3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(7):1618-1627. doi:10.7150/ijms.50804 This issue Cite
Research Paper
Hypoxia protects H9c2 cells against Ferroptosis through SENP1-mediated protein DeSUMOylation
1. Qinghai Provincial People's Hospital, Xining, 810001, PR China.
2. Beijing Institute of Radiation Medicine, Beijing, 100850, PR China.
3. Research Center for High Altitude Medicine, Qinghai University, Xining, 810001, PR China.
4. Department of Molecular Diagnosis and Regenerative Medicine, Medical Research Center, the Affiliate Hospital of Qingdao University, Qingdao 266000, PR. China.
Abstract
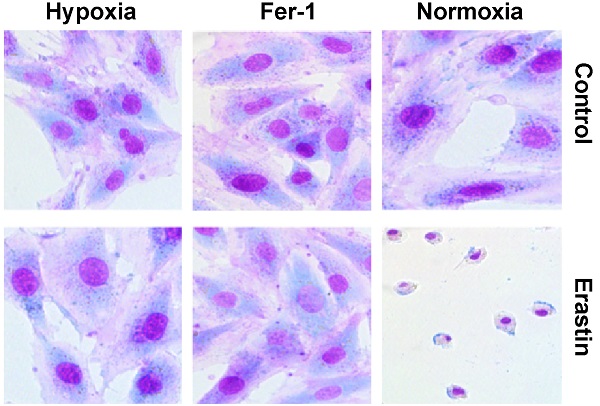
Hypoxia affects proliferation, differentiation, as well as death of cardiomyocyte, and plays an important role in the development of myocardial ischemia. However, the detailed mechanisms through which hypoxia regulates cardiomyocyte ferroptosis have not been explored. In this study, we revealed that hypoxia suppresses the proliferation, migration, and erastin-induced ferroptosis of H9c2 cells. First, we confirmed the upregulation of SENP1 in H9c2 cells cultured under hypoxic conditions. Through adenovirus-mediated SENP1 gene transfection, we demonstrated that SENP1 overexpression could enhance H9c2 cell proliferation and migration while also protecting H9c2 cells from erastin-induced ferroptosis. Furthermore, through immunoprecipitation and western blotting, we confirmed that SENP1 mediated deSUMOylation of HIF-1α and ACSL4 in H9c2 cells. In conclusion, this study describes the underlying mechanism through which hypoxia upregulates SENP1 expression, in turn protecting against ferroptosis via the regulation of HIF-1α and ACSL4 deSUMOylation. Our findings provide a theoretical foundation for the development of novel therapeutics for ischemic heart diseases.
Keywords: Ferroptosis, Hypoxia, SENP1

 Global reach, higher impact
Global reach, higher impact