Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(2):520-527. doi:10.7150/ijms.50705 This issue Cite
Research Paper
The role of chest CT in management of asymptomatic SARS-CoV-2 infections: A longitudinal multi-center study in Chongqing, China
1. Department of Radiology, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
2. Department of Radiology, the Second Affiliated Hospital of Chongqing Medical University at Fengjie, Chongqing, China.
3. Department of Radiology, Yongchuan Hospital of Chongqing Medical University, Chongqing, China.
4. Department of Radiology, Chongqing Three Gorges Central Hospital, Chongqing, China.
5. Department of Radiology, People's Hospital of Changshou Chongqing, Chongqing, China.
Received 2020-7-15; Accepted 2020-11-17; Published 2021-1-1
Abstract
Background: Multiple societies including the Fleischner Society do not recommend that CT is routinely used in asymptomatic SARS-CoV-2 infections; however, this advice is based on the limited evidence. In this study, we aim to confirm whether it is necessary to do CT scans in SARS-CoV-2 asymptomatic infections by summarizing the longitudinal chest CT and clinical features of asymptomatic SARS-CoV-2 infections.
Methods: A total of 33 individuals (14 men and 19 women) with asymptomatic SARS-CoV-2 infections were retrospectively enrolled. Clinical data of CT positive and negative groups were compared. Longitudinal chest CT scans were reviewed for CT features and analyzed for temporal change.
Results: Thirty-two (97%) individuals had positive results for first RT-PCR testing. For clinical data, only monocyte count showed a significant difference between CT positive and negative groups. For first chest CT, only eighteen (54.5%) individuals had abnormal manifestations, common CT features were GGO (88.9%) and consolidation (33.3%), the median number of segments involved was 3.0 (1.0-7.5). No case in CT negative group was abnormal on the follow-up CT. Three patterns of evolution throughout series of CT were observed in CT positive group, including gradual improvement (12, 66.7%), mismatch to improvement (3, 16.7%) and mild progression to improvement (3, 16.7%). On last CT scans, most cases had radiographic improvement but residual abnormalities. Significant differences were exhibited in density, long diameter, number of lung segments involved, and percentage of consolidation between the first and last CT scans. All cases had stable conditions and finally confirmed negative for SARS-CoV-2 RT-PCR tests without developing into severe pneumonia.
Conclusion: Considering poor performance of CT in screening, stable conditions during followup, and good outcomes in asymptomatic SARS-CoV-2 infections, we confirm that it is unnecessary to do CT scans in asymptomatic SARS-CoV-2 infections.
Keywords: SARS-CoV-2, COVID-19, Asymptomatic infections, Chest CT
Introduction
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing human disease officially named Corona Virus Disease 2019 (COVID-19), was found in Wuhan, Hubei Province, China [1-5]. Currently, human-to-human transmission of the virus accounts for most infections worldwide [6]. And this disease has spread to an increasing number of countries, areas or territories around the globe; there were more confirmed cases reported outside China than inside China, and many new epicentres of spread have emerged [7]. On the basis of “alarming levels of spread and severity, and by the alarming levels of inaction”, on March 11, 2020, the COVID-19 situation was characterized as a pandemic by the Director-General of WHO [8,9]. Up to 2 November 2020, WHO has officially reported over 46 million confirmed COVID-19 cases and 1.1 million confirmed deaths [7].
Asymptomatic SARS-CoV-2 infections have been evaluated to comprise 18%-46% of all infections [10-12]. As asymptomatic infections are very covert and may be a vital contagious source [13-19]. Besides early studies showed low sensitivity of SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) test (the golden standard for confirmation of SARS-CoV-2 infection) and high sensitivity of chest CT [20-23]. So numerous studies recommend that combining assessment of chest CT and RT-PCR could facilitate early diagnosis of SARS-CoV-2 infections [20, 21, 24-26], consequently, the number of CTs performed in persons under investigation for SARS-CoV-2 infections has increased [27]. However, according to recent statements of multiple societies including the Fleischner Society [28], CT is not routinely indicated as a screening test for COVID-19 in asymptomatic individuals, based on the limited evidence. For this reason, we summarized the longitudinal chest CT and clinical features of asymptomatic SARS-CoV-2 infections to confirm whether it is necessary to do CT scans in asymptomatic infections.
Materials and Methods
This retrospective study was approved by the Institutional Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University, and the requirement for informed consent was waived.
