Impact Factor
ISSN: 1449-1907
Int J Med Sci 2023; 20(3):366-375. doi:10.7150/ijms.80189 This issue Cite
Review
Role of Obesity in Female Reproduction
1. Center Laboratory of the Fourth Affiliated Hospital, China Medical University, Shenyang 110032, China.
2. Affiliated Hospital of Changchun University of Chinese Medicine, Changchun 130021, China.
3. Third Affiliated Clinical Hospital to Changchun University of Chinese Medicine, Changchun 130021, China.
4. Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China.
# These authors contributed equally to this work.
Received 2022-10-26; Accepted 2023-1-19; Published 2023-1-31
Abstract
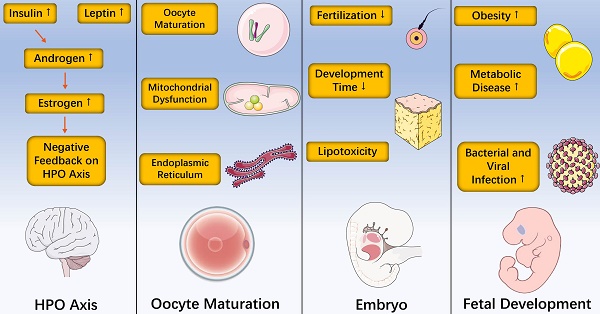
Contemporary scientists need no “p value” and “relative risk” statistics to be exquisitely aware of the increasing prevalence of obesity and complications posed by obesity. It is now well recognized that obesity is strongly associated with type 2 diabetes, hypertension, vascular disease, tumors and reproductive disorders. Obese women show lower levels of gonadotropin hormones, reduced fecundity, higher miscarriage rates and poorer outcomes of in vitro fertilization, revealing that obesity affects female reproduction. In addition, adipose tissue contains special immune cells and obesity-induced inflammation is a chronic, low-grade inflammatory response. Herein, we mainly review detrimental influences of obesity in the complete process of female reproduction, including hypothalamic-pituitary-ovarian axis, oocyte maturation, embryo and fetal development. In the latter part, we view obesity-induced inflammation and discuss related epigenetic impact on female reproduction.
Keywords: obesity, female reproduction, infertility, oocytes, adipokines
Introduction
The obvious but unfortunate trend towards widespread epidemic of obesity has posed a serious threat to public health worldwide. Over the past few decades, the number of overweight and obese individuals has reached alarming levels, with about 40% of adults worldwide now overweight or obese. For example, the prevalence of obesity and overweight was only 7.8% for Chinese adults in 1985, more than half of adults in China are now categorized as overweight or obese [1]. Even worsen, by 2030, the prevalence of overweight and obesity might reach 65.3% in adults if this trend would continue [2]. Body mass index (BMI) is an important indicator to measure obesity and standard weight (Normal: 18.5 ≤ BMI ≤ 24.9 kg/m2; Overweight: 25.0 ≤ BMI ≤ 29.9 kg/m2; Obese: BMI ≥30.0 kg/m2), and the risk of obesity complications will increase with the rise of BMI [3].
Adipose tissue, the main storage and transport of energy, plays an important role in the regulation of energy balance. Obesity occurs when the body stores too much energy in the form of fat. Considerable evidence has demonstrated that obesity is not only directly harmful to health, but also is strongly related to a number of chronic diseases such as type 2 diabetes, cardiovascular diseases, respiratory diseases and reproductive disorders [4-6]. Obesity reduces fertility, with a 2.7-fold increased risk of infertility in women with a BMI of >30 kg/m2 and a 25-37% increased risk of miscarriage in pregnant women compared with normal-weight counterparts [7]. Obese women also respond less well to infertility treatments and have a higher risk of early miscarriage after in vitro fertilization (IVF), with the live-birth rate reduced by 20% [6-9]. Despite clinical impacts of obesity on female reproduction have been well characterized, the underlying mechanisms remain to be elucidated. This article aims to review the relationship between obesity and the complete female reproduction including hypothalamic-pituitary-ovarian axis, oocyte maturation, embryo and fetal development, adding obesity-induced inflammation and related epigenetic impact.
Effects of Obesity on the Hypothalamic-pituitary-ovarian (HPO) Axis
The hypothalamic-pituitary-ovarian (HPO) axis, a complete and coordinated neuroendocrine system, is essential for female reproductive function. The hypothalamus regulates the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) in the pituitary by secreting gonadotrophin releasing hormone (GnRH), thus controlling gonadal development and secretion of sex hormones. Obese women showed obviously reduced amplitude, mean LH and higher circulating levels of insulin compared with normal-weight women [9, 10]. Insulin promoted the synthesis of androgens, which are aromatized into estrogen in response to excess adipose tissue and ultimately cause negative feedback on the HPO axis [11]. Mainly produced by white adipose tissue, leptin is related to modifications of reproductive function. It has been reported that leptin affected GnRH pulse neurons and LH secretion by the pituitary [12-14]. A randomized trial mimicking the metabolic syndrome of obesity found that there was no significant difference in serum inflammatory signal and endoplasmic reticulum stress markers, revealing that the endocrine disruption and adverse reproductive outcomes caused by obesity may be mediated by HPO axis directly [15]. In support of this central mechanism, it has been demonstrated that the significant reduction in natural pregnancy rate of obese mice induced by a high fat diet can be overcome after exogenous gonadotrophin stimulation [16, 17].
