3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(4):367-375. doi:10.7150/ijms.18288 This issue Cite
Research Paper
In vivo application of Granulocyte-Macrophage Colony-stimulating Factor enhances postoperative qualitative monocytic function
1. Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Germany
2. Sostana GmbH Berlin, Germany
* These authors contributed equally to this work.
Received 2016-11-10; Accepted 2017-1-30; Published 2017-4-8
Abstract
BACKGROUND: Granulocyte macrophage colony-stimulating factor (GM-CSF) can be used as a potent stimulator for immune suppressed patients as defined by a decrease of human leukocyte antigen-D related expression on monocytes (mHLA-DR) after surgery. However, the exact role of GM-CSF on monocytic and T cell function is unclear. METHODS: In this retrospective randomized controlled trial (RCT) subgroup analysis, monocytic respectively T cell function and T cell subspecies of 20 immune suppressed (i.e. mHLA-DR levels below 10,000 monoclonal antibodies (mAb) per cell at the first day after surgery) patients after esophageal or pancreatic resection were analyzed. Each 10 patients received either GM-CSF (250 μg/m²/d) or placebo for a maximum of three consecutive days if mHLA-DR levels remained below 10,000 mAb per cell. mHLA-DR and further parameters of immune function were measured preoperatively (od) until day 5 after surgery (pod5). Statistical analyses were performed using nonparametric statistical procedures. RESULTS: In multivariate analysis, mHLA-DR significantly differed between the groups (p < 0.001). mHLA-DR was increased on pod2 (p < 0.001) and pod3 (p = 0.002) after GM-CSF application. Tumor necrosis factor-α (TNF-α) release of lipopolysaccharide (LPS) stimulated monocytes multivariately significantly differed between the groups (p < 0.008) and was increased in the GM-CSF group on pod2 (p < 0.001) and pod3 (p = 0.046). Th17/regulatory T (Treg) cell ratio was higher after GM-CSF treatment on pod2 (p = 0.041). No differences were seen in lymphocytes and T helper cell (Th)1/Th2 specific cytokine production after T cell stimulation with Concanavalin (Con) A between the groups. CONCLUSIONS: Postoperative application of GM-CSF significantly enhanced qualitative monocytic function by increased mHLA-DR and TNF-α release after LPS stimulation and apparently enhanced Th17/Treg ratio.
Clinical trial registered with
Keywords: GM-CSF, HLA-DR, immune suppression, immune stimulation, monocytic function, T cell function, Th17/Treg ratio
Background
Monocytic human leukocyte antigen-D related (mHLA-DR) is crucial for antigen-presentation and reflects the functional state of immune competence since T cells are activated through recognition of antigens presented by mHLA-DR [1].
Postoperative immune suppression is frequently observed after surgery due to surgical stress and tissue damage and can be measured by a decreased mHLA-DR [2, 3]. Various further immune alterations occur after surgery [4], not at least an impairment of T cell function associated with an elevated risk for postoperative infections. A recent study of our research group aimed to stimulate postoperative immune function using GM-CSF and found an increase of mHLA-DR as well as a decrease of infection days after application of GM-CSF [5]. However, the exact role of GM-CSF on monocytic function and alterations of T cells after in vivo biomarker-guided postoperative immune stimulation is unclear. Therefore, monocytic and T cell function such as mHLA-DR, tumor necrosis factor alpha (TNF-α) after LPS stimulation, T cell counts, Th1/Th2 specific cytokine production and the ratio of the number of Th17 cells to the number of Treg cells were studied after postoperative GM-CSF application.
Patients and Methods
Study Participants and Treatment
This retrospective subgroup analysis is a further analysis of a previously published study [5] which studied further parameters of monocytic and T cell immune function between the GM-CSF and placebo group (Figure 1) that were determined in 10 patients of each study group (no selection of the patients, determination of further immune cells stopped after 32 out of 61 patients of the bigger cohort due to economic reasons). 10 of the analyzed immune suppressed patients of each group after elective esophageal or pancreatic resection were treated for at least 24 hours with either GM-CSF (250 µg/m² body surface) or placebo in a double-blind fashion. If mHLA-DR levels remained below 10,000 mAb per cell on day 2 and day 3 after surgery (pod2 and pod3), the application of the study drugs was continued for a maximum of 72 hours (Figure 2). All patients received guideline-based anesthesiological and surgical treatment according to our standard operating procedures [6].
This clinical trial was approved by the Ethics Committee of the Landesamt für Gesundheit und Soziales Berlin (LaGeSo), Germany (ref ZSEK15287/08) on September 01, 2008 and meets the requirements set out by the ICH-GCP, Declaration of Helsinki and the German Drug Law (AMG). Written informed consent was obtained.