Study population
According to the Prevention and Control of COVID-19 of China (version sixth) [29], all individuals confirmed as asymptomatic SARS-CoV-2 infections by RT-PCR from January 2020 to February 2020 were recruited in this study. The inclusion criteria were as follows: 1) all cases were asymptomatic when they were confirmed as SARS-CoV-2 infections and were subsequently hospitalized isolation; 2) all cases underwent first chest CT (obtained within two days of first positive SARS-CoV-2 result) and last CT (obtained within two days of first negative SARS-CoV-2 result from two consecutive detection) examinations; 3) all cases were followed up to discharge. The exclusion criteria: having severe artifacts on their CT images. Finally, a total of 33 individuals (14 men and 19 women) were included in the study with mean age of 43.2 years (SD 14.3), including 10 individuals from the Second Affiliated Hospital of Chongqing Medical University at Fengjie, 10 individuals from Yongchuan Hospital of Chongqing Medical University, nine individuals from Chongqing Three Gorges Central Hospital, and four individuals from People's Hospital of Changshou Chongqing.
According to the first chest CT manifestations, the eligible individuals were split into two groups: CT positive and negative groups. The clinical parameters included age, gender, signs, laboratory findings, and time of PT-PCR conversion (calculated from the day when SARS-CoV-2 was detected by RT-PCR to the first day of two consecutive negative results of RT-PCR) were collected and evaluated.
CT examinations and imaging evaluation
All chest CT scans were obtained using four multi-detector CT scanners: SOMATOM go.Top (Siemens Healthineers, Germany), SOMATOM Sensation 16 (Siemens Healthineers, Germany), Light Speed16 (GE Medical Systems, USA) and Asteion (TOSHIBA, Japan). Two chest radiologists with 10 and 8 years of experience who were blinded to the clinical data evaluated the CT findings in consensus. For each of the individuals, the first and last chest CT images were evaluated for the following characteristics based on the Fleischner Society Nomenclature recommendations [30] and similar studies [24,31]: ground-glass opacity (GGO), consolidation, linear opacities, interlobular septal thickening, crazy-paving pattern, “spider web sign”, subpleural curvilinear line, thickening of the adjacent pleura, lymphadenopathy, pleural effusion and pericardial effusion. What's more, the margin definition of the max lesion, distribution, location and extent of abnormalities were recorded [24].
All the follow-up CT images were evaluated for: 1) the patterns of evolution throughout the series of CT scans [24], 2) the long diameter (cm) and density (HU) of the max lesion of the lung, 3) the number of segments involved.
The median volume CT dose index and dose-length product for CT acquisition were 11.1 mGy (range, 5.5-18.4) and 364.8 mGy∙cm (range, 215-750), respectively, corresponding to an effective radiation dose of 5.1 mSv (range, 3.0-10.5) (using a standard conversion factor for chest CT of 0.014 mSv/mGy∙cm).
Statistical Analysis
Day 0 was defined as the day of first positive SARS-CoV-2 result. Categorical variables were expressed as number (%), and quantitative variables were expressed as mean (SD) or median (interquartile range, IQR) values. Comparisons of clinical features between CT positive and CT negative groups, χ2 test and Fisher exact test were used for categorical variables. Quantitative variables were tested for normality by using Shapiro-Wilk tests, normally distributed data were analyzed by independent sample t test; otherwise, the Mann-Whitney U test was used. Comparisons of CT features between first and last CT scans were performed by using paired Student t test or Wilcoxon sign-rank test for continuous data and the McNemar test or Marginal Homogeneity test for categorical data. Differences with p < 0.05 were considered statistically significant. All statistical analyses were done by using SPSS statistical software (version 20.0 IBM).
Results
Subject characteristics
All individuals had some contact with SARS-CoV-2 infections. Thirty-two (97%) individuals had positive results for first RT-PCR testing, one individual had positive results for second RT-PCR testing. Multiple laboratory indicators were abnormal; the common features were increased procalcitonin (57.6%) and decreased lymphocyte count (36.4%). Individuals were assigned to two groups on the basis of CT findings: CT positive group (18/33, 54.5%) and CT negative group (15/33, 45.5%). The clinical characteristics and laboratory results of individuals by group were summarized in the Table 1. No significant differences in age (p = 0.276) or sex distribution (p = 0.062) between groups were identified. For all the signs of admission, no significant differences were found between the two groups (p > 0.05). The monocyte count of CT positive group was significantly higher compared with that of CT negative group; however the other laboratory parameters were not significantly different for the two groups. More details were summarized in the Table 1.
During the follow up, although five individuals (27.8%) in CT positive group and four individuals (26.7%) in CT negative group occurred mild symptoms after an average of three days from first CT scans, all individuals had stable conditions and finally confirmed negative for SARS-CoV-2 RT-PCR tests without developing into severe pneumonia. The time for RT-PCR conversion of CT positive group and CT negative group was 13 days (IQR, 8-9) and 11 days (IQR, 6-14), respectively; and there was no statistical difference between the two groups.