Effects of Obesity on the Oocyte Maturation
In bisexual reproduction, female gametes bear the main accumulation of energy and matter during the later zygote development, and the development female gametes are more specific and complicated. Follicles are the structural basis of female gamete development in higher animals, especially mammals. During follicular development, on the one hand, the germ cell realizes the orderly arrest and restart of development, and completes the maturation of nucleus and cytoplasm of oocyte. On the other hand, follicular cells undergo multiple differentiation and proliferation, and the orderly development of germ cells is regulated while completing its endocrine function. An important feature of the follicular development initiation stage is the growth of oocyte, which determines the embryo potential. The oocyte in primordial follicles was blocked in the prophase of meiosis I and formed primary oocytes. When oocytes grew to certain sizes, they gained the ability to restore meiosis. At this time, oocytes had large nucleuses, highly loose chromatins, and complete nuclear membranes called germinal vesicle (GV). If completely grown oocytes released from follicular inhibition, spontaneous meiotic recovery may happen, which is called germinal vesicle breakdown (GVBD). After GVBD occurs, oocytes completed the first meiosis, homologous chromosomes were separated, and the first body was discharged [18-20]. Then, spindles were assembled again and oocytes entered the metaphase II until fertilization. However, oocytes collected from both obese women and obese mice show poor quality, indicating oocytes negatively impact the oocyte maturation [21-24].
Meiotic maturation
Complete meiotic maturation contains resumption of meiosis and the correct segregation of chromosomes [25]. The oocyte at meiotic arrest is characterized by nucleus having a complete nuclear membrane structure called GV. The most important feature of meiotic resumption is GVBD. After GVBD occurs, the oocyte completed first meiosis, homologous chromosomes were separated, and the first polar body was expelled. Subsequently, the spindle reassembled and entered the second meiotic metaphase until fertilization. In high-fat diet induced obesity mouse models, 39.45% of oocytes from obese mice completed GVBD, whereas 89.46% of control oocytes completed this maturational stage, and oocytes of obese mice were smaller than control oocytes as well [26]. Similarly, the maturation of obese mice in IVF was delayed [27].
The spindle is the core of chromosome separation, a dynamic machine mainly composed of a large number of longitudinally arranged microtubules, whose job is to collect and sort chromosomes and distribute them to daughter cells as they divide [28]. During meiosis I, GVBD occurs and microtubules form bipolar spindles around chromosomes. At the end of meiosis I stage, spindle migrate to the cortex and cortical reorganization begins. After first polar body extrusion, oocytes enter meiosis II stage and spindles quickly form below the first polar body [29]. The integrity of spindles determines the correctness of chromosome segregation, and abnormalities in chromosome segregation can lead to aneuploidy, which contributes to early pregnancy loss [30]. The polarized light microscopy can be used to detect spindle abnormalities of oocytes, and compared with the normal BMI groups, the odds of oocytes with disarranged spindles and non-aligned chromosomes was dramatically greater in severely obese groups [31-33]. Studies in high fat diet (HFD)-induced obesity models correlated with clinical findings in humans. Both percentages of abnormal spindle morphologies and chromosome misalignment were increased evidently in HFD mice oocytes [30]. Furthermore, examination of abnormal oocytes in diet-induced obesity (DIO) mice revealed high rates of meiotic aneuploidy characterized by disorganized spindles and chromosomes improperly aligned on the metaphase plate [34].
Mitochondrial dysfunction
In general, oocyte maturation involves two different levels of maturation: One is the aforementioned resumption of oocyte meiosis during nuclear maturation. Another one is the cytoplasmic maturation of oocyte, which directly determines the fertilization ability of late oocyte and the ability of early embryo development. It bears mentioning that mitochondria, the principal maternally-inherited organelles in the cytoplasm of oocytes, plays an important role in maturation, fertilization and embryonic development of oocytes, with the main function of manufacturing 5'-adenosine triphosphate (ATP) through actions of the tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) [35]. Generally, mitochondrial DNA (mtDNA) copy numbers increase over 30-fold from primary oocytes to MII stage oocytes [36]. Oocytes with low mtDNA copy numbers are more likely to be damaged than oocytes with high mtDNA copy numbers in mice and human oocytes which are successfully fertilized contain more mtDNA copies than oocytes which are unsuccessfully fertilized, suggesting that the mtDNA copy number in oocyte corrects with oocyte quality [37-40]. Another intriguing feature of mitochondria during oocyte maturation is that the membrane potential of mitochondria increased significantly, which is associated with a concomitant increase in OXPHOS, and the lack of membrane potential may cause the decrease of oocyte development potential [41-43]. In addition, the increasing of ATP levels plays a critical role during oocyte maturation and oocytes with higher ATP levels showed a better fertilization rate and embryo development than oocytes with lower ATP levels [44-46].
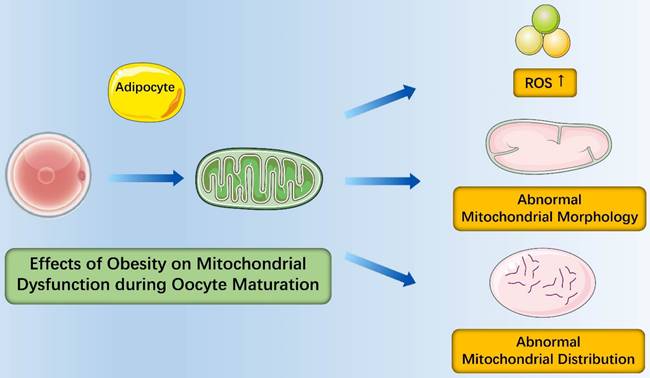
In the context of obesity, the number of oocyte mtDNA copies was dramatically higher in obese mice than in those from lean counterparts and the expression of mitochondrial biogenesis and fission markers (PGC-1α and Drp-1) increased in obese mice [34]. Similarly, the expression of mtTFAM and NRF1 (nuclear genes encoding mtDNA transcription factors) elevated as well [47]. These findings suggested that oxidative stress from obesity induced mitochondrial damage that contributed to compensatory responses of increased mitochondrial biogenesis and fission [35]. It is important to note that mitochondria of mice oocytes had fewer cristae which were more disarrayed, decreased electron density of the matrix, increased swelling and a growing number of vacuoles and distribution of mitochondria from obese females was in an unorganized clumping pattern instead of being distributed evenly throughout the ooplasm in mitochondria from normal females [34]. Igosheva et al. detected that the membrane potentiala dramatic increased in oocytes and zygotes of C57BL/6J DIO mice by using a potentiometric fluorescent dye with low toxicity [47]. Alternatively, most studies found that membrane potential of oocytes mitochondria from obese mice was lower than that of oocytes mitochondria from normal mice [48, 49]. To evaluate the redox state of oocytes, studies were performed and found that oocytes from obese mice were more oxidized and had higher rate of reactive oxygen species (ROS) production. Taken together, obesity causes abnormalities of mitochondrial morphology, mitochondrial distribution of oocytes and oocyte metabolism, which ultimately has a negative effect on oocyte maturation (Figure 1).
