3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(11):1469-1478. doi:10.7150/ijms.83808 This issue Cite
Review
Research Progress of Exosomal Non-Coding RNAs in Cardiac Remodeling
1. Department of Geriatrics, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China.
2. Department of Clinical laboratory Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China.
3. Hunan Clinical Medical Research Center for Geriatric Syndrome, Changsha, Hunan 410011, China.
Received 2023-2-23; Accepted 2023-8-18; Published 2023-9-11
Abstract
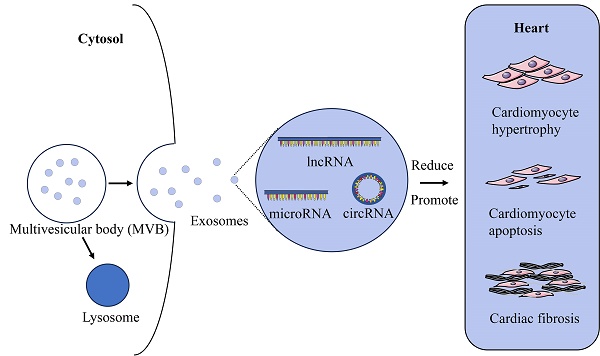
Exosomes are vesicles with a size range of 50 to 200 nm and released by different cells, which are essential for the exchange of information between cells. They have attracted a lot of interest from medical researchers. Exosomal non-coding RNAs play an important part in pathological cardiac remodelings, such as cardiomyocyte hypertrophy, cardiomyocyte apoptosis, and cardiac fibrosis. This review summarizes the origins and functions of exosomes, the role of exosomal non-coding RNAs in the process of pathological cardiac remodeling, as well as their theoretical basis for clinical application.
Background
Cardiovascular diseases (CVDs) continue to be the main causes of morbidity and mortality globally. As CVDs worsen, irreversible cardiac remodeling will occur, ultimately leading to heart failure (HF) [1]. Early cardiac remodeling is originally thought to be an active compensatory alteration of the body in response to increased stress, recent research showed it is directly associated with the morbidity and mortality of CVDs [2]. Human cardiac tissues are composed of various cell types including cardiomyocytes, cardiac fibroblasts, endothelial cells (ECs), smooth muscle cells, and a few cardiac stem cells. Additionally, there are a number of transitory cells that are linked to CVDs, including mast cells, macrophages, and lymphocytes. The presence and interation of several cardiac cells form a complex intercellular network comprised of numerous signaling pathways, regulating cell-cell connections and/or cell-extracellular matrix interactions, as well as autocrine, paracrine, endocrine, etc [3, 4].
Exosomes have recently been identified as having an important role in cellular communication. Apart from abundant physiological and signaling tasks, exosomes are necessary for removing cellular wastes [5]. Exosomes are released by various type of cells, including stem cells, ECs, fibroblast cells, and even tumor cells. Through the intercellular connections, multicellular organisms remain homeostatic maintenance [6]. Exosomes are round-shaped particles that form by the inward budding and fission of late endosomal vesicle membranes and have been found to contain a wide range of biomolecules, such as proteins, cytokines, messenger RNAs (mRNAs), and non-coding RNAs (ncRNAs) [7]. Carrying these informative molecules, exosomes are considered to serve as key cell-to-cell messengers that regulate homeostasis and have an impact on the pathophysiology of several diseases, including CVDs [4].
Based on the structure, length of nucleotide and function, ncRNAs are divided into microRNAs (miRNAs or miRs), long noncoding RNAs (lncRNAs), circular noncoding RNAs (circRNAs), transfer RNAs, ribosomal RNAs, small nuclear RNAs, small nucleolar RNAs, guide RNAs, piwi-interacting RNAs, and small interfering RNAs [8]. MiRNAs are short ncRNAs with a length of 19-25 nucleotides which are highly conserved and regulate gene expression. By binding to the 3' untranslated region (3'-UTR) of target mRNA, it inhibits the translation of mRNA or promotes its degradation. Many miRNAs control the same mRNA, and one miRNA is capable of controlling multiple mRNAs. MiRNAs are involve in the process of various diseases, including CVDs [9]. LncRNAs are defined as ncRNAs of over 200 nucleotides. According to the position of lncRNAs relative to protein-coding genes, they can be divided into five groups: sense, antisense, bidirectional, intergenic and intronic. LncRNAs have the ability to attach to proteins, RNA, and DNA and regulate their functions. Endogenous cavernous RNAs, namely competing endogenous RNAs (ceRNAs), can interact with miRNAs to affect the expression of mRNAs [10]. CircRNAs are a class of endogenous ncRNAs that form continuous closed loops with strong tissue specificity, which are mainly derived from exons and introns, and generated from back-splicing of premRNA. Compared with linear RNAs, circRNAs are characterized by the absence of free 3' or 5' ends. CircRNAs act as sponges of miRNAs, regulating selective splicing and parental gene expression. CircRNAs appear to be implicated in the formation and development of CVDs and tumors, and have the possibility to become new targets of disease treatment and new biomarkers for clinical diagnosis and prognosis [11].
As a stable carrier of information communication between cells, exosomal ncRNAs can precisely regulate cellular signaling pathways and participate in multiple pathophysiological processes such as vascular remodeling, myocardial ischemia, cardiac hypertrophy, and inflammatory immune response [4]. Regarding these significant roles of ncRNAs in regulating compensatory and non-compensatory cardiac remodeling, along with the roles of exosomes as carriers of ncRNAs, exosomal ncRNAs are considered a promising new therapeutic target of CVDs [12]. This review highlights the function of various exosomal ncRNA in the pathogenesis of CVDs as well as advanced therapeutic procedures.
