent a health interview, a general physical examination, and biochemical tests of blood and urine. Data on lifestyle practices, dietary habits, environmental exposures, genomic characteristics, and long-term health outcomes were collected. Ethical approval for TWB was granted by the Ethics and Governance Council of the TWB and the Institutional Review Board on Biomedical Science Research, Academia Sinica, Taiwan.
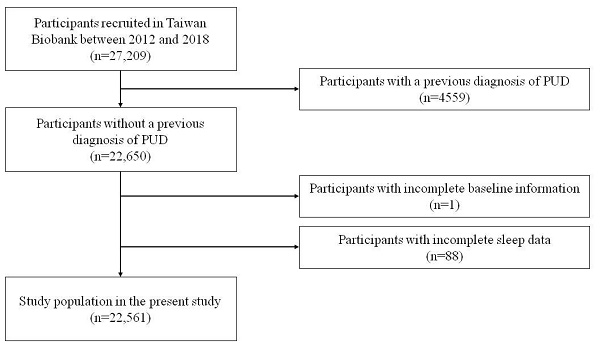
A total of 27,209 participants with long-term follow-up data were enrolled in the study. Initially, the participants were screened for PUD, and those with known PUD were excluded (n = 4559). Participants with missing baseline information (n = 1) and those with incomplete sleep data (n = 88) were also excluded, and the remaining 22,561 participants were enrolled (Figure 1). The mean follow-up period was 43 months. Written informed consent was obtained from all included participants. This study was conducted according to the Declaration of Helsinki and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058).
Baseline data including age, sex, body mass index (BMI), smoking status, alcohol status, physical activity, marital status, education status, living alone, systolic/diastolic blood pressure, fluid intake, gravity, parity, induced abortion, breastfeeding, hormone therapy, menopause etiology, and histories of hypertension, dyslipidemia, diabetes mellitus, gout, and chronic kidney disease were recorded. BMI was calculated as body weight/height (kg/m2). Systolic and diastolic blood pressure measurements were also recorded. Hypertension was defined as a self-reported history of hypertension, and a systolic blood pressure of ≥ 140 mmHg and/or diastolic blood pressure of ≥ 90 mmHg. Chronic kidney disease was defined as a glomerular filtration rate lower than 60 mL/min/1.73 m2 estimated according to the 2021 Chronic Kidney Disease Epidemiology Collaboration creatinine equation [30].
Flowchart of study population (PUD = Peptic ulcer disease).
Sleep assessment
The participants were asked two questions to assess their sleep duration and sleep quality through self-reported questionnaires. For sleep duration, an open-ended question was asked, “How many hours did you sleep on average every day in the past month?” The participants were divided into three groups based on sleep duration per day: “less than 7 hours”, “7 hours”, and “more than 7 hours”. The cut point of 7 hours per day was based on previous research that reported the lowest risk of overall mortality in those who slept over 7 hours per night [31].
For sleep quality, the participants were asked, “How was your sleep quality in the past month?” The participants were categorized into five groups according to their answer as: “very poor”, “poor”, “normal”, “good”, and “very good”.
Study outcome, incident PUD
The primary endpoint of the study was the self-reported development of PUD diagnosed by a physician. As mentioned above, participants with a prior history of PUD were excluded initially, and none of the participants in this study had a record of PUD at baseline. During follow-up, the participants were asked, “Have you been diagnosed with peptic ulcer disease?” The development of PUD was defined as the subject responding “Yes” to this question.
Statistical analysis
Statistical analysis was performed using SPSS v20.0 for Windows (IBM Inc., Armonk, NY). Data were expressed as percentages or means ± standard deviations. One-way analysis of variance followed by a Bonferroni post hoc test was used to compare variables among the study groups. Survival curves for incident PUD survival were illustrated using the Kaplan-Meier method. The time to the development of incident PUD and covariates of risk factors were modeled using a multivariable Cox proportional hazards model, adjusted for age, sex, systolic and diastolic blood pressure, smoking status, alcohol status, exercise habits, marriage status, educational status, as well as a history of hypertension, diabetes, dyslipidemia, depression, drug abuse, bipolar disorder, schizophrenia, epilepsy, migraine, multiple sclerosis, dementia, chronic kidney disease, sleep duration and quality. The participants with sleep duration < 7 hours/day and very poor sleep quality group were treated as the reference group, which was at the lowest risk of incident PUD. A difference was considered significant at p < 0.05.
Results
Baseline characteristics of the three sleep duration groups
Baseline characteristics of the three sleep duration groups are presented in Table 1. A total of 22,561 participants (14,877 women and 7,684 men) without known PUD were enrolled, excluding those without complete baseline information and those with missing sleep data. The mean age was 50.77 ± 10.46 years, and the mean BMI was 24.12 ± 3.59 kg/m2. The participants were categorized into three groups based on sleep duration: less than 7 hours (n = 10,906, 48%), 7 hours (n = 6536, 29%), and more than 7 hours (n = 5119, 23%).