Consort diagram
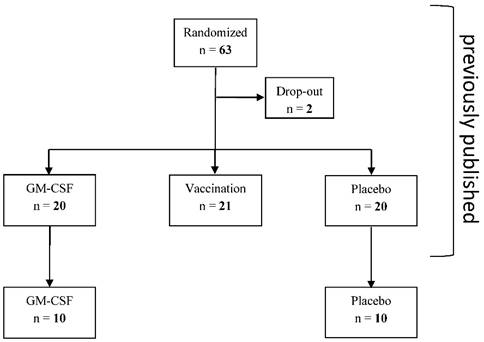
Trial Flowchart
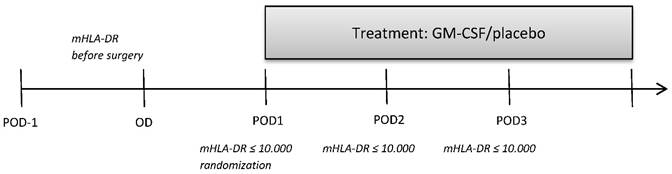
Measurement of parameters of immune function
Blood samples were drawn from od until pod5 and intraoperative parameters (blood loss, blood glucose, blood lactate, systolic blood pressure and surgical time) were documented. mHLA-DR and further parameters of immune function were measured from od until pod5. Expression of mHLA-DR on monocytes was determined by flow cytometry using a highly standardized quantitative assay as described earlier [7]. For determination of soluble mediators, ethylenediaminetetraacetic acid (EDTA) and heparin plasma samples were collected and stored at -80°C until assayed. Plasma levels of C reactive protein (CRP) were measured using an immunoturbidometric assay (Roche Diagnostics). Procalcitonin (PCT) concentration was measured using a commercial immunoluminometric assay (Brahms, Hennigsdorf, Germany). All immunologic parameters were analyzed in collaboration with the Institute of Medical Immunology and Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité - Universitätsmedizin Berlin, Berlin, Germany. White blood cell differential count was measured on a standard hematology analyzer (Sysmex). For flow cytometry analysis, the following fluorescence-labeled mouse anti-human monoclonal antibodies (BD Biosciences, Heidelberg, Germany) were used: cluster of differentiation (CD)2, CD4 and CD16 fluorescein isothiocyanate (FITC); CD3, CD19 phycoerythrin (PE); CD45, CD4 peridinin chlorophyll (PerCP); CD8 allophycocyanine (APC). Lymphocyte subpopulations were identified using following antibody combinations: CD45 for leukocytes, CD3+ for T lymphocytes, CD3+CD4+ for Th cells, CD3+CD8+ for cytotoxic T cells, CD2+ CD3-CD16+ for natural killer (NK) cells, CD19+ for B lymphocytes. Cell phenotyping was performed by flow cytometry/fluorescence-activated cell sorting (FACS) on a FACSCalibur ™ using CELLQuest™ Software (BD Biosciences).
Stimulation of whole blood cultures with LPS and Con A
For analysis of ex vivo endotoxin-induced cytokine synthesis, heparinized blood was diluted 1:10 with RPMI 1640 medium (Biochrom KG, Berlin, Germany) and stimulated with endotoxin (500 pg/mL; Milenia Ex Vivo Whole Blood Stimulation kit, Milenia Biotec, Giessen, Germany) at 37°C and 5% CO2. TNF-α, and interleukin (IL)-10 secretion was measured in culture supernatants after 4 and 24 hours, respectively. Con A-induced lymphocytic interferon (IFN)-γ, TNF-α, IL-2, IL-4, IL-5 and IL-10 secretion was analyzed in 24 hours stimulated whole blood sample supernatants by Cytometric Bead Array (BD Biosciences).
Flow cytometric analysis of Treg and Th17 cells
Th17 and Treg cell concentrations were analyzed at the research laboratory of the Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin using four-color flow cytometry on a FACSCanto II™ and FACS™DIVA Software (BD Biosciences). For the quantification of Treg cells, the following antibodies were added to EDTA-blood samples: CD4PerCP, CD3 pacific blue for identifying Th cells and CD 25 APC, CD 127 PE to extract Treg cells (low CD 127, high CD 25). For the analysis of Th17 cells, peripheral mononuclear cells (PBMC) were separated from citrate anticoagulated blood. After counting the cells, cytokine production was stimulated for two hours using phorbol-12-myristate-13-acetate (PMA) and inomycin (Sigma Aldrich, Taufkirchen, Germany). GolgiStop™ (BD Biosciences) was then added for further 2 hours to ensure intracellular accumulation of cytokines before staining the superficial antigens CD3 and CD8 using CD3 pacific blue and CD8 APC. For determination of intracellular IL-17, cells were fixated and permeabilized using FACS™Fix/Perm Solution (BD Biosciences) and stained using IL-17 PE. All used antibodies were achieved from BD Biosciences.
Statistical analysis
Data were expressed according to their scaling as arithmetic mean ± standard deviation (SD), median [25%, 75% quartiles], or frequencies [%], respectively. After exploratory data analysis, all tests were accomplished by means of non-parametric exact statistical tests. Basic patient characteristics and post intervention characteristics were evaluated for group differences using the exact Mann-Whitney test for continuous variables and Fisher's exact test for categorical variables. Changes in interesting clinical outcomes with respect to time were analyzed using multivariate nonparametric analysis of longitudinal data in a two-factorial design (1st factor: groups, 2nd factor: repetitions in time) (nonparametric MANOVA for repeated measures). Therefore, we compared all time points simultaneously on the corresponding response curves. This analysis proves hypotheses about group differences between GM-CSF and placebo, systemic changes in time, and interactions between groups and time, as well as tests for systemic time changes for every treatment separately. In cases of relevant differences at baseline, a multivariate nonparametric analysis of covariance (MANCOVA) for repeated measures with the baseline as covariate was applied.