Endoplasmic reticulum stress
The endoplasmic reticulum (ER) is a cellular organelle playing a critical role in protein biosynthesis, trafficking, folding and Ca2+ homeostasis. ER stress occurs when misfolded proteins accumulated in the ER of liver and adipose tissue. Accumulating evidence has revealed that obesity can cause ER stress in mammalian cells [50]. ER stress markers inositol requiring enzyme 1α (IRE1α), glucose-related protein 78 (GRP78) and X-box binding protein 1 (XBP1) were significantly increased in adipose tissue of obese compared to lean pregnant women. ER stress was also increased in adipose tissue of women with gestational diabetes mellitus compared to BMI-matched normal glucose tolerant women [51]. In placental tissue, transcript levels of ER stress factors XBP1, activating transcription factor 4 (Atf4), and the molecular chaperone calnexin, were decreased with maternal HFD intake [52].
Functional protein synthesis occurs through translation of maternal mRNA and is necessary for oocyte maturation [53-56]. During these processes, ER plays significant roles in meeting increased protein demand of oocytes, which is accomplished by correct protein synthesis, folding and modification [57]. Therefore, regulation of ER stress is a key mechanism during oocyte maturation. Lipid is deposited not only in adipose tissues, but also in nonadipose tissues, which leads to a high level of free fatty acids and triggering lipotoxicity characterized by ER stress, and ER stress links mitochondrial damage [58]. Theres is evidence of ER stress in oocyte that cumulus-oocyte complexes (COCs) treated with thapsigargin (a strong ER stress inducer) decreased cumulus cell expansion (a marker of oocyte quality) and led to poor pre-implantation development rates [59]. Similarly, treatment of COCs with palmitic acid (another strong ER stress inducer) decreased cumulus expansion and reduced pre-implantation development [60]. To demonstrate that ER stress damages oocyte quality, when cultured with ER stress inhibitor salubrinal, COCs treated with an ER stress inducer or collected from obese mice were surprisingly reversed the oocyte quality by increasing levels of mitochondrial replication factors mitochondrial transcription factor A (TFAM) and dynamin related protein 1 (DRP1) as well as mtDNA in obese mice oocytes [48]. Overall, these findings suggest that ER stress plays a crucial role in cumulus-oocyte complex interactions as well as oocyte quality.
Effects of Obesity on the Embryo
Embryo quality is one of the most critical factors assessing the success of implantation process and subsequent pregnancy [61]. In mammals, mature oocytes combine with the sperm to form fertilized eggs. After formation of fertilized eggs, embryos enter the cleavage stage and form solid cell clusters similar to mulberry called morula. As cells divide, fluid-filled cavities appear inside the embryo and cells differentiate into inner cell mass (ICM) and trophoblast cells (TE) [62]. The embryo at this stage is called blastocyst. With the development of embryos, they hatch out of the crack in the zona pellucida, and trophoblast cells make contact with the inner wall of the uterus and implant into the uterine tissue, which is called preimplantation embryo development. After implantation, the embryo begins to absorb nutrients in order to maintain development, and gradually differentiates into various cells such as ectoderm, mesoderm and endoderm through gastrula, eventually forming tissues and organs [63-65]. To evaluate the association between pregnancy performance and obesity, Zheng et al. used 10,252 frozen-thawed cycles with single blastocyst transfer, and patients were divided into four groups: underweight, normal-weight, overweight, and obesity. Miscarriage rate was higher in the obese group (27.51%), compared with the normal-BMI group (20.91%). Meanwhile, Using the normal-BMI group as reference, the incidence of preterm birth (11.19% vs. 16.87%) and the proportion of women needing a cesarean section (87.91% vs. 93.98%) were higher in the obese group [66]. In a retrospective analysis, comparison of human IVF/ICSI cycles with normal BMI groups showed that obesity had adverse effects on the mean embryo grade, the embryo utilization rate and the number of embryos discarded and cryopreserved [67]. Similarly, Leary et al. found that embryos from overweight or obese women (BMI ≥25 kg/m2) were less likely to accomplish development post-fertilization and more quickly developed to the morula stage with fewer cells in the trophectoderm, reduced glucose consumption, improved amino acid metabolism and increased levels of endogenous triglyceride [68].
Effects of obesity on mitochondrial dysfunction during oocyte maturation. Obesity causes abnormalities of oocyte metabolism with more oxidized oocytes and higher rate of reactive oxygen species (ROS) production; Obesity causes abnormalities of mitochondrial morphology with increased swelling and a growing number of vacuoles; Obesity causes abnormalities of mitochondrial distribution in an unorganized clumping pattern.
As discussed earlier with regard to oocytes, embryos are susceptible to lipotoxicity as well [69, 70]. Culturing in excess palmitic acid (PA, the most abundant saturated free fatty acids in human serum and ovarian follicular fluid), murine blastocysts had altered embryonic IGF1 receptor expression, increased glutamic pyruvate transaminase activity and decreased nuclei count. Murine trophoblastic stem cells exposed to PA in vitro proliferated less and underwent increased dose-dependent apoptosis. When these embryos transferred into foster mice, fetuses of PA-exposed blastocysts were smaller than controls [68]. Apart from this central acting, elevated levels of leptin in obese women exerted a direct negative effect on embryos [71]. In vitro, leptin stimulated human trophoblastic stem cell growth, and its inhibition modified proliferation and improved apoptosis. Altogether, an obese environment affects embryonic growth and lasted adverse effects on offspring.
