3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(14):3333-3341. doi:10.7150/ijms.62621 This issue Cite
Research Paper
The Incidence and Effect of Cytomegalovirus Disease on Mortality in Transplant Recipients and General Population: Real-world Nationwide Cohort Data
1. Divison of Infectious Disease, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
2. Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea
Received 2021-5-11; Accepted 2021-7-19; Published 2021-7-25
Abstract
Background: In addition to the conventional opportunistic infections in solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT) recipients, cytomegalovirus (CMV) infection is associated with various chronic inflammatory diseases or poor outcomes in non-immunocompromised critically ill patients. To evaluate the burden or outcome of CMV replication in non-transplant individuals, we compared the incidence rates (IRs) for CMV disease and all-cause mortality between SOT recipients, HSCT recipients, and non-transplant population.
Methods: The SOT (N=16,368) and HSCT (N=10,206) cohorts between 2010 and 2015 were established using the WHO ICD-10 from the whole population-based large database of the Health Insurance Review & Assessment Service (HIRA). CMV cases, defined as symptomatic disease with isolation of virus, DNA, pp65 antigen, and pathology except CMV syndrome, were extracted with the unique codes for relief of medical costs of HIRA in the same dataset. Cox's proportional hazard regression analyses and log-rank test in the Kaplan-Meier curves were performed to compare all-cause mortality between the three groups.
Results: The CMV IRs adjusted by age and sex were significantly higher in the SOT (adjusted IR [95% confidence intervals], 33.1 [28.8-38.0] per 1,000 person-years) and HSCT recipients (5.1 [4.6-6.1] per 1,000 person-years) than in the whole population (0.58 [0.49-0.67] per 100,000 person-years). However, SOT recipients with CMV (18/283, 6.4%) had significantly lower all-cause mortality than non-transplant individuals with CMV (207/1,258, 16.5%) (adjusted hazard ratio [95% CI], 0.42 [0.25-0.67], log-rank P < 0.001).
Conclusion: These data suggest that CMV disease in patients without transplants is associated with poor outcomes.
Keywords: Cytomegalovirus, Disease, Incidence, Mortality, Population, Solid organ transplantation, Hematopoietic stem cell transplantation
Introduction
The ubiquitous cytomegalovirus (CMV) can lead to diverse clinical outcomes through acute direct or chronic indirect pathogenesis in populations with different comorbidities [1-3]. In the era of growing aging population and immunomodulation drugs, CMV could exert an influence on public health in various ways, including chronic inflammatory vascular disorders besides tissue-invasive end-organ disease [1]. Recently, several studies have reported the association of CMV with neurocognitive function, metabolic diseases, stroke, myocardial infarction, autoimmune diseases, or frailty in addition to well-known conventional opportunistic infections in severely immunocompromised patients or congenital CMV infection [4-13]. The non-transplant, non-immunocompromised, critically ill patients in intensive care unit are another risk group, who have a higher rate of CMV reactivation on mortality (approximately 15-20% of incidence), even though it is not obvious whether active CMV replication has a causal relationship with life-threatening outcomes or represents the bystander effect by hyperactivation of immune system in severe diseases [14-17]. Taken together, the burden of potential CMV infection or disease caused by life-long periodic reactivation necessitates the development of CMV vaccine for widespread elimination [18-20].
Despite the clinical significance and impact, the precise incidence rate (IR) per person-years of CMV infection or disease in solid organ transplantation (SOT) or hematopoietic stem cell transplantation (HSCT) recipients as well as non-transplant individuals has not been evaluated [1]. Most studies assessing post-SOT CMV epidemiology presented only the frequency (percentage) of CMV infection or disease in a single institution with relatively small recipients (6.2-60%, 1-65%, 19-72.1%, and 15-33.9% in kidney, liver, heart, and pancreas transplantation, respectively) in clinical settings applying various preventive strategies against CMV [21-31]. Similarly, the overall incidence of CMV infection or disease after allo-HSCT was reported to range from 3% to 40% [32-37]. In addition, the definition of CMV infection or disease as well as the cut-off threshold for clinically significant DNAemia were not uniform in publications [21-23,27,28,30,32]. Some studies defined CMV infection as asymptomatic viremia, DNAemia, or antigenemia, while others used suspected or probable end-organ disease [22,23,32,38]. The most recent systemic review of real-world data in kidney transplant recipients showed that the estimated pooled incidences of CMV infection, including acute viral syndrome or end-organ disease, were quite heterogeneous according to the various risk factors, severity of immunosuppression, or methods of CMV prevention, including the dosage of anti-CMV agents [39].
The association between CMV and various comorbidities or mortality in general population has been evaluated previously using CMV seropositivity rates or immunoglobulin G (IgG) concentrations, but do not demonstrate the presence of virus in the host body as shown by methods, such as the measurement of CMV DNA, virion, or pp65 antigen [12,13,40-43]. However, the clear definition of CMV disease should be based on virus isolation by culture, nucleic acid amplification, or protein tests [30,44]. The result of CMV IgG concentration may represent the past exposure history and/or the current status of humoral immunity, but does not indicate active lytic CMV replication [30,44,45].
Therefore, we assessed the difference in IRs for CMV per person-years between SOT recipients, HSCT recipients, and non-transplant individuals and the effect of CMV end-organ disease except CMV syndrome on all-cause mortality in these groups to evaluate the clinical impact and burden of active CMV replication, defined as CMV detection by polymerase chain reaction, culture, shell viral assay, antigen test, or histopathology, using a general population-based large cohort dataset.