After such global tests over time domain, differences in immune cells and cytokines were ascertained with univariate post-hoc analyses to detect specific differences with respect to groups for fixed times (exact Mann-Whitney tests). A two-tailed p-value < 0.05 was considered statistically significant. All tests should be understood as constituting exploratory data analysis, such that no adjustments for multiple testing have been made. Numerical calculations were performed with IBM© SPSS© Statistics, Version 23, and SAS™ (Version 9.1) software and The R Project for Statistical Computing, Version 3.0.2 (2013-09-25), Copyright (C) 2013 The R Foundation for Statistical Computing.
Results
Study population and groups
Basic patient characteristics, intraoperative und pre-interventional parameters did not differ between the groups (Table 1). In the GM-CSF group, all patients received study medication on pod1, one patient on pod2 and two patients on pod3 since mHLA-DR was < 10,000 mAb per cell in these patients. In the placebo group, all patients were treated on pod1 and on pod2 and nine patients on pod3.
GM-CSF significantly enhanced qualitative monocytic function
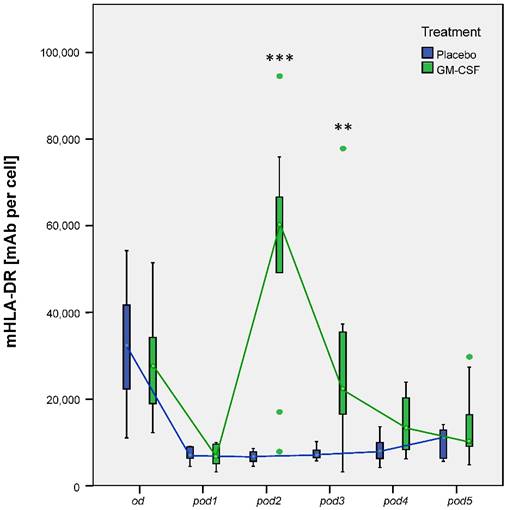
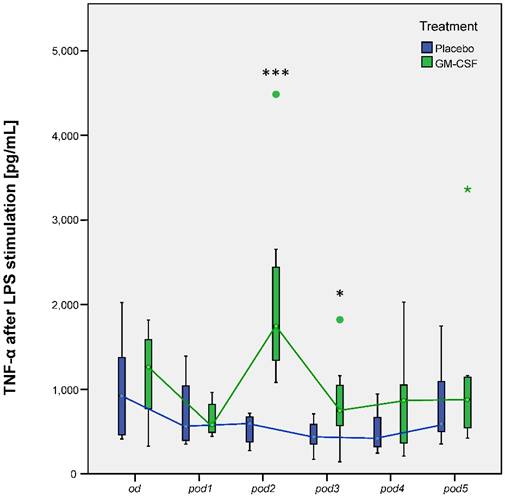
Our research group could already show that postoperative application of GM-CSF could increase mHLA-DR [5]. Our subanalyses now showed the same results: Nonparametric MANOVA for repeated measures regarding mHLA-DR showed significant differences over time between the groups (p < 0.001). mHLA-DR significantly decreased after surgery in all 20 patients (p < 0.001 between od and pod1). mHLA-DR was increased on pod2 (p < 0.001), pod3 (p = 0.002) and slightly on pod4 (p = 0.052) after GM-CSF application (Figure 3). TNF-α release of LPS stimulated monocytes significantly differed between the groups (p < 0.008) in multivariate MANOVA and was increased in the GM-CSF group on pod2 (p < 0.001) and pod3 (p = 0.046) (Figure 4). IL-10 release of stimulated monocytes and absolute monocyte counts showed no significant differences (data not shown).
GM-CSF enhanced the ratio of Th17/Treg cells
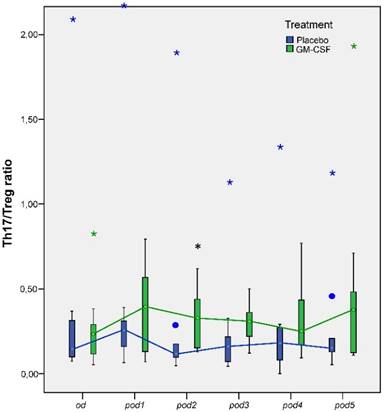
Univariate testing revealed no significant differences between the groups for the concentrations of Th17 and Treg cells, although we found a tendency for elevated Th17 concentration in GM-CSF treated patients on pod2 (p = 0.05; Table 2). However, multivariate nonparametric analysis of covariance for repeated measures with the baseline as covariate showed significant differences between the groups for Treg cells over time (p = 0.036). Furthermore, Th17/Treg ratio on pod2 (p = 0.041) was significantly higher in patients treated with GM-CSF (Figure 5).