Effects of Obesity on Fetal Development
The placenta, an important organ for the exchange of materials between fetus and mother, is formed by the membrane of the embryo and the endometrium of mother [72]. Fetus develops in womb and relies on placenta to obtain nutrients, while maintaining considerable independence [73]. Maternal obesity affects both the placenta and the fetus, leading to fetal overgrowth and a higher frequency of large for gestational age (LGA) infants [74]. Placental transport was proved to be increased in rodent models of maternal obesity and is closely associated with birth weight of humans [75-77]. Children born to obese mothers have higher risks of developing childhood obesity and metabolic disease [78]. According to the developmental origins of health and disease (DOHaD) hypothesis, maternal obesity is linked with detrimental cardiometabolic and neurocognitive outcomes in the offspring [79-83]. Animal studies have revealed that maternal obesogenic diets induced insulin resistance and increased levels of fetal blood glucose, resulting in accelerated pancreatic β-cell maturation and reduced glucose tolerance in the offspring [84]. It has been reported that maternal HFD resulted in decreased number of oocytes and increased apoptosis in fetal ovaries in the rat at Embryonic Day 20 (E20), increased numbers of primordial and transitioning follicles at Postnatal Day 4 (P4), small secondary follicles and increased follicular atresia in prepubertal offspring, and impaired ovarian follicle growth in adult offspring [85]. More recent studies have also reported that newborns born to obese mothers had a higher incidence of bacterial and viral infections and required admission to neonatal intensive care units, indicating maternal obesity impacts the fetal immune system [86, 87].
Inflammation in Female Reproduction with Obesity
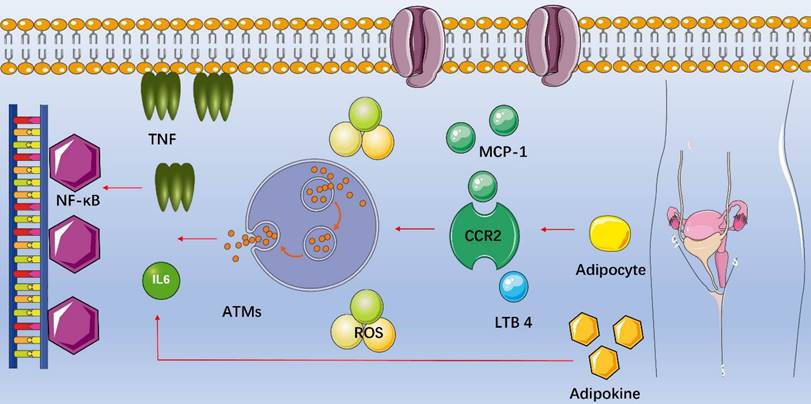
Fat is not only a storage organ but also one of the largest endocrine organs in human body, secreting important signaling molecules and expressing a variety of receptors to sense endocrine signals (Table 1). Of interest, adipose tissue contains special immune cells and obesity-induced inflammation is a chronic, low-grade inflammatory response caused by excess nutrients in the metabolic cell [88]. Macrophages are functionally the most important immune cells in the adipose tissue [89], and the recruitment of macrophages into adipose tissues is the initial event of obesity-induced inflammation. The number of macrophages in adipose tissue increases in obesity and participates in inflammatory pathways activated in fat of obese individuals. Increasing in circulating triglycerides causes adipocyte secreting the chemokine such as monocyte chemotactic protein-1 (MCP-1/CCL2), leukotriene B4 (LTB4) or others, attracting monocytes into adipose tissues and becoming adipose tissue macrophages (ATMs) [90, 91]. MCP-1 is a critical chemokine secreted by enlarging adipocytes and stimulates macrophage migration by binding to the C-C chemokine receptor type 2 (CCR2) on macrophages. LTB4, a product of arachidonic acid metabolism, is produced by adipocytes and promotes ATMs infiltration [92]. As soon as proinflammatory ATMs move into adipose tissues, they can secrete their own chemokines and attract additional macrophages. In addition, increased secretion of leptin also contributes to macrophage accumulation through stimulating transport of macrophages to fat and adhesion between macrophages and endothelial cells [93]. Subsequently, macrophages secrete cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and IL-1β, activating the nuclear factor-κB (NF-κB) signal transduction pathway and producing more cytokines [94]. Adipocytes produce adipokines as well, which promotes additional release of TNF-α and IL-6 [95] (Figure 2). Chronic inflammation induces oxidative stress as well because of increased production of ROS, and ROS are chemically reactive chemicals containing oxygen, mainly including superoxide anion (O2-), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). Studies showed that excessive ROS in the ovary inhibited the oocyte maturation, decreased the quality of oocytes, caused granular cell (GC) apoptosis and accelerated degeneration of the corpus luteum [96, 97].
Inflammation in female reproduction with obesity. Increasing in circulating triglycerides causes adipocyte secreting chemotactic protein-1 (MCP-1/CCL2), leukotriene B4 (LTB4) or others, attracting monocytes into adipose tissues and becoming adipose tissue macrophages (ATMs). As soon as proinflammatory ATMs move into adipose tissues, they can secrete their own chemokines and attract additional macrophages. Subsequently, macrophages secrete cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6) and IL-1β, activating the nuclear factor-κB (NF-κB) signal transduction pathway and producing more cytokines. Adipocytes produce adipokines as well, which promotes additional release of TNF-α and IL-6.