Baseline characteristics of the study subjects in the Taiwan Biobank cohort divided by sleep duration
| All | < 7 hours | 7 hours | > 7 hours | ||
|---|---|---|---|---|---|
| Characteristics | n = 22,561 | n = 10,906 | n = 6536 | n = 5119 | p |
| Age, years | 50.77 ± 10.46 | 51.37 ± 10.35 | 50.17 ± 10.44* | 50.24 ± 10.66* | < 0.001 |
| Female, n (%) | 14,877 (65.9) | 7212 (66.1) | 4283 (65.5) | 3382 (66.1) | 0.704 |
| Body mass index, kg/m2 | 24.12 ± 3.59 | 24.21 ± 3.64 | 24.00 ± 3.46* | 24.07 ± 3.65* | < 0.001 |
| Living alone, yes, n (%) | 1,435 (6.4) | 734 (6.7) | 367 (5.6) | 334 (6.5) | 0.012 |
| Married status, ever, n (%) | 20,503 (90.9) | 9954 (91.3) | 5935 (90.8) | 4614 (90.1) | 0.064 |
| Ever smoking, yes, n (%) | 5232 (23.2) | 2518 (23.1) | 1457 (22.3) | 1257 (24.6) | 0.015 |
| Alcohol status, ever, n (%) | 1810 (8.0) | 832 (7.6) | 527 (8.1) | 451 (8.8) | 0.037 |
| Exercise habits, yes, n (%) | 10,252 (45.4) | 5066 (46.5) | 2942 (45.0) | 2244 (43.8) | 0.006 |
| Educational status, n (%) | < 0.001 | ||||
| Elementary school, n (%) | 1656 (7.3) | 907 (8.3) | 359 (5.5) | 390 (7.6) | |
| Middle to high school, n (%) | 9961 (44.2) | 4867 (44.6) | 2834 (43.4) | 2260 (44.1) | |
| University and above, n (%) | 10,944 (48.5) | 5132 (47.1) | 3343 (51.1) | 2469 (48.2) | |
| Comorbidities, n (%) | |||||
| Hypertension | 4573 (20.3) | 2260 (20.7) | 1250 (19.1) | 1063 (20.8) | 0.024 |
| Diabetes | 1159 (5.1) | 576 (5.3) | 306 (4.7) | 277 (5.4) | 0.133 |
| Dyslipidemia | 1541 (6.8) | 772 (7.1) | 425 (6.5) | 344 (6.7) | 0.323 |
| Chronic kidney disease | 168 (0.7) | 73 (0.7) | 46 (0.7) | 49 (1.0) | 0.128 |
| Depression | 638 (2.8) | 331 (3.0) | 150 (2.3) | 157 (3.1) | 0.009 |
| Substance use disorder | 2 (0.0) | 1 (0.0) | 1 (0.0) | 0 (0.0) | 0.684 |
| Bipolar disorder | 104 (0.5) | 50 (0.5) | 31 (0.5) | 23 (0.4) | 0.979 |
| Schizophrenia | 33 (0.1) | 11 (0.1) | 5 (0.1) | 17 (0.3) | < 0.001 |
| Epilepsy | 71 (0.3) | 28 (0.3) | 21 (0.3) | 22 (0.4) | 0.188 |
| Migraine | 565 (2.5) | 260 (2.4) | 159 (2.4) | 146 (2.9) | 0.190 |
| Multiple sclerosis | 6 (0.0) | 5 (0.0) | 1 (0.0) | 0 (0.0) | 0.202 |
| Parkinson's disease | 17 (0.1) | 11 (0.1) | 3 (0.0) | 3 (0.1) | 0.389 |
| Dementia | 7 (0.0) | 3 (0.0) | 2 (0.0) | 2 (0.0) | 0.927 |
*p < 0.05 compared with sleep duration < 7 hours group.
Compared to the < 7 hours/day group, the 7 hours and > 7 hours/day groups had a lower age and BMI.
Association between sleep duration and incident PUD
Table 2 shows the multivariable Cox regression analysis for incident PUD according to sleep duration. Over an average 43 months of follow-up, 1325 participants (5.9% of the study population) developed PUD, including 682 (6.3%), 382 (5.8%), and 261 (5.1%) in the < 7 hours, 7 hours, and > 7 hours/day groups, respectively. After adjusting for confounders, sleep duration > 7 hours/day (vs. < 7 hours/day; hazard ratio [HR], 0.771; 95% confidence interval [CI], 0.668 to 0.890; p < 0.001) was significantly associated with incident PUD, whereas a duration of 7 hours/day was not (p = 0.201). Furthermore, sleep duration (per 1 hour/day; HR, 0.933; 95% CI, 0.889 to 0.979; p = 0.005) was also significantly associated with incident PUD.
Multivariate-adjusted hazard ratios for incident peptic ulcer disease according to sleep duration (n = 22,561)
| Sleep duration | Peptic ulcer cases / subjects n, %) | HR (95% CI) | p |
|---|---|---|---|
| < 7 hours/day | 682/10,906 (6.3) | reference | - |
| 7 hours/day | 382/6536 (5.8) | 0.921 (0.812 - 1.045) | 0.201 |
| > 7 hours/day | 261/5119 (5.1) | 0.771 (0.668 - 0.890) | < 0.001 |
| Per hour of sleep | - | 0.933 (0.889 - 0.979) | 0.005 |
Values expressed as hazard ratio (HR) and 95% confidence interval (CI).