Basic patient characteristics and pre-interventional course.
| Placebo (n = 10) | GM-CSF (n = 10) | p-value | |
|---|---|---|---|
| Age [years] | 62 (55-69) | 64 (54-69) | 0.796 |
| Gender male/female [n] | 7/3 | 9/1 | 0.582 |
| Body Mass Index [kg/m²] | 25.5 (24.2-27.5) | 25.8 (23.3-28.0) | 0.912 |
| Pancreatic/esophageal resection [n] | 6/4 | 4/6 | 0.656 |
| ASA score II/III [n] | 7/3 | 5/5 | 0.650 |
| smokers/non-smokers [n] | 4/6 | 3/7 | 1.000 |
| AUDIT score | 3 (0-6) | 4 (2-4) | 0.708 |
| metabolic equivalent (MET) <4/4-10/>10 | 0/8/2 | 0/10/0 | 0.474 |
| NRS at rest dBs | 1 (0-3) | 0 (0-1) | 0.166 |
| NRS at rest pod1 | 2 (0-3) | 3 (0-5) | 0.055 |
| NRS during movement pod1 | 5 (0-7) | 5 (3-7) | 0.416 |
| surgical time [min] | 308 (280-378) | 346 (272-390) | 0.529 |
| blood loss [mL] | 600 (313-950) | 650 (575-875) | 0.696 |
| blood glucose [mg/dL] | 127 (122-142) | 138 (120-145) | 0.739 |
| blood lactate [mmol/L] (max.) | 1.0 (0.8-1.3) | 1.1 (0.9-1.7) | 0.529 |
| systolic blood pressure [mmHg] | 113 (109-117) | 116 (112-126) | 0.190 |
| APACHE II score on admission to ICU | 12 (9-16) | 12 (11-13) | 0.500 |
| SAPS II score on admission to ICU | 22 (12-27) | 28 (17-30) | 0.097 |
| SOFA score on admission to ICU | 2 (1-4) | 1 (0-4) | 1.000 |
| TISS 28 score on admission to ICU | 32 (27-36) | 29 (23-36) | 0.169 |
Continuous quantities in median (25%-75% percentiles), frequencies with n (%); NRS, Numeric Rating Scale; dBs, day before surgery; pod1, day 1 after surgery; ASA, American Society of Anesthesiologists; AUDIT score, Alcohol Use Disorders Identification Test; PONV, postoperative nausea and vomiting; APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; TISS, Therapeutic Intervention Scoring System; ICU, Intensive Care Unit.
Treg and Th17 cells.
| Placebo (n = 10) | GM-CSF (n = 10) | p-value | |
|---|---|---|---|
| Th17 [% of CD4+ cells] | |||
| od | 0.9 (0.6-1.7) | 0.8 (0.6-1.9) | 0.970 |
| pod1 | 1.5 (1.0-2.5) | 1.6 (1.1-2.6) | 0.970 |
| pod2 | 0.8 (0.7-1.7) | 1.7 (1.2-2.9) | 0.050 |
| pod3 | 1.3 (0.6-2.3) | 1.9 (1.5-2.4) | 0.227 |
| pod4 | 1.5 (0.8-2.0) | 1.3 (1.1-2.6) | 0.479 |
| pod5 | 1.2 (1.0-2.0) | 1.7 (1.0-3.0) | 0.289 |
| Treg [% of CD4+ cells] | |||
| od | 5.6 (4.8-6.1) | 5.3 (3.1-7.0) | 0.940 |
| pod1 | 5.7 (5.0-8.4) | 5.6 (3.2-7.6) | 0.579 |
| pod2 | 7.9 (5.1-8.6) | 6.1 (4.5-7.8) | 0.273 |
| pod3 | 8.1 (6.0-8.9) | 5.7 (4.7-7.2) | 0.140 |
| pod4 | 7.2 (5.6-9.1) | 5.2 (4.5-7.4) | 0.130 |
| pod5 | 6.1 (5.8-10.1) | 5.7 (4.6-8.0) | 0.287 |
Continuous quantities in median (25%-75% percentiles); od, day of surgery before surgery; pod, day after surgery
mHLA-DR from day of surgery before surgery (od) until day 5 after surgery (pod5) between the groups. Nonparametric MANOVA for repeated measures regarding mHLA-DR showed significant differences over time between the groups (p < 0.001). mHLA-DR significantly decreased after surgery in all 20 patients (p < 0.001 between od and pod1). mHLA-DR significantly increased on pod2 (***p < 0.001), pod3 (**p = 0.002) and slightly on pod4 (p = 0.052) after GM-CSF application.
TNF-α release of LPS stimulated monocytes from day of surgery before surgery (od) until day 5 after surgery (pod5) between the groups. TNF-α significantly differed between the groups (p < 0.008) in multivariate MANOVA and was increased on pod2 (***p < 0.001) and pod3 (*p = 0.046) after stimulation with GM-CSF.
GM-CSF did not change Th1/Th2 specific cytokine production
After stimulation of whole blood cultures for 24 h with Con A, the cytokines for Th1 and Th2 responsiveness IL-4, IL-5, IL-10, IFN-γ and TNF-α showed no differences between the groups (Table 3). Noteworthy, IL-2 slightly increased after GM-CSF application on pod2 (p = 0.073).