Introduction of exosomes
The source of exosomes
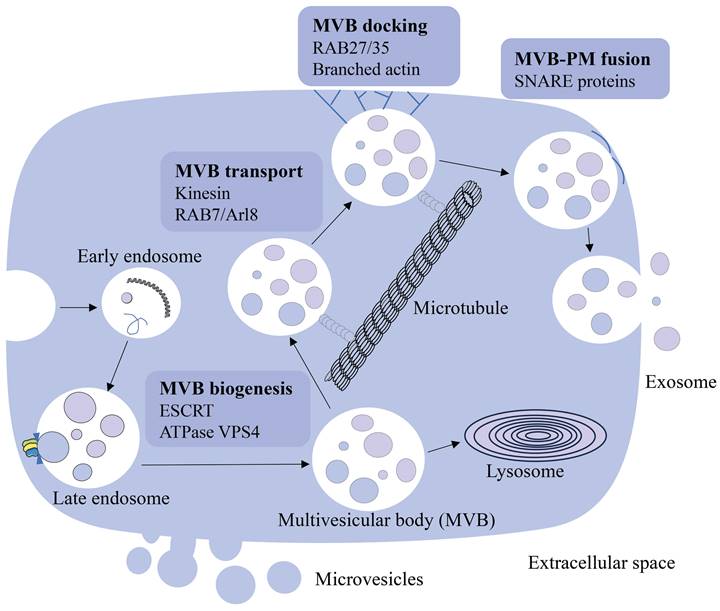
Cells communicate with each other and work as a collective in vivo through direct interactions and soluble molecules such as cytokines. Pan et al. first described the vesicles released by sheep reticulocytes as exosomes in 1987, and later proved their presence using electron microscopy [13]. In early reports, extracellular vesicles, including exosomes, were considered as cellular waste [14, 15]. Recent studies have shown that extracellular vesicles are also involved in intercellular communication. Exosomes are membrane-bound vesicles with sizes ranging from 50 to 200 nm and are found in virtually all biological fluids, including blood [16]. They are created by the early endosomes' limiting membranes budding inward, and mature into multivesicular bodies (MVBs) [16-18]. Endosomal sorting complexes required for transport (ESCRT) regulate MVBs (including exosomes) synthesis and release [19, 20]. This process is initiated by ESCRT-0, which recognizes and retains specific proteins in the late endosomal membrane. After ESCRT-I/II triggers the degradation of the limiting membrane into the MVB cavity, ESCRT-III forms a spiral structure, constricts the budding neck and ATPase VPS4 drives the membrane scission [21-23]. MVBs are involved in the endocytosis and transportation of the cell's material [24], eventually either delivered to the lysosome and degraded with all their components, or fuse with the cell membrane and discharge the contents (including exosomes) into the extracellular environment [17, 25]. MVBs destined for exocytosis are transported to the plasma membrane along microtubules by the molecular motor kinesin. MVBs transport is mediated by a variety of kinesin isoforms and can be regulated by Arl8- and RAB7-dependent protein complexes [26, 27]. MVB docking to the plasma membrane is regulated by RABs, such as RAB27 and RAB35, and the docking site is formed by stabilizing the branched actin filaments [28-30]. After transport and docking to the plasma membrane, secreted MVB fuses with the plasma membrane via soluble N-ethylmaleimide-sensitive component attachment protein receptor (SNARE). Humans have a variety of SNARE proteins that locate in different intracellular membranes and mediate the fusion of cell compartments by forming different SNARE complexes [31, 32]. When MVBs fuse with the plasma membrane, exosomes are released into the extracellular space, where they interact with the extracellular matrix to affect cells in the microenvironment, and also can enter the circulation through lymph or blood (Figure 1). The elements that influence the fate of a certain MVB are not fully known [18], however, it has been shown it is associated with the level of cholesterol in MVB. Specifically, cholesterol rich vesicles were secreted, whereas cholesterol deficient vesicles were directed to the lysosome for degradation [33].
Formation and role of exosomes
Exosomes are released into the extracellular space by most cells after fusion with the plasma membrane. The main components of exosome membranes are lipids and proteins, which are enriched with lipid rafts, tetraspanins such as CD9 and CD63, and membrane transport proteins [16, 25, 34, 35]. Because the formation of exosomal and the transport of MVB are regulated by ESCRT proteins, these proteins and their accessory proteins (Alix, TSG101, HSC70, and HSP90β) can be found in exosomes regardless of the cell type [36]. In addition to lipids and proteins, various nucleic acids, such as mRNAs, miRNAs, circRNAs, and other ncRNAs, have recently been discovered in exosomes [37, 38]. When exosomes circulate, these exosomal RNAs can be taken up by neighboring or distant cells and modulate recipient cells. Exosomes have received increased attention because of the discovery of their role in cell-to-cell genetic exchange.
Initially, researchers thought that exosomes were vectors during reticulocyte maturation. Subsequently, some studies have revealed that exosomes play an important role in variety of pathophysiological processes, such as mediating information communication between cells, participating in immune response, signal transduction, and waste removal [39]. Recently, studies have shown that exosomes deliver proteins, RNAs, and other contents to the recipient cells by autocrine, paracrine, or endocrine and then affect the biological functions of the recipient cells. The way that the exosomes bind to recipient cells include: releasing active components near the recipient cells; binding to surface-specific receptors of the target cell; fusion with plasma membrane and endocytosis of receptor cells [40]. Exosomal ncRNAs offer some benefits in the diagnosis and treatment of diseases.
