Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(11):1377-1385. doi:10.7150/ijms.86773 This issue Cite
Research Paper
22q11.2 Deletion Syndrome in Taiwan: Clinical Presentation and Immune System Status of Patients
1. Department of Pediatrics, MacKay Memorial Hospital, Taipei, Taiwan.
2. Institute of Clinical Medicine, National Yang-Ming Chiao-Tung University, Taipei, Taiwan.
3. Department of Rare Disease Center, MacKay Memorial Hospital, Taipei, Taiwan.
4. Department of Medicine, Mackay Medical College, New Taipei City, Taiwan.
5. Mackay Junior College of Medicine, Nursing, and Management, Taipei, Taiwan.
6. Division of Genetics and Metabolism, Department of Medical Research, MacKay Memorial Hospital, Taipei, Taiwan.
7. College of Medicine, Fu-Jen Catholic University, Taipei, Taiwan.
8. Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan.
9. Department of Infant and Child Care, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan.
Chung-Lin Lee and Shan-Miao Lin contributed equally to this study.
Received 2023-6-3; Accepted 2023-8-23; Published 2023-9-4
Abstract
Background: 22q11.2 deletion syndrome (22q11.2DS) is a microdeletion syndrome exhibiting significant clinical phenotype variability. This study aimed to investigate the clinical features, immune profiles, and cognitive abilities of 22q11.2DS patients receiving treatment at MacKay Memorial Hospital in Taipei, Taiwan.
Methods: This is a cross-sectional analysis between January 2001 and December 2022. We recruited 27 patients with 22q11.2DS using fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification (MLPA) and array comparative genomic hybridization (aCGH). Our evaluation included patient history, physical examination, laboratory analysis, and cardiac and cognitive assessment.
Results: We included 27 patients with 22q11.2DS, 7 (25.9%) of whom were female. The median age of the patients was 17.9 yr. Ninety-three percent of the patients exhibited the characteristic facial features associated with the syndrome. A family history of 22q11.2DS was found in 11.1% of the patients. Furthermore, 74.1% of the patients had a congenital heart defect, the most common of which was tetralogy of Fallot (40.7%). Hypocalcemia was observed in 40.7% of the patients. A low T-cell count was observed in 66.7% of the patients, whereas 18.5% had low immunoglobulin levels. Cognitive assessments revealed that four out of six evaluated patients (66.7%) had an intellectual disability, as evidenced by intellectual quotient scores less than 70. The remaining two patients (33.3%) had a borderline intellectual function.
Conclusion: Tetralogy of Fallot, hypocalcemia, immunologic defects, and cognitive impairment were common among our patients. To address the potential multisystem involvement, we recommend that all affected individuals undergo a comprehensive evaluation by a multidisciplinary care team.
Keywords: 22q11.2 deletion syndrome, congenital heart disease, intellectual disability, T-cell defect, Taiwan
Introduction
22q11.2 deletion syndrome (22q11.2DS) is the most common microdeletion syndrome in humans [1]. It is caused by deletions on the long arm of chromosome 22 [2]. 22q11.2DS was previously known as a velocardiofacial syndrome, conotruncal anomaly face syndrome, Cayler cardiofacial syndrome, and, in some cases, autosomal dominant Opitz G/BBB syndrome [3,4]. Fluorescence in situ hybridization (FISH), multiplex ligation-dependent probe amplification (MLPA) and array comparative genomic hybridization (aCGH) are often used diagnostic test for 22q11.2DS [2,5].
Two separate studies have reported that the estimated prevalence of 22q11.2DS ranges from 1 in 4,000 to 1 in 7,092 live births [1,6]. Around 90% of cases were caused by de novo mutations, whereas the remaining cases were caused by inherited chromosome 22 with deletion at the q11.2 arm from one parent in an autosomal dominant pattern [2,7,8]. Patients with 22q11.2DS have a wide range of clinical manifestations, including a conotruncal congenital heart defect, immunodeficiency, characteristic facial features, palatal defects, developmental and/or learning disabilities, hypocalcemia, hypoparathyroidism, and hypothyroidism. Additional abnormalities linked to 22q11.2DS include renal anomalies, hearing loss, growth retardation, psychiatric disorders, and feeding and swallowing difficulties. Proposed practical guidelines for managing patients with 22q11.2DS suggest strategies for identifying, assessing, monitoring, and treating associated morbidities [3,9]. These guidelines recommend that all individuals affected by the condition undergo regular comprehensive evaluations [9].
Due to the lack of comprehensive, integrated studies on 22q11.2DS in Taiwan, this study aimed to outline the clinical characteristics, immunological features, and intellectual status of patients with 22q11.2DS at MacKay Memorial Hospital to facilitate early identification and intervention of the condition in an effort to reduce morbidity and mortality.
Materials and Methods
The medical records of patients at MacKay Memorial Hospital from 2001 to 2022 were analyzed using a hospital-based, cross-sectional design. The study identified 27 patients with 22q11.2DS, which were confirmed by FISH, MLPA or aCGH analysis. Between January 2001 and December 2022, electronic medical records of patients were reviewed, as well as clinical history interviews and physical examinations to evaluate growth and development, family history, facial malformation, congenital heart diseases, history of hypocalcemia, urological imaging, feeding difficulties, infections, and vaccinations. All 27 participants were also invited to undergo further evaluation of their intellectual status and laboratory tests.