Th1/Th2 specific cytokine production.
| Placebo (n = 10) | GM-CSF (n = 10) | p-value | |
|---|---|---|---|
| IL-2 [pg/mL] | |||
| od | 152 (146-271) | 223 (100-481) | 1.000 |
| pod1 | 121 (81-172) | 231 (90-354) | 0.181 |
| pod2 | 110 (79-216) | 190 (117-414) | 0.073 |
| pod3 | 137 (79-199) | 245 (112-467) | 0.138 |
| pod4 | 152 (133-207) | 176 (77-505) | 0.699 |
| pod5 | 168 (109-223) | 233 (123-415) | 0.366 |
| IL-4 [pg/mL] | |||
| od | 3.0 (2.0-12.0) | 2.5 (2.0-9.8) | 0.731 |
| pod1 | 7.0 (2.0-12.0) | 6.5 (2.0-28.8) | 1.000 |
| pod2 | 4.0 (2.0-11.0) | 8.0 (3.5-21.3) | 0.295 |
| pod3 | 7.0 (2.0-13.0) | 8.5 (5.0-19.0) | 0.731 |
| pod4 | 8.0 (5.3-16.3) | 10.5 (2.0-17.3) | 1.000 |
| pod5 | 2.0 (2.0-9.0) | 9.0 (4.3-14.3) | 0.234 |
| IL-5 [pg/mL] | |||
| od | 6.0 (2.0-26.0) | 23.5 (2.0-33.8) | 0.628 |
| pod1 | 9.0 (4.0-65.0) | 19.5 (5.5-69.3) | 0.628 |
| pod2 | 5.0 (3.0-31.0) | 11.0 (2.8-29.8) | 0.836 |
| pod3 | 11.0 (7.0-20.0) | 26.5 (2.0-59.8) | 0.628 |
| pod4 | 11.0 (5.3-28.0) | 15.0 (3.5-38.8) | 1.000 |
| pod5 | 7.0 (4.0-17.0) | 14.0 (7.3-48.3) | 0.534 |
| IL-10 [pg/mL] | |||
| od | 71 (34-91) | 90 (37-130) | 0.731 |
| pod1 | 80 (46-136) | 86 (59-131) | 0.731 |
| pod2 | 48 (40-104) | 86 (39-148) | 0.628 |
| pod3 | 56 (32-72) | 75 (51-125) | 0.445 |
| pod4 | 47 (32-115) | 100 (50-112) | 0.394 |
| pod5 | 39 (30-67) | 89 (58-143) | 0.101 |
| IFN-γ [pg/mL] | |||
| od | 757 (275-1,784) | 1,553 (721-3,594) | 0.295 |
| pod1 | 296 (95-1,045) | 509 (166-719) | 1.000 |
| pod2 | 259 (163-1,084) | 587 (191-913) | 1.000 |
| pod3 | 374 (88-1,237) | 765 (491-986) | 0.295 |
| pod4 | 318 (230-1,719) | 509 (281-1,462) | 0.589 |
| pod5 | 345 (43-1,874) | 724 (289-1,268) | 0.628 |
| TNF-α [pg/mL] | |||
| od | 378 (107-468) | 571 (220-710) | 0.234 |
| pod1 | 110 (18-202) | 167 (82-272) | 0.628 |
| pod2 | 70 (39-105) | 162 (31-242) | 0.534 |
| pod3 | 116 (87-154) | 167 (117-245) | 0.366 |
| pod4 | 152 (78-370) | 116 (78-292) | 0.818 |
| pod5 | 209 (29-573) | 193 (155-274) | 1.000 |
Continuous quantities in median (25%-75% percentiles); od, day of surgery before surgery; pod, day after surgery
Impact of GM-CSF on inflammatory mediators and peripheral blood leukocytes
As shown in Table 4, there were no significant changes concerning the total counts of granulocytes, monocytes and lymphocytes between the groups. Slight increases were seen for total leukocyte (p = 0.063) and granulocyte count (p = 0.075) on pod2 after GM-CSF application. Lymphocyte subspecies such as NK cells, CD3+, CD4+ and CD8+ T lymphocytes did not differ between the groups (Table 5). However, B lymphocytes also showed slight increases in the GM-CSF group on pod4 (p = 0.052) and pod5 (p = 0.053). CRP and PCT did not differ between the groups either (data not shown).
Peripheral blood leukocytes.
| Placebo (n = 10) | GM-CSF (n = 10) | p-value | |
|---|---|---|---|
| Leukocytes [1/nL] | |||
| od | 5.7 (4.0-7.2) | 5.7 (5.0-7.5) | 0.481 |
| pod1 | 10.0 (9.3-13.5) | 8.1 (7.2-13.0) | 0.315 |
| pod2 | 11.5 (8.2-12.6) | 16.7 (9.0-20.5) | 0.063 |
| pod3 | 9.4 (7.4-11.4) | 11.2 (6.5-13.7) | 0.912 |
| pod4 | 9.3 (7.0-11.7) | 10.7 (8.0-13.0) | 0.684 |
| pod5 | 10.6 (6.2-11.9) | 11.8 (7.4-15.3) | 0.447 |
| Granulocytes [1/nL] | |||
| od | 3.4 (2.7-3.9) | 3.8 (2.6-5.6) | 0.631 |
| pod1 | 8.7 (7.8-10.6) | 6.6 (5.8-11.0) | 0.393 |
| pod2 | 9.9 (7.4-10.4) | 15.1 (7.6-18.4) | 0.075 |
| pod3 | 7.6 (6.3-9.4) | 9.5 (5.4-11.6) | 0.853 |
| pod4 | 7.5 (6.1-9.7) | 7.6 (5.3-9.7) | 0.853 |
| pod5 | 7.6 (4.6-9.8) | 9.6 (5.6-12.4) | 0.278 |
| Monocytes [1/nL] | |||
| od | 0.49 (0.41-0.67) | 0.60 (0.52-0.76) | 0.105 |
| pod1 | 0.73 (0.62-1.25) | 0.75 (0.62-1.11) | 0.529 |
| pod2 | 0.73 (0.52-1.02) | 0.89 (0.64-1.22) | 0.447 |
| pod3 | 0.67 (0.55-0.88) | 0.75 (0.61-0.93) | 0.123 |
| pod4 | 0.77 (0.60-1.18) | 0.87 (0.78-1.09) | 0.143 |
| pod5 | 1.06 (0.68-1.50) | 1.25 (0.92-1.72) | 0.829 |
| Lymphocytes [1/nL] | |||
| od | 1.00 (0.77-2.52) | 1.36 (1.20-1.72) | 0.796 |
| pod1 | 0.86 (0.55-1.05) | 0.74 (0.60-0.82) | 0.393 |
| pod2 | 0.74 (0.55-1.36) | 0.76 (0.69-0.82) | 0.912 |
| pod3 | 0.64 (0.47-1.20) | 0.77 (0.67-1.04) | 0.579 |
| pod4 | 0.54 (0.39-1.10) | 0.76 (0.61-1.21) | 0.247 |
| pod5 | 0.66 (0.42-1.37) | 0.98 (0.67-1,32) | 0.278 |
Continuous quantities in median (25%-75% percentiles); od, day of surgery before surgery; pod, day after surgery.