First chest CT findings
Eighteen (54.5%) individuals had abnormal CT manifestations of infections. The common CT features included GGO (16/18, 88.9%) and consolidation (6/18, 33.3%) (Table 2). Two (11.1%) individuals had linear opacities, and one (5.6%) individual had subpleural curvilinear line. Lymphadenopathy, pleural effusion and pericardial effusion were absent in all individuals. Max lesions of 12 (66.7%) individuals were ill-defined margins. The median long diameter and density of the max lesion were 2.3 cm and -305.6HU, respectively.
Clinical characteristics and laboratory findings of asymptomatic SARS-CoV-2 infections
| Parameter | Total (n=33) | CT positive (n=18) | CT negative (n=15) | p |
|---|---|---|---|---|
| Age (y) | 43.2 (14.3) | 45.7 (15.0) | 40.2 (13.3) | 0.276 |
| Sex | 0.062 | |||
| Male | 14 (42.4%) | 5 (27.8%) | 9 (60.0%) | |
| Female | 19 (57.6%) | 13 (72.2%) | 6 (40.0%) | |
| Temperature (°C) | 36.50 (36.30-36.60) | 36.50 (36.20-36.60) | 36.40 (36.40-36.60) | 0.929 |
| Heart rate (bpm) | 79.58 (8.23) | 81.50 (8.75) | 77.27 (7.17) | 0.144 |
| Respiratory rate | 20.00 (19.50-20.00) | 20.00 (19.75-20.25) | 20.00 (19.00-20.00) | 0.421 |
| White blood cell count (×109/L) | 6.35 (5.24-7.02) | 6.11 (5.25-6.64) | 6.90 (5.18-7.47) | 0.343 |
| Increased | 3 (9.1%) | 2 (11.1%) | 1 (6.7%) | 1.000 |
| Decreased | 1 (3.0%) | 0 (0.0%) | 1 (6.7%) | 0.455 |
| Neutrophil ratio (%) | 65.20 (60.25-75.75) | 62.60 (59.23-76.00) | 69.80 (63.70-75.60) | 0.274 |
| Increased | 10 (30.3%) | 6 (33.3%) | 4 (26.7%) | 0.927 |
| Lymphocyte ratio (%) | 23.68 (9.21) | 22.64 (9.83) | 24.93 (8.57) | 0.487 |
| Decreased | 10 (30.3%) | 7 (38.9%) | 3 (20.0%) | 0.426 |
| Monocyte ratio (%) | 7.07 (3.09) | 7.99 (3.46) | 5.97 (2.24) | 0.061 |
| Increased | 5 (15.2%) | 4 (22.2%) | 1 (6.7%) | 0.346 |
| Decreased | 1 (3.0%) | 1 (5.6%) | 0 (0.0%) | 1.000 |
| Neutrophil count (×109/L) | 4.10 (2.93-4.98) | 3.94 (2.90-4.86) | 4.39 (3.03-5.75) | 0.442 |
| Increased | 4 (12.1%) | 3 (16.7%) | 1 (6.7%) | 0.607 |
| Decreased | 1 (3.0%) | 0 (0.0%) | 1 (6.7%) | 0.455 |
| Lymphocyte count (×109/L) | 1.35 (0.46) | 1.34 (0.44) | 1.37 (0.50) | 0.878 |
| Decreased | 12 (36.4%) | 7 (38.9%) | 5 (33.3%) | 0.741 |
| Monocyte count (×109/L) | 0.38 (0.14) | 0.43 (0.15) | 0.32 (0.11) | 0.023 |
| Increased | 2 (6.1%) | 2 (11.1%) | 0 (0.0%) | 0.489 |
| Procalcitonin (ng/mL) | 0.050 (0.030-0.068) | 0.050 (0.038-0.075) | 0.050 (0.030-0.060) | 0.682 |
| Increased | 19 (57.6%) | 11 (61.1%) | 8 (53.3%) | 0.653 |
| Mild symptoms on during the follow-up | 9 (27.3%) | 5 (27.8%) | 4 (26.7%) | 1.000 |
| Time with mild symptoms onset after first CT (d) | 3.0 (2.5-7.0) | 3.0 (2.5-5.5) | 5.5 (2.0-7.5) | 0.556 |
| Time of RT-PCR conversion (d) | 12.0 (7.5-15.5) | 13.0 (8.0-19.0) | 11.0 (6.0-14.0) | 0.215 |
Data are n (%), mean (SD), median (IQR). Increased means over the upper limit of the normal range and decreased means below the lower limit of the normal range. bpm, beats per minute; RT-PCR, reverse transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The common lung segments involved were lateral basal segments of bilateral lower lobes and posterior segment of right lower lobe. The median number of segments involved was 3.0 (IQR, 1.0-7.5). Ten (55.6%) individuals had bilateral lung involvement, 12 (66.7%) individuals showed subpleural distribution, 12 (66.7%) individuals showed posterior distribution, and eight (44.4%) showed diffuse distribution of CT abnormalities.