The source and formation of exosomes. Early endosomes are generated by the plasma membrane budding inward and mature into the late endosome. Late endosome becomes multivesicular body (MVB) through regulation of ESCRT and ATPase VPS4. MVBs release exosomes into the extracellular space through MVB transport, docking and MVB-PM fusion processes, or are degraded by the lysosome.
Exosomes in cardiac remodeling
It is considered that cardiac stress can lead to fibroblast proliferation, extracellular matrix protein secretion, cardiomyocyte hypertrophy, cardiomyocyte death, and the production of proinflammatory cytokines [4]. Notably, 28% cells are cardiomyocytes and 70% is cardiac fibroblasts in human heart [41]. Therefore, cardiac myocytes and cardiac fibroblasts are the main determinants of cardiac remodeling. We concentrate principally on two forms of cardiac remodeling in this review: reactive interstitial remodeling and focal or diffuse replacement remodeling. Reactive interstitial remodeling is characterized by increased collagen production and diffused collagen deposition without a loss of myocytes. This type of remodeling happens gradually with increased pressure and/or volume loads, such as in hypertension and aortic stenosis. Eliminating the harmful stimuli or using specific therapy may be possible to reverse the condition. Diffuse or focal replacement remodeling is caused by the death of cardiomyocytes and usually occurs after myocardial infarction (MI). Since the affected myocardium does not survive and the contractile abilities are unable to recover, this type of remodeling is irreversible. We review the role of exosomal ncRNAs in cardiac remodeling to provide fresh perspectives for the mechanism and treatment of cardiac remodeling.
Exosomal miRNAs in cardiac remodeling
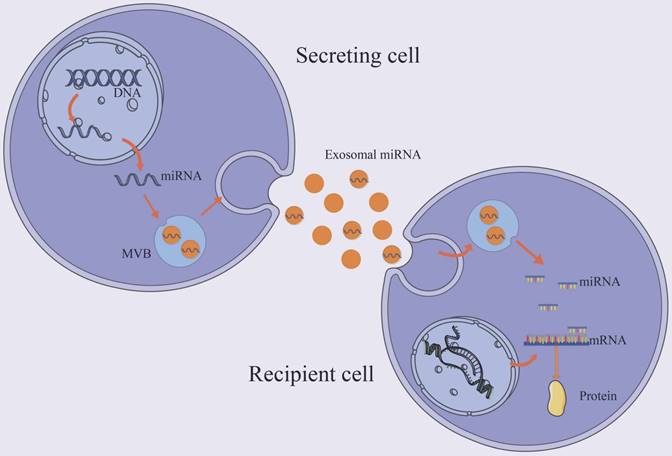
MiRNAs are small endogenous oligonucleotides of 21-25 nucleotides, which are essential for regulation of post-transcriptional gene by attaching to or inhibiting target mRNAs [42]. The syntheses of majority miRNAs are encoded by their own genes, transcribed by RNA polymerase II. A small number of miRNA sequences, on the other hand, are encoded in other RNA molecules. Exosomal miRNAs enter the target cell and, based on partial sequence complementarity, bind the target mRNA [43] (Figure 2). Because miRNA regulation is sequence-dependent, one mRNA can be silenced by multiple miRNAs, and one miRNA can target multiple mRNAs. Exosomes miRNAs have been found to play a a critical role in cardiac pathology [44, 45].
Trafficking and biological function of exosomal miRNAs. The majority of miRNAs are coded by their own genes and transported to the cytoplasm and enters the exosome. Exosomal miRNA regulates post-transcriptional gene expression after taken up by a neighboring or distant cell. It binds the target mRNA according to partial sequence complementarity. By forming a complex with specific proteins, miRNAs inhibit protein synthesis.
Exosomes contain a large number of miRNAs, which are diversified from cell types and in response to disease. Mir-21 levels are higher in failing hearts, suggesting a possible role in cardiac remodeling [46]. However, inhibiting or overexpressing miR-21 in cardiomyocytes has no effect on the morphology or size of cardiomyocytes [47]. Further evidence suggests that miR-21 levels are much higher in fibroblast-derived exosomes than in cells, implying that miR-21 is packed into exosomes [48]. During cardiac stress, exosomal miR-21-3p from angiotensin II (Ang II)-stimulated cardiac fibroblasts induced cardiomyocyte hypertrophy by inhibiting sorbin and SH3 domain-containing protein 2 (SORBS2), PDZ and LIM domain 5 (PDLIM5). In cardiomyocytes, silencing or inhibiting miR-21-3p attenuated hypertrophy. Thus, exosomal miR-21, rather than intracelluar miR-21, plays an important role in the transfer of pro-hypertrophy information from cardiac fibroblasts to cardiomyocytes [48]. Another study found that Ang II induced exosomes secreted by cardiac myofibroblasts can also enhance the activity of the renin-angiotensin system in cardiomyocytes and promote the hypertrophy of cardiomyocytes by activating mitogen-activated protein kinases (MAPKs) and Akt; exosome suppressor could effectively reduce this pathological process [49]. Exosomes derived from macrophages with enriched miR-155 can promote cardiac fibrosis and hypertrophy in uremic mice by activating the pro-hypertrophic pathway FOXO3a [50]. MiRNA-enriched exosomes derived from TNF-treated fibroblasts not only inhibited the Nrf2/ARE signaling pathway, but also promoted the expression of cardiac hypertrophy-related genes, implying that these exosomes can inhibit Nrf2 translation and subsequent transcription of downstream targeting genes that contribute to cardiac hypertrophy [51]. Cardiac progenitor cells (CPC) -derived exosomal miR-21 inhibited cardiomyocyte's apoptosis pathway through downregulating programmed cell death 4 (PDCD4) [52]. Exosomal miRNA-24 derived from rat plasma were also found to have antiapoptotic effects after remote ischemic preconditioning [53]. In addition, exosomal miR-19a-3p, miRNA-214, miR-146a, miR-210 and miR-338 were associated with cardiomyocyte apoptosis [54-58].