Materials and methods
Cohort construction and data extraction
The Korean National Health Insurance System (NHIS) organized by the government provides a single healthcare insurance service to the entire nation, covering approximately 50 million people. The Health Insurance Review & Assessment Service (HIRA), managed by the NHIS, establishes the whole database consisting of general information, diagnosis, drug, and healthcare services subset to operate the efficient claim process to healthcare providers [46]. Using the nationwide database warehouse in the HIRA, we constructed SOT and HSCT recipient cohorts who received transplantation between January 2010 and December 2015. For the retrieval of recipients, we used the unique HIRA codes (V084, V085, V086, V087, V088 for SOT, and V081, V082, V083 for HSCT) to identify the rare incurable diseases for the direct relief co-payment policy [47], which are perfectly matched with the International Statistical Classification of Disease and Related Health Problems 10th Revision (ICD-10) codes version 2016 by the World Health Organization (Z94.0, Z94.1, Z.94.2, Z.94.3, Z.94.4, and Z94.8) [48]. The SOT and HSCT cohorts for 6 years included 16,368 and 10,206 recipients, respectively.
The HIRA includes CMV disease except CMV syndrome as rare incurable diseases and manages a strict review process for the relief of medical costs [47]. For application of the unique code V104 for CMV disease except CMV syndrome, physicians should submit the results of CMV detection in blood, body fluid, or tissues using histopathologic findings, nucleic amplification test of polymerase chain reaction, pp65 antigen test, virus culture, and shell viral assay to the HIRA. The definition of CMV disease in the HIRA process is matched by the ICD-10 codes of B25 (cytomegaloviral disease), B25.0 (cytomegaloviral pneumonitis), B25.1 (cytomegaloviral hepatitis), B25.2 (cytomegaloviral pancreatitis), B25.8 (other cytomegaloviral diseases), and B25.9 (cytomegaloviral disease, unspecified), and does not include the P35.1 (congenital CMV infection) or B27.1 (cytomegaloviral mononucleosis) [48]. The asymptomatic CMV isolation as CMV viremia, DNAemia, and pp65 antigenemia in the regular screening or surveillance for post-transplant CMV prevention is not acceptable to submission for the HIRA review. We used the term 'CMV cases' in this study, defined as CMV disease except CMV syndrome [30,44].
The co-morbid diseases were identified by ICD-10 codes: (1) type 2 diabetes mellitus (DM) (E11), (2) end stage renal disease (ESRD) on dialysis (N18.5), (3) chronic heart diseases including congestive heart failure (CHF) (I50.0), left ventricular failure (I50.1), heart failure, unspecified (I50.9), myocardial infarction (I21, I22, I25.2), hypertensive heart disease with CHF (I11.0), and cardiomyopathy (I42, I25.5), (4) chronic liver diseases including hepatic failure (K72, K70.4), chronic viral hepatitis (B18.0, B18.1, B18.2, B18.8, B18.9), and liver cirrhosis (K74, K70.3), (5) chronic lung diseases including chronic obstructive pulmonary diseases (J44), chronic emphysema (J43.8, J43.9), and interstitial lung diseases (J84), (6) human immunodeficiency virus-1 (HIV-1) infection (B20-B24, Z21).
This study was approved by the NHIS and the Gangnam Severance Institutional Review Board, and informed consent was waived because of the anonymous data (Study No: REQ0000017664 and IRB No: 3-2017-0341).
Statistical analyses
The data are expressed as numbers (percentage) or means ± standard deviations, as well as IRs or hazard ratios (HRs) (95% confidence intervals [CI]). The standardized IRs and HRs per person-years were obtained by adjusting for age and sex using the 2010 South Korea Population and Housing Census data. We used the ANOVA and chi-square test to compare the continuous and nominal variables, respectively, between the three groups. Cox proportional hazards regression analysis was performed to compare the mortality rates between SOT recipients, HSCT recipients, and non-transplant individuals. Kaplan-Meier curves were constructed to compare the incidence probability of CMV cases or all-cause death. All statistical analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC, USA), and a two-tailed P-value of < 0.05, was considered statistically significant.
Results
Incidence rates of CMV cases in SOT and HSCT recipients
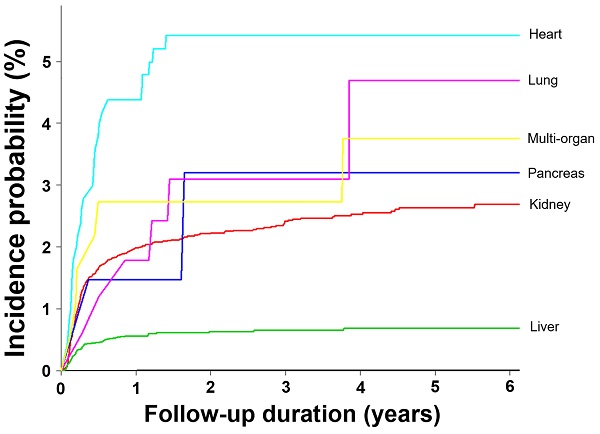
The percentages of patients with CMV cases were 0.003%, 1.7%, and 0.44% in non-transplant individuals, SOT recipients, and HSCT recipients, respectively. The adjusted IR of CMV in the whole-population cohort, including 50 million individuals per year between 2010 and 2015 was 0.58/100,000 person-years (Table 1). The SOT (adjusted IR [95% CI], 33.1 [28.8-38.0] per 1,000 person-years) and HSCT recipients (5.1 [4.6-6.1] per 1,000 person-years) had significantly higher IRs than that in the whole population (5,517- and 850-fold, respectively). In the SOT cohort, the liver transplant recipients had the lowest IR (11.1 [7.7-16.3] per 1,000 person-years). The IR of CMV in heart transplant recipients (104.2 [66.4-163.7] per 1,000 person-years) was the highest, followed by multi-organ (72.7 [30.2-175.4]) and kidney transplantation (44.3 [37.7-52.1] per 1,000 person-years) with 8.6, 6.3, and 3.8, respectively, compared to liver transplantation. The lung transplant recipients had a relatively low IR of 17.3 (2.4-123.2) per 1,000 person-years (Table 2 and Figure 1).
