Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(10):1358-1362. doi:10.7150/ijms.88022 This issue Cite
Research Paper
Adverse Events of Everolimus in Patients with Tuberous Sclerosis Complex Treated for Renal Angiomyolipoma/Subependymal Giant Cell Astrocytoma
1. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Institute of Medical Education, Chi Mei Medical Center, Tainan, Taiwan.
3. Department of Urology, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. Department of Neurosurgery, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. Department of Medical Radiology, Chung Shan Medical University Hospital, Taichung, Taiwan.
6. Department of Pediatrics, Chung Shan Medical University Hospital, Taichung, Taiwan.
Received 2023-7-12; Accepted 2023-8-25; Published 2023-9-4
Abstract
Background: Although regarded as a potentially efficient approach to address tuberous sclerosis complex (TSC)-associated complications, the adverse event profile of everolimus has not yet been fully elucidated. The present study aimed to clarify the adverse event spectrum in patients with TSC who are using everolimus for common indications, in comparison to those who do not use everolimus.
Materials and Methods: We recruited patients with TSC who were followed up annually at TSC integrated clinics or referred for medical assistance. Medical reviews and laboratory investigations were performed at baseline and annually by clinical physicians. The adverse events were assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events.
Results: Common adverse events in everolimus users included hypercholesterolemia (55%), gingivostomatitis (50%), proteinuria (50%), and hyperglycemia (40%). Compared with everolimus nonusers, the occurrence of gingivostomatitis and proteinuria was significantly higher in everolimus users (gingivostomatitis, p=0.02; proteinuria, p=0.02). Among the everolimus users, 12 patients had level I CTCAE, and five had level II CTCAE. None of the everolimus users presented with CTCAE level III or higher.
Conclusion: Patients with TSC who are everolimus users had a higher tendency to develop gingivostomatitis and proteinuria compared to nonusers. However, no differences were observed in the occurrence of other adverse events between everolimus users and nonusers.
Introduction
As an autosomal-dominant disease, tuberous sclerosis complex (TSC) results in symptomatic involvement of various organ systems, including dermatological, renal, and central nervous systems [1, 2]. In a recent population-based study from France, the incidence of TSC was reported to be 0.44 per 100,000 person-years [3]. The presence of TSC can significantly negatively influence patients' quality of life due to the impairment of the psychological and physical domains [4, 5].
In recent years, as the pathophysiology of gene abnormalities of TSC1 and TSC2 and their subsequent influence on the downstream mTOR pathway have been gradually clarified, the therapeutic role of mTOR inhibitors in TSC treatment has also been evaluated [6]. It has been reported that everolimus, an mTOR inhibitor, presents significant clinical efficacy in improving subependymal giant cell astrocytoma (SEGA), angiomyolipoma (AML), lymphangioleiomyomatosis (LAM), and facial angiofibroma caused by TSC [7-9].
Considered as a potentially efficient approach to address TSC-associated complications, the adverse event profile of everolimus has been examined under different clinical contexts in large scale studies [10-12]. Results of the EXIST-1 and EXIST-2 studies suggested that elevation of serum creatinine and proteinuria were observed as potential adverse events in patients taking everolimus [10]. However, to the best of our knowledge, the adverse event profile of everolimus users with TSC in Taiwan is lacking. To bridge the knowledge gap, we conducted a clinical study to clarify the adverse event spectrum in patients with TSC who are using everolimus for common indications, in comparison to those who do not use everolimus.