Exosomal miRNAs can aggravate or reduce cardiac fibrosis, inflammation, and scar formation by regulating gene expression. Macrophage-derived miR-155 as a paracrine regulator can promote cardiac fibroblast proliferation and collagen production [59]. Exosomal miR-24-3p derived from mesenchymal stem cells (MSCs) can protect myocardial function after MI, reduce inflammation and fibrosis, inhibit cardiac fibroblast differentiation, promote cardiomyocyte proliferation, and reduce apoptosis [53, 60]. Exosomal miR-132 has the ability to reduce scarring, infarct zone size, inflammation and myofibroblast proliferation [61]. MiR-378 exosomes from cardiac myocytes entered cardiac fibroblasts, resulting in increased miR-378 levels, which can inhibit the myocardial fibrosis by regulating p38 MAPK signaling pathways [62]. Increased exosomal miR-146a-5p expression from cardiosphere-derived cells (CDCs) was related to a decrease in myocardial fibrosis via inhibition of proinflammatory cytokines and transcripts [63]. One study had shown that the levels of serum exosomal miR-425 and miR-744 were reduced in 31 patients with HF and Ang II treated cardiac fibroblasts; further investigation revealed these two miRNAs suppressed angiotensin-induced collagen and cellulose synthesis, as well as myocardial remodeling by targeting transforming growth factor-β (TGF-β) [64]. For myocardial fibrosis caused by acute MI (AMI), previous studies have shown that MSC-derived exosome miR-22 can inhibit myocardial cell fibrosis by binding methylated CpG binding protein 2 and reduce the degree of myocardial necrosis and fibrosis [65]. In addition, increased expression of miR-24 and miR-29 and decreased expression of miR-15, miR-21, miR-34, miR-130, and miR-378 in bone marrow mesenchymal stem cell-derived exosomes inhibited myocardial fibrosis and improved cardiac function in mice after MI [66]. Exosomal miR-27a, miR-28, and miR-34a in a rat HF model derived from cardiac fibroblasts contribute to dysregulation of Nrf2/ARE signaling pathway, which resulted in myocardial dysfunction and heart inflammation [51]. Exosomal miR-208a was discovered to cause and exacerbate cardiac fibrosis [67].
Reported exosomal miRNA that associated with cardiac remodeling are presented in Figure 3 and Table 1.
Exosomal lncRNAs in cardiac remodeling
LncRNA has been discovered for years, its significance and universality are well known due to the development of high throughput sequencing technologies and the completion of large human genome sequencing projects [68]. Accumulating evidence suggests that lncRNAs play a role in the regulation of CVDs and pathological remodeling [69, 70]. Despite their large size, some lncRNAs can be transported through extracellular vesicles (EVs), where they are protected by RNase without being degraded [71, 72]. Exosomes could deliver cardioprotective and anti-fibrotic lncRNA to the desired site with specificity [73].
In a study, exosomal lncRNAs of AMI patients and controls were sequenced, a total of 518 lncRNAs were found to be differentially expressed over a two-fold change, and circulating exosomal lncRNAs ENST00000556899.1 and ENST00000575985.1 were noticeably elevated in AMI patients compared with controls [74]. Hypoxia upregulated the level of exosomal AK139128 from cardiomyocytes and exacerbated MI in the rat model. Moreover, exosomal AK139128 stimulated apoptosis and inhibited proliferation, migration, and invasion of cardiac fibroblasts [75]. The exosomal lncRNA KLF3-AS1 secreted by MSCs can regulate Sirt1 to suppress cardiomyocyte viability, apoptosis, and pyroptosis by acting as a ceRNA to sponge miR-138-5p; transfection of miR-138-5p inhibitor and incubation of KLF3-AS1 exosome contribute to reduce pyroptosis both in vivo and in vitro, whereas sh-Sirt1 transfection may reverse the protective effect of exosomal KLF3-AS1 on hypoxia cardiomyocytes [76]. Exosomal lncRNA MALAT1 prevented aging-induced cardiac dysfunction and lncRNA H19 protected cardiomyocytes by enhancing neovascularization [77, 78]. Exosomal lncRNA ZFAS1 induced heart fibrosis via the wnt4/β-catenin signal pathway [79].
Currently, there are few studies in exosomal lncRNA and cardiac remodeling, further experiments are needed to find out its potential mechanism. Reported exosomal lncRNA that associated with cardiac remodeling are presented in Table 2.
Role of exosomal miRNAs in cardiac remodeling. Cardiac remodeling mainly includes cardiomyocyte hypertrophy, cardiomyocyte apoptosis, cardiac fibrosis and cardiac immunity. Different exosomal miRNAs cause cardiac remodeling through different mechanisms and even multiple mechanisms.