Difference in incidence probability for development of CMV disease except CMV syndrome according to the transplant organ in solid organ transplantation recipients
Incidence of CMV disease except CMV syndrome in solid organ and hematopoietic stem cell transplantation recipients
| Populations | Year | ||||||
|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
| SOT | |||||||
| Total | Total Pop. | 2,360 | 2,858 | 3,072 | 2,971 | 3,142 | 3,411 |
| CMV casesa | 31 | 25 | 45 | 60 | 69 | 53 | |
| Crude IRb | 1.31 (0.85-1.77) | 0.87 (0.53-1.22) | 1.46 (1.04-1.89) | 2.02 (1.51-2.53) | 2.20 (1.68-2.71) | 1.55 (1.14-1.97) | |
| Std. IRb | 1.34 (0.67-2.01) | 1.02 (0.39-1.64) | 2.07 (1.07-3.07) | 2.97 (1.76-4.17) | 3.16 (2.05-4.26) | 2.14 (1.26-3.01) | |
| Kidney | Total Pop. | 1,258 | 1,604 | 1,741 | 1,716 | 1,775 | 1,875 |
| CMV casesa | 24 | 19 | 36 | 41 | 42 | 35 | |
| Crude IRb | 1.91 (1.15-2.66) | 1.18 (0.65-1.71) | 2.07 (1.40-2.74) | 2.39 (1.67-3.11) | 2.37 (1.66-3.07) | 1.87 (1.25-2.48) | |
| Std. IRb | 1.71 (0.86-2.55) | 1.19 (0.37-2.01) | 2.44 (1.14-3.73) | 7.63 (0-17.08) | 2.86 (0.95-4.78) | 2.43 (0.51-4.35) | |
| Liver | Total Pop. | 1,012 | 1,131 | 1,191 | 1,084 | 1,181 | 1,307 |
| CMV casesa | 4 | 3 | 7 | 12 | 17 | 10 | |
| Crude IRb | 0.40 (0.01-0.78) | 0.27 (0-0.57) | 0.59 (0.15-1.02) | 1.11 (0.48-1.73) | 1.44 (0.76-2.12) | 0.77 (0.29-1.24) | |
| Std. IRb | 0.36 (0-0.89) | 0.10 (0-0.22) | 1.76 (0-3.8) | 3.65 (0.54-6.76) | 2.72 (0.65-4.79) | 1.19 (0.27-2.10) | |
| Heart | Total Pop. | 69 | 92 | 103 | 117 | 112 | 141 |
| CMV casesa | 2 | 3 | 2 | 6 | 5 | 8 | |
| Crude IRb | 2.90 (0-6.96) | 3.26 (0-6.96) | 1.94 (0-4.65) | 5.13 (1.07-9.18) | 4.46 (0.58-8.35) | 5.67 (1.81-9.54) | |
| Std. IRb | 2.12 (0-5.59) | 5.94 (0-15.78) | 1.32 (0-3.15) | 4.87 (0-9.82) | 3.16 (0.13-6.18) | 5.78 (0.19-11.37) | |
| Lung | Total Pop. | 18 | 33 | 35 | 43 | 54 | 63 |
| CMV casesa | 0 | 0 | 0 | 0 | 2 | 0 | |
| Crude IRb | — | — | — | — | 3.70 (0-8.91) | — | |
| Std. IRb | — | — | — | — | 8.30 (0-21.66) | — | |
| Pancreas alone | Total Pop. | 25 | 39 | 35 | 60 | 54 | 59 |
| CMV casesa | 1 | 1 | 1 | 0 | 3 | 1 | |
| Crude IRb | 4.00 (0-12.26) | 2.56 (0-7.75) | 2.86 (0-8.66) | — | 5.56 (0-11.87) | 1.69 (0-5.09) | |
| Std. IRb | 1.64 (0-4.84) | 0.67 (0-1.99) | 0.90 (0-2.65) | — | 6.99 (0-17.18) | 0.52 (0-1.54) | |
| HSCT | |||||||
| Total Pop. | 1,263 | 1,522 | 1,639 | 1,740 | 1,863 | 2,179 | |
| CMV casesa | 4 | 2 | 7 | 5 | 11 | 16 | |
| Crude IRb | 0.32 (0.01-0.63) | 0.13 (0-0.31) | 0.43 (0.11-0.74) | 0.29 (0.04-0.54) | 0.59 (0.24-0.94) | 0.73 (0.38-1.09) | |
| Std. IRb | 0.35 (0-0.73) | 0.10 (0-0.24) | 0.36 (0.09-0.63) | 0.27 (0.03-0.51) | 0.49 (0.19-0.78) | 0.70 (0.35-1.06) | |
| Whole population | |||||||
| Total Pop. | 50,165,317 | 50,443,562 | 50,761,374 | 51,011,717 | 51,279,732 | 51,571,506 | |
| CMV casesa | 159 | 206 | 205 | 259 | 359 | 398 | |
| Crude IRc | 0.32 (0.27-0.37) | 0.41 (0.35-0.46) | 0.40 (0.35-0.46) | 0.51 (0.45-0.57) | 0.70 (0.63-0.77) | 0.77 (0.70-0.85) | |
| Std. IRc | 0.32 (0.27-0.37) | 0.41 (0.35-0.47) | 0.40 (0.35-0.46) | 0.50 (0.44-0.56) | 0.68 (0.61-0.76) | 0.75 (0.68-0.82) | |
Data are expressed as number or IR (95% CI). aIndicate CMV disease except CMV syndrome. bPer 100 person-years. cPer 100,000 person-years. Crude IR means the unadjusted incidence rate. Standardized IRs are adjusted by age and sex using 2010 South Korea Population and Housing Census data. Aberrations: CI, confidence interval; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplantation; IR, incidence rate; Pop., population; SOT, solid organ transplantation, Std., standardized