First and last CT features of CT positive group with asymptomatic SARS-CoV-2 infections
| First CT scan (n = 18) | Last CT scan (n = 18) | p | |
|---|---|---|---|
| Margin definition | 0.102 | ||
| Well-defined | 4 (22.2%) | 3 (16.7%) | |
| Ill-defined | 12 (66.7%) | 12 (66.7%) | |
| Partial ill-defined | 2 (11.1%) | 1 (5.6%) | |
| No lesion | / | 2 (11.1%) | |
| CT value (HU) | -305.6 ([-519.9]- [-108.1]) | -701.9 ([-743.5]-[-540.3]) | 0.003 |
| Long diameter (cm) | 2.3 (1.6-4.2) | 1.7 (1.2-3.1) | 0.001 |
| Ground glass opacity | 16 (88.9%) | 16 (88.9%) | 1.000 |
| Consolidation | 6 (33.3%) | 0 (0.0%) | 0.031 |
| Linear opacities | 2 (11.1%) | 4 (22.2%) | 0.625 |
| Interlobular septal thickening | 2 (11.1%) | 0 (0.0%) | 0.500 |
| Crazy-paving pattern | 1 (5.6%) | 0 (0.0%) | 1.000 |
| Spider web sign | 4 (22.2%) | 0 (0.0%) | 0.125 |
| Subpleural curvilinear line | 1 (5.6%) | 0 (0.0%) | 1.000 |
| Thickening of the adjacent pleura | 4 (22.2%) | 1 (5.6%) | 0.375 |
Data are n (%), median (IQR). Percentages may not total 100 because of rounding. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Follow-up chest CT findings
No case in CT negative group was abnormal on the follow-up CT. Eighteen individuals of CT positive group had at least one follow-up chest CT examination. The mean interval time from first CT to last CT was 12.4 days (SD, 6.6). Three patterns of evolution throughout the series of CT scans were observed among these 18 individuals: gradual improvement (type-GI), followed by mismatch to improvement (type-MI), and mild progression to improvement (type-PI).
The CT findings of 12 (66.7%) individuals showed type-GI, that is decrease in extent and density from the first to last CT scans (Fig. 1). The median interval time from first CT scans to last CT scans was 7.5 days (range, 3-18). Three of them had mild coughs, and the period from the date of first CT scans to the symptom onset ranged from 2 to 3 days. All of the 12 individuals had RT-PCR conversion with a median interval of 9.5 days (range, 5-19).
Three (16.7%) individuals showed type-MI, that is the trends of the extent and density were not similar to each other in the early stage and then improvement (Fig. 2). Two individuals showed the increase in long diameter and decrease in density on the second CT scans (2-3 days after the first positive SARS-CoV-2 result), followed by improvement. What's more, one individual showed the decrease in long diameter and increase in density on the second CT scan (7 days after the first positive SARS-CoV-2 result), followed by improvement. The interval time from first CT scans to last CT scans ranged from 14 to 24 days. The time for RT-PCR conversion of the three individuals ranged 14 to 26 days.
Three (16.7%) individuals showed type-PI, that is the mild increase in extent and density on the second CT scans (3 days [range, 2-4 days] after first positive SARS-CoV-2 result) and then improvement (Fig. 3). The interval time from first CT scans to last CT scans ranged from 21 to 23 days. One individual had a mild cough at the time of progress. The time for RT-PCR conversion of the three individuals ranged 19 to 21 days.
Last chest CT findings
On the last CT scans, two individuals had complete resolution of lung abnormalities, and the other 16 individuals had residual lesions including GGO (88.9%) and linear opacities (22.2%). The percentage of consolidation showed a decrease on the first CT to last CT, and the difference was statistically significant (p = 0.031). The median long diameter and density of the max lesion on the last CT were 1.7cm and -701.9HU, respectively, which were statistically smaller than those of the first CT (Table 2).