Methods and Materials
Patient selection and study design
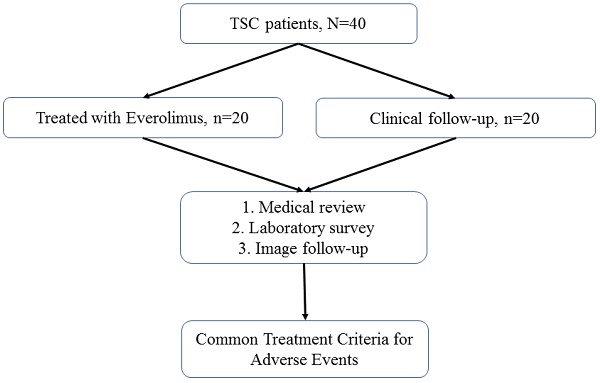
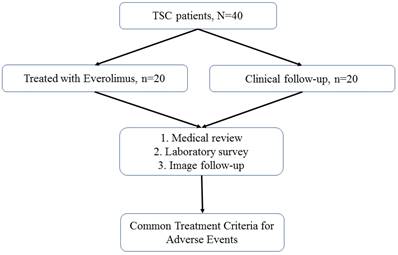
Patients with TSC were recruited for this study. All patients were followed up annually at TSC-integrated clinics or referred for medical assistance at the three branches of Chung Shan Medical University Hospital, a tertiary center in central Taiwan. The detailed patient selection process is illustrated in Figure 1. The diagnostic criteria for TSC were based on the updated International Tuberous Sclerosis Complex Diagnostic Criteria [15]. The indications for everolimus included TSC-associated SEGA, AML, and LAM. For each everolimus user, the initial dose of everolimus was 2.5 mg per day for all enrolled patients; this was titrated up to 5.0 mg per day.
Patient selection flowchart
Assessment of adverse effects
Adverse events were assessed as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version .0), which is widely utilized in clinical studies [16]. Medical reviews and laboratory investigations were performed at baseline and annually by clinical physicians. To monitor changes in the laboratory data and identify potential adverse events, regular laboratory investigations were conducted. These investigations included routine blood tests, biochemistry profiles, HBV/HCV titers, and routine urinary tests.
Statistical methods
Categorical variables are presented as frequencies (n) and percentages (%). The chi-squared test was used to compare categorical variables. Statistical significance was defined as p < 0.05. All statistical analyses were conducted using SPSS for Windows (version 26.0; SPSS Inc., Chicago, IL, USA).
Ethnic Statement
The study was conducted in accordance with The Declaration of Helsinki and approved by the Institutional Review Board of Chung Shan Medical University (CSMUH No.CS1-22076).
Results
Baseline characteristics
Among all the enrolled patients with TSC, 20 were treated with everolimus and 20 were not (Figure 1). No statistically significant differences in age, sex, or genotype were observed between everolimus users and nonusers (Table 1).
Demographic data of TSC patients, n=40
| Variable | All | TSC with everolimus | P value | ||
|---|---|---|---|---|---|
| Yes (group A) | No (group B) | ||||
| Numbers | 40 | 20 | 20 | ||
| Age (year, range) | 5-54 | 31.7 (9.7) | 21.4 (31.7) | > 0.05 | |
| Gender | |||||
| male | 13 | 4 | 9 | > 0.05 | |
| female | 29 | 16 | 11 | ||
| Gene | |||||
| TSC 1 | 7 | 4 | 3 | > 0.05 | |
| TSC 2 | 21 | 9 | 12 | ||
| NMI/ND | 12 | 7 | 5 | ||
NMI: no mutation identified; ND: not done. NS: not significant.
Medical utilization profiles
Among the enrolled everolimus users, the indication for everolimus use was mostly for the treatment of AML (n=12; 60% of everolimus users), whereas approximately 30% of everolimus users were prescribed medication for the treatment of SEGA (n=6). The mean age at administration was 26.6 years, and the mean duration was 5.5 years. The mean daily dose was 4.6 mg, while the mean trough level was 7.6 ng/ml (Table 2).