Genetic testing
FISH analysis was performed using Vysys LSI TUPLE1 SpectrumOrange/LSI ARSA SpectrumGreen probes, which bind to band 22q11.2 loci D22s553, D22S609, and D22S942 (LSI DiGeorge/VCFS, TUPLE1-HIRA locus SpectrumOrange, Abbott®), as well as band 22q13 (LSI ASRA SpectrumGreen, Abbott®), to identify a deletion of chromosome 22q11.2. At least 20 metaphases were analyzed in each FISH assay. We utilized the MLPA P034/P035 kit (MRC, Holland). The DNA amplification products were then analyzed through capillary electrophoresis using the ABI 3500Dx Genetic Analyzer (Applied Biosystems Life Technologies, Singapore). Subsequently, the obtained results were analyzed with the MLPA Cofalyser.net software (MRC, Holland). For aCGH, we employed the Affymetrix GeneChip Genome-Wide Human SNP array 6.0 (Affymetrix, Santa Clara, CA, USA). This array comprises 750,000 probes with a resolution of 200 kb throughout the genome to detect copy number variation. The array data were analyzed using Affymetrix Genotyping Console™ version 3.0.1, following the instructions provided by the manufacturers to handle all samples.
Intellectual testing
A developmental-behavioral pediatrician and a licensed clinical psychologist evaluated the intellectual status of the patients, and the patients were divided into four age-based categories for assessment using four different tools to evaluate intellectual quotient (IQ): the Bayley Scales of Infant and Toddler Development third edition (Bayley-III), the Stanford-Binet Intelligence scales, the Wechsler Intelligence Scale for Children (WISC)-fourth edition, and the Wechsler Adult Intelligence Scale (WAIS)-fourth edition. These tools were administered to patients aged birth to 3, 3-7, 7-17, and >17 yr, respectively. A psychiatric diagnosis was also recorded. An IQ score of less than 70 (2 standard deviation below the mean) was considered intellectually disabled, whereas an IQ score of 70-84 was classified as borderline intelligence (1 to 2 SD below the mean) [10].
Laboratory testing
The laboratory tests obtained included the complete blood count, as well as measurements of various lymphocyte subpopulations, such as CD3+, CD4+, and CD8+, levels of immunoglobulins (IgG, IgA, IgM, and IgE), serum calcium and phosphate levels, thyroid hormone measurements (thyroid-stimulating hormone and free T4), as well as parathyroid hormone levels. After comparing them to the normal 95% confidence limits for the individual's age, low total lymphocyte and immunoglobulin levels were defined as lymphopenia and low immunoglobulin levels. The absolute counts and percentages of CD3+, CD4+, and CD8+ were also found to be low, with values below the 10th percentile based for age [11,12]. Hypocalcemia (corrected for serum albumin level), hypophosphatemia, hypothyroidism, and hypoparathyroidism were below the 10th percentile of the age distribution [13].
Statistical analysis
MedCalc version 20.218 (MedCalc Software Ltd, Ostend, Belgium) was used for statistical analysis. The Wilcoxon signed‐rank test was used for small, numbered groups. A descriptive analysis was used to characterize the study population. Continuous variables were expressed as the median, whereas categorical variables were reported as a percentage or ratio, as deemed appropriate. The statistical data were presented as mean ± standard deviation, with a significance level of p < 0.05. A p value <0.05 was statistically significant.
Results
Based on genetic testing, the study included 27 patients with 22q11.2DS, with males accounting for 74.1% (20 patients). The clinical characteristics of these patients are shown in Table 1. At the time of the study, the median age of the patients was 17.9 yr (interquartile range [IQR], 14.1-20.3). The majority of patients had clinical symptoms during their newborn period, and the median age at diagnosis was 0.8 yr (IQR, 0.3-7.2). Depending on specific symptoms, the age of onset, age at diagnosis, and diagnostic criteria differed significantly. Furthermore, 11.1% of patients had a confirmed family history of the deletion, including two siblings.
Historical and clinical profiles (n = 27)
| Clinical characteristics | n (%) |
|---|---|
| Gender: male | 20 (74.1) |
| Median age (yr [IQR]) | 17.9 (14.1-20.3) |
| Median age of diagnosis (yr [IQR]) | 0.8 (0.3-7.2) |
| Parental history of 22q11.2 DS | |
| Confirmed 22q11 deletion | 2 (7.4) |
| Facial malformation | 2 (7.4) |
| Facial dysmorphic features | |
| Typical facial malformation | 25 (92.6) |
| Cleft lip | 1 (3.7) |
| Cleft palate | 7 (25.9) |
| Cleft lip and palate | 1 (3.7) |
| History of cardiac surgery | 11 (40.7) |
| Presence of thymus (n = 11) | |
| Normal | 7 (63.6) |
| Absence or ectopic | 4 (36.4) |
| History of recurrent infection | 11 (40.7) |
| Recurrent pneumonia | 3 (11.1) |
| Acute otitis media | 5 (18.5) |
| Chronic otitis media | 1 (3.7) |
| Septicemia | 1 (3.7) |
| History of vaccine adverse effects | 1 (3.7) |
Typical facial malformations, such as palpebral fissure narrowing, hypertelorism, widened nasal dorsum, hypoplastic nasal alae, nasal voice, short philtrum, micrognathia, and malar flattening, were found in 25 patients (92.6%). Seven patients (25.9%) had submucosal cleft palate, whereas one patient (3.7%) had a cleft lip. Only one patient (3.7%) had both cleft lip and submucosal cleft palate. Congenital heart disease was the most common presenting symptom, and most patients were referred to MacKay Memorial Hospital for cardiac evaluation and treatment.