Incidence rates of CMV disease except CMV syndrome in transplant recipients
| Total number | CMV casesa | F/U duration (years) | Unadjusted IR | Adjusted IRb | Adjusted HRb | |
|---|---|---|---|---|---|---|
| Whole population | 50,165,317 | 1,586 | 45,494.48 | 0.005 (0.004-0.006) | 0.006 (0.005-0.007) | 1 (Reference) |
| SOT | 16,368 | 283 | 8124.10 | 33.7 (29.5-38.6) | 33.1 (28.8-38.0) | 5,517 (4,114-7,600)c |
| Kidney | 9,381 | 192 | 4645.58 | 44.3 (37.9-51.9) | 44.3 (37.7-52.1) | 3.78 (2.54-5.85) |
| Liver | 6,066 | 56 | 3024.34 | 11.5 (7.9-16.7) | 11.1 (7.6-16.3) | 1 (Reference) |
| Heart | 502 | 25 | 246.06 | 107.4 (68.5-168.4) | 104.2 (66.4-163.7) | 8.60 (4.71-15.39) |
| Lung | 168 | 2 | 83.78 | 18.2 (2.6-129.4) | 17.3 (2.4-123.2) | 1.30 (0.07-6.09) |
| Pancreas alone | 68 | 2 | 33.87 | 47.7 (6.7-338.6) | 52.9 (7.4-379.9) | 3.51 (0.20-16.94) |
| Multi-organ | 183 | 6 | 90.47 | 70.3 (29.3-169.0) | 72.7 (30.2-175.4) | 6.33 (2.13-15.24) |
| HSCT | 10,206 | 45 | 1291.82 | 9.0 (8.0-10.4) | 5.1 (4.6-6.1) | 850 (657-920)c |
Incidence rates are expressed as cases as per 1,000 person-years. aIndicate CMV disease except CMV syndrome. bAdjusted for age and sex. cComparison to the whole population. Aberrations: CMV, cytomegalovirus; F/U, follow up; HR, hazard ratio; IR, incidence rate
Characteristics of patients with CMV case
Among the 1,586 patients with CMV cases, 283 (17.9%) and 45 (2.8%) patients received SOT and HSCT, respectively. A total of 1,258 (79.3%) patients had no transplantation history (neither SOT nor HSCT). The SOT recipients, HSCT recipients, and non-transplant groups had similar mean age, male percentage, and ICD-10 codes for CMV diagnosis (Table 3). According to transplant organs, the percentage of CMV among total recipients was highest in heart transplantation (25 CMV cases/502 total recipients, 5.0%) followed by multi-organ (6/183, 3.3%), pancreas (2/68, 2.9%), kidney (192/9,381, 2.0%), lung (2/168, 1.2%), and liver transplantation (56/6,066, 0.9%) (Table 3, Figure 1, and Supplementary Table 1). The percentage of SOT recipients aged ≥ 40 years (68.9%) was significantly higher than that in HSCT recipients (55.6%) or non-transplant recipients (60.8%) (P = 0.026). The non-transplant population had the significantly higher rates of co-morbid diseases compared to SOT and HSCT recipients (P = 0.031). The post-transplantation duration before diagnosis of CMV cases was significantly shorter in HSCT recipients than in SOT recipients (2.2 ± 2.0 vs 3.4 ± 2.7 months, P = 0.007) (Table 3).
All-cause mortalities in patients with CMV cases
The percentage of all-cause death was significantly higher in HSCT recipients (28.9%) than in SOT recipients (6.4%) and non-transplant recipients (16.5%) (P < 0.001) (Table 3 and Figure 2). Cox's proportional hazards regression analysis showed that SOT recipients had a significantly lower mortality rate (adjusted IR [95% CI] per 1,000 person-years, 53.2 [33.4-84.8]; adjusted HR [95% CI], 0.4 [0.3-0.7] compared to that of non-transplant individuals with adjusted IR of 130 per 1,000 person-years). But, the adjusted mortality rate in the HSCT recipients (329.4 [190.8-568.7] per 1.000 person-years) was significantly higher with the adjusted HR of 2.4 (1.3-4.0) compared to the non-transplant individuals (log-rank P < 0.001). Among transplant organs, only kidney transplantation (33.4 [16.7-67.0] per 1,000 person-years) was associated with a significantly lower mortality rate (adjusted HR of 0.3 [0.1-0.5]) (Table 4).