Key adipokines and their functions
| Adipokine | Function |
|---|---|
| TNF [93] | Predominantly paracrine, inflammation, insulin resistance and downregulating of anti-inflammatory pathways |
| Leptin [102] | Appetite control and stimulating fatty acid oxidation |
| Adiponectin [103] | Stimulating fatty acid oxidation, reducing gluconeogenesis and anti-inflammatory |
| Resistin [104] | Promoting insulin resistance and inflammation |
| IL-6 [105] | Limiting expression of genes encoding inflammatory cytokines and augmenting responsiveness of macrophages to IL-4 |
| IL-8 [106] | Greater cardiovascular risk |
| IL-10 [107] | Anti-inflammatory and immunosuppressive cytokine |
| IL-18 [108] | Insulin resistance |
| Visfatin [109] | Enhancing effects of IL-7 and the stem cell factor |
| Apelin [110] | Regulating cardiovascular homeostastis, cell proliferation, food intake, and angiogenesis |
| MCP-1/CCL2 [91] | Stimulating macrophage migration |
| RBP4 [111] | Insulin resistance |
TNF: tumor necrosis factor; IL: interleukin; MCP-1: monocyte chemotactic protein-1; CCL2: Chemokine (C-C motif) ligand 2; RBP4: retinol-binding protein 4.
There is concern that an altered inflammatory state can impact the growing fetus indirectly by altering a variety of placental functions (e.g., trophoblast invasion or nutrient transport) [98]. Aye et al. found that increased maternal BMI was associated with activation of placental p38-MAPK and STAT signaling, demonstrating that inflammation associated with maternal obesity is regulated by altered placental function [99]. In addition, gut microbiota differs between obese and normal individuals and plays a role in obesity during female reproduction [100]. It has been reported that maternal diet-induced obesity resulted in maternal intestinal infammation, altered fetal glucose metabolism at mid-gestation, and increased risk of metabolic dysfunction in offspring [101].
The Epigenetic Impact of Obesity on Female Reproduction
All levels of epigenetic regulation seem to have a wide range of effects on development and health [112]. For example, epigenetic modification, including acetylation, phosphorylation, methylation, glycosylation and ubiquitination, plays critical roles in the process of female reproduction [113-116]. Generally, lysine acetylation of histones is controlled by histone acetyl transferases (HATs) and histone deacetylases (HDACs) and has essential roles in follicle development and oocyte maturation [117]. DNA methylation usually involves the addition of a methyl group to the fifth carbon atom of cytosine to form 5-methyl cytosine. Protein phosphorylation occurs most on serine, threonine, or tyrosine residues and regulates cell cycle in various signal transduction pathways [118]. The ubiquitination is essential for degradation of proteins, cell cycle process and transcriptional regulation, which is critical for oocyte maturation [119].
However, epigenetic regulation can be altered by diet, BMI, inflammation, oxidative stress and so on [120]. In obesity, stable epigenetic changes occur and have detrimental influences during female reproduction [121]. It is well established that oxidative stress induces DNA damage reducing the ability of DNA to be methylated by DNA methyltransferases and resulting in global hypomethylation [122]. Related factors in obesity can induce epigenetic alterations in adult target cells and epigenetic phenotypes in germline cells that not only impede gamete function, but can also be passed on to the next generation [123]. Specific patterns of epigenetic factors were also found to correct with obesity itself in an analysis in leucocytes and adipose tissue [124].
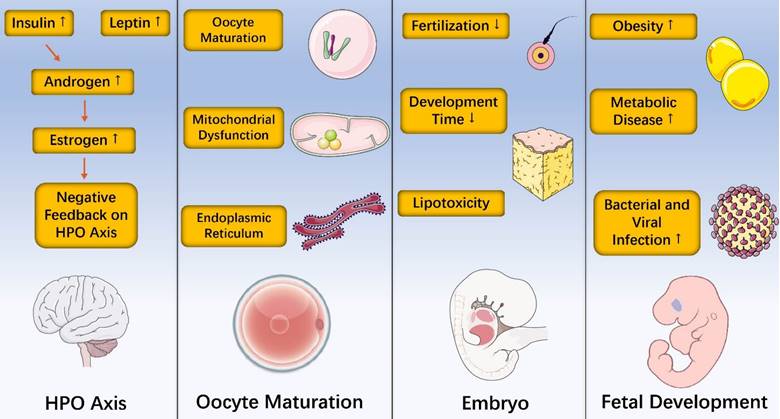
Effects of obesity on hypothalamic-pituitary-ovarian axis, oocyte maturation, embryo and fetal development.
Conclusion
Obesity has become a major public health problem worldwide. It is not only a major risk factor for many common diseases, but also leads directly or indirectly to an increase in health care resources. Compared with lean counterparts, obese women show reduced amplitude, higher circulating levels of insulin, poor oocyte quality, higher miscarriage rates and offspring who are more likely to be sick. In addition, obesity-induced inflammation is a chronic, low-grade inflammatory response and epigenetic changes caused by obesity occur and have detrimental influences during female reproduction. Furthermore, recent data found that losing gut microbes in Drosophila melanogaster suppressed oogenesis [125]. This finding, along with observations that polycystic ovarian syndrome (PCOS) was associated with reduced diversity of the gut microbiome in patients from different countries [126-128], collectively support the idea that gut microbiome changes may impact fertility. Taken together, this review discusses effects of obesity on hypothalamic-pituitary-ovarian axis, oocyte maturation, embryo and fetal development (Figure 3) with related inflammatory and epigenetic impact. However, more translational work will be needed to better understand the relationship between obesity and female reproduction.