Discussion
The major finding of this subgroup analysis of our previously published study is that postoperative GM-CSF treatment enhanced monocytic function (mHLA-DR and ability of TNF-α release) but not the absolute count of monocytes. Furthermore, Th17/Treg ratio was increased. To the best of our knowledge, no other studies investigated monocytic function and the balance between Th17 and Treg cells after postoperative in vivo biomarker-guided application of GM-CSF in immune compromised patients.
Th17/Treg ratio from day of surgery before surgery (od) until day 5 after surgery (pod5) between the groups. Th17/Treg ratio significantly increased on pod2 (*p = 0.041) after stimulation with GM-CSF.
Lymphocyte subspecies.
| Placebo (n = 10) | GM-CSF (n = 10) | p-value | |
|---|---|---|---|
| B lymphocytes [1/nL] | |||
| od | 0.08 (0.04-0.26) | 0.11 (0.08-0.13) | 0.529 |
| pod1 | 0.09 (0.04-0.15) | 0.07 (0.06-0.10) | 0.796 |
| pod2 | 0.08 (0.04-0.14) | 0.11 (0.08-0.12) | 0.218 |
| pod3 | 0.07 (0.04-0.12) | 0.11 (0.10-0.11) | 0.105 |
| pod4 | 0.06 (0.03-0.10) | 0.10 (0.08-0.14) | 0.052 |
| pod5 | 0.06 (0.04-0.11) | 0.13 (0.09-0.19) | 0.053 |
| CD3+ T lymphocytes [1/nL] | |||
| od | 0.79 (0.61-2.20) | 1.10 (0.91-1.33) | 0.796 |
| pod1 | 0.61 (0.39-0.87) | 0.55 (0.43-0.69) | 0.739 |
| pod2 | 0.62 (0.42-1.02) | 0.59 (0.52-0.63) | 0.529 |
| pod3 | 0.52 (0.33-0.97) | 0.60 (0.50-0.78) | 0.796 |
| pod4 | 0.45 (0.31-1.13) | 0.58 (0.48-0.92) | 0.529 |
| pod5 | 0.49 (0.32-1.09) | 0.77 (0.51-1.00) | 0.315 |
| CD4+ T lymphocytes [1/nL] | |||
| od | 0.44 (0.25-1.01) | 0.73 (0.47-0.80) | 0.684 |
| pod1 | 0.37 (0.20-0.47) | 0.32 (0.22-0.42) | 0.684 |
| pod2 | 0.34 (0.18-0.45) | 0.35 (0.25-0.43) | 0.739 |
| pod3 | 0.25 (0.20-0.54) | 0.43 (0.31-0.49) | 0.684 |
| pod4 | 0.28 (0.17-0.68) | 0.41 (0.26-0.52) | 0.684 |
| pod5 | 0.28 (0.21-0.75) | 0.48 (0.31-0.65) | 0.356 |
| CD8+ T lymphocytes [1/nL] | |||
| od | 0.25 (0.17-0.43) | 0.30 (0.20-0.51) | 1.000 |
| pod1 | 0.19 (0.10-0.35) | 0.16 (0.10-0.24) | 0.579 |
| pod2 | 0.16 (0.09-0.30) | 0.17 (0.13-0.27) | 0.796 |
| pod3 | 0.14 (0.09-0.28) | 0.17 (0.12-0.27) | 1.000 |
| pod4 | 0.14 (0.06-0.61) | 0.18 (0.11-0.35) | 0.739 |
| pod5 | 0.14 (0.08-0.42) | 0.22 (0.11-0.42) | 0.780 |
| NK cells [1/nL] | |||
| od | 0.15 (0.10-0.20) | 0.18 (0.09-0.27) | 0.353 |
| pod1 | 0.11 (0.09-0.16) | 0.10 (0.04-0.13) | 0.579 |
| pod2 | 0.09 (0.06-0.13) | 0.12 (0.05-0.12) | 0.739 |
| pod3 | 0.06 (0.04-0.08) | 0.09 (0.05-0.11) | 0.190 |
| pod4 | 0.07 (0.05-0.11) | 0.08 (0.06-0.12) | 0.218 |
| pod5 | 0.09 (0.05-0.14) | 0.11 (0.06-0.15) | 0.720 |
Continuous quantities in median (25%-75% percentiles); od, day of surgery before surgery; pod, day after surgery
In general, GM-CSF is often used in neutropenic patients resulting in mobilization of myeloid cells and of those an increasing survival and activation [8]. On monocytes, 190 genes are upregulated and 212 genes are downregulated after GM-CSF application [9]. A role more as an immune modulator than a growth factor is assumed [10]. During the last decade, studies focused on immune stimulation in immune compromised patients [11-13]. Nierhaus et al. [14] as well as Meisel et al. [11] were the first who stratified severe septic patients as immune compromised using mHLA-DR expression as biomarker threshold for in vivo GM-CSF immune stimulation. Both studies could increase mHLA-DR whereas Meisel at al. could also decrease the time of ventilation and shorten the ICU and hospital stay.