Covariates in the multivariable-adjusted model included sleep duration, age, sex, smoking status, alcohol status, exercise habits, marriage status, educational status, as well as the history of hypertension, diabetes, dyslipidemia, depression, drug abuse, bipolar disorder, schizophrenia, epilepsy, migraine, multiple sclerosis, dementia, and chronic kidney disease.
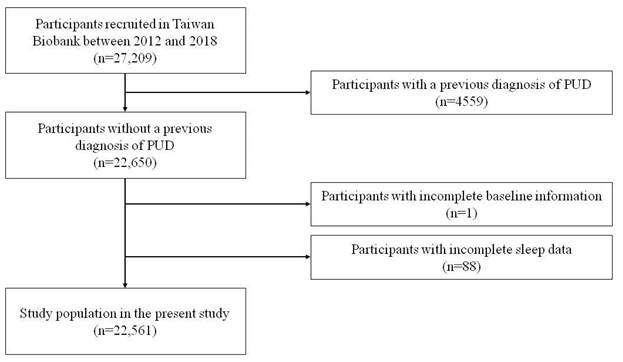
Figure 2 illustrates the Kaplan-Meier analysis of incident PUD among the three study groups. The time to incident PUD was longer in the > 7 hours/day group compared to the < 7 hours/day group.
Association between sleep quality and incident PUD
Table 3 shows the multivariable Cox regression analysis for incident PUD according to sleep quality. After adjusting for confounders, the participants with poor sleep quality (vs. very poor quality; HR, 0.649; 95% CI, 0.491 to 0.858; p = 0.002), normal sleep quality (vs. very poor quality; HR, 0.611; 95% CI, 0.469 to 0.795; p < 0.001), good sleep quality (vs. very poor quality; HR, 0.507; 95% CI, 0.382 to 0.671; p < 0.001), and very good sleep quality (vs. very poor quality; HR, 0.493; 95% CI, 0.367 to 0.662; p < 0.001) were significantly associated with incident PUD. Progressively lower HRs were observed in groups with better sleep quality compared to the group with very poor sleep quality.
Multivariate-adjusted hazard ratios for incident peptic ulcer disease according to sleep quality (n = 22,561)
| Sleep quality | Peptic ulcer cases / subjects (n, %) | Adjusted HR (95% CI) | p |
|---|---|---|---|
| Very poor | 62/570 (10.9) | reference | - |
| Poor | 247/3969 (6.2) | 0.649 (0.491 - 0.858) | 0.002 |
| Normal | 601/9932 (6.1) | 0.611 (0.469 - 0.795) | < 0.001 |
| Good | 247/4872 (5.1) | 0.507 (0.382 - 0.671) | < 0.001 |
| Very good | 168/3218 (5.2) | 0.493 (0.367 - 0.662) | < 0.001 |
Values expressed as hazard ratio (HR) and 95% confidence interval (CI).
Covariates in the multivariable-adjusted model included sleep quality, age, sex, smoking status, alcohol status, exercise habits, marriage status, educational status, as well as the history of hypertension, diabetes, dyslipidemia, depression, drug abuse, bipolar disorder, schizophrenia, epilepsy, migraine, multiple sclerosis, dementia, and chronic kidney disease.
Figure 3 illustrates the Kaplan-Meier analysis of incident PUD among the five sleep quality groups. The time to incident PUD was longer in the participants with very good sleep quality compared to those with very poor sleep quality. Similar trends were observed when comparing good, normal, and normal sleep quality to very poor sleep quality.
Subgroup analysis: the association between sleep duration and incident peptic ulcer disease
In a subgroup analysis of the association between sleep duration (sleep duration > 7 hours/day vs. < 7 hours /day) and the risk of incident PUD, significant variations in HR were observed across different demographics and lifestyle factors (Table 4 and Figure 4). In participants with sleep duration > 7 hours/day, female sex, BMI < 25 kg/m2, non-living alone, non-smokers, non-drinkers, no diabetes, no dyslipidemia, and no chronic kidney disease demonstrated a significantly reduced risk of PUD compared to sleep duration < 7 hours/day.
Time to incident peptic ulcer disease (PUD) was longer in participants with a sleep duration of more than 7 hours per day compared to those with less than 7 hours per day. Kaplan-Meier plot depicting the development of incident PUD based on sleep duration in 22,561 participants with follow-up data.
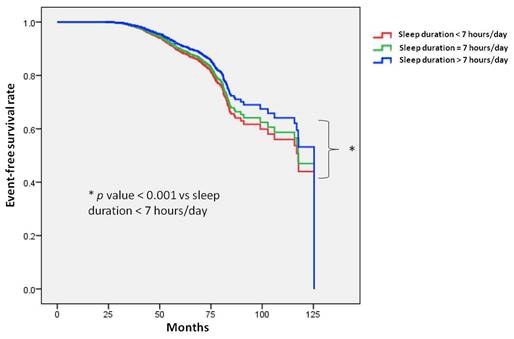
Time to incident peptic ulcer disease (PUD) was longer in participants with very good sleep quality compared to those with very poor sleep quality. Similar trends were observed when comparing good, normal, and normal sleep quality to very poor sleep quality. Kaplan-Meier plot depicting the development of incident PUD based on sleep quality in 22,561 participants with follow-up data.