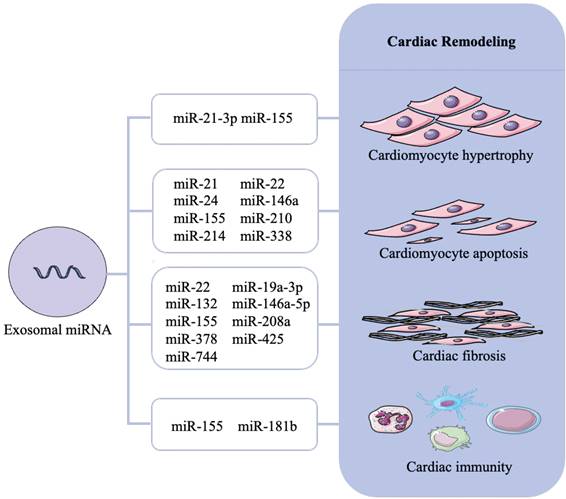
Exsomal miRNA which are identified to modulate cardiac remodeling
| Exsomal cargo | Communication | Targets | Functions | References |
|---|---|---|---|---|
| miRNAs | ||||
| miR-19a-3p | Between endothelial cells and heart | ND | Promotes angiogenesis, decrease myocardial fibrosis, and improve left ventricular function | [54] |
| miR-21-3p | Between cardiomyocytes and cardiac fibroblasts | SORBS2, PDLIM5 | Promotes cardiomyocytes hypertrophy | [48] |
| miR-21 | Between CPCs and cardiomyocytes | PDCD4 | Reduces cardiomyocytes apoptosis | [52] |
| miR-24 | Between plasma and cardiomyocytes | ND | Reduces cardiomyocytes apoptosis | [53] |
| miR-146a | Between cardiosphere-derived cells and cardiomyocytes | ND | Reduces apoptosis and promote proliferation of cardiomyocytes | [56] |
| miR-155 | Between macrophages and cardiomyocytes | FOXO3a | Promotes cardiomyocytes hypertrophy and apoptosis | [50] |
| miR-210 | Between MSCs and cardiomyocytes | ND | Reduces cardiomyocytes apoptosis | [57] |
| miR-214 | Between adipose cells and cardiomyocytes | ND | Reduces cardiomyocytes apoptosis | [55] |
| miR-338 | Between MSCs and cardiomyocytes | MAP3K2 | Reduces cardiomyocytes apoptosis | [58] |
| miR-155 | Between macrophages and cardiac fibroblasts | Son of Sevenless 1 | Promotes cardiac fibroblast proliferation and and collagen production | [59] |
| miR-24-3p | Between MSCs and heart | ND | Inhibits cardiac fibroblasts differentiation, promotes cardiomyocytes proliferation | [53, 60] |
| miR-132 | Between CPC and heart | RasGAP-p120 | Inhibits cardiac fibrosis | [61] |
| miR-378 | Between cardiomyocytes and cardiac fibroblasts | MKK6 | Inhibits cardiac fibrosis | [62] |
| miR-425 | Between plasma and heart | TGFβ1 | Inhibits cardiac fibrosis | [64] |
| miR-744 | Between plasma and heart | TGFβ1 | Inhibits cardiac fibrosis | [64] |
| miR-146a-5p | Between CDCs and heart | ND | Inhibits cardiac fibrosis | [63] |
| miR-22 | Between MSCs and heart | Mecp2 | Inhibits cardiac fibrosis and necrosis | [65] |
| miR-208a | Between cardiomyocytes and cardiac fibroblasts | Dyrk2 | Increases fibroblast proliferation and differentiation | [67] |
“ND” (Not Determined) indicates that a target has not yet been reported. Abbreviations: CPCs, cardiac progenitor cells; MSCs, mesenchymal stem cells; CDCs, cardiosphere-derived cells.
Exsomal lncRNA and circRNA which are identified to modulate cardiac remodeling
| Exsomal cargo | Communication | Targets | Functions | References |
|---|---|---|---|---|
| lncRNAs | ||||
| AK139128 | Between cardiomyocytes and cardiac fibroblasts | ND | Promotes cardiac fibroblasts apoptosis and inhibits proliferation, migration, and invasion | [75] |
| KLF3-AS1 | Between MSCs and cardiomyocytes | miR-138-5p/sirt1 | Reduces cardiomyocytes apoptosis | [76] |
| MALAT1 | Between UMSCs and cardiomyocytes | NF-κB/TNF-α | Prevents aging-induced cardiac dysfunction | [77] |
| H19 | Between MSCs and endothelial cells | ND | Protects cardiomyocytes by enhancing neovascularization | [78] |
| ZFAS1 | Between cardiomyocytes and heart | miR-4711-5p/wnt4 | Promotes cardiac fibrosis | [79] |
| circRNAs | ||||
| hsa_circ_0097435 | Between plasma and heart | ND | Promotes cardiomyocyte apoptosis | [85] |
| circ-0001273 | Between UMSCs and cardiomyocytes | ND | Reduces cardiomyocytes apoptosis | [86] |
“ND” (Not Determined) indicates that a target has not yet been reported. Abbreviations: MSCs, mesenchymal stem cells; UMSCs, umbilical cord mesenchymal stem cells.
Exosomal circRNAs in cardiac remodeling
CircRNA is a type of ncRNA that is produced during the backsplicing of exons or from lariat introns [80, 81]. In contrast to linear RNAs, the 3' and 5' ends of a circRNA strand are linked together by covalent bonds to form a stable and conserved circular structure. CircRNAs are mainly present in the cytoplasm, but also found in the nucleus and EVs.
CircRNAs may be vital for cardiac disease progression and treatment. By microarray assay and quantitative PCR, it was found that 29 circRNAs were up-regulated and 34 were down-regulated in a post-MI model of mice. The expression of circRNA-010567 was up-regulated and predicted by bioinformatics software that it could sponge miR-141 [82]. In cardiac fibroblasts, silencing circRNA-010567 could increase miR-141 expression, decrease TGF-β1 expression, and inhibit collagen I, collagen III, and SMA expression. CircRNA-010567/miR-141/TGF-β1 axis is important in the diabetic myocardial fibrosis mouse model [83].
