ISSN: 1449-1907
Int J Med Sci 2023; 20(10):1358-1362. doi:10.7150/ijms.88022 This issue Cite
Research Paper
Adverse Events of Everolimus in Patients with Tuberous Sclerosis Complex Treated for Renal Angiomyolipoma/Subependymal Giant Cell Astrocytoma
1. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Institute of Medical Education, Chi Mei Medical Center, Tainan, Taiwan.
3. Department of Urology, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. Department of Neurosurgery, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. Department of Medical Radiology, Chung Shan Medical University Hospital, Taichung, Taiwan.
6. Department of Pediatrics, Chung Shan Medical University Hospital, Taichung, Taiwan.
Abstract
Background: Although regarded as a potentially efficient approach to address tuberous sclerosis complex (TSC)-associated complications, the adverse event profile of everolimus has not yet been fully elucidated. The present study aimed to clarify the adverse event spectrum in patients with TSC who are using everolimus for common indications, in comparison to those who do not use everolimus.
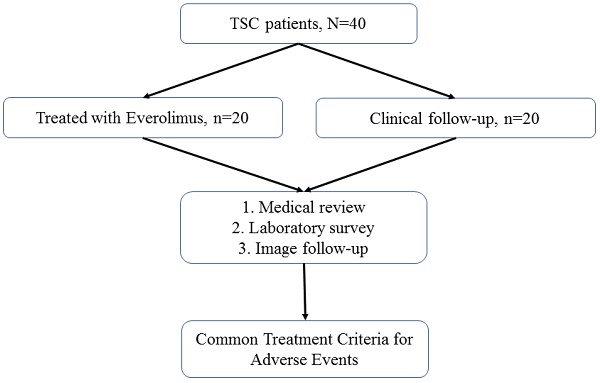
Materials and Methods: We recruited patients with TSC who were followed up annually at TSC integrated clinics or referred for medical assistance. Medical reviews and laboratory investigations were performed at baseline and annually by clinical physicians. The adverse events were assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events.
Results: Common adverse events in everolimus users included hypercholesterolemia (55%), gingivostomatitis (50%), proteinuria (50%), and hyperglycemia (40%). Compared with everolimus nonusers, the occurrence of gingivostomatitis and proteinuria was significantly higher in everolimus users (gingivostomatitis, p=0.02; proteinuria, p=0.02). Among the everolimus users, 12 patients had level I CTCAE, and five had level II CTCAE. None of the everolimus users presented with CTCAE level III or higher.
Conclusion: Patients with TSC who are everolimus users had a higher tendency to develop gingivostomatitis and proteinuria compared to nonusers. However, no differences were observed in the occurrence of other adverse events between everolimus users and nonusers.

 Global reach, higher impact
Global reach, higher impact