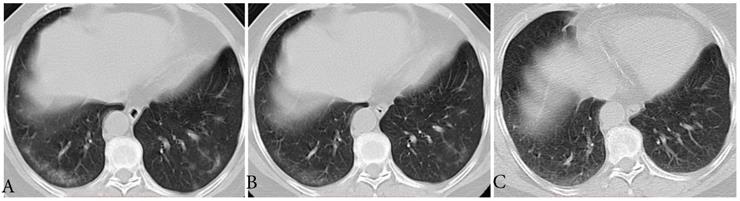
Typical evolution of type-GI in a 61-year-old female with asymptomatic SARS-CoV-2 infection. A, Day 0, the first chest CT showed multifocal lesions of subpleural GGO in bilateral lower lobe. B, Day 3, obvious resolution of the first GGO was observed. C, Day 6, continued resolution with minimal residual GGO was observed, the patient had two consecutive negative results of RT-PCR (day 6 and 7). GGO, ground-glass opacity; GI, gradual improvement; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
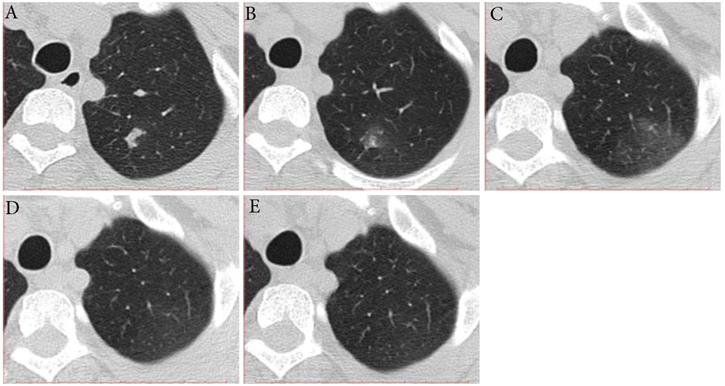
Typical evolution of type-MI in a 39-year-old female with asymptomatic SARS-CoV-2 infection. A, Day 0, the first chest CT showed a focal consolidation in apical posterior segment of left upper lobe. B-C, Day 2 and 6, the second to third CT scans showed increase in extent and decrease in density. D, Day 10, obvious resolution of the previous GGO was observed. E, Day 14, full resolution of the lesion was observed; and day 14 and 15, the patient had two consecutive negative results of RT-PCR. GGO, ground-glass opacity; MI, mismatch to improvement; RT-PCR, reverse transcriptase-polymerase chain reaction.
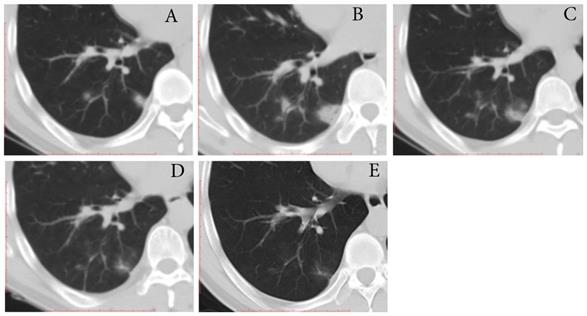
Typical evolution of type-PI in a 48-year-old male with asymptomatic SARS-CoV-2 infection. A, Day 0, the first chest CT showed multifocal lesions of consolidation in right lower lobe. B, Day 2, mild progression was showed on the second CT scan. C-E, Day 8-21, gradual resolution was observed on the third to last CT scans, and minimal residual GGO and linear opacities were observed on the last CT scan; And day 21 and 22, the patient had two consecutive negative results of RT-PCR. GGO, ground-glass opacity; PI, progression to improvement; RT-PCR, reverse transcriptase-polymerase chain reaction.
The median number of segments involved on last CT (1.0 [IQR, 1.0-3.2]) was significantly smaller than that of the first CT (3.0 [IQR, 1.0-7.5], p = 0.005). The percent of bilateral distribution, random distribution, both anterior and posterior distribution, and diffuse involvement showed decreased on the first CT to last CT (Table 3).
Discussion
Although the literature on SARS-CoV-2 infections has grown exponentially, most of them focused on symptomatic patients [24,32-35], especially on severe and critical patients [31,35,36]. Recently, several studies have reported chest CT of asymptomatic SARS-CoV-2 infections. However, most of these asymptomatic studies were cross-sectional and focused on summarizing of CT features or screening [25,37-39]. A comprehensive and longitudinal study (including screening, CT features and evolution of asymptomatic SARS-CoV-2 infections) was scarcely reported. Besides, the chest CT role in early studies may be overestimated and the overuse of CT will inevitably increase the risk of radiation damage and cross-infection. So multiple societies including the Fleischner Society do not recommend that CT is routinely used in asymptomatic individuals [28], however, this advice is based on the limited evidence. In this context, we conducted this study to confirm whether it is necessary to do CT scans of screening and follow-up in asymptomatic infections. We found low sensitivity of initial CT, no obvious progression of lung lesions and stable conditions during followup, and good outcomes in asymptomatic SARS-CoV-2 infections. And we found a novel pattern (type-MI) of evolution in asymptomatic SARS-CoV-2 infections.