Interestingly, through RNA sequence analysis, exosomal circRNAs were found to be more plentiful and stable than those weren't encapsulated in exosomes [84]. Next-generation sequencing of exosomes from HF patients and a healthy control group found that the exosomal hsa circ-0097435 was elevated in HF patients [85]. Human umbilical cord mesenchymal stem cells (UMSCs) derived exosomes can improve the cardiac function after MI by delivering circ-0001273. Compared with the circ-0001273-exosome-treated rats, the cardiac structure and function was remarkably deteriorative in the si-circ-0001273-exosome-treated group; circ-0001273 can remarkably inhibit the occurrence of myocardial cell apoptosis and improve cardiac function in the ischemic environment [86].
Research about the role of exosomal circRNA in cardiac remodeling is in early stage. Reported exosomal circRNA that associated with cardiac remodeling are presented in Table 2.
Exosomes mediated cardiac immunity in cardiac remodeling
Neurohumoral factors are the major stimulating factors in cardiac remodeling. Various inflammatory cytokines and immune cells are involved in this process, including interleukin 6 (IL-6), macrophages, monocytes, and T cells. Exosomes can encapsulate inflammatory cytokines and act on other cells to participate in the inflammatory process of cardiac remodeling. Exosomal miRNA-181b secreted by cardiomyocytes can inhibit the invasion process of macrophages and mediate immune response [87]. In the exosomes secreted by macrophages, miRNA-155 was found to target cardiac fibroblasts and promoted the inflammatory response of fibroblasts [59]. Exosomes secreted by hypoxic dendritic cells could upregulate the expression of inflammatory factor IFN-γ and TNF-α in CD4+T cells, and thus promote the activation of T cells [88]. Another study found that the plasma exosomes were increased in HF patients compared with the control group, and the exosomal miRNA promoted inflammatory responses through the TLR9-NF-κB signaling pathway [89]. These results suggest that exosomal ncRNA can act as vectors to regulate the expression of inflammatory factors and participate in the inflammatory response in the process of cardiac remodeling.
Conclusion
Given the global aging of populations and the associated rise in HF prevalence, there is an urgent need to develop therapeutic interventions that prevent cardiac remodeling. Since the discovery of exosomal ncRNAs in the past decades, their role in the process of cardiac remodeling development has received increasing attention. Cellular and animal experiments have confirmed that exosomal ncRNAs can aggravate or alleviate cardiac remodeling. In clinical experiments, there are only some exosomal miRNAs was used for the diagnosis of cardiac remodeling [90-94] (Table 3), no ncRNA has been used as clinical drug yet. The research on exosomal lncRNA and circRNA in cardiac remodeling is limited. Further and in-depth research on the mechanism and role of exosomal ncRNAs can help to identify new interacting molecules and signal transduction pathways for cardiac remodeling, providing new ideas and methods for diagnosis and treatment for CVDs.
Clinical studies of exsomal miRNAs in cardiac remodeling
| Exsomal miRNAs | Pathology | Biospecimen | References |
|---|---|---|---|
| miR-1 miR-24 miR-133a miR-133b miR-210 | CABG surgery | plasma | [90] |
| miR-1 miR-133b miR-208b miR-499 | MI | plasma | [91] |
| miR-210-3p | atrial fibrillation | atrial myocytes, serum | [92] |
| miR-136-5p miR-144-5p miR-624-3p miR-624-5p miR-1284-5p | cardiac sarcoidosis | plasma, serum | [93] |
| miR-29a | Obesity related cardiomyopathy | plasma | [94] |
Abbreviations: CABG, coronary artery bypass graft; MI, myocardial infarction.
Abbreviations
CVDs: cardiovascular diseases; HF: heart failure; ECs: endothelial cells; mRNAs: messenger RNAs; ncRNAs: non-coding RNAs; miRNAs: microRNAs; lncRNAs: long noncoding RNAs; circRNAs: circular noncoding RNAs; ceRNAs: endogenous RNAs; MVBs: multivesicular bodies; ESCRT: endosomal sorting complexes required for transport; SNARE: soluble N-ethylmaleimide-sensitive component attachment protein receptor; MI: myocardial infarction; Ang II: angiotensin II; SORBS2: sorbin and SH3 domain-containing protein 2 (SORBS2); PDLIM5: PDZ and LIM domain 5; MAPKs: mitogen-activated protein kinases; CPC: cardiac progenitor cells; PDCD4: programmed cell death 4; MSCs: mesenchymal stem cells; CDCs: cardiosphere-derived cells; TGF-β: transforming growth factor-β; EVs: extracellular vesicles; UMSCs: umbilical cord mesenchymal stem cells.
Acknowledgements
Funding
This work is supported by grants from the National Natural Science Foundation of China (No. 82271625), Key Research and Development Plan of Human Province (No. 2022SK2013), Natural Science Foundation of Hunan Province (No. 2022JJ70063, No.2022JJ70051).