Congenital cardiovascular anomalies were diagnosed in 20 patients (74.1%), with details of the findings presented in Table 2. The most common cardiac defect was tetralogy of Fallot (TOF), found in 11 patients (40.7%), followed by ventricular septal defect (VSD, 40.7%), atrial septal defect (14.8%), and patent ductus arteriosus (11.1%). These primary anomalies were frequently associated with subpulmonic VSD (25.9%), right-sided aortic arch (22.2%), major aortopulmonary collateral arteries (14.8%), aberrant subclavian artery (11.1%), and double aortic arch (11.1%).
After reviewing the electronic medical records, it was found that 11 patients (40.7%) had a history of hypocalcemia. All four patients who underwent hypoparathyroid hormone level testing (14.8%) had a history of hypocalcemia. Additionally, one patient (3.7%) had primary hypothyroidism, and three (11.1%) had primary hyperthyroidism.
The immunological evaluation was conducted for all our patients. Five patients (18.5%) had low serum immunoglobulin levels, including two with pan hypogammaglobulinemia, one with low IgA and IgG levels, and one with low IgA and IgE levels. Lymphopenia was observed in 29.6% of patients compared with age-specific normal values. T-cell deficiency were found in 11 patients (40.7%), as determined by lymphocyte-subset enumerations using flow cytometry. Of these patients, seven (25.9%) had low absolute CD3+ counts or percentages, one (3.7%) had low CD4+ counts or percentages, and three (11.1%) had low CD8+ counts or percentages. None of the patients required intravenous immunoglobulin replacement therapy. There was no evidence of an autoimmune disease. The laboratory profiles for both immunologic and hormonal parameters can be found in Table 3. The thymus was evaluated in 11 patients using a computed tomography scan or a surgical procedure. Four of the 11 (36.4%) assessed were determined to have a nonvisualized or hypoplastic thymus.
Cardiovascular observations (n = 27)
| The type of cardiovascular anomalies | N (%) | Associated lesions: n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| RAA | Double aortic arch | ASCA | Bilateral SVC | LSVC | MAPCAs | Subpulmonic VSD | ||
| Congenital heart disease | 20 (74.1) | |||||||
| Tetralogy of Fallot and variants | ||||||||
| TOF/PS | 5 (18.5) | 1 (3.7) | 1 (3.7) | 1 (3.7) | 1 (3.7) | — | — | 2 (7.4) |
| TOF/PA | 6 (22.2) | 1 (3.7) | — | — | — | 1 (3.7) | 1 (3.7) | — |
| Interrupted aortic arch type B | 1 (3.7) | — | — | — | — | — | 1 (3.7) | — |
| Ventricular septal defect | 11 (40.7) | 2 (7.4) | 1 (3.7) | 1 (3.7) | 1 (3.7) | — | 1 (3.7) | 2 (7.4) |
| Atrial septal defect | 4 (14.8) | 1 (3.7) | 1 (3.7) | 1 (3.7) | - | 1 (3.7) | 1 (3.7) | 2 (7.4) |
| Patent ductus arteriosus | 3 (11.1) | — | — | — | — | — | — | 1 (3.7) |
| Vascular anomalies | ||||||||
| Aberrant subclavian artery | 1 (3.7) | 1 (3.7) | — | — | — | — | — | — |
| No congenital heart disease | 7 (25.9) | |||||||
Note. ASCA, aberrant subclavian artery; LSVC, left superior vena cava; MAPCAs, major aortopulmonary collateral arteries; PA, pulmonary atresia; PS, pulmonary stenosis; RAA, right-sided aortic arch; SVC, superior vena cava; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
Immunological and laboratory characteristics (n = 27)
| Parameters | N (%) |