Abbreviations
BMI: Body mass index; IVF: In vitro fertilization; HPO: Hypothalamic-pituitary-ovarian; LH: Luteinizing hormone; FSH: Follicle stimulating hormone; GnRH: gonadotrophin releasing hormone; GV: Germinal vesicle; GVBD: Germinal vesicle breakdown; HFD: High fat diet; DIO: Diet-induced obesity; ATP: Adenosine triphosphate; TCA: tricarboxylic acid; OXPHOS: oxidative phosphorylation; mtDNA: Mitochondrial DNA; ROS: Reactive oxygen species; ER: Endoplasmic reticulum; COCs: Cumulus-oocyte complexes; DRP1: Dynamin related protein 1; ICM: Inner cell mass; TE: Trophoblast cells; PA: Palmitic acid; LGA: Large for gestational age; DOHaD: Origins of health and disease; MCP-1: Monocyte chemotactic protein-1; LTB4: Leukotriene B4; ATMs: Adipose tissue macrophages; TNF: Tumor necrosis factor; IL: Interleukin; MCP-1: Monocyte chemotactic protein-1; CCL2: Chemokine (C-C motif) ligand 2; RBP4: retinol-binding protein 4; O2-: Uperoxide anion; ·OH: Hydroxyl radical; H2O2: hydrogen peroxide; GC: Granular cell; HATs: Histone acetyl transferases; HDACs: Histone deacetylases; PCOS: Polycystic ovarian syndrome.
Acknowledgements
Thanks for the help of Jiaying Qin.
Funding
This review was funded by the National Natural Science Foundation of China (Nos. 81273662 and 81473592).
Availability of data and materials
The current study was based on the results of relevant published studies.
Author contributions
WY and HW contributed to writing and editing of this review. JW, LL and YL collected the information. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Brown A, Aristides M, FitzGerald P, Davey P, Bhalla S, Kielhorn A. Pcn19 Examining Preferences and Timetrade-Off Utility for Gemcitabine Plus Cisplatin in the Treatment of Bladder Cancer:A Survey Using Discrete Choice Conjoint Analysis in the Uk. Value in Health. 2002;5:543-4
2. Hu G. More vigorous efforts are needed to fight obesity, a serious public health problem in China. Obesity (Silver Spring). 2021;29:1580-1
3. Rittenberg V, Seshadri S, Sunkara SK, Sobaleva S, Oteng-Ntim E, El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23:421-39
4. Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res. 2016;118:1723-35
5. Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755-67
6. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1
7. Jungheim ES, Travieso JL, Carson KR, Moley KH. Obesity and reproductive function. Obstet Gynecol Clin North Am. 2012;39:479-93
8. Jungheim ES, Schon SB, Schulte MB, DeUgarte DA, Fowler SA, Tuuli MG. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. 2013;28:2720-7
9. Rachon D, Teede H. Ovarian function and obesity-interrelationship, impact on women's reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316:172-9
10. Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G. et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468-73
11. Jungheim ES, Moley KH. Current knowledge of obesity's effects in the pre- and periconceptional periods and avenues for future research. Am J Obstet Gynecol. 2010;203:525-30
12. Zuure WA, Roberts AL, Quennell JH, Anderson GM. Leptin signaling in GABA neurons, but not glutamate neurons, is required for reproductive function. J Neurosci. 2013;33:17874-83
13. Sanchez-Garrido MA, Tena-Sempere M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm Behav. 2013;64:187-94
14. Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129-38
15. Tannous A, Bradford AP, Kuhn K, Fought A, Schauer I, Santoro N. A randomised trial examining inflammatory signaling in acutely induced hyperinsulinemia and hyperlipidemia in normal weight women-the reprometabolic syndrome. PLoS One. 2021;16:e0247638
16. Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238-47
17. Goldsammler M, Merhi Z, Buyuk E. Role of hormonal and inflammatory alterations in obesity-related reproductive dysfunction at the level of the hypothalamic-pituitary-ovarian axis. Reprod Biol Endocrinol. 2018;16:45
18. Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol. 2019;17:8
19. Sun QY, Miao YL, Schatten H. Towards a new understanding on the regulation of mammalian oocyte meiosis resumption. Cell Cycle. 2009;8:2741-7
20. Solc P, Schultz RM, Motlik J. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod. 2010;16:654-64
21. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity's impact. Fertil Steril. 2017;107:840-7
22. Colton SA, Humpherson PG, Leese HJ, Downs SM. Physiological changes in oocyte-cumulus cell complexes from diabetic mice that potentially influence meiotic regulation. Biol Reprod. 2003;69:761-70
23. Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149:2646-56
24. Pohlmeier WE, Xie F, Kurz SG, Lu N, Wood JR. Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol Reprod Dev. 2014;81:735-47
25. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158:R79-R90
26. Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858
27. Zhang JJ, Feret M, Chang L, Yang M, Merhi Z. Obesity adversely impacts the number and maturity of oocytes in conventional IVF not in minimal stimulation IVF. Gynecol Endocrinol. 2015;31:409-13
28. Keefe D, Kumar M, Kalmbach K. Oocyte competency is the key to embryo potential. Fertil Steril. 2015;103:317-22
29. Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update. 2011;17:68-75
30. Howe K, FitzHarris G. Recent Insights into Spindle Function in Mammalian Oocytes and Early Embryos. Biology of Reproduction. 2013 89
31. Machtinger R, Combelles CM, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27:3198-207
32. Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reproductive biomedicine online. 2003;7:24-9
33. Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34-45
34. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N. et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217
35. Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol Hum Reprod. 2013;19:486-94
36. Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA. Rearranged mitochondrial genomes are present in human oocytes. American journal of human genetics. 1995;57:239-47
37. Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H. et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012;79:392-401
38. Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F. et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Molecular Human Reproduction. 2001;7:425-9
39. Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584-91
40. Cao L, Shitara H, Horii T, Nagao Y, Imai H, Abe K. et al. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat Genet. 2007;39:386-90