Meisel et al. [11] further studied immune cells and cytokines and similar to our results they found an increase in stimulated monocytic TNF-α. However, main differences to our results were seen in increasing monocytes and T lymphocytes in their analyses. We saw stable monocyte and T lymphocyte count. Nonetheless, monocytic function was restored in both studies as mHLA-DR and TNF-α of stimulated monocytes increased. Most likely, differences in T cells between both studies are due to the study design: Meisel et al. stimulated for 8 days whereas we stimulated at least for one but maximum for 3 days. Thus, interactions between monocytes and T cells seem to be more pronounced after long-term stimulation. However, we already saw interactions on day 2 after surgery by an increased Th17/Treg ratio. Surprisingly, we did not see increasing monocytes after GM-CSF stimulation. Though GM-CSF is capable of generating both cells from bone marrow [15, 16], postoperative increases of monocytes after GM-CSF application failed to materialize. In contrast to Meisel at el., Nierhaus et al. [14] found no increase in monocytes after GM-CSF treatment in severe septic patients. Inferentially, contradiction persists about behavior of monocytes after stimulation during severe sepsis. The underlying cause of the absences of increasing monocytes in our study is unclear. One explanation might be a lack of progenitor cells in the bone marrow due to a previous release intraoperatively caused by surgical stress and trauma. The major effect of GM-CSF therefore seems to be more the enhanced function than the absolute count of monocytes, i.e. GM-CSF enhanced qualitative but not quantitative monocytic function.
GM-CSF did not change the amount of peripheral blood leukocytes and Th1/Th2 specific cytokine production after Con A stimulation. Lymphocytes and NK cells generally do not express GM-CSF receptors, thus could not directly be influenced by GM-CSF [17]. Increasing lymphocyte counts after GM-CSF application by Meisel et al. [11] and an increased Th17/Treg ratio in our study indicate that GM-CSF might have indirect effects on lymphocytes, likely through interactions with monocytes [10, 18]. Immune stimulatory effects of GM-CSF thus covers far more than monocytic function. We found an increased Th17/Treg ratio in patients receiving GM-CSF but only a tendencially increased Th17 concentration occurred, which is likely due to the small sample size. Th17 cells represent a pro-inflammation, whereas Treg cells have an anti-inflammatory effect. Their developmental pathways are reciprocally interconnected and there is an important plasticity between Th17 and Treg cells [19, 20]. Changes of the Th17/Treg ratio during surgery may contribute to an imbalance between pro- and anti-inflammatory immune response and factors recovering the balance between Th17 and Treg cells may generate potential therapeutic targets. Major interest of further research could be NK and cytotoxic T cell function after GM-CSF application, particularly after surgery when immune function of these cells is essential to avoid infections, cancer metastases and recurrence.
In our previous published study [5] we found a reduced duration of infections after postoperative GM-CSF application. We previously postulated that this reduction is due to the increase of mHLA-DR and associated with a restored monocytic function. In the current analyses, we could reinforce this assumption: we have additionally seen increased TNF-α levels after monocytic stimulation and, particularly noteworthy an increased Th17/Treg ratio. This restoration most likely shortened the duration of infection but did not prevent patients from developing infections. Restoration of monocytic function is of major relevance as sepsis is characterized by a reduced ability of monocytes to secrete pro-inflammatory cytokines, and an impaired antigen presentation by mHLA-DR [8]. Interestingly, effects of GM-CSF lasted only for time of application and declined after stop. The exact mechanism is unclear - perhaps monocytes remain in an immune suppressive state being just boostered and declining to initial values.
This study reveals several limitations. First of all, it is a retrospective subgroup analysis. Second, study drugs were administered only for a maximum of consecutive 3 days. The effect of longer duration of therapy on monocytes and immunity is unclear. Third, we analyzed only a small sample size of 10 patients in each group. Hence, some results might possibly be not significant but show a tendency. Fourth, we analyzed patients only until pod5. The impact of GM-CSF application on immune function after this time is unclear. Finally, the threshold level for mHLA-DR (we had ≤ 10,000 mAb/cell) used to stratify patients with severe surgery-induced immunosuppression is unclear. Studies suggest that values between 5,000 and 10,000 mAb/cell are an indicator of severely impaired immune function in critically ill patients [7, 11, 21].