Indications and administration profile of TSC patients treated with everolimus, n=20
| Indication | Patient amount | Percentage (%) | ||
|---|---|---|---|---|
| AML | 12 | 60 | ||
| SEGA | 6 | 30 | ||
| LAM | 2 | 10 | ||
| Administration | Mean | SD | ||
| Age of administration (years old) | 26.6 | 9.5 | ||
| Duration of administration (year) | 5.5 | 3.2 | ||
| Daily dose (mg) | 4.6 | 0.9 | ||
| Mean trough levels (ng/ml) | 7.6 | 5.7 | ||
AML, angiomyolipoma; SEGA, subependymal giant cell astrocytoma; LAM, lymphangioleiomyomatosis; SD, standard difference
Adverse events of TSC patients with or without administration of everolimus
| Variable | Administration of everolimus (%) | P value | ||
|---|---|---|---|---|
| Yes (n=20) | No (n=20) | |||
| Immune system disorder | ||||
| Gingivostomatitis | 10 (50) | 3 (15) | 0.02 | |
| Skin disorder | ||||
| Acnes | 7 (35) | 9 (45) | 0.37 | |
| Infection disorder | ||||
| URI | 3 (15) | 1 (5) | 0.30 | |
| Pneumonia | 4 (20) | 1 (5) | 0.17 | |
| HBV | 4 (20) | 3 (15) | 0.50 | |
| HCV | 0 (0) | 0 (0) | - | |
| Gastrointestinal disorders | ||||
| Diarrhea | 4 (20) | 1 (5) | 0.17 | |
| Endocrine disorder | ||||
| Amenorrhea | 4 (20) | 0 (0) | 0.10 | |
| Metabolic disorder | ||||
| Hypertriglyceridemia | 5 (25) | 4 (20) | 0.50 | |
| Hypercholesterolemia | 11 (55) | 6 (30) | 0.10 | |
| DM | 3 (15) | 1 (5) | 0.30 | |
| Hyperglycemia | 8 (40) | 5 (25) | 0.25 | |
| Renal and urinary disorder | ||||
| UTI | 1 (5) | 0 (0) | ||
| Pyuria | 4 (20) | 3 (15) | 0.50 | |
| Hematuria | 6 (30) | 2 (10) | 0.77 | |
| Proteinuria | 10 (50) | 3 (15) | 0.02 | |
| CTCAE | ||||
| 0 | 3 (15) | 6 (30) | ||
| I | 12 (60) | 12 (60) | ||
| II | 5 (25) | 2 (10) | ||
| III | 0 (0) | 0 (0) | ||
| IV | 0 (0) | 0 (0) | ||
URI: upper airway respiratory infection; UTI: urinary tract infection; HPF, high-powered field; CTCAE: Common Terminology Criteria for Adverse Events
Adverse events
Adverse events in everolimus users and nonusers are presented in Table 3. Common adverse events, including hypercholesterolemia (55%), gingivostomatitis (50%), proteinuria (50%), and hyperglycemia (40%), were observed in everolimus users. Compared with everolimus nonusers, the occurrence of gingivostomatitis and proteinuria was significantly higher in everolimus users (gingivostomatitis, p=0.02; proteinuria, p=0.02). Among the everolimus users, 12 patients had level I CTCAE, and five had level II CTCAE. None of the everolimus users presented with CTCAE level III or above. Among everolimus nonusers, 12 patients were categorized as level I, while two patients were categorized as level II. Similar to the everolimus users, none of the patients in this group presented with CTCAE levels III or IV.
Discussion
In this study, we reported the adverse event profiles of everolimus users with different indications, including TSC-associated AML, SEGA, and LAM. Most adverse events in the different organ systems did not show statistically significant differences between everolimus users and nonusers. However, incident gingivostomatitis and proteinuria were more frequently observed in the everolimus users than in the nonusers. Considering that studies investigating the adverse event profiles of everolimus are scarce and are generally limited to a small number of participants or lack control groups [13, 14], the results reported in this study could potentially be useful for clinicians when considering management strategies in patients with TSC.
Sirolimus and everolimus are the two most well-known mTOR inhibitors for TSC management; they target mTORC1 and separate its connections with the cofactor FKB12 [17]. The mTORC1 inhibitor-associated adverse events such as infection, proteinuria, and stomatitis were widely reported in randomized controlled trials and single-arm studies [6, 10, 18]. The most common adverse events of mTOR inhibitors, such as stomatitis, were reported to be mild and did not seem to influence the continuation of medication prescriptions [6]. However, severe infectious adverse events have been reported in previous randomized controlled trials (EXIST-3 study) among mTOR inhibitor users, resulting in severe pneumonia, septic shock, and subsequent participant deaths [19]. In the current study, infectious adverse events, including upper airway respiratory infection, pneumonia, and hepatitis virus B infection, were numerically high in the everolimus group. However, compared with nonusers, the difference in the percentage of infectious events was insignificant. Moreover, no adverse events above CTCAE level III were noted, with most participants presenting with a CTCAE level I (60%). These findings indicated that in Taiwanese patients with TSC who are everolimus users, the severity and categories of adverse events are similar to those reported in previous international studies.