|---|---|
| Abnormal thyroid function | |
| Hypothyroidism | 1 (3.7) |
| Hyperthyroidism | 3 (11.1) |
| Hypoparathyroidism | 4 (14.8) |
| Hypocalcemia | 11 (40.7) |
| Immunologic parameters | |
| Low white blood cell count | 0 (0.0) |
| Low absolute lymphocyte count | 18 (66.7) |
| T-cell deficiency | |
| Low CD3 | 3 (11.1) |
| Low CD4 | 3 (11.1) |
| Low CD8 | 4 (14.8) |
| Low immunoglobulin | |
| Low IgG | 4 (14.8) |
| Low IgA | 5 (18.5) |
| Low IgM | 4 (14.8) |
| Low IgE | 3 (11.1) |
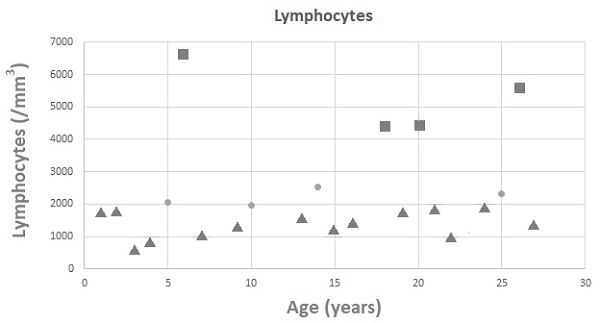
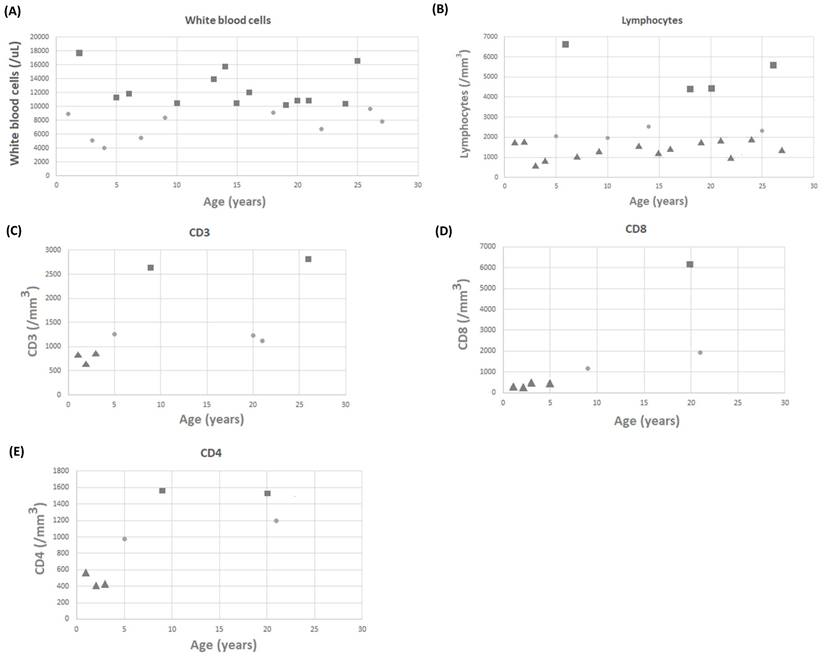
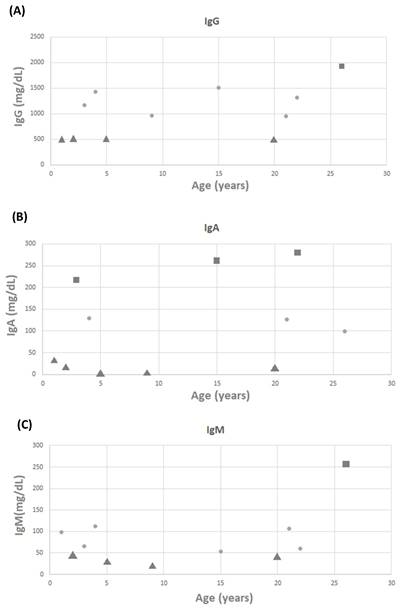
White blood cell counts for 22 patients were recorded, with a mean value of 10.32 ± 3.51 k/mL (Figure 1A). Lymphocyte counts for 22 patients were recorded, with a mean value of 3,010.87 ± 1,708.24 lymphocytes/mm3 (Figure 1B). There were no patients with low white blood cell counts; however, 18 of 22 patients had low lymphocyte counts. Three (11.1%) and four (14.8%) of the 27 patients with available data on CD3 and CD8 levels had low levels, respectively (Figure 1C and 1D). Three (11.1%) of the 27 patients with available data on CD4 levels had low levels (Figure 1E). It is worth noting that none of the patients required any specific treatment for these laboratory abnormalities. IgG, IgM, and IgA levels were measured in 27 patients, with mean values of 1,024.45 ± 498.22, 80.27 ± 66.28, and 107.36 ± 105.46 mg/dL, respectively. Four patients had low IgG levels, five had low IgA levels, and four had low IgM levels (Figure 2A-2C).
The formal intellectual analyses were conducted on six patients, representing 22.2% of the total sample (Table 4). The median age at evaluation was 5.6 yr, with an interquartile range (IQR) of 3.4-8.1. The median full-scale IQ (FSIQ) of all evaluated patients was 67, ranging from 54 to 83. The Bayley-III scale was used for patients under 3 years old, and the Stanford-Binet Intelligence scales were used for those aged 3-7 years old. WISC-IV was used to evaluate patients aged 7-17 yr, and WAIS-IV was used to assess patients older than 17 yr. According to the previously mentioned definitions, four (66.7%) of the six patients were diagnosed with intellectual disability, and the remaining two (33.3%) were classified as having borderline intelligence (FSIQ, 70-84). In terms of the factors that can affect the IQ score, no statistical difference was found between the group with major cardiovascular diseases and the group with minor cardiovascular diseases. The median FSIQ score for patients with major cardiovascular diseases was 66.0 (IQR, 63.5-71.0) and 68.0 (IQR, 61.0-75.5) for those with minor cardiovascular diseases.
Discussion
This report presents the clinical characteristics of patients with 22q11.2DS in Taiwan, with no discernable gender bias. Although the onset of symptoms occurred during the newborn period, the genetic diagnosis was not confirmed until the average age of 18 months. Most patients were referred to MacKay Memorial Hospital for congenital heart disease, with TOF being the most common cardiac condition. All patients who were evaluated intellectually had developmental delays or intellectual impairments. These findings confirm that 22q11.2DS affects multiple systems and has a diverse range of phenotypic expressions.