Comparison of characteristics between solid organ, hematopoietic stem cell transplantation recipients, and non-transplant individuals among total 1,586 patients with CMV disease except CMV syndrome
| Characteristics | No transplantation (N = 1,258) | SOT (N = 283) | HSCT (N = 45) | P-value |
|---|---|---|---|---|
| Age, years | 42.1 ± 26.8 | 43.9 ± 17.0 | 40.9 ± 16.9 | 0.501 |
| ≥ 40-year-old | 765 (60.8) | 195 (68.9) | 25 (55.6) | 0.026 |
| Sex, male | 699 (55.6) | 148 (52.3) | 21 (46.7) | 0.331 |
| Co-morbid illnessa | 636 (50.6) | 97 (34.3) | 18 (40.0) | 0.031 |
| Type 2 DM | 375 (29.8) | 78 (27.6) | 14 (31.1) | 0.849 |
| ESRD on dialysis | 83 (6.6) | 2 (0.7) | 1 (2.2) | 0.017 |
| Chronic heart diseases | 64 (5.1) | 8 (2.8) | 3 (6.7) | 0.125 |
| Chronic liver diseases | 38 (3.0) | 10 (3.5) | 4 (8.9) | 0.367 |
| Chronic lung diseases | 93 (7.4) | 5 (1.8) | 1 (2.2) | 0.025 |
| HIV-1 infection | 20 (1.6) | 0 (0) | 0 (0) | — |
| Transplant organ | — | |||
| Single | — | 277 (97.9) | — | |
| Kidney | — | 192 (67.8) | — | |
| Liver | — | 56 (19.8) | — | |
| Heart | — | 25 (8.8) | — | |
| Lung | — | 2 (0.7) | — | |
| Pancreas alone | — | 2 (0.7) | — | |
| Multi-organ | — | 6 (2.1) | — | |
| CMV diagnosisb | 0.248 | |||
| B25 | 3 (0.2) | 0 (0) | 0 (0) | |
| B25.0 | 89 (7.1) | 6 (2.1) | 0 (0) | |
| B25.1 | 101 (8.0) | 4 (1.4) | 0 (0) | |
| B25.2 | 4 (0.3) | 0 (0) | 0 (0) | |
| B25.8 | 465 (37.0) | 80 (28.3) | 20 (44.4) | |
| B25.9 | 596 (47.4) | 193 (68.2) | 25 (55.6) | |
| Intervals between transplantation and CMV casesc, months | — | 3.4 ± 2.7 | 2.2 ± 2.0 | 0.007 |
| Transplant organs | ||||
| Kidney | — | 3.4 ± 2.7 | — | |
| Liver | — | 3.4 ± 2.8 | — | |
| Heart | — | 3.4 ± 2.5 | — | |
| Lung | — | 8.1 ± 3.0 | — | |
| Pancreas alone | — | 5.0 ± 0.8 | — | |
| Multi-organ | — | 2.9 ± 2.3 | — | |
| CMV diagnosisb | ||||
| B25.0 | — | 6.2 ± 3.5 | — | |
| B25.1 | — | 2.0 ± 1.8 | — | |
| B25.8 | — | 3.9 ± 2.7 | 2.2 ± 1.2 | |
| B25.9 | — | 3.2 ± 2.6 | 2.3 ± 1.9 | |
| All-cause death | 207 (16.5) | 18 (6.4) | 13 (28.9) | <0.001 |
Data are expressed as mean ± standard deviation or number (percentage). aSome patients had several illnesses. bBy WHO ICD-10 codes, version 2016 (B25: cytomegaloviral disease, B25.0: cytomegaloviral pneumonitis, B25.1: cytomegaloviral hepatitis, B25.2: cytomegaloviral pancreatitis, B25.8: other cytomegaloviral diseases, B25.9: cytomegaloviral disease, unspecified). cIndicate CMV disease except CMV syndrome. Aberrations: CMV, cytomegalovirus; DM, diabetes mellitus; ESRD, end stage renal disease; HIV-1, human immunodeficiency virus-1; HSCT, hematopoietic stem cell transplantation; f/u, follow-up; ICD-10, International Statistical Classification of Diseases and Related-Health Problems 10th Revision; SOT, solid organ transplantation, WHO, World Health Organization
All-cause mortality rates in solid organ transplantation recipients, hematopoietic stem cell transplantation recipients, and non-transplant individuals with CMV disease except CMV syndrome. *Log rank test. Aberrations: HSCT, hematopoietic stem cell transplantation; SOT, solid organ transplantation
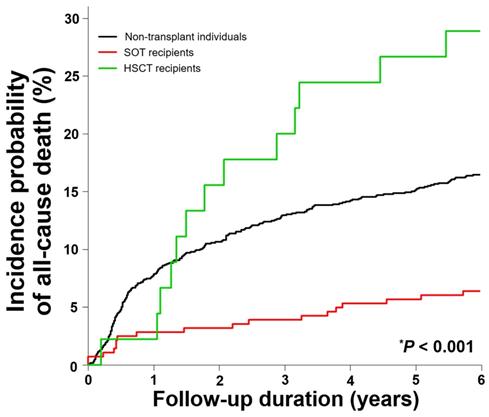
All-cause mortality rates in solid organ transplantation recipients, hematopoietic stem cell transplantation recipients, and non-transplant individuals with CMV disease except CMV syndrome in Cox's proportional hazards regression model
| No. | All-cause death | Total f/u Duration (years) | Unadjusted | Adjusteda | |||
|---|---|---|---|---|---|---|---|
| IR (95% CI) | HR (95% CI) | IR (95% CI) | HR (95% CI) | ||||
| Non-transplant individuals | 1,258 | 207 | 1111.27 | 186.3 (162.6-213.5) | 1 (Reference) | 129.9 (107.5-156.8) | 1 (Reference) |
| SOT | 283 | 18 | 271.45 | 66.3 (41.8-105.2) | 0.37 (0.22-0.57) | 53.2 (33.4-84.8) | 0.42 (0.25-0.67) |
| Kidney | 192 | 8 | 186.91 | 42.8 (21.4-85.6) | 0.23 (0.10-0.43) | 33.4 (16.7-67.0) | 0.25 (0.11-0.48) |
| Liver | 56 | 8 | 50.93 | 157.0 (78.6-314.1) | 0.84 (0.38-1.58) | 139.7 (69.6-280.4) | 1.04 (0.47-1.98) |
| Heart | 25 | 2 | 23.61 | 84.7 (21.2-338.7) | 0.45 (0.08-1.40) | 69.4 (17.3-277.8) | 0.51 (0.09-1.60) |
| Lung | 2 | 0 | — | — | — | — | — |
| Pancreas alone | 2 | 0 | — | — | — | — | — |
| Multi-organ | 6 | 0 | — | — | — | — | — |
| HSCT | 45 | 13 | 36.91 | 352.2 (204.5-606.5) | 1.81 (0.98-3.04) | 329.4 (190.8-568.7) | 2.37 (1.28-4.01) |
Incidence rates indicate cases as per 1,000 person-years. aAdjusted by age and sex using 2010 South Korea Population and Housing Census data. Aberrations: CI, confidence interval; CMV, cytomegalovirus; f/u, follow-up; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; IR, incidence rate; No., number; SOT, solid organ transplantation
Discussion
The new finding in our data from a large entire population-based cohort was that the recipients with post-SOT CMV cases had lower (approximately 60% decrease) all-cause mortality rate than that of non-transplant individuals, although the SOT recipient group had a significantly higher IR (nearly 5,000-fold) of CMV cases after transplantation compared to that of non-transplant individuals. The reduction in mortality rate was distinct in kidney transplant recipients (approximately 75% decrease). The comparison of IR or mortality of CMV cases in transplant recipients and non-transplant group has not been performed yet. Interestingly, the patients with CMV in each group were relatively young with a mean age of 40-44 years. This may be because non-transplant patients with various comorbidities may have a higher risk of adverse outcomes with CMV end-organ disease than SOT recipients, although post-SOT CMV disease could also be attributable to morbidity and mortality [30,31,49]. The relatively high all-cause mortality in individuals without transplantation might be related to the various co-morbid illnesses, especially ESRD on dialysis, chronic lung diseases, and HIV-1 infection, and/or the indirect effects of CMV disease, including the risk of infections by other bacteria or fungi [20,30,50].