The adverse events associated with everolimus have been widely evaluated in clinical studies. In a recent Dutch study based on electronic medical records, it was reported that in TSC patients, total cholesterol, LDL- and HDL-cholesterol levels at baseline were similar to patients without TSC. However, the use of everolimus could lead to elevation of these lab data [20]. In our current study, we report that the occurrence of hypercholesterolemia did not present statistically significant difference between everolimus users and non-users. Another international real-world evidence revealed that 14 of 174 patients with TSC who were everolimus users (7.8%) presented with stomatitis during the follow-up period, whereas 6.1% of the participants presented with hypercholesterolemia [21]. In a Japanese clinical study, Hatano et al. reported that more than 25% of patients with TSC prescribed low-dose everolimus (5 mg QD) presented with adverse events including stomatitis, irregular menstruation, and nasopharynxgitis. When comparing the difference in adverse event profiles between low-dose and conventional dosage users (10 mg QD), only the presence of stomatitis was statistically significant; low-dose everolimus users presented with stomatitis less frequently after using everolimus [13]. The results of the current study correspond with previous evidence reporting a high prevalence of gingivostomatitis and hypercholesterolemia in patients with TSC who are everolimus users. However, among all evaluated adverse events, only gingivostomatitis and proteinuria in the everolimus group showed significant differences compared with the control group. Further large-scale case-control studies are necessary to compare the prevalence of adverse events in other organ systems, including the endocrine and gastrointestinal systems, between everolimus users and nonusers.
The strength of this study lies in its ability to provide information on the adverse event profiles of everolimus users and compare them with those of everolimus nonusers for common TSC-associated indications. By comparing the adverse event percentage of nonusers, we were able to provide a clearer view of the adverse event profiles in everolimus users, compared with those obtained from single-arm studies. However, there are some limitations to this study that should be considered when interpreting the results. First, considering that this study was conducted at a medical center in central Taiwan, the results may not be generalizable. Second, the number or participants in this study could be too small to show differences in critical parameters such as serum lipids (total cholesterol, LDL and HDL, etc.) between everolimus users and non-users. In this case, we might not be able to perform further analyses regarding these parameters and evaluate their detailed interplay with the use of everolimus. Future large-scale studies are warranted to compare the incidence of adverse events among everolimus users from different populations and ethnicities.
In conclusion, patients with TSC who are everolimus users had a higher tendency to develop gingivostomatitis and proteinuria than nonusers. However, no differences in the occurrence of other adverse events were noted between everolimus users and nonusers. Based on the results of the current study, the safety profile of everolimus should be considered by clinicians when caring for patients with TSC.
Guarantor of the article
Dr. Jeng-Dau Tsai is the corresponding author who is responsible for the accuracy and integrity of data analysis.
Specific author contributions
All the authors involved in drafting or revising the article and approved of the submitted version.
Study conception and design: Gau SY, Tsai JD, Chen SL and Tsao TF.
Data acquisition: Gau SY, Tsai JD.
Data analysis and demonstration: Gau SY, Tsai JD.
Original draft preparation: Gau SY, Tsai JD, Chen SL and Tsao TF.
Statement of ethics
The hospital's Institutional Review Board of Chung Shan Medical University Hospital approved this study (CSMUH No. CS1-22076) and the study was performed according to the Declaration of Helsinki. All study participants were provided of informed consent.
Data sharing statement
The original data analyzed in the present study can be provided by the authors upon reasonable request.
Competing Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, or publication of this article.