Subgroup analyses between sleep duration (sleep duration > 7 hours/day vs. < 7 hours /day) and the risk of incident peptic ulcer disease.
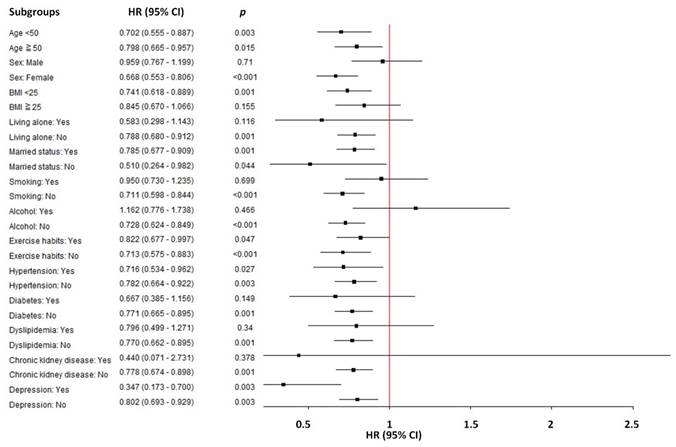
Subgroup analysis: the association between sleep duration and incident peptic ulcer disease
| Subgroup | Sleep duration > 7 hours/day vs. < 7 hours /day HR (95% CI) | p |
|---|---|---|
| Age | ||
| < 50 years | 0.702 (0.555 - 0.887) | 0.003 |
| ≥ 50 years | 0.798 (0.665 - 0.957) | 0.015 |
| Sex | ||
| Male | 0.959 (0.767 - 1.199) | 0.710 |
| Female | 0.668 (0.553 - 0.806) | <0.001 |
| Body mass index | ||
| < 25 kg/m2 | 0.741 (0.618 - 0.889) | 0.001 |
| ≥ 25 kg/m2 | 0.845 (0.670 - 1.066) | 0.155 |
| Living alone | ||
| Yes | 0.583 (0.298 - 1.143) | 0.116 |
| No | 0.788 (0.680 - 0.912) | 0.001 |
| Married status | ||
| Yes | 0.785 (0.677 - 0.909) | 0.001 |
| No | 0.510 (0.264 - 0.982) | 0.044 |
| Smoking | ||
| Yes | 0.950 (0.730 - 1.235) | 0.699 |
| No | 0.711 (0.598 - 0.844) | <0.001 |
| Alcohol | ||
| Yes | 1.162 (0.776 - 1.738) | 0.466 |
| No | 0.728 (0.624 - 0.849) | <0.001 |
| Exercise habits | ||
| Yes | 0.822 (0.677 - 0.997) | 0.047 |
| No | 0.713 (0.575 - 0.883) | <0.001 |
| Hypertension | ||
| Yes | 0.716 (0.534 - 0.962) | 0.027 |
| No | 0.782 (0.664 - 0.922) | 0.003 |
| Diabetes | ||
| Yes | 0.667 (0.385 - 1.156) | 0.149 |
| No | 0.771 (0.665 - 0.895) | 0.001 |
| Dyslipidemia | ||
| Yes | 0.796 (0.499 - 1.271) | 0.340 |
| No | 0.770 (0.662 - 0.895) | 0.001 |
| Chronic kidney disease | ||
| Yes | 0.440 (0.071 - 2.731) | 0.378 |
| No | 0.778 (0.674 - 0.898) | 0.001 |
| Depression | ||
| Yes | 0.347 (0.173 - 0.700) | 0.003 |
| No | 0.802 (0.693 - 0.929) | 0.003 |
Values expressed as hazard ratio (HR) and 95% confidence interval (CI).
Discussion
In this longitudinal study of a representative sample of the Taiwanese population, we explored associations between sleep duration and sleep quality with the development of PUD in 22,561 participants over a 43-month period. The results showed that longer sleep duration and better sleep quality were significantly associated with a lower risk of incident PUD. Moreover, for every additional hour of sleep, there was a 0.93-fold decrease in the risk of developing PUD.
The first notable aspect of this research is that a long sleep duration was associated with a low risk of incident PUD. Previous studies have reported associations between sleep duration and adverse health outcomes including cardiovascular disease, obesity, hypertension, type 2 diabetes, metabolic syndrome, immunosuppression, decline in renal function, and overall mortality [2, 8-15]. Gastrointestinal diseases, including gastroesophageal reflux disease, irritable bowel syndrome, inflammatory bowel disease, colorectal cancer, and liver diseases, have also been reviewed in relation to sleep dysfunction [25, 32]. However, little research has investigated the associations between sleep duration and sleep quality with the development of PUD. Ko et al. reported that women who slept less than 7 hours a night had nearly twice the risk of having PUD compared to those who slept more than 9 hours in a Korean population [29]. In addition, a longer sleep duration (≥ 9 hours) tended to protect against PUD in men, but without significance [29].