Litjens et al. recently reported that donor-derived memory-like NKG2C+ natural killer cells and Vδ2negγδ T lymphocytes could be expanded by CMV replication, and the terminally differentiated TCR αβ+ T lymphocytes with poor alloimmunity, NKG2C gene expression, and resistance of the adaptive immune system driven by interferon could be enhanced during CMV infection [51]. These immunological alterations by active CMV replication may be associated with allograft acceptance in kidney, liver, or heart transplant recipients, as well as protection against post-HSCT leukemic relapse in patients with acute myeloid leukemia [27,51-57]. Even though further studies are needed to explore more evidence for causal relationships, some studies suggest that CMV infection or disease after transplantation might have beneficial effects to avoid various adverse morbidities or mortality in special recipients [51,58,59]. Our epidemiologic results might provide an opportunity to contemplate the large burden of CMV disease in non-transplant settings as well as the potential effect of CMV replication on a poor final outcome in transplant recipients with certain characteristics in actual clinical settings with CMV prevention strategies.
In this analysis, the IR of CMV in the HSCT cohort did not differ considerably from that in the SOT recipients, which may be associated with proper prevention, especially in HSCT recipients with a generally higher risk of adverse effects caused by CMV reactivation. Unlike the prior reports on CMV risk factors, our SOT cohort had a relatively low IR for CMV in lung transplant recipients [30,31]. The IR for CMV in lung transplantation may be underestimated due to the exclusion of CMV syndrome in our study. However, all previous studies presenting CMV epidemiology with percentage did not assess IR, taking into consideration the follow-up duration [21-29,31]. This first analysis assessing direct comparison of CMV IRs according to transplant organs in a specific cohort showed that liver or lung transplant recipients had lower IRs for CMV, and heart transplant recipients had the highest IRs. The CMV cases in the kidney, liver, and heart transplant recipients occurred at a similar time after SOT (mean of 3.4 months). During the follow-up period, most CMV cases developed in the early post-transplantation period in both SOT and HSCT recipients (mean of 3 and 2 months, respectively, and majority cases within 6 months) (Supplementary Figure 1A and 1B).
This large cohort study is limited by the lack of information on CMV preventive strategies in the SOT and HSCT cohorts. Regardless of the consensus guidelines for CMV prevention in transplant settings, standardized protocols, including dosage or duration of anti-CMV drugs, strategies or surveillance of prevention (especially, application of recent hybrid approaches), cut-off thresholds of CMV viral load, monitoring methods for preemptive therapy, or management of refractory/recurrent/relapsed cases would be quite heterogeneous for each institution or physician [30,35,45,60]. This is because sophisticated strategies are based on tailored management in accordance with several risk factors and clinical conditions [30,45]. Unfortunately, it was not possible to extract detailed data on CMV preventive strategies from the national database, and this process revealed only a few CMV cases in various subgroups. Additionally, we could not evaluate the IRs of CMV according to the serostatus of donors and recipients, because almost all adult Koreans among general population and transplant recipients are seropositive for CMV [61-63]. The small number of CMV cases in the childhood and adolescent groups would assure the extraction of symptomatic CMV cases with the exclusion of congenital CMV infection, CMV mononucleosis, or asymptomatic CMV detection in the total cohort (Supplementary Tables 2 and 3). However, the absence of CMV syndrome in our dataset could underestimate the IR of CMV, especially in the transplant recipients.
Nevertheless, our analysis is the first attempt to report the difference in IR and mortality between non-transplant individuals and SOT or HSCT recipients to assess the burden of CMV cases in clinical practice. Two previous cohort studies evaluated the association of CMV seropositivity with all-cause mortality in the adult general population without transplants, except in the pregnant women group. In 14,153 American adults, CMV seropositive rates were related to higher adjusted all-cause mortality (overall HR of 1.2) [42]. Another cohort of 13,000 adults in the UK confirmed that CMV seropositive status (59% seroprevalence) was associated with increased all-cause mortality (adjusted HR of 1.2) [43]. However, these studies with small HRs did not compare mortality in intensively immunocompromised patients. Another strength of our study is that we did not use the seroprevalence rates or CMV IgG titers to identify the CMV group. Furthermore, our CMV cohort could exclude asymptomatic CMV detection in blood or body fluids.
Conclusions
Our large cohort from the entire population-based database showed that non-transplant individuals with CMV, regardless of low incidence of CMV disease, had higher all-cause mortality than SOT recipients. This finding suggests that active CMV replication causing CMV disease may be associated with a potentially large burden because of life-threatening outcomes in other patient groups besides severely immunocompromised transplant recipients.
Supplementary Material
Supplementary figures and tables.