References
1. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372(9639):657-668
2. Gau SY, Sheu JN, Wang XA, Tsai JD. Unilateral renal agenesis and contralateral hydroureteronephrosis in a boy with tuberous sclerosis complex. Pediatr Neonatol. 2023
3. Fagnani F, Laurendeau C, de Zelicourt M, Marshall J. Epidemiology and disease burden of tuberous sclerosis complex in France: A population-based study based on national health insurance data. Epilepsia Open. 2022;7(4):633-644
4. Amin S, Mallick AA, Lux A, O'Callaghan F. Quality of life in patients with Tuberous Sclerosis Complex (TSC). Eur J Paediatr Neurol. 2019;23(6):801-807
5. Fong CY, Ng K, Kong AN, Ong LC, Rithauddin MA, Thong MK, Ganesan V, Heng HS, Teh CM, Yahya N. Quality of life of children with tuberous sclerosis complex. Arch Dis Child. 2019;104(10):972-978
6. Luo C, Ye WR, Shi W, Yin P, Chen C, He YB, Chen MF, Zu XB, Cai Y. Perfect match: mTOR inhibitors and tuberous sclerosis complex. Orphanet J Rare Dis. 2022;17(1):106
7. Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817-824
8. Curran MP. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs. 2012;14(1):51-60
9. Wei CC, Hsiao YP, Gau SY, Wu YT, Wu CT, Wu MH, Tsai JD. The Efficacy of Everolimus for Facial Angiofibromas in Tuberous Sclerosis Complex Patients Treated for Renal Angiomyolipoma/Subependymal Giant Cell Astrocytoma. Dermatology. 2021;237(3):444-449
10. Bissler JJ, Budde K, Sauter M, Franz DN, Zonnenberg BA, Frost MD, Belousova E, Berkowitz N, Ridolfi A, Christopher Kingswood J. Effect of everolimus on renal function in patients with tuberous sclerosis complex: evidence from EXIST-1 and EXIST-2. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2019;34(6):1000-1008
11. Davies M, Saxena A, Kingswood JC. Management of everolimus-associated adverse events in patients with tuberous sclerosis complex: a practical guide. Orphanet J Rare Dis. 2017;12(1):35
12. Franz DN, Agricola K, Mays M, Tudor C, Care MM, Holland-Bouley K, Berkowitz N, Miao S, Peyrard S, Krueger DA. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015;78(6):929-938
13. Hatano T, Endo K, Tamari M. Efficacy and safety of low-dose everolimus treatment for renal angiomyolipoma associated with tuberous sclerosis complex. Int J Clin Oncol. 2021;26(1):163-168
14. Robles NR, Peces R, Gomez-Ferrer A, Villacampa F, Alvarez-Ossorio JL, Perez-Segura P, Morote J, Herrera-Imbroda B, Nieto J, Carballido J. et al. Everolimus safety and efficacy for renal angiomyolipomas associated with tuberous sclerosis complex: a Spanish expanded access trial. Orphanet J Rare Dis. 2016;11(1):128
15. Northrup H, Aronow ME, Bebin EM, Bissler J, Darling TN, de Vries PJ, Frost MD, Fuchs Z, Gosnell ES, Gupta N. et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr Neurol. 2021;123:50-66
16. Amitay-Laish I, Prag-Naveh H, Ollech A, Davidovici B, Leshem YA, Snast I, Popovtzer A, Purim O, Flex D, David M. et al. Prophylactic Topical Treatment for EGFR Inhibitor-Induced Papulopustular Rash: A Randomized Clinical Trial. Dermatology. 2021;237(6):988-994
17. Zhao W, Xie C, Zhang X, Liu J, Liu J, Xia Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023;13(6):e2995
18. Cabrera-López C, Martí T, Catalá V, Torres F, Mateu S, Ballarín J, Torra R. Assessing the effectiveness of rapamycin on angiomyolipoma in tuberous sclerosis: a two years trial. Orphanet J Rare Dis. 2012;7:87
19. French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, Curatolo P, de Vries PJ, Dlugos DJ, Berkowitz N. et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10056):2153-2163
20. Mulder FVM, Peeters E, Westerink J, Zwartkruis FJT, de Ranitz-Greven WL. The long-term effect of mTOR inhibition on lipid and glucose metabolism in tuberous sclerosis complex: data from the Dutch TSC registry. Orphanet J Rare Dis. 2022;17(1):252
21. Ruiz-Falco Rojas ML, Feucht M, Macaya A, Wilken B, Hahn A, Maamari R, Hirschberg Y, Ridolfi A, Kingswood JC. Real-World Evidence Study on the Long-Term Safety of Everolimus in Patients With Tuberous Sclerosis Complex: Final Analysis Results. Front Pharmacol. 2022;13:802334
Author contact
![]() Corresponding author: Jeng-Dau Tsai, MD, PhD. Department of Paediatrics, Chung Shan Medical University Hospital, Taichung, Taiwan. No. 110, Section 1, Jianguo North Road, Taichung 402, Taiwan. E-mail: fernand.tsaihinet.net.
Corresponding author: Jeng-Dau Tsai, MD, PhD. Department of Paediatrics, Chung Shan Medical University Hospital, Taichung, Taiwan. No. 110, Section 1, Jianguo North Road, Taichung 402, Taiwan. E-mail: fernand.tsaihinet.net.

 Global reach, higher impact
Global reach, higher impact