22q11.2DS is a disorder caused by haploinsufficiency, which means that an affected parent has a 50% probability of passing the condition on to their offspring [7]. Nonetheless, most cases of 22q11.2DS are caused by spontaneous or de novo mutations. Peer-reviewed studies indicate that only 10% of cases are inherited from affected parents [1-3], which is consistent with our finding that 11.1% of patients had a history of parents with confirmed chromosomal deletions. Because not all families of the participants were evaluated for 22q11.2 deletions, the reported proportions of inherited chromosomal deletions may be underestimated. In addition, predicting the phenotype in a sibling inheriting the 22q11.2 deletion is unreliable due to the substantial intrafamilial clinical variability linked to 22q11.2 deletion syndrome [14]. In the three cases of patients with affected parents, the patients exhibited the characteristic facial phenotypes of 22q11.2DS, similar to their affected parents. However, distinct variations were observed between generations, with one patient presenting with a cleft palate and another experiencing seizure, neither of which were observed in the parents.
The gold standard technique for detecting this microdeletion is the SNP microarray with a probe targeting the 22q11.2 chromosome region [5,14]. This method is widely used and can identify the proximal and common deletion in more than 90% of patients with 22q11.2DS [5,16]. Despite its high sensitivity, a negative FISH result does not completely rule out the possibility of 22q11.2DS, as some patients may have minor or atypical deletions not detected by this technique. In such cases, chromosomal microarray analysis can be a valuable tool for diagnosing clinically suspected patients who have tested negative for FISH [1,5]. The 22q11.2DS phenotype is believed to be primarily influenced by several genes. TBX1, HIRA, UFD1L, and CRKL are some genes associated with developing cardiac and palatal anomalies [16]. We were unable to pinpoint the exact genes involved because we used the FISH technique, which resulted in the missing identification of patients with atypical deletions.
The scattergram of age and (A) white blood cells (WBCs), (B) lymphocytes, (C) CD3 cells, (D) CD8 cells, and (E) CD4 cells. Circle symbols represent biomarker concentrations within the normal range, square symbols represent concentrations above the upper limit of normal, and triangle symbols represent concentrations below the lower limit of normal.
The scattergram of age and (A) IgG, (B) IgA, and (C) IgM. Circle symbols represent biomarker concentrations within the normal range, square symbols represent concentrations above the upper limit of normal, and triangle symbols represent concentrations below the lower limit of normal.
Cognitive function (n = 6)
| Intellectual status | N (%) |
|---|---|
| Median age of evaluation (yr [IQR]) | 5.6 (3.4-8.1) |
| Median full-scale IQ/DQ level (IQR) | 67 (62-74) |
| -1 to -2 SD | 3 (50.0) |
| Less than -2 SD | 1 (16.7) |
According to a previous study, the most common cardiac malformations associated with 22q11.2DS include truncus arteriosus, TOF, and interrupted aortic arch, all of which fall under the category of conotruncal heart defects [17]. In this study, the proportion of TOF among conotruncal heart defects was found to be 72%, which is notably higher than the percentages reported in Western countries (ranging from 13% to 43%) [17, 18]. This finding implies a higher incidence of TOF in Asian patients with 22q11.2DS than in Caucasian patients [15].
22q11.2DS has a wide range of clinical manifestations, and its heterogeneity of causes often leads to confusion [3, 18]. Hypocalcemia due to hypoparathyroidism is a common presentation that can develop at any age but is more common after puberty [9]. In this study, 40.7% of the patients had a history of hypocalcemia, which is consistent with a large study of adults [19] but higher than most previous studies [3, 9]. Four percent of the patients showed abnormal serum thyroid hormone levels. Previous studies have found hypothyroidism and hyperthyroidism in patients with 22q11.2DS [3, 9], although the underlying mechanisms are unclear, and it is unknown whether they are caused by autoimmune or developmental factors [1].
The prevalence of T-cell and T-cell subset deficiencies in our cohort was high, with a notably higher incidence of CD4+ deficiency before the age of 2 yr. These findings are consistent with existing literature demonstrating various T-cell deficiencies in patients with 22q11.2DS due to thymic hypoplasia [20, 21], which is primarily prevalent in the pediatric age group [22, 23]. A study of 34 individuals with 22q11.2DS reported that CD4+ T-cell levels normalized in most individuals up to the age of 3 yr [24]. In contrast to previous studies reporting higher rates of IgM deficiency in patients with 22q11.2DS [25-27], our study found that the most prevalent humoral deficiency was IgA deficiency.