Author contributions
Xiangyu Zhang and Yang Liu developed the concept of the project and wrote the manuscript. Xiangyu Zhang, Yang Liu, Xing Lyu, and Shengyu Tan were involved in the manuscript writing, including discussion of content and writing, and editing of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37:3232-45
2. Nadruz W. Myocardial remodeling in hypertension. J Hum Hypertens. 2015;29:1-6
3. Gartz M, Strande JL. Examining the Paracrine Effects of Exosomes in Cardiovascular Disease and Repair. J Am Heart Assoc. 2018 7
4. Iaconetti C, Sorrentino S, De Rosa S, Indolfi C. Exosomal miRNAs in Heart Disease. Physiology (Bethesda). 2016;31:16-24
5. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208
6. Calway T, Kim GH. Harnessing the Therapeutic Potential of MicroRNAs for Cardiovascular Disease. J Cardiovasc Pharmacol Ther. 2015;20:131-43
7. Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 2015;11:238-45
8. Bayoumi AS, Aonuma T, Teoh JP, Tang YL, Kim IM. Circular noncoding RNAs as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol Sin. 2018;39:1100-9
9. Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res. 2015;116:751-62
10. Li B, Huang N, Wei S, Xv J, Meng Q, Aschner M. et al. lncRNA TUG1 as a ceRNA promotes PM exposure-induced airway hyper-reactivity. J Hazard Mater. 2021;416:125878
11. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK. et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384-8
12. Gunthel M, Barnett P, Christoffels VM. Development, Proliferation, and Growth of the Mammalian Heart. Mol Ther. 2018;26:1599-609
13. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942-8
14. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-97
15. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269-88
16. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066
17. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11
18. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83
19. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3:271-82
20. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864-9
21. Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci. 2017;42:42-56
22. Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445-52
23. Schoneberg J, Lee IH, Iwasa JH, Hurley JH. Reverse-topology membrane scission by the ESCRT proteins. Nat Rev Mol Cell Biol. 2017;18:5-17
24. Borges FT, Reis LA, Schor N. Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res. 2013;46:824-30
25. Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575-81
26. Guardia CM, Farias GG, Jia R, Pu J, Bonifacino JS. BORC Functions Upstream of Kinesins 1 and 3 to Coordinate Regional Movement of Lysosomes along Different Microtubule Tracks. Cell Rep. 2016;17:1950-61
27. Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW. et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234-8
28. Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N. et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159-68
29. Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223-32
30. Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M. et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197-213
31. Hong W, Lev S. Tethering the assembly of SNARE complexes. Trends Cell Biol. 2014;24:35-43
32. Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474-7
33. Mobius W, Ohno-Iwashita Y, van Donselaar EG, Oorschot VM, Shimada Y, Fujimoto T. et al. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J Histochem Cytochem. 2002;50:43-55
34. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-20
35. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036-45
36. Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019 8
37. Conigliaro A, Fontana S, Raimondo S, Alessandro R. Exosomes: Nanocarriers of Biological Messages. Adv Exp Med Biol. 2017;998:23-43
38. Sato-Kuwabara Y, Melo SA, Soares FA, Calin GA. The fusion of two worlds: non-coding RNAs and extracellular vesicles-diagnostic and therapeutic implications (Review). Int J Oncol. 2015;46:17-27
39. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
40. Frontiers Production O. Erratum: Exosomes: A Rising Star in Failing Hearts. Front Physiol. 2017;8:620
41. van Amerongen MJ, Engel FB. Features of cardiomyocyte proliferation and its potential for cardiac regeneration. J Cell Mol Med. 2008;12:2233-44
42. Pu M, Chen J, Tao Z, Miao L, Qi X, Wang Y. et al. Regulatory network of miRNA on its target: coordination between transcriptional and post-transcriptional regulation of gene expression. Cell Mol Life Sci. 2019;76:441-51
43. Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y. et al. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front Cell Dev Biol. 2020;8:616161
44. Greco S, Gorospe M, Martelli F. Noncoding RNA in age-related cardiovascular diseases. J Mol Cell Cardiol. 2015;83:142-55
45. Dutka M, Bobinski R, Korbecki J. The relevance of microRNA in post-infarction left ventricular remodelling and heart failure. Heart Fail Rev. 2019;24:575-86
46. Ding H, Wang Y, Hu L, Xue S, Wang Y, Zhang L. et al. Combined detection of miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217 for screening of early heart failure diseases. Biosci Rep. 2020 40
47. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980-4
48. Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136-46
49. Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M. et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol. 2015;89:268-79
50. Wang B, Wang ZM, Ji JL, Gan W, Zhang A, Shi HJ. et al. Macrophage-Derived Exosomal Mir-155 Regulating Cardiomyocyte Pyroptosis and Hypertrophy in Uremic Cardiomyopathy. JACC Basic Transl Sci. 2020;5:148-66
51. Tian C, Gao L, Zimmerman MC, Zucker IH. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol. 2018;314:H928-H39
52. Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH. et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277
53. Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W. et al. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring miR-24. Cell Death Dis. 2018;9:320
54. Gollmann-Tepekoylu C, Polzl L, Graber M, Hirsch J, Nagele F, Lobenwein D. et al. miR-19a-3p containing exosomes improve function of ischaemic myocardium upon shock wave therapy. Cardiovasc Res. 2020;116:1226-36
55. Eguchi S, Takefuji M, Sakaguchi T, Ishihama S, Mori Y, Tsuda T. et al. Cardiomyocytes capture stem cell-derived, anti-apoptotic microRNA-214 via clathrin-mediated endocytosis in acute myocardial infarction. J Biol Chem. 2019;294:11665-74
56. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606-19
57. Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J. et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018;46:1659-70
58. Fu DL, Jiang H, Li CY, Gao T, Liu MR, Li HW. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24:10107-17
59. Wang C, Zhang C, Liu L, A X, Chen B, Li Y. et al. Macrophage-Derived mir-155-Containing Exosomes Suppress Fibroblast Proliferation and Promote Fibroblast Inflammation during Cardiac Injury. Mol Ther. 2017;25:192-204
60. Qian L, Van Laake LW, Huang Y, Liu S, Wendland MF, Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549-60
61. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530-41
62. Yuan J, Liu H, Gao W, Zhang L, Ye Y, Yuan L. et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics. 2018;8:2565-82
63. Hirai K, Ousaka D, Fukushima Y, Kondo M, Eitoku T, Shigemitsu Y. et al. Cardiosphere-derived exosomal microRNAs for myocardial repair in pediatric dilated cardiomyopathy. Sci Transl Med. 2020 12
64. Wang L, Liu J, Xu B, Liu YL, Liu Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J Med Sci. 2018;34:626-33
65. Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685
66. Moghaddam AS, Afshari JT, Esmaeili SA, Saburi E, Joneidi Z, Momtazi-Borojeni AA. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019;285:1-9
67. Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res. 2018;10:4350-66
68. Zhou H, Wang B, Yang YX, Jia QJ, Zhang A, Qi ZW. et al. Long Noncoding RNAs in Pathological Cardiac Remodeling: A Review of the Update Literature. Biomed Res Int. 2019;2019:7159592
69. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298-307
70. Kolat D, Hammouz R, Bednarek AK, Pluciennik E. Exosomes as carriers transporting long non-coding RNAs: Molecular characteristics and their function in cancer (Review). Mol Med Rep. 2019;20:851-62
71. Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L. et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36:2007-12
72. Viereck J, Thum T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ Res. 2017;120:381-99
73. Liang H, Pan Z, Zhao X, Liu L, Sun J, Su X. et al. LncRNA PFL contributes to cardiac fibrosis by acting as a competing endogenous RNA of let-7d. Theranostics. 2018;8:1180-94
74. Zheng ML, Liu XY, Han RJ, Yuan W, Sun K, Zhong JC. et al. Circulating exosomal long non-coding RNAs in patients with acute myocardial infarction. J Cell Mol Med. 2020;24:9388-96
75. Wang L, Zhang J. Exosomal lncRNA AK139128 Derived from Hypoxic Cardiomyocytes Promotes Apoptosis and Inhibits Cell Proliferation in Cardiac Fibroblasts. Int J Nanomedicine. 2020;15:3363-76
76. Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10:393
77. Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y. et al. Stem Cell-Derived Exosomes Prevent Aging-Induced Cardiac Dysfunction through a Novel Exosome/lncRNA MALAT1/NF-kappaB/TNF-alpha Signaling Pathway. Oxid Med Cell Longev. 2019;2019:9739258
78. Huang P, Wang L, Li Q, Tian X, Xu J, Xu J. et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116:353-67
79. Wang Y, Cao X, Yan L, Zheng Y, Yu J, Sun F. et al. WITHDRAWN: Exosome-derived long non-coding RNA ZFAS1 controls cardiac fibrosis in chronic kidney disease. Aging (Albany NY). 2021 13
80. Greco S, Cardinali B, Falcone G, Martelli F. Circular RNAs in Muscle Function and Disease. Int J Mol Sci. 2018 19
81. Carrara M, Fuschi P, Ivan C, Martelli F. Circular RNAs: Methodological challenges and perspectives in cardiovascular diseases. J Cell Mol Med. 2018;22:5176-87
82. Wu HJ, Zhang CY, Zhang S, Chang M, Wang HY. Microarray Expression Profile of Circular RNAs in Heart Tissue of Mice with Myocardial Infarction-Induced Heart Failure. Cell Physiol Biochem. 2016;39:205-16
83. Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem Biophys Res Commun. 2017;487:769-75
84. Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J. et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981-4
85. Han J, Zhang L, Hu L, Yu H, Xu F, Yang B. et al. Circular RNA-Expression Profiling Reveals a Potential Role of Hsa_circ_0097435 in Heart Failure via Sponging Multiple MicroRNAs. Front Genet. 2020;11:212
86. Li CX, Song J, Li X, Zhang T, Li ZM. Circular RNA 0001273 in exosomes derived from human umbilical cord mesenchymal stem cells (UMSCs) in myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24:10086-95
87. de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF. et al. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation. 2017;136:200-14
88. Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L. et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4(+) T lymphocytes after myocardial infarction. J Mol Cell Cardiol. 2016;91:123-33
89. Ye W, Tang X, Yang Z, Liu C, Zhang X, Jin J. et al. Plasma-derived exosomes contribute to inflammation via the TLR9-NF-kappaB pathway in chronic heart failure patients. Mol Immunol. 2017;87:114-21
90. Emanueli C, Shearn AI, Laftah A, Fiorentino F, Reeves BC, Beltrami C. et al. Coronary Artery-Bypass-Graft Surgery Increases the Plasma Concentration of Exosomes Carrying a Cargo of Cardiac MicroRNAs: An Example of Exosome Trafficking Out of the Human Heart with Potential for Cardiac Biomarker Discovery. PLoS One. 2016;11:e0154274
91. Deddens JC, Vrijsen KR, Colijn JM, Oerlemans MI, Metz CH, van der Vlist EJ. et al. Circulating Extracellular Vesicles Contain miRNAs and are Released as Early Biomarkers for Cardiac Injury. J Cardiovasc Transl Res. 2016;9:291-301
92. Hao H, Yan S, Zhao X, Han X, Fang N, Zhang Y. et al. Atrial myocyte-derived exosomal microRNA contributes to atrial fibrosis in atrial fibrillation. J Transl Med. 2022;20:407
93. Crouser ED, Julian MW, Bicer S, Ghai V, Kim TK, Maier LA. et al. Circulating exosomal microRNA expression patterns distinguish cardiac sarcoidosis from myocardial ischemia. PLoS One. 2021;16:e0246083
94. Li F, Zhang K, Xu T, Du W, Yu B, Liu Y. et al. Exosomal microRNA-29a mediates cardiac dysfunction and mitochondrial inactivity in obesity-related cardiomyopathy. Endocrine. 2019;63:480-8
Author contact
![]() Corresponding author: Xiangyu Zhang, Department of Geriatrics, the Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China. Email: xiangyuzhangedu.cn.
Corresponding author: Xiangyu Zhang, Department of Geriatrics, the Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China. Email: xiangyuzhangedu.cn.

 Global reach, higher impact
Global reach, higher impact