Another notable finding of our research is that good sleep quality was associated with a low risk of incident PUD. Fang et al. also investigated the association between sleep quality and peptic ulcer recurrence in older patients after H. pylori eradication, and found that poor objective sleep quality, defined as longer sleep onset latency and more nighttime awakenings, increased the risk of peptic ulcer recurrence [26]. Similar results were observed in another study of patients who had recovered from peptic ulcer bleeding after endoscopic or medical treatment, in whom the risk of peptic ulcer rebleeding was found to be higher in those with poorer objective sleep quality [27]. In contrast, longer sleep duration and better sleep efficiency have been reported to protect against ulcer recurrence and rebleeding [27]. Patients with sleep apnea have also been shown to be at risk of sleep disturbance [33]. Episodes of intermittent nocturnal hypoxemia result in systemic inflammation, oxidative stress, and sympathetic activation [34]. Increasing evidence has shown a link between moderate-to-severe sleep apnea and cardiovascular diseases including hypertension, coronary artery disease, atrial fibrillation, heart failure, and stroke [35]. Some research has indicated an association between sleep apnea and gastroesophageal reflux disease, and its impact on PUD is still being studied [36]. Shiao et al. reported a 2.4-fold higher risk of peptic ulcer bleeding in patients with sleep apnea in a study using data from the Taiwan National Health Insurance Research Database [28]. Zha et al. conducted a Mendelian randomization analysis to clarify the causal relationship between sleep quality and PUD, and found that an increased incidence of insomnia was related to a higher risk of PUD, without evidence of reverse causality [37]. The result indicated that insomnia was a risk factor for PUD, rather than a complication of PUD.
However, the mechanism between sleep and PUD remains unclear. Possible factors determining how sleep influences the risk of PUD include gastric mucosal blood flow, circadian rhythm, superoxide generation, the immune system, and the levels of key hormones such as melatonin and leptin [38-42]. Gastric mucosal blood flow, which increases during rapid eye movement (REM) sleep, is currently considered to be the primary factor in gastric mucosal protection [39]. Mucosal blood flow is regulated by systemic and local metabolic factors such as prostaglandins and leukotrienes [39]. Increased mucosal blood flow supports the proper structure of the mucosa, promotes the secretion of mucus and bicarbonate ions, and consequently accelerates peptic ulcer healing [43]. In an animal study, gastric mucosal epithelial cell loss and ischemic damage to the epithelium were observed in rats deprived of REM sleep [38]. The resulting mucosal ischemia weakens gastric mucosal defense and repair mechanisms.
Another possible explanation is melatonin secretion. Melatonin has emerged as another pivotal factor in gastroprotection, possibly due to its stimulation of bicarbonate, antioxidative actions, and the ability to increase angiogenesis and accelerate mucosal microcirculation [44-46]. Melatonin is synthesized in the pineal gland as an endocrine hormone, and is produced by intestinal enterochromaffin cells as a paracrine hormone [47]. The pineal gland secretes melatonin in a circadian pattern, with the highest amounts released during the nighttime, and this may play an important role in the nocturnal control of duodenal alkaline secretion and mucosal protection [40]. Melatonin also scavenges free radicals, and thereby has an anti-inflammatory effect [48, 49]. The addition of oral melatonin and its precursor L-tryptophan to proton pump inhibitor therapy has been shown to significantly accelerate ulcer healing in humans [42].
Another associated key hormone, leptin, has also been shown to have gastroprotective and ulcer-healing activities, possibly due to its angiogenetic properties [50]. A previous study reported that plasma leptin levels were significantly increased in patients who were treated with a regimen including melatonin for H. pylori-related peptic ulcers [41]. The angiogenesis effect has been postulated to be induced by the stimulation of nitric oxide, endogenous prostaglandins, and growth factors such as transforming growth factor-alpha and vascular endothelial growth factor, leading to an increase in mucosal blood flow [50].
Another possible mechanism relates to the gut-brain axis, which involves bidirectional interactions between the central autonomic and enteric nervous systems [51]. The gut-brain axis regulates intestinal immune activation, intestinal permeability, enteric reflex, and entero-endocrine signaling [40]. Sleep disturbance may disrupt circadian physiology and subsequently affect the brain-gut axis. The circadian rhythm is governed by the suprachiasmatic nucleus (SCN) in the anterior hypothalamus, and it regulates the sleep-wake cycle, body temperature, blood pressure, immune response, and hormone secretion [1, 52, 53]. The circadian system also influences gastric acid secretion, with variations observed during different sleep stages [54]. Acid secretion decreases with deeper sleep stages, and only minimal amounts occur during REM phases, possibly resulting from decreased plasma noradrenaline and histamine release [55]. Arousal or waking periods have been shown to correlate with increased acid output [56].