Immunodeficiency develops due to malformation of the third and fourth pharyngeal pouches in the early stages of embryonic development, which can cause underdevelopment or absence of the thymus and parathyroid glands. The thymus produces T-cell lymphocytes and decreased thymic volume is associated with lymphopenia. Immune deficiencies vary in severity but typically involve reduced levels of CD3+, CD4+, and CD8+ T lymphocytes, resulting in mildly impaired cellular immunity [28-30]. The correlation between immune status and phenotypic characteristics yielded conflicting results [1,3,9]. According to Suksawat et al. [31], hypocalcemia patients exhibited higher odds ratios for CD4 lymphopenia. Our study found no correlation between immune status and heart disease, palate anomalies, hormonal function tests, or hypocalcemia. However, we observed that all patients noted to have thymic hypoplasia had low absolute counts or percentages of T cells. In the present study, 66.7% of patients exhibited low absolute lymphocyte counts, while 37.0% had decreased absolute counts or percentages of T cells. A previous study reported decreased CD4+ T cells in 61% of patients, with 52% and 45% exhibiting reductions in CD3+ and CD8+ T cells, respectively [30]. Given the high frequency of T cell lymphopenia observed in 22q11.2DS in both our study and previous reports, we recommend immunologic evaluation including enumeration of lymphocyte subsets by flow cytometry for all patients with this syndrome [1,3].
Some 22q11.2DS patients may experience transiently low IgG levels or selective IgM or IgA deficiency [28,29]. Our study found that 59.3% of patients had decreased immunoglobulin levels, consistent with previous reports [29-30]. However, a prior investigation in Bangkok by Suksawat et al. [31] observed abnormal immunoglobulin levels in just 14% of individuals with 22q11.2DS, which normalized by 1.4 years of age. Additionally, B cell abnormalities have been frequently documented in other studies of 22q11.2DS [32]. As patients with 22q11.2DS get older, their immune system tends to improve, and most of them eventually have normal levels of functional T lymphocytes [9,31,33]. Nevertheless, the clinical course may be unpredictable, and T-cell counts may not reliably predict the likelihood of infection [9, 34].
Less than 1% of 22q11.2DS patients have a severe T-cell deficiency, which can lead to severe combined immunodeficiency disease due to congenital athymia [28]. This condition requires thymic transplant/implant for treatment [28]. Early diagnosis is crucial in ensuring the implementation of appropriate management and improving patient outcomes. According to previous studies, a frequent manifestation of the condition is recurring infections, primarily affecting the sinopulmonary system, such as recurring pneumonia and otitis media [3]. Our study found no cases of severe infections in individuals with 22q11.2DS. Instead, most only experienced relatively minor infections.
Although live vaccines are typically well tolerated in patients with 22q11.2DS, they should be avoided in those with severe T cell deficiencies [1,3]. The current study population exhibited no adverse reactions to live vaccines, likely due to the absence of patients with severely low T cell counts (minimum CD3, CD4, and CD8 levels observed were 607, 395, and 219/cu.mm, respectively). However, the sample size was small and may have precluded inclusion of the approximately 0.5% of 22q11.2DS patients with profound T cell lymphopenia, as estimated previously [28].
Previous studies have demonstrated that individuals with 22q11.2DS can exhibit various intellectual abilities, ranging from borderline intelligence to mild learning disabilities [33,35]. Reports suggest that individuals with 22q11.2DS frequently experience cognitive impairment, learning difficulties, intellectual disabilities, speech, and language deficits, as well as mood, behavioral, and psychiatric disorders [1,3,9]. The findings of this study confirmed the previously observed decrease in FSIQ [35-37]. Furthermore, there was a notable discrepancy between VIQ and FSIQ scores, with the 7- to 17-year-old group having a higher FSIQ than VIQ and the >17-year-old group having a higher VIQ score than FSIQ scores. Our findings revealed no significant correlation between cardiovascular disease and intellectual status in the studied patient population. Interestingly, patients above the age of 17 years old had a lower median FSIQ than those in the younger age group, possibly indicating underdiagnosis or previous inadequate interventions. However, these results should be interpreted with caution due to the small sample size. Each of our patients had a documented history of delayed global development, with 80% presenting with intellectual disabilities (FSIQ, <70).
Congenital heart disease has been shown to impact cognitive function and neurological development. However, our analysis revealed no statistically significant difference in intellectual status between patients with major and minor cardiovascular diseases. Although one adult patient in our study had a history of psychiatric disorders, the sample size was too small to draw statistically significant conclusions regarding psychiatric comorbidities in 22q11.2DS. Nonetheless, as more data on adult populations become available, the incidence of such comorbidities is increasingly being recognized [9]. One in every four adults with 22q11.2DS develops schizophrenia [38,39].
Recent clinical practice guidelines on the immunological management of 22q11.2DS recommend an initial evaluation of T, B, and natural killer (NK) cell counts along with naïve and memory T cell subsets [40]. Follow-up assessments at periodic intervals are advised to monitor changes in immune function over time. Patients with 22q11.2DS face an increased risk of humoral immune deficiencies later in childhood and adulthood, highlighting the importance of ongoing immune surveillance [40]. Administration of live attenuated vaccines including measles-mumps-rubella (MMR) and varicella is endorsed when T cell numbers satisfy minimum criteria, as these vaccines confer protection against wild-type infections [40]. A minority of patients with 22q11.2DS have congenital athymia and may benefit from thymic transplantation, a procedure shown to be effective for immune reconstitution and survival in this population [40].
Because this was a cross-sectional study, there were significant limitations due to the lack of longitudinal data on the patient's immunologic, hormonal, and intellectual evaluation. As the 22q11.2DS progresses throughout the lifespan, new syndrome-related conditions may emerge, immunologic and hormonal conditions may improve with age, and psychological problems may appear later in adult life [3,9]. There was a selection bias because only patients with a suspected clinical phenotype underwent FISH evaluation, and most of the sample was obtained from a pediatric cardiology clinic. As a result, determining the actual prevalence of 22q11.2DS and discerning clinical differences between patients with detected and undetected 22q11.2 deletions cannot be statistically validated. Further prospective longitudinal studies with more diverse groups are necessary to obtain more precise data on patients with 22q11.2DS in our region.