41. Van Blerkom J, Davis P. Mitochondrial signaling and fertilization. Mol Hum Reprod. 2007;13:759-70
42. Acton BM, Jurisicova A, Jurisica I, Casper RF. Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod. 2004;10:23-32
43. Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Human reproduction (Oxford, England). 2000;15(Suppl 2):129-47
44. Nagano M, Katagiri S, Takahashi Y. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote. 2006;14:53-61
45. Duran HE, Simsek-Duran F, Oehninger SC, Jones HW Jr, Castora FJ. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil Steril. 2011;96:384-8
46. Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB. et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biology of Reproduction. 2001;64:904-9
47. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR. et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074
48. Wu LL, Russell DL, Wong SL, Chen M, Tsai TS, St John JC. et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development. 2015;142:681-91
49. Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ. et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438-45
50. Ajoolabady A, Liu S, Klionsky DJ, Lip GYH, Tuomilehto J, Kavalakatt S. et al. ER stress in obesity pathogenesis and management. Trends Pharmacol Sci. 2022;43:97-109
51. Liong S, Lappas M. Endoplasmic reticulum stress is increased in adipose tissue of women with gestational diabetes. PLoS One. 2015;10:e0122633
52. Gohir W, Kennedy KM, Wallace JG, Saoi M, Bellissimo CJ, Britz-McKibbin P. et al. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J Physiol. 2019;597:3029-51
53. Lee MT, Bonneau AR, Giraldez AJ. Zygotic genome activation during the maternal-to-zygotic transition. Annu Rev Cell Dev Biol. 2014;30:581-613
54. Barckmann B, Simonelig M. Control of maternal mRNA stability in germ cells and early embryos. Biochim Biophys Acta. 2013;1829:714-24
55. Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859-70
56. Gosden RG. Oogenesis as a foundation for embryogenesis. Molecular and Cellular Endocrinology. 2002;186:149-53
57. Guzel E, Arlier S, Guzeloglu-Kayisli O, Tabak MS, Ekiz T, Semerci N. et al. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int J Mol Sci. 2017 18
58. Scha. Lipotoxicity: when tissues overeat. Current Opinion in Lipidology. 2003:281-7
59. Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol. 2012;26:562-73
60. Sutton-McDowall ML, Wu LLY, Purdey M, Abell AD, Goldys EM, MacMillan KL. et al. Nonesterified Fatty Acid-Induced Endoplasmic Reticulum Stress in Cattle Cumulus Oocyte Complexes Alters Cell Metabolism and Developmental Competence. Biology of Reproduction. 2016 94
61. Hourvitz A, Lerner-Geva L, Elizur SE, Baum M, Levron J, David B. et al. Role of embryo quality in predicting early pregnancy loss following assisted reproductive technology. Reproductive Biomedicine Online. 2006;13:504-9
62. Gardner DK. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology. 1998;49:83-102
63. Farin PW, Crosier AE, Farin CE. Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology. 2001;55:151-70
64. Thompson JG. In vitro culture and embryo metabolism of cattle and sheep embryos - a decade of achievement. Animal Reproduction Science. 2000;60:263-75
65. Vanderwall DK. Early embryonic development and evaluation of equine embryo viability. The Veterinary clinics of North America Equine practice. 1996;12:61-83
66. Zheng Y, Dong X, Chen B, Dai J, Yang W, Ai J. et al. Body mass index is associated with miscarriage rate and perinatal outcomes in cycles with frozen-thawed single blastocyst transfer: a retrospective cohort study. BMC Pregnancy Childbirth. 2022;22:118
67. Metwally M, Cutting R, Tipton A, Skull J, Ledger WL, Li TC. Effect of increased body mass index on oocyte and embryo quality in IVF patients. Reproductive Biomedicine Online. 2007;15:532-8
68. Leary C, Leese HJ, Sturmey RG. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Human Reproduction. 2015;30:122-32
69. Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertility and Sterility. 2011;96:880-3
70. Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship Between Serum Polyunsaturated Fatty Acids and Pregnancy in Women Undergoing In vitro Fertilization. Journal of Clinical Endocrinology & Metabolism. 2013;98:E1364-E8
71. Magarinos MP, Sanchez-Margalet V, Kotler M, Calvo JC, Varone CL. Leptin promotes cell proliferation and survival of trophoblastic cells. Biology of Reproduction. 2007;76:203-10
72. Myatt L, Maloyan A. Obesity and Placental Function. Semin Reprod Med. 2016;34:42-9
73. Howell KR, Powell TL. Effects of maternal obesity on placental function and fetal development. Reproduction. 2017;153:R97-R108
74. Stang J, Huffman LG. Position of the Academy of Nutrition and Dietetics: Obesity, Reproduction, and Pregnancy Outcomes. J Acad Nutr Diet. 2016;116:677-91
75. Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring). 2015;23:1663-70
76. Jones HN, Woollett LA, Barbour N, Prasad PD, Powell TL, Jansson T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009;23:271-8
77. Lager S, Ramirez VI, Gaccioli F, Jang B, Jansson T, Powell TL. Protein expression of fatty acid transporter 2 is polarized to the trophoblast basal plasma membrane and increased in placentas from overweight/obese women. Placenta. 2016;40:60-6
78. Santangeli L, Sattar N, Huda SS. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract Res Clin Obstet Gynaecol. 2015;29:438-48
79. Gaillard R, Felix JF, Duijts L, Jaddoe VW. Childhood consequences of maternal obesity and excessive weight gain during pregnancy. Acta Obstet Gynecol Scand. 2014;93:1085-9
80. Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond). 2015;39:677-85
81. Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH. et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303-13
82. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8:e61627
83. Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VWV, Eriksson JG. et al. Influence of maternal obesity on the long-term health of offspring. The Lancet Diabetes & Endocrinology. 2017;5:53-64
84. Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW. et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297:R835-43
85. Tsoulis MW, Chang PE, Moore CJ, Chan KA, Gohir W, Petrik JJ. et al. Maternal High-Fat Diet-Induced Loss of Fetal Oocytes Is Associated with Compromised Follicle Growth in Adult Rat Offspring. Biol Reprod. 2016;94:94