First and last CT distribution and extent of lung lesions of CT positive group with asymptomatic SARS-CoV-2 infections
| The lung segment involved | First CT scan(n = 18) | Last CT scan(n = 18) | p |
|---|---|---|---|
| Left upper lobe | |||
| Apical posterior | 7 (38.9%) | 3 (16.7%) | 0.125 |
| Anterior | 3 (16.7%) | 1 (5.6%) | 0.500 |
| Superior lingula | 3 (16.7%) | 0 (0.0%) | 0.250 |
| Inferior lingula | 3 (16.7%) | 0 (0.0%) | 0.250 |
| Left lower lobe | |||
| Superior | 5 (27.8%) | 1 (5.6%) | 0.125 |
| Medial Anterior basal | 5 (27.8%) | 1 (5.6%) | 0.125 |
| Lateral basal | 9 (50.0%) | 7 (38.9%) | 0.500 |
| Posterior | 4 (22.2%) | 3 (16.7%) | 1.000 |
| Right upper lobe | |||
| Apical | 3 (16.7%) | 0 (0.0%) | 0.250 |
| Posterior | 5 (27.8%) | 3 (16.7%) | 0.500 |
| Anterior | 3 (16.7%) | 1 (5.6%) | 0.500 |
| Right middle lobe | |||
| Lateral | 4 (22.2%) | 1 (5.6%) | 0.250 |
| Medial | 2 (11.1%) | 0 (0.0%) | 0.500 |
| Right lower lobe | |||
| Superior | 5 (27.8%) | 3 (16.7%) | 0.500 |
| Medial basal | 0 (0.0%) | 0 (0.0%) | / |
| Anterior | 3 (16.7%) | 0 (0.0%) | 0.250 |
| Lateral basal | 9 (50.0%) | 8 (44.4%) | 1.000 |
| Posterior | 8 (44.4%) | 7 (38.9%) | 1.000 |
| Number of segments involved | 3.0 (1.0-7.5) | 1.0 (1.0-3.2) | 0.005 |
| Lung involvement | 0.102 | ||
| Unilateral | 8 (44.4%) | 8 (44.4%) | |
| Bilateral | 10 (55.6%) | 8 (44.4%) | |
| No lesion | / | 2 (11.1%) | |
| Distribution | 0.059 | ||
| Subpleural | 12 (66.7%) | 13 (72.2%) | |
| Peribronchovasular | 1 (5.6%) | 0 (0.0%) | |
| Random | 5 (27.8%) | 3 (16.7%) | |
| No lesion | / | 2 (11.1%) | |
| Location | 0.046 | ||
| Anterior | 0 (0.0%) | 0 (0.0%) | |
| Posterior | 12 (66.7%) | 14 (77.8%) | |
| Both anterior and posterior | 6 (33.3%) | 2 (11.1%) | |
| No lesion | / | 2 (11.1%) | |
| Extent of lesion involvement | 0.020 | ||
| Focal | 6 (33.3%) | 7 (38.9%) | |
| Multifocal | 4 (22.2%) | 5 (27.8%) | |
| Diffuse | 8 (44.4%) | 4 (22.2%) | |
| No lesion | / | 2 (11.1%) |
Data are n (%), median (IQR). Percentages may not total 100 because of rounding. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In this study, only eighteen (54.5%) of asymptomatic SARS-CoV-2 infections had abnormal chest CT findings, which indicates that normal chest CT cannot exclude the diagnosis of SARS-CoV-2 infection. This is in line with low sensitivity of chest CT for asymptomatic SARS-CoV-2 infections from the cruise ship “Diamond Princess” [40]. The superior sensitivity in earlier literature was likely biased toward symptomatic patients imaged in later stages of disease [41]. The most common abnormal CT feature in the asymptomatic SARS-CoV-2 infections was GGO with predominantly bilateral, subpleural and diffuse involvement, which was similar to those of the symptomatic SARS-CoV-2 infections [24,31]. But the median number of segments involved in our study was less than that of symptomatic infections [24,31] and the consolidation in the asymptomatic SARS-CoV-2 infections was focal, which suggests that lung involvements of asymptomatic SARS-CoV-2 infections are less extensive than those of symptomatic SARS-CoV-2 infections. Although the CT findings of asymptomatic SARS-CoV-2 infections were characteristic, the sensitivity of CT was only modest and definite diagnosis still requires a positive RT-PCR test, this suggests limited value of chest CT as a screening test. Fortunately, the sensitivity of initial RT-PCR test reached up to 97% in our study. While early studies of test performance in Wuhan showed significantly lower sensitivities [21-23], this could be explained by different enrollment criteria, sample sizes, testing capacities, kit performances, and stages of infections [28]. Even in this scenario, multiple RT-PCR testing should be the first choice to exclude the diagnosis if no constraint on RT-PCR testing exists [28,42]. And a recent study [43] found that antibody testing may provide additional value on identification of asymptomatic infections with negative RT-PCR results.