Author Contributions
S.H.H and K.H.L designed concept and supervised the study. K.D.H retrieved the cohort data from the large database and performed the statistical analysis. S.G.Y, D.E.K, Y.L and S.H.H summarized the detailed data and wrote the main manuscript text. All authors revised the manuscript critically for important intellectual content and approved the final version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Griffiths PD. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect Dis. 2012;12(10):790-8
2. Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4(12):725-38
3. Siegmund B. Cytomegalovirus infection associated with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2017;2(5):369-76
4. Letendre S, Bharti A, Perez-Valero I, Hanson B, Franklin D, Woods SP. et al. Higher Anti-Cytomegalovirus Immunoglobulin G Concentrations Are Associated With Worse Neurocognitive Performance During Suppressive Antiretroviral Therapy. Clin Infect Dis. 2018;67(5):770-7
5. Zhen J, Zeng M, Zheng X, Qiu H, Cheung BMY, Xu A. et al. Human cytomegalovirus infection is associated with stroke in women: the US National Health and Nutrition Examination Survey 1999-2004. Postgrad Med J. 2021 doi:10.1136/postgradmedj-2020-139201
6. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a Stroke Risk Factor and Determinant of Outcome After Stroke. Stroke. 2020;51(10):3156-68
7. Ji YN, An L, Zhan P, Chen XH. Cytomegalovirus infection and coronary heart disease risk: a meta-analysis. Mol Biol Rep. 2012;39(6):6537-46
8. Gugliesi F, Pasquero S, Griffante G, Scutera S, Albano C, Pacheco SFC. et al. Human Cytomegalovirus and Autoimmune Diseases: Where Are We? Viruses. 2021 13(2)
9. Yoo SG, Han KD, Lee KH, La Y, Kwon DE, Han SH. Impact of Cytomegalovirus Disease on New-Onset Type 2 Diabetes Mellitus: Population-Based Matched Case-Control Cohort Study. Diabetes Metab J. 2019;43(6):815-29
10. Zhang J, Liu YY, Sun HL, Li S, Xiong HR, Yang ZQ. et al. High Human Cytomegalovirus IgG Level is Associated with Increased Incidence of Diabetic Atherosclerosis in Type 2 Diabetes Mellitus Patients. Med Sci Monit. 2015;21:4102-10
11. Stebbins RC, Noppert GA, Yang YC, Dowd JB, Simanek A, Aiello AE. Immune Response to Cytomegalovirus and Cognition in the Health and Retirement Study. Am J Epidemiol. 2020 doi:10.1093/aje/kwaa238
12. Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B. et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171(10):1144-52
13. Schmidt L, Nelson HH, Thyagarajan B, Hunter-Schlichting D, Pankow JS, Capistrant B. et al. Association between cytomegalovirus seropositivity and Type 2 diabetes is explained by age and other demographic characteristics: the National Health and Nutrition Examination Survey. Diabet Med. 2018;35(12):1722-6
14. Schildermans J, De Vlieger G. Cytomegalovirus: A Troll in the ICU? Overview of the Literature and Perspectives for the Future. Front Med (Lausanne). 2020;7:188
15. Limaye AP, Boeckh M. CMV in critically ill patients: pathogen or bystander? Rev Med Virol. 2010;20(6):372-9
16. Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009;37(8):2350-8
17. Papazian L, Hraiech S, Lehingue S, Roch A, Chiche L, Wiramus S. et al. Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016;42(1):28-37
18. Inoue N, Abe M, Kobayashi R, Yamada S. Vaccine Development for Cytomegalovirus. Adv Exp Med Biol. 2018;1045:271-96
19. Plotkin SA, Wang D, Oualim A, Diamond DJ, Kotton CN, Mossman S. et al. The Status of Vaccine Development Against the Human Cytomegalovirus. J Infect Dis. 2020;221(Suppl 1):S113-s22
20. Griffiths P. The direct and indirect consequences of cytomegalovirus infection and potential benefits of vaccination. Antiviral Res. 2020;176:104732
21. Bosch W, Heckman MG, Diehl NN, Shalev JA, Pungpapong S, Hellinger WC. Association of cytomegalovirus infection and disease with death and graft loss after liver transplant in high-risk recipients. Am J Transplant. 2011;11(10):2181-9
22. Parsaik AK, Bhalla T, Dong M, Rostambeigi N, Dierkhising RA, Dean P. et al. Epidemiology of cytomegalovirus infection after pancreas transplantation. Transplantation. 2011;92(9):1044-50
23. Linares L, Sanclemente G, Cervera C, Hoyo I, Cofán F, Ricart MJ. et al. Influence of cytomegalovirus disease in outcome of solid organ transplant patients. Transplant Proc. 2011;43(6):2145-8
24. Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transpl. 2005;11(6):700-4
25. Brennan DC. Cytomegalovirus in renal transplantation. J Am Soc Nephrol. 2001;12(4):848-55
26. Liu PY, Cheng SB, Lin CC, Lin CH, Chang SN, Cheng CY. et al. Cytomegalovirus disease after liver transplantation: a nationwide population-based study. Transplant Proc. 2014;46(3):832-4
27. Boutolleau D, Coutance G, Désiré E, Bouglé A, Bréchot N, Leprince P. et al. Association between cytomegalovirus infection and allograft rejection in a large contemporary cohort of heart transplant recipients. Transpl Infect Dis. 2021 doi:10.1111/tid.13569.e13569
28. Klimczak-Tomaniak D, Roest S, Brugts JJ, Caliskan K, Kardys I, Zijlstra F. et al. The Association Between Cytomegalovirus Infection and Cardiac Allograft Vasculopathy in the Era of Antiviral Valganciclovir Prophylaxis. Transplantation. 2020;104(7):1508-18
29. Schachtner T, Zaks M, Otto NM, Kahl A, Reinke P. Simultaneous pancreas/kidney transplant recipients are predisposed to tissue-invasive cytomegalovirus disease and concomitant infectious complications. Transpl Infect Dis. 2017 19(5)
30. Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L. et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018;102(6):900-31
31. Beam E, Razonable RR. Cytomegalovirus in solid organ transplantation: epidemiology, prevention, and treatment. Curr Infect Dis Rep. 2012;14(6):633-41
32. Chen PM, Hsiao LT, Tang JL, Yen CC, Liu JH, Lin KH. et al. Haematopoietic stem cell transplantation in Taiwan: past, present, and future. Hong Kong Med J. 2009;15(3 Suppl 3):13-6
33. Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003-8