Trefoil factor family 2 (TFF2) protein may also play a role in the interaction between sleep and PUD. TFF2 protein is produced in the gastrointestinal mucosa, and it inhibits gastric acid secretion and affects healing of the epithelium [57]. The diurnal variation of TFF2 concentration in the alimentary tract, with high levels during the night and early morning, suggests that the cytoprotective effects mainly occur during sleep [57]. Sleep deprivation has been shown to disrupt the normal TFF2 rhythm and potentially predispose to increased gastric morbidity [58].
Subgroup analyses revealed that the protective effect of sleep duration was more pronounced among specific subgroups, including female sex, BMI < 25 kg/m2, non-living alone, non-smokers, non-drinkers, no diabetes, no dyslipidemia, and no chronic kidney disease. This sensitivity analysis reinforces our conclusion that sleep duration > 7 hours/day is significantly associated with a reduced risk of PUD. Some sub-groups are not listed in Table 4 because the number of cases is too small, such as dementia, Parkinson's disease and substance use disorder. However, it can be seen from Figure 4 that almost all sub-groups are to the left. In other words, no matter what sub-group, sleep has a tendency to reduce PUD. These findings provide valuable insights into lifestyle factors influencing PUD risk and lay the groundwork for future research in this area.
The main strengths of this study lie in its incorporation of a large community-based cohort, offering comprehensive follow-up data and detailed information. However, there are also some limitations. First, the presence of PUD was assessed using self-reports of a previous diagnosis made by a physician. Since the definite diagnosis of peptic ulcers involves direct visualization of the ulcer on endoscopy, the incidence of the disease may have been overestimated. In addition, some of the patients may have had asymptomatic ulcers and thus not been diagnosed with PUD. However, the use of self-reported diagnoses has been validated in other studies [59]. Second, information on sleep duration and sleep quality was obtained through questionnaires. However, a previous study demonstrated that self-reported sleep duration was sufficient, given the concordance between self-reported sleep duration and that derived from actigraphy [60]. To investigate more detailed aspects of sleep quality, including sleep latency and sleep sufficiency, further research may be conducted with information acquired through established assessment tools such as the Pittsburgh Sleep Quality Index. Third, data on H. pylori status and use of NSAIDs, steroids, chemotherapy agents, aspirin and antiplatelet therapy, which are established risk factors for PUD development, are not included in the TWB database. However, some previous studies demonstrated that sleep quality is significantly associated with PUD, even adjusted for NSAIDs use [61] and H. pylori [26].
In conclusion, longer sleep duration and good sleep quality were significantly associated with low risks of incident PUD. Furthermore, for every additional hour of sleep, there was a 0.93-fold decrease in the risk of PUD development. The effect of both sleep duration and quality on subsequent PUD development suggests the importance of optimizing the quantity and quality of sleep in the general population.
Acknowledgements
Funding
This work was supported partially by the Research Center for Precision Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and by Kaohsiung Medical University Research Center Grant (KMU-TC113A01), and Kaohsiung Municipal Siaogang Hospital (kmhk-113-001).
Ethical approval
The study was conducted according to the Declaration of Helsinki, and it was granted approval by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058), and the TWB was granted approval by the IRB on Biomedical Science Research, Academia Sinica, Taiwan and the Ethics and Governance Council of the TWB.
Authors contributions
Conceptualization, methodology, validation, formal analysis, writing—review and editing, and supervision: Y-HH, J-HG, C-HK and S-CC. Software and investigation: J-HG, and S-CC. Resources, project administration, and funding acquisition: S-CC. Data curation: Y-HH, H-YH, J-IL, T-IW, S-PH, J-HG, C-HK and S-CC. Writing—original draft preparation: Y-HH, J-HG, and S-CC. Visualization: J-HG, C-HK and S-CC. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The data underlying this study are from the Taiwan Biobank. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to: Szu-Chia Chen, PhD, MD. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59-69
2. Svensson T, Saito E, Svensson AK, Melander O, Orho-Melander M, Mimura M. et al. Association of Sleep Duration With All- and Major-Cause Mortality Among Adults in Japan, China, Singapore, and Korea. JAMA Netw Open. 2021;4:e2122837
3. Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L. et al. National Sleep Foundation's updated sleep duration recommendations: final report. Sleep Health. 2015;1:233-43
4. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D. et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843-4
5. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10-7
6. Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: Diagnosis and management. J Gen Fam Med. 2017;18:61-71
7. Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, Daly FJ. et al. National Sleep Foundation's sleep quality recommendations: first report. Sleep Health. 2017;3:6-19
8. Park SE, Kim HM, Kim DH, Kim J, Cha BS, Kim DJ. The association between sleep duration and general and abdominal obesity in Koreans: data from the Korean National Health and Nutrition Examination Survey, 2001 and 2005. Obesity (Silver Spring). 2009;17:767-71
9. Lao XQ, Liu X, Deng HB, Chan TC, Ho KF, Wang F. et al. Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. J Clin Sleep Med. 2018;14:109-17
10. Cai H, Shu XO, Xiang YB, Yang G, Li H, Ji BT. et al. Sleep duration and mortality: a prospective study of 113 138 middle-aged and elderly Chinese men and women. Sleep. 2015;38:529-36
11. Stefani KM, Kim HC, Kim J, Oh K, Suh I. The influence of sex and age on the relationship between sleep duration and metabolic syndrome in Korean adults. Diabetes Res Clin Pract. 2013;102:250-9
12. Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. 2012;25:335-41
13. Joundi RA, Patten SB, Williams JVA, Smith EE. Association Between Excess Sleep Duration and Risk of Stroke: A Population-Based Study. Can J Neurol Sci. 2023;50:17-22
14. Jang JH, Kim W, Moon JS, Roh E, Kang JG, Lee SJ. et al. Association between Sleep Duration and Incident Diabetes Mellitus in Healthy Subjects: A 14-Year Longitudinal Cohort Study. J Clin Med. 2023;12:2899
15. Daghlas I, Dashti HS, Lane J, Aragam KG, Rutter MK, Saxena R. et al. Sleep Duration and Myocardial Infarction. J Am Coll Cardiol. 2019;74:1304-14
16. Cheng M, Lei X, Zhu C, Hou Y, Lu M, Wang X. et al. The association between poor sleep quality and anxiety and depression symptoms in Chinese patients with coronary heart disease. Psychol Health Med. 2022;27:1347-56
17. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-24
18. Kavitt RT, Lipowska AM, Anyane-Yeboa A, Gralnek IM. Diagnosis and Treatment of Peptic Ulcer Disease. Am J Med. 2019;132:447-56
19. Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T. et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. 2021;56:303-22
20. Azhari H, King JA, Coward S, Windsor JW, Ma C, Shah SC. et al. The Global Incidence of Peptic Ulcer Disease Is Decreasing Since the Turn of the 21st Century: A Study of the Organisation for Economic Co-Operation and Development (OECD). Am J Gastroenterol. 2022;117:1419-27
21. Zhang Z, Yan W, Zhang X, Wang J, Zhang Z, Lin Z. et al. Peptic ulcer disease burden, trends, and inequalities in 204 countries and territories, 1990-2019: a population-based study. Therap Adv Gastroenterol. 2023;16:17562848231210375
22. Malfertheiner P, Schulz C. Peptic Ulcer: Chapter Closed? Dig Dis. 2020:1-5
23. Quan S, Frolkis A, Milne K, Molodecky N, Yang H, Dixon E. et al. Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes. World J Gastroenterol. 2014;20:17568-77
24. Christensen S, Riis A, Nørgaard M, Sørensen HT, Thomsen RW. Short-term mortality after perforated or bleeding peptic ulcer among elderly patients: a population-based cohort study. BMC Geriatr. 2007;7:8
25. Orr WC, Fass R, Sundaram SS, Scheimann AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:616-24
26. Fang B, Liu H, Yang S, Xu R, Chen G. Effect of Subjective and Objective Sleep Quality on Subsequent Peptic Ulcer Recurrence in Older Adults. J Am Geriatr Soc. 2019;67:1454-60
27. Fang B, Li D, Liu H, Yang S, Xu R, Chen G. et al. Impact of Subjective and Objective Sleep Quality on Peptic Ulcer Rebleeding in Older Adults. Psychosom Med. 2021;83:995-1003
28. Shiao TH, Liu CJ, Luo JC, Su KC, Chen YM, Chen TJ. et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am J Med. 2013;126:249-55 55.e1
29. Ko SH, Baeg MK, Ko SY, Han KD. Women Who Sleep More Have Reduced Risk of Peptic Ulcer Disease; Korean National Health and Nutrition Examination Survey (2008-2009). Sci Rep. 2016;6:36925
30. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y. et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737-49
31. Lin Y, Wu Y, Lin Q, Wing YK, Xu L, Ge J. et al. Objective Sleep Duration and All-Cause Mortality Among People With Obstructive Sleep Apnea. JAMA Netw Open. 2023;6:e2346085
32. Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108:213-21
33. Ong JC, Crawford MR. Insomnia and Obstructive Sleep Apnea. Sleep Med Clin. 2013;8:389-98
34. Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgraduate Medical Journal. 2009;85:693
35. Bauters F, Rietzschel ER, Hertegonne KB, Chirinos JA. The Link Between Obstructive Sleep Apnea and Cardiovascular Disease. Curr Atheroscler Rep. 2016;18:1
36. El Hage Chehade N, Fu Y, Ghoneim S, Shah S, Song G, Fass R. Association between obstructive sleep apnea and gastroesophageal reflux disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2023;38:1244-51