Conclusions
Postoperative application of GM-CSF significantly enhanced qualitative but not quantitative monocytic function seen in an increased expression of mHLA-DR and TNF-α release after LPS stimulation whereas counts of monocytes did not increase. Furthermore, we found an increased Th17/Treg ratio after GM-CSF application. The balance of Th17 and Treg cells is regarded as a key factor in immune homeostasis. These findings should be the subject of further studies to determine whether Th17/Treg may serve as a potential therapeutic target to manage immune suppressed patients.
Acknowledgements
We are very grateful to Kathrin Scholtz for monitoring this study, to Anja-Vanessa Philippeit, Dominik Stöber, Julia Schäfer, Carolyn Geipel and Kay Dittrich for data acquisition and help with the data base. Preliminary data of this manuscript were presented at ASA 2013 Annual Meeting, San Francisco, CA as an e-poster.
Author Contributions
Conceived and designed the experiments: CS, KDW. Performed the experiments: GL, JK, CVH, FY. Analyzed the data: GL, CVH, JK, KDW. Contributed materials / analysis tools: CVH, KDW. Wrote the paper: GL, JK, CVH, CS, KDW.
Competing Interests
Deutsche Forschungsgemeinschaft (DFG SP432-1, http://www.dfg.de/, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript), Charité - Universitätsmedizin Berlin (www.charite.de, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript), statistical analysis has been supervised by Professor Klaus-Dieter Wernecke, PhD, former head of the Institute of Medical Biometry at Charité - Universitätsmedizin Berlin and owner of Sostana GmbH. We confirm that it does not alter our adherence to all policies on sharing data and materials.
References
1. Mokart D, Textoris J, Chow-Chine L, Brun JP, Sannini A, Turrini O. et al. HLA-DR and B7-2 (CD86) monocyte expressions after major cancer surgery: profile in sepsis. Minerva anestesiologica. 2011;77:522-7
2. Veenhof AA, Sietses C, von Blomberg BM, van Hoogstraten IM, vd Pas MH, Meijerink WJ. et al. The surgical stress response and postoperative immune function after laparoscopic or conventional total mesorectal excision in rectal cancer: a randomized trial. International journal of colorectal disease. 2011;26:53-9
3. Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W. et al. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infection and immunity. 1997;65:2283-91
4. Bartal I, Melamed R, Greenfeld K, Atzil S, Glasner A, Domankevich V. et al. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain, behavior, and immunity. 2010;24:376-86
5. Spies C, Luetz A, Lachmann G, Renius M, von Haefen C, Wernecke KD. et al. Influence of Granulocyte-Macrophage Colony-Stimulating Factor or Influenza Vaccination on HLA-DR, Infection and Delirium Days in Immunosuppressed Surgical Patients: Double Blind, Randomised Controlled Trial. PloS one. 2015;10:e0144003
6. Spies C, Kox W, Kastrup M, Melzer-Gartzke C. SOPs in Intensivmedizin und Notfallmedizin: Alle relevanten Standards und Techniken für die Klinik. Stuttgart. Georg Thieme Verlag. 2013
7. Docke WD, Hoflich C, Davis KA, Rottgers K, Meisel C, Kiefer P. et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clinical chemistry. 2005;51:2341-7
8. Hutchins NA, Unsinger J, Hotchkiss RS, Ayala A. The new normal: immunomodulatory agents against sepsis immune suppression. Trends in molecular medicine. 2014;20:224-33
9. Dabritz J, Weinhage T, Varga G, Wirth T, Walscheid K, Brockhausen A. et al. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J Immunol. 2015;194:2424-38
10. Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine. 2015;75:261-71
11. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J. et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. American journal of respiratory and critical care medicine. 2009;180:640-8
12. Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG. et al. Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. American journal of respiratory and critical care medicine. 2012;186:838-45
13. Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD. et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive care medicine. 2011;37:525-32
14. Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R. et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive care medicine. 2003;29:646-51
15. Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56:947-58
16. Yasui K, Sekiguchi Y, Ichikawa M, Nagumo H, Yamazaki T, Komiyama A. et al. Granulocyte macrophage-colony stimulating factor delays neutrophil apoptosis and primes its function through Ia-type phosphoinositide 3-kinase. J Leukoc Biol. 2002;72:1020-6
17. Rosas M, Gordon S, Taylor PR. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. European journal of immunology. 2007;37:2518-28
18. Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L. et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398-408
19. Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Seminars in immunology. 2013;25:305-12
20. Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Current opinion in immunology. 2009;21:274-80
21. Cheron A, Floccard B, Allaouchiche B, Guignant C, Poitevin F, Malcus C. et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care. 2010;14:R208
Author contact
![]() Corresponding author: Claudia Spies, Professor of Anesthesiology and Intensive Care Medicine, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany. Phone: +49 30 450 551 001; Fax: +49 30 450 551 909; Email: claudia.spiesde
Corresponding author: Claudia Spies, Professor of Anesthesiology and Intensive Care Medicine, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany. Phone: +49 30 450 551 001; Fax: +49 30 450 551 909; Email: claudia.spiesde

 Global reach, higher impact
Global reach, higher impact