Compared with the CT negative group, only monocyte count was observed significantly higher in the CT positive group. This could be explained by compensation to infiltration of pulmonary monocytes [44]. The other indicators especially including time of RT-PCR conversion showed no statistical differences between CT positive and negative groups. This suggests that initial CT can provide little additional value in clinical practice for asymptomatic SARS-CoV-2 infections.
No case in CT negative group was abnormal on the follow-up CT. Three patterns of evolution throughout the longitudinal CT scans were observed among these 18 CT positive individuals: type-GI, followed by type-MI, and type-PI. Noticeably, the type-MI was a novel pattern of evolution that we found in asymptomatic SARS-CoV-2 infections. The type-MI infers that progression and improvement occur simultaneously during the mismatch stage from different dimensions. What's more, we found that asymptomatic SARS-CoV-2 infections had lower incidence and milder progression of type-PI compared with symptomatic SARS-CoV-2 infections of previous studies [24,32,33]. In addition, for type-MI or type-PI in our study, the persistence of high levels for lung lesions in asymptomatic SARS-CoV-2 infections was not observed, while it was very often seen in symptomatic SARS-CoV-2 infections; after that, the asymptomatic SARS-CoV-2 infections in our study showed a faster decrease in lung lesions [32,34]. Besides all cases in our study had stable conditions and finally confirmed negative for SARS-CoV-2 RT-PCR testing without developing into severe pneumonia. Thus, we can infer that the asymptomatic SARS-CoV-2 infections have less severity and more favourable outcomes than those of symptomatic SARS-CoV-2 infections. So we suggest that a different strategy with no need for CT follow-up in asymptomatic individuals should be taken for avoiding unnecessary radiation damage and reducing the risk of cross-infection, compared with the symptomatic patients.
Noticeably, although the individuals in our study already confirmed negative for RT-PCR before discharge from isolation, the last CT of most cases still showed abnormalities mainly including GGO. A previous study [45] found that some recovered individuals who met criteria for hospital discharge had positive results for SARS-CoV-2 infections five to 13 days later. A recent pathological examination confirmed SARS-CoV-2-viruses remaining in pneumocytes and virus-caused pathological changes in the lungs of a ready-for-discharge patient [46]. This indicates that self-monitoring of health status, isolation at home, and further follow-up including RT-PCR testing will be required after discharge.
This study has several limitations. Firstly, the sample size of the asymptomatic SARS-CoV-2 infections was relatively small. Statistical tests and p values should be interpreted with caution because of the small sample size. Secondly, because SARS-CoV-2 infection is a sudden novel emergency and highly contagious, in the early stage with no experience for reference, chest CT followup scans of some individuals were frequent in our study. Thirdly, the CT scans for the included patients might have different time intervals from the date of being infected.
In conclusion, considering poor performance of CT in screening, no obvious progression of lung lesions and stable conditions during followup, and good outcomes in asymptomatic SARS-CoV-2 infections, we confirm that it is unnecessary to do CT scans in asymptomatic infections.