34. Asano-Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H. et al. Clinical features of late cytomegalovirus infection after hematopoietic stem cell transplantation. Int J Hematol. 2008;87(3):310-8
35. El Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128(23):2624-36
36. Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170(1):22-48
37. Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM. et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119-27
38. Lehto JT, Lemström K, Halme M, Lappalainen M, Lommi J, Sipponen J. et al. A prospective study comparing cytomegalovirus antigenemia, DNAemia and RNAemia tests in guiding pre-emptive therapy in thoracic organ transplant recipients. Transpl Int. 2005;18(12):1318-27
39. Raval AD, Kistler KD, Tang Y, Murata Y, Snydman DR. Epidemiology, risk factors, and outcomes associated with cytomegalovirus in adult kidney transplant recipients: A systematic literature review of real-world evidence. Transpl Infect Dis. 2020 doi:10.1111/tid.13483.e13483
40. Di Benedetto S, Gaetjen M, Müller L. The Modulatory Effect of Gender and Cytomegalovirus-Seropositivity on Circulating Inflammatory Factors and Cognitive Performance in Elderly Individuals. Int J Mol Sci. 2019 20(4)
41. Blum A, Peleg A, Weinberg M. Anti-cytomegalovirus (CMV) IgG antibody titer in patients with risk factors to atherosclerosis. Clin Exp Med. 2003;3(3):157-60
42. Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6(2):e16103
43. Gkrania-Klotsas E, Langenberg C, Sharp SJ, Luben R, Khaw KT, Wareham NJ. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis. 2013;56(10):1421-7
44. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G. et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87-91
45. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA. et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260-e72
46. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data Configuration and Publication Trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J. 2020;44(5):671-8
47. Yoo KB, Ahn HU, Park EC, Kim TH, Kim SJ, Kwon JA. et al. Impact of co-payment for outpatient utilization among Medical Aid beneficiaries in Korea: A 5-year time series study. Health Policy. 2016;120(8):960-6
48. World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision, Version: 2016. Revised April 20, 2021. https://icd.who.int/browse10/2016/en
49. Pérez-Nadales E, Gutiérrez-Gutiérrez B, Natera AM, Abdala E, Reina Magalhães M, Mularoni A. et al. Predictors of mortality in solid organ transplant recipients with bloodstream infections due to carbapenemase-producing Enterobacterales: The impact of cytomegalovirus disease and lymphopenia. Am J Transplant. 2019 doi:10.1111/ajt.15769
50. Freeman RB Jr. The 'indirect' effects of cytomegalovirus infection. Am J Transplant. 2009;9(11):2453-8
51. Litjens NHR, van der Wagen L, Kuball J, Kwekkeboom J. Potential Beneficial Effects of Cytomegalovirus Infection after Transplantation. Front Immunol. 2018;9:389
52. Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S. et al. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol. 2009;20(4):238-42
53. Tu W, Potena L, Stepick-Biek P, Liu L, Dionis KY, Luikart H. et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation. 2006;114(15):1608-15
54. Shi XL, de Mare-Bredemeijer EL, Tapirdamaz Ö, Hansen BE, van Gent R, van Campenhout MJ. et al. CMV Primary Infection Is Associated With Donor-Specific T Cell Hyporesponsiveness and Fewer Late Acute Rejections After Liver Transplantation. Am J Transplant. 2015;15(9):2431-42
55. Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B. et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30(2):456-63
56. Ito S, Pophali P, Co W, Koklanaris EK, Superata J, Fahle GA. et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48(10):1313-6
57. Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T. et al. Cytomegalovirus Reactivation after Allogeneic Hematopoietic Stem Cell Transplantation is Associated with a Reduced Risk of Relapse in Patients with Acute Myeloid Leukemia Who Survived to Day 100 after Transplantation: The Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant. 2015;21(11):2008-16
58. Bigley AB, Baker FL, Simpson RJ. Cytomegalovirus: an unlikely ally in the fight against blood cancers? Clin Exp Immunol. 2018;193(3):265-74
59. Manjappa S, Bhamidipati PK, Stokerl-Goldstein KE, DiPersio JF, Uy GL, Westervelt P. et al. Protective effect of cytomegalovirus reactivation on relapse after allogeneic hematopoietic cell transplantation in acute myeloid leukemia patients is influenced by conditioning regimen. Biol Blood Marrow Transplant. 2014;20(1):46-52
60. Bruminhent J, Bushyakanist A, Kantachuvesiri S, Kiertiburanakul S. A Nationwide Survey of Cytomegalovirus Prevention Strategies in Kidney Transplant Recipients in a Resource-Limited Setting. Open Forum Infect Dis. 2019;6(9):ofz322
61. La Y, Kwon DE, Yoo SG, Lee KH, Han SH, Song YG. Human cytomegalovirus seroprevalence and titres in solid organ transplant recipients and transplant donors in Seoul, South Korea. BMC Infect Dis. 2019;19(1):948
62. Choi R, Lee S, Lee SG, Lee EH. Seroprevalence of CMV IgG and IgM in Korean women of childbearing age. J Clin Lab Anal. 2021 doi:10.1002/jcla.23716.e23716
63. Park M, Lee YH, Lee SH, Yoo KH, Sung KW, Koo HH. et al. Cytomegalovirus infection in seropositive unrelated cord blood recipients: a study of 349 Korean patients. Ann Hematol. 2015;94(3):481-9
Author contact
![]() Corresponding author: Sang Hoon Han, M.D., Ph.D. Department of Internal Medicine, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul, 06273, Republic of Korea. Tel: +82-2-2019-3319, Fax: +82-2-3463-3882, E-mail: shhan74ac
Corresponding author: Sang Hoon Han, M.D., Ph.D. Department of Internal Medicine, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul, 06273, Republic of Korea. Tel: +82-2-2019-3319, Fax: +82-2-3463-3882, E-mail: shhan74ac

 Global reach, higher impact
Global reach, higher impact