37. Zha LF, Dong JT, Wang JL, Chen QW, Wu JF, Zhou YC. et al. Effects of Insomnia on Peptic Ulcer Disease Using Mendelian Randomization. Oxid Med Cell Longev. 2021;2021:2216314
38. Beltran NE, Garcia-Lorenzana M, Gomez E Y, Velazquez-Moctezuma J. Effect of REM sleep deprivation on gastric mucosa. International Journal of Clinical and Experimental Medicine. 2016;9:1964-1974
39. Kawano S, Tsuji S. Role of mucosal blood flow: a conceptional review in gastric mucosal injury and protection. J Gastroenterol Hepatol. 2000;15(Suppl):D1-6
40. Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62:139-50
41. Celinski K, Konturek PC, Konturek SJ, Slomka M, Cichoz-Lach H, Brzozowski T. et al. Effects of melatonin and tryptophan on healing of gastric and duodenal ulcers with Helicobacter pylori infection in humans. J Physiol Pharmacol. 2011;62:521-6
42. Celinski K, Konturek SJ, Konturek PC, Brzozowski T, Cichoz-Lach H, Slomka M. et al. Melatonin or L-tryptophan accelerates healing of gastroduodenal ulcers in patients treated with omeprazole. J Pineal Res. 2011;50:389-94
43. Abdel-Salam OM, Czimmer J, Debreceni A, Szolcsányi J, Mózsik G. Gastric mucosal integrity: gastric mucosal blood flow and microcirculation. An overview. J Physiol Paris. 2001;95:105-27
44. Sjöblom M, Flemström G. Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion. J Pineal Res. 2003;34:288-93
45. Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M. et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58:381-405
46. Bandyopadhyay D, Bandyopadhyay A, Das PK, Reiter RJ. Melatonin protects against gastric ulceration and increases the efficacy of ranitidine and omeprazole in reducing gastric damage. J Pineal Res. 2002;33:1-7
47. Rezzani R, Franco C, Franceschetti L, Gianò M, Favero G. A Focus on Enterochromaffin Cells among the Enteroendocrine Cells: Localization, Morphology, and Role. Int J Mol Sci. 2022;23:3758
48. Hardeland R, Reiter RJ, Poeggeler B, Tan DX. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993;17:347-57
49. Ma N, Zhang J, Reiter RJ, Ma X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med Res Rev. 2020;40:606-32
50. Konturek PC, Brzozowski T, Sulekova Z, Brzozowska I, Duda A, Meixner H. et al. Role of leptin in ulcer healing. Eur J Pharmacol. 2001;414:87-97
51. Mayer EA, Nance K, Chen S. The Gut-Brain Axis. Annu Rev Med. 2022;73:439-53
52. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190-8
53. Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432-7
54. Vaughn B, Rotolo S, Roth H. Circadian rhythm and sleep influences on digestive physiology and disorders. ChronoPhysiology and Therapy. 2014;2014:67
55. Khanijow V, Prakash P, Emsellem HA, Borum ML, Doman DB. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol Hepatol (N Y). 2015;11:817-25
56. Watanabe M, Nakazawa S, Yoshino J, Yamao K, Inui K, Yamachika H. et al. [A study of the relationship between nocturnal intragastric pH and sleep stages of peptic ulcer]. Nihon Shokakibyo Gakkai Zasshi. 1995;92:1241-9
57. Semple JI, Newton JL, Westley BR, May FE. Dramatic diurnal variation in the concentration of the human trefoil peptide TFF2 in gastric juice. Gut. 2001;48:648-55
58. Johns CE, Newton JL, Westley BR, May FE. The diurnal rhythm of the cytoprotective human trefoil protein TFF2 is reduced by factors associated with gastric mucosal damage: ageing, Helicobacter pylori infection, and sleep deprivation. Am J Gastroenterol. 2005;100:1491-7
59. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9:e112257
60. Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175-83
61. Shiao TH, Liu CJ, Luo JC, Su KC, Chen YM, Chen TJ. et al. Sleep apnea and risk of peptic ulcer bleeding: a nationwide population-based study. Am J Med. 2013;126:249-55 55 e1
Author contact
![]() Corresponding authors: Jiun-Hung Geng, Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung, Taiwan. No. 482, Shanming Rd, Xiaogang District, Kaohsiung City 812, Taiwan. Tel.: +886(7)3208212; Fax: +886(7)3211033; E-mail: u9001090com. Chao-Hung Kuo, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, 482, Shan-Ming Rd., Hsiao-Kang Dist., 812 Kaohsiung, Taiwan, R.O.C. Tel.: 886-7-8036783 ext. 3440; Fax: 886-7-8063346; E-mail: kjh88kmucom. Szu-Chia Chen, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, 482, Shan-Ming Rd., Hsiao-Kang Dist., 812 Kaohsiung, Taiwan, R.O.C. Tel.: 886-7-8036783 ext. 3440; Fax: 886-7-8063346; E-mail: scarchenonecom.tw.
Corresponding authors: Jiun-Hung Geng, Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung, Taiwan. No. 482, Shanming Rd, Xiaogang District, Kaohsiung City 812, Taiwan. Tel.: +886(7)3208212; Fax: +886(7)3211033; E-mail: u9001090com. Chao-Hung Kuo, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, 482, Shan-Ming Rd., Hsiao-Kang Dist., 812 Kaohsiung, Taiwan, R.O.C. Tel.: 886-7-8036783 ext. 3440; Fax: 886-7-8063346; E-mail: kjh88kmucom. Szu-Chia Chen, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, 482, Shan-Ming Rd., Hsiao-Kang Dist., 812 Kaohsiung, Taiwan, R.O.C. Tel.: 886-7-8036783 ext. 3440; Fax: 886-7-8063346; E-mail: scarchenonecom.tw.

 Global reach, higher impact
Global reach, higher impact