86. Rajappan A, Pearce A, Inskip HM, Baird J, Crozier SR, Cooper C. et al. Maternal body mass index: Relation with infant respiratory symptoms and infections. Pediatr Pulmonol. 2017;52:1291-9
87. Sureshchandra S, Marshall NE, Messaoudi I. Impact of pregravid obesity on maternal and fetal immunity: Fertile grounds for reprogramming. J Leukoc Biol. 2019;106:1035-50
88. Denison FC, Roberts KA, Barr SM, Norman JE. Obesity, pregnancy, inflammation, and vascular function. Reproduction. 2010;140:373-85
89. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112:1796-808
90. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363-74
91. Gerhardt CC, Romero IA, Cancello R, Camoin L, Strosberg AD. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Molecular and Cellular Endocrinology. 2001;175:81-92
92. Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H. et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol Endocrinol Metab. 2011;300:E175-87
93. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219-46
94. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97
95. Xie F, Anderson CL, Timme KR, Kurz SG, Fernando SC, Wood JR. Obesity-Dependent Increases in Oocyte mRNAs are associated with Increases in Proinflammatory Signaling and Gut Microbial Abundance of Lachnospiraceae in Female Mice. Endocrinology. 2016;157:1630-43
96. Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y. et al. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front Pharmacol. 2020;11:617843
97. Al-Zubaidi U, Adhikari D, Cinar O, Zhang QH, Yuen WS, Murphy MP. et al. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum Reprod. 2021;36:771-84
98. Denizli M, Capitano ML, Kua KL. Maternal obesity and the impact of associated early-life inflammation on long-term health of offspring. Front Cell Infect Microbiol. 2022;12:940937
99. Aye IL, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc Natl Acad Sci U S A. 2015;112:12858-63
100. Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M. et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8:77
101. Wallace JG, Bellissimo CJ, Yeo E, Fei Xia Y, Petrik JJ, Surette MG. et al. Obesity during pregnancy results in maternal intestinal inflammation, placental hypoxia, and alters fetal glucose metabolism at mid-gestation. Sci Rep. 2019;9:17621
102. Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends in Endocrinology and Metabolism. 2001;12:65-72
103. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1-16
104. Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. British Journal of Pharmacology. 2012;165:622-32
105. Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature Immunology. 2014;15:423 -+
106. Straczkowski M, Dzienis-Straczkowska S, Stepien A, Kowalska I, Szelachowska M, Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. Journal of Clinical Endocrinology & Metabolism. 2002;87:4602-6
107. Liu Y, Xu D, Yin C, Wang S, Wang M, Xiao Y. IL-10/STAT3 is reduced in childhood obesity with hypertriglyceridemia and is related to triglyceride level in diet-induced obese rats. Bmc Endocrine Disorders. 2018 18
108. Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. European Journal of Endocrinology. 2007;157:465-71
109. Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Molecular and cellular biology. 1994;14:1431-7
110. Krist J, Wieder K, Kloting N, Oberbach A, Kralisch S, Wiesner T. et al. Effects of Weight Loss and Exercise on Apelin Serum Concentrations and Adipose Tissue Expression in Human Obesity. Obesity Facts. 2013;6:57-69
111. Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356-62
112. He M, Zhang T, Yang Y, Wang C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front Cell Dev Biol. 2021;9:654028
113. Allfrey VG, Faulkner R, Mirsky AE. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proceedings of the National Academy of Sciences of the United States of America. 1964;51:786-94
114. Gutierrez RM, Hnilica LS. Tissue specificity of histone phosphorylation. Science (New York, NY). 1967;157:1324-5
115. Kim JH, Park KC, Chung SS, Bang O, Chung CH. Deubiquitinating enzymes as cellular regulators. Journal of Biochemistry. 2003;134:9-18
116. Stowell SR, Ju T, Cummings RD. Protein Glycosylation in Cancer. In: Abbas AK, Galli SJ, Howley PM, editors. Annual Review of Pathology: Mechanisms of Disease, Vol 10. 2015 p. 473-510
117. Zhang K, Lu Y, Jiang C, Liu W, Shu J, Chen X. et al. HDAC8 functions in spindle assembly during mouse oocyte meiosis. Oncotarget. 2017;8:20092-102
118. Schatten H, Sun Q-Y. Posttranslationally Modified Tubulins and Other Cytoskeletal Proteins: Their Role in Gametogenesis, Oocyte Maturation, Fertilization and Pre implantation Embryo Development. In: Sutovsky P, editor. Posttranslational Protein Modifications in the Reproductive System. 2014 p. 57-87
119. Mtango NR, Sutovsky M, Vandevoort CA, Latham KE, Sutovsky P. Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation. Journal of Cellular Physiology. 2012;227:2022-9
120. Milagro FI, Mansego ML, De Miguel C, Martinez JA. Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Molecular Aspects of Medicine. 2013;34:782-812
121. Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489-98
122. Wachsman JT. DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutation research. 1997;375:1-8
123. Crujeiras AB, Casanueva FF. Obesity and the reproductive system disorders: epigenetics as a potential bridge. Hum Reprod Update. 2015;21:249-61
124. Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aissi D, Wahl S. et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383:1990-8
125. Elgart M, Stern S, Salton O, Gnainsky Y, Heifetz Y, Soen Y. Impact of gut microbiota on the fly's germ line. Nature Communications. 2016 7
126. Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y. et al. Association between Polycystic Ovary Syndrome and Gut Microbiota. Plos One. 2016 11
127. Lindheim L, Bashir M, Muenzker J, Trummer C, Zachhuber V, Leber B. et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. Plos One. 2017 12
128. Insenser M, Murri M, del Campo R, Angeles Martinez-Garcia M, Fernandez-Duran E, Escobar-Morreale HF. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. Journal of Clinical Endocrinology & Metabolism. 2018;103:2552-62
Author contact
![]() Corresponding author: Han Wang, E-mail: dxzouac.cn.
Corresponding author: Han Wang, E-mail: dxzouac.cn.

 Global reach, higher impact
Global reach, higher impact