Conclusion
Our study in Taiwan has demonstrated a diverse range of clinical manifestations of 22q11.2DS. Therefore, in any individual presenting with suspected associated symptoms, such as cardiac defects, abnormal facial features, thymic hypoplasia, cleft palate, or hypocalcemia, genetic testing for 22q11.2DS should be considered to confirm the diagnosis and allow for appropriate genetic counseling. Early diagnosis and intervention are crucial in minimizing morbidity and improving the quality of life for affected individuals. Given the multisystem nature of 22q11.2DS, we recommend that patients be evaluated regularly by a multidisciplinary care team for comprehensive management.
Acknowledgements
Our gratitude goes out to all individuals and families affected by 22q11.2DS and the dedicated clinical and research laboratory staff who have contributed to this study.
Funding Information
The preparation and editing of this manuscript were supported by research grants from MacKay Memorial Hospital (MMH-MM-112-14, MMH-E-112-13, and MMH-E-111-13) and the Ministry of Science and Technology, Executive Yuan, Taiwan (NSTC-112-2314-B-195-014-MY3, NSTC-112-2811-B-195-001, NSTC-112-2314-B-195-003, NSTC-111-2314-B-195-017, NSTC-111-2811-B-195-002, NSTC-111-2811-B-195-001, NSTC-110-2314-B-195-014, NSTC-110-2314-B-195-010-MY3, and NSTC-110-2314-B-195-029).
Data Availability Statement
All data are present within the article.
Institutional Review Board Statement
The present study adhered to the guidelines set forth by the Declaration of Helsinki and was approved by the Institutional Review Board of MacKay Memorial Hospital (21MMHIS109e, 2021/10/01) and was authorized for publication.
Informed Consent Statement
Informed consent was obtained from all study participants, and patients provided written informed consent for the publication of this study.
Author Contributions
The manuscript was drafted by C.-L.L. and S.-M.L., with S.-P.L., M.-R.C., and H.-Y.L. contributing to patient follow-up and aiding in manuscript preparation. Biochemical analyses were conducted by C.-K.C. and R.-Y.T., who also revised the manuscript. Patient screening and manuscript revision were the responsibility of Y.-T.L., Y.-H.C., and H.-C.C. All authors thoroughly reviewed and approved the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sullivan KE. Chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Immunol Rev. 2019;287(1):186-201
2. Driscoll DA. Molecular and genetic aspects of DiGeorge/velocardiofacial syndrome. Methods Mol Med. 2006;126:43-55
3. Bassett AS, McDonald-McGinn DM, Devriendt K. et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159(2):332-9 e1
4. Matsuoka R, Takao A, Kimura M. et al. Confirmation that the conotruncal anomaly face syndrome is associated with a deletion within 22q11.2. Am J Med Genet. 1994;53(3):285-9
5. Sgardioli IC, Paoli Monteiro F, Fanti P. et al. Testing criteria for 22q11.2 deletion syndrome: preliminary results of a low-cost strategy for public health. Orphanet J Rare Dis. 2019;14(1):123
6. Panamonta V, Wichajarn K, Chaikitpinyo A. et al. Birth Prevalence of Chromosome 22q11.2 Deletion Syndrome: A Systematic Review of Population-Based Studies. J Med Assoc Thai. 2016;99(Suppl 5):S187-93
7. Maggadottir SM, Sullivan KE. The diverse clinical features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome). J Allergy Clin Immunol Pract. 2013;1(6):589-94
8. Emanuel BS. Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Dev Disabil Res Rev. 2008;14(1):11-8
9. Fung WL, Butcher NJ, Costain G. et al. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet Med. 2015;17(8):599-609
10. Purugganan O. Intellectual Disabilities. Pediatr Rev. 2018;39(6):299-309
11. Garcia-Prat M, Alvarez-Sierra D, Aguilo-Cucurull A. et al. Extended immunophenotyping reference values in a healthy pediatric population. Cytometry B Clin Cytom. 2019;96(3):223-33
12. Jyonouchi S. Normal ranges for lymphocyte subset counts. In: Sullivan K, Stiehm E, editors. Stiehm's immunodeficiencies. San Diego: Elsevier Saunder. 2014 p. 1111-3
13. Adeli K, Higgins V, Nieuwesteeg M. et al. Complex reference values for endocrine and special chemistry biomarkers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian Health Measures Survey. Clin Chem. 2015;61(8):1063-74
14. McDonald-McGinn DM, Sullivan KE, Marino B. et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1:15071
15. Wichajam K, Kampan J. Difference of clinical phenotypes and immunological features of 22q11.2 deletion syndrome in north-eastern Thai children compare to western countries. J Med Assoc Thai. 2014;97(Suppl 10):S59-66
