3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2022; 19(14):2071-2079. doi:10.7150/ijms.77287 This issue Cite
Review
Current understanding of gliomagenesis: from model to mechanism
1. School of Public Health and Management, Chongqing Medical University, Chongqing 400016, China.
2. Guangdong Provincial Key Laboratory of Infectious Disease and Molecular Immunopathology, Shantou University Medical College, Shantou 515041, China.
3. University of Chinese Academy of Sciences, Beijing 100049, China.
#These authors contributed equally to this work.
Abstract
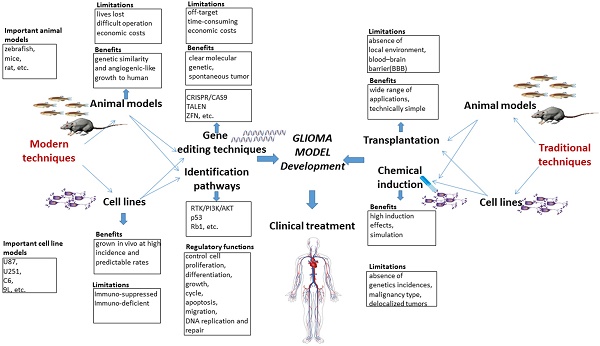
Glioma, a kind of central nervous system (CNS) tumor, is hard to cure and accounts for 32% of all CNS tumors. Establishing a stable glioma model is critically important to investigate the underlying molecular mechanisms involved in tumorigenesis and tumor progression. Various core signaling pathways have been identified in gliomagenesis, such as RTK/RAS/PI3K, TP53, and RB1. Traditional methods of establishing glioma animal models have included chemical induction, xenotransplantation, and genetic modifications (RCAS/t-va system, Cre-loxP, and TALENs). Recently, CRISPR/Cas9 has emerged as an efficient gene editing tool with high germline transmission and has extended the scope of stable and efficient glioma models that can be generated. Therefore, this review will highlight the documented evidence about the molecular characteristics, critical genetic markers, and signaling pathways responsible for gliomagenesis and progression. Moreover, methods of establishing glioma models using gene editing techniques and therapeutic aspects will be discussed. Finally, the prospect of applying gene editing in glioma by using CRISPR/Cas9 strategy and future research directions to establish a stable glioma model are also included in this review. In-depth knowledge of glioma signaling pathways and use of CRISPR/Cas9 can greatly assist in the development of a stable, efficient, and spontaneous glioma model, which can ultimately improve the effectiveness of therapeutic responses and cure glioma patients.
Keywords: Glioma, Tumorigenesis, Signaling pathways, CRISPR/Cas9, Model

 Global reach, higher impact
Global reach, higher impact