Abbreviations
COVID-19: coronavirus disease 2019; GGO: ground-glass opacity; GI: gradual improvement; MI: mismatch to improvement; PI: progression to improvement; RT-PCR: reverse transcriptase-polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81971608).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506
2. WHO. World experts and funders set priorities for COVID-19 research. https://www.who.int/news-room/detail/12-02-2020-world-experts-and-funders-set-priorities-for-covid-19-research/
3. Zhu N, Zhang D, Wang W. et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733
4. Gorbalenya AE, Baker SC, Baric RS. et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses - a statement of the Coronavirus Study Group. bioRxiv. 2020
5. WHO. Clinical management of COVID-19. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected/
6. Li Q, Guan X, Wu P. et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207
7. WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/
8. WHO. Virtual press conference on COVID-19 - 11 March 2020. https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2/
9. Bedford J, Enria D, Giesecke J. et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015-1018
10. Mizumoto K, Kagaya K, Zarebski A. et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25:2000180
11. Nishiura H, Kobayashi K, Suzuki S. et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). International journal of infectious diseases. 2020;94:154-155
12. He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J Med Virol. 2020 May 29, Epub ahead of print
13. Kim H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? European radiology. 2020;30:3266-3267
14. Bai Y, Yao L, Wei T. et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406-1407
15. Rothe C, Schunk M, Sothmann P. et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971
16. Huang R, Xia J, Chen Y. et al. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. The Lancet Infectious Diseases. 2020;20:534-535
17. Ng OT, Marimuthu K, Chia PY. et al. SARS-CoV-2 Infection among Travelers Returning from Wuhan, China. The New England journal of medicine. 2020;382:1476-1478
18. Arons MM, Hatfield KM, Reddy SC. et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020;382:2081-2090
19. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid-19. N Engl J Med. 2020;382:2158-2160
20. Meng H, Xiong R, He R. et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020;81:e33-e39
21. Ai T, Yang Z, Hou H. et al. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40
22. Li Y, Yao L, Li J. et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92:903-908
23. Fang Y, Zhang H, Xie J. et al. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115-E117
24. Shi H, Han X, Jiang N. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infectious Diseases. 2020;20:425-434
25. Hu Z, Song C, Xu C. et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Science China. Life sciences. 2020;63:706-711
26. Zhao W, Zhong Z, Xie X. et al. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR. American journal of roentgenology. 2020;214:1072-1077
27. Simpson S, Kay FU, Abbara S. et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020;35:219-227
28. Rubin GD, Ryerson CJ, Haramati LB. et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology. 2020;296:172-180
29. National Health Commission of the People's Republic of China. Prevention and Control of COVID-19 of China (version sixth). http://www.nhc.gov.cn/xcs/zhengcwj/202003/4856d5b0458141fa9f376853224d41d7.shtml
30. Hansell DM, Bankier AA, MacMahon H. et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722
31. Li K, Wu J, Wu F. et al. The Clinical and Chest CT Features Associated with Severe and Critical COVID-19 Pneumonia. Investigative Radiology. 2020;55:327-331
32. Wang Y, Dong C, Hu Y. et al. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology. 2020;296:E55-E64
33. Xiong Y, Sun D, Liu Y. et al. Clinical and High-Resolution CT Features of the COVID-19 Infection: Comparison of the Initial and Follow-up Changes. Investigative Radiology. 2020;55:332-339
34. Pan F, Ye T, Sun P. et al. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020;295:715-721
35. Yang X, Yu Y, Xu J. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481
36. Wang D, Hu B, Hu C. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069
37. Puylaert CAJ, Scheijmans JCG, Borgstein ABJ. et al. Yield of Screening for COVID-19 in Asymptomatic Patients Prior to Elective or Emergency Surgery using Chest CT and RT-PCR (SCOUT): Multicenter Study. Ann Surg. 2020;272:919-924
38. Liu Z, Wu Q, Zou Z. et al. Investigation of a family cluster outbreak of COVID-19 indicates the necessity of CT screening for asymptomatic family members in close contact with confirmed patients. J Thorac Dis. 2020;12:3673-3681
39. Zhou J, Tan Y, Li D. et al. Observation and analysis of 26 cases of asymptomatic SARS-COV2 infection. J Infect. 2020;81:e69-e70
40. Inui S, Fujikawa A, Jitsu M. et al. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19). Radiology: Cardiothoracic Imaging. 2020
41. Isikbay M, Hope MD, Raptis CA. et al. CT on the Diamond Princess: What might this tell us about sensitivity for COVID-19? Radiology: Cardiothoracic Imaging. 2020;2:e200155
42. Nair A, Rodrigues JCL, Hare S. et al. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75:329-334
43. Long Q, Liu B, Deng H. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845-848
44. Xu Z, Shi L, Wang Y. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine. 2020;8:420-422
45. Lan L, Xu D, Ye G. et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502-1503
46. Yao X, He Z, Li T. et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Research. 2020;30:541-543
Author contact
![]() Corresponding author: Kunhua Li, MS. Department of Radiology, The Second Affiliated Hospital of Chongqing Medical University, No. 74 Linjiang Rd, Yuzhong District, Chongqing, China 400010. Email: likunhuacqmu.edu.cn. ORCID iD: https://orcid.org/0000-0002-2360-0397.
Corresponding author: Kunhua Li, MS. Department of Radiology, The Second Affiliated Hospital of Chongqing Medical University, No. 74 Linjiang Rd, Yuzhong District, Chongqing, China 400010. Email: likunhuacqmu.edu.cn. ORCID iD: https://orcid.org/0000-0002-2360-0397.

 Global reach, higher impact
Global reach, higher impact