16. Rozas MF, Benavides F, Leon L. et al. Association between phenotype and deletion size in 22q11.2 microdeletion syndrome: systematic review and meta-analysis. Orphanet J Rare Dis. 2019;14(1):195
17. Peyvandi S, Lupo PJ, Garbarini J. et al. 22q11.2 deletions in patients with conotruncal defects: data from 1,610 consecutive cases. Pediatr Cardiol. 2013;34(7):1687-94
18. Botto LD, May K, Fernhoff PM. et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1):101-7
19. Cheung EN, George SR, Costain GA. et al. Prevalence of hypocalcaemia and its associated features in 22q11.2 deletion syndrome. Clin Endocrinol (Oxf). 2014;81(2):190-6
20. Sullivan KE. Chromosome 22q11.2 deletion syndrome: DiGeorge syndrome/velocardiofacial syndrome. Immunol Allergy Clin North Am. 2008;28:353-66
21. McLean-Tooke A, Barge D, Spickett GP. et al. Immunologic defects in 22q11.2 deletion syndrome. J Allergy Clin Immunol. 2008;122:362-7 e1-4
22. Zemble R, Prak EL, McDonald K. et al. Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clin Immunol. 2010;136:409-18
23. Piliero LM, Sanford AN, McDonald-McGinn DM. et al. T-cell homeostasis in humans with thymic hypoplasia due to chromosome 22q11.2 deletion syndrome. Blood. 2004;103:1020-5
24. Sediva A, Bartunkova J, Zachova R. et al. Early development of immunity in DiGeorge syndrome. Med Sci Monit. 2005;11:CR182-C187
25. Giardino G, Radwan N, Koletsi P. et al. Clinical and immunological features in a cohort of patients with partial DiGeorge syndrome followed at a single center. Blood. 2019;133:2586-96
26. Mahe P, Nagot N, Portales P. et al. Risk factors of clinical dysimmune manifestations in a cohort of 86 children with 22q11.2 deletion syndrome: a retrospective study in France. Am J Med Genet A. 2019;179:2207-13
27. Nissan E, Katz U, Levy-Shraga Y. et al. Clinical Features in a Large Cohort of Patients With 22q11.2 Deletion Syndrome. J Pediatr. 2021;238:215-220.e5
28. Gennery AR. Immunological features of 22q11 deletion syndrome. Curr Opin Pediatr. 2013;25(6):730-5
29. Jawad AF, McDonald-Mcginn DM, Zackai E. et al. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). J Pediatr. 2001;139(5):715-23
30. Kuo CY, Signer R, Saitta SC. Immune and Genetic Features of the Chromosome 22q11.2 Deletion (DiGeorge Syndrome). Curr Allergy Asthma Rep. 2018;18(12):75
31. Suksawat Y, Sathienkijkanchai A, Veskitkul J. et al. Resolution of Primary Immune Defect in 22q11.2 Deletion Syndrome. J Clin Immunol. 2017;37(4):375-82
32. Gennery AR, Barge D, O'Sullivan JJ. et al. Antibody deficiency and autoimmunity in 22q11.2 deletion syndrome. Arch Dis Child. 2002;86(6):422-5
33. Cancrini C, Puliafito P, Digilio MC. et al. Clinical features and follow-up in patients with 22q11.2 deletion syndrome. J Pediatr. 2014;164(6):1475-80 e2
34. Sullivan KE, Jawad AF, Randall P. et al. Lack of correlation between impaired T cell production, immunodeficiency, and other phenotypic features in chromosome 22q11.2 deletion syndromes. Clin Immunol Immunopathol. 1998;86(2):141-6
35. Zhao Y, Guo T, Fiksinski A. et al. Variance of IQ is partially dependent on deletion type among 1,427 22q11.2 deletion syndrome subjects. Am J Med Genet A. 2018;176(10):2172-81
36. Schneider M, Van der Linden M, Menghetti S. et al. Predominant negative symptoms in 22q11.2 deletion syndrome and their associations with cognitive functioning and functional outcome. J Psychiatr Res. 2014;48(1):86-93
37. Ua-Areechit T, Varnado P, Tengsujaritkul M. et al. Clinical characteristics and immunological status of patients with 22q11.2 deletion syndrome in Northern Thailand. Asian Pac J Allergy Immunol. 2023;41(1):89-95
38. Schneider M, Debbane M, Bassett AS. et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171(6):627-39
39. Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940-5
40. Mustillo PJ, Sullivan KE, Chinn IK. et al. Clinical Practice Guidelines for the Immunological Management of Chromosome 22q11.2 Deletion Syndrome and Other Defects in Thymic Development. J Clin Immunol. 2023;43(2):247-270
Author contact
![]() Corresponding authors: Hsiang-Yu Lin and Shuan-Pei Lin, Department of Pediatrics, MacKay Memorial Hospital, No. 92, Sec. 2, Chung-Shan North Road, Taipei 10449, Taiwan. Tel: +886-2-2543-3535 (ext. 3089); Fax: +886-2-2543-3642; Email: lxc46199hinet.net; 4535lincom.
Corresponding authors: Hsiang-Yu Lin and Shuan-Pei Lin, Department of Pediatrics, MacKay Memorial Hospital, No. 92, Sec. 2, Chung-Shan North Road, Taipei 10449, Taiwan. Tel: +886-2-2543-3535 (ext. 3089); Fax: +886-2-2543-3642; Email: lxc46199hinet.net; 4535lincom.

 Global reach, higher impact
Global reach, higher impact