3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(3):268-275. doi:10.7150/ijms.7769 This issue Cite
Research Paper
Role of overexpression of MACC1 and/or FAK in predicting prognosis of hepatocellular carcinoma after liver transplantation
1. Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health.
2. Key Laboratory of Organ Transplantation, Zhejiang Province.
3. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
* These authors contributed equally to this work.
Received 2013-9-27; Accepted 2013-12-24; Published 2014-1-21
Abstract
Background: Metastasis-associated in colon cancer-1 (MACC1) acts as a promoter of tumor metastasis; however, the predictive value of MACC1 for hepatocellular carcinoma (HCC) after liver transplantation (LT) remains unclear.
Methods: We examined the expression of MACC1 and its target genes MET and FAK by quantitative PCR in 160 patients with HCC that was undergone LT.
Results: The patients with MACC1high or FAKhigh in HCCs showed a significantly shorter overall survival and higher cumulative recurrence rates after liver transplantation (LT), compared with MACC1low or FAKlow group. Multivariate analysis indicated that MACC1 alone or combination of MACC1/FAK was an independent prognostic factor for overall survival and cumulative recurrence.
Conclusions: MACC1 or combination of MACC1/FAK could serve as a novel biomarker in predicting the prognosis of HCC after LT.
Keywords: hepatocellular carcinoma, metastasis-associated in colon cancer-1, metastasis, prognosis, liver transplantation
Introduction
Hepatocellular carcinoma (HCC) is a highly aggressive cancer, characterized by the activation of multiple molecular pathways [1, 2]. It is the sixth most common cancer and the third most common cause of cancer-related deaths worldwide [3]. China alone accounts for 55% of the world's cases because of the high prevalence of chronic hepatitis B virus (HBV) infection and liver cirrhosis, both significant risk factors for the disease [3]. Currently, liver transplantation (LT) is the only potentially curative therapeutic modality that can treat both the cancer and the associated liver dysfunction simultaneously. But while LT offers a reasonable survival benefit for selected patients with HCC and end-stage liver disease [4, 5], the long-term survival of patients following surgery remains unsatisfactory because of the high frequency of recurrence due to metastasis - principally attributed to the presence of microscopic extrahepatic metastatic foci before LT [6]. Therefore, a better understanding of the molecular mechanisms underlying HCC recurrence may offer improved diagnostic and prognostic capabilities in addition to the development of effective novel therapeutic strategies.
The hepatocyte growth factor (HGF)-MET pathway is primarily involved in regulating cell proliferation, motility, and invasion [7]. Dysregulation of HGF-MET signaling leading to tumorigenesis and metastasis has been described in various types of cancer, including HCC [8, 9]. Emerging evidence suggests that aberrant activation of the HGF-MET signaling pathway is closely associated with malignant transformation and the metastatic potential of HCC [2]. So understanding events both upstream and downstream of HGF-MET signaling might reveal new strategies to tackle HCC.
Recent investigations have revealed that the metastasis-associated in colon cancer-1 (MACC1) is a key regulator of HGF-MET signaling in colorectal cancer cells; regulation is achieved by transcriptionally upregulating the expression of MET [10]. MACC1-mediated activation of the HGF-MET signaling cascade could enhance the metastatic ability of colon cancer cells. MACC1 is a marker for advanced colorectal cancer and peritoneal disseminated gastric carcinoma, and it could be used to identify patients with a poor prognosis [10, 11]. Our previous study identified that MACC1 promotes cell survival and metastasis in HCC and MACC1 acts as a key factor regulating HGF/MET and FAK signaling network. MACC1 expression has been shown to correlate with vascular invasive HCC and other studies suggest that its expression correlates with prognosis in HBV-related HCC [11-13]. However, the predictive value of MACC1, combined with its target gene MET and FAK, for HCC after LT remains unclear.
In this study, we investigated the expression pattern of MACC1, MET and FAK in HCC and analyzed its clinicopathological significance, and determined whether MACC1, MET and FAK could be important prognostic factors for predicting clinical outcomes in HCC patients treated with LT.
Materials and methods
Patients and samples
One hundred and sixty HCC patients who underwent LT during 2001 and 2010 in the First Affiliated Hospital of Zhejiang University School of Medicine were enrolled in this study. The eligibility criteria of the study patients were as follows: (a) HCC was diagnosed either before or after transplantation (as an incidental finding). The diagnoses were confirmed by pathologic examination; (b) all patients were HBV infected; (c) clinicopathologic variables, such as vascular invasion, preoperative alpha-fetoprotein (AFP), and tumor size were completely recorded; (d) pretransplant tumor therapies included liver resection, transarterial chemoembolization, radiofrequency ablation, and ethanol ablation. The clinicopathologic characteristics of the patients were shown in Table 1. Specimens of cancer tissues and clinical information were available from these patients after obtaining informed consent. This study was approved by the ethical review committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Follow up
The follow-up course and diagnostic criteria of recurrence have been described as previous [14], briefly, the patients were followed up closely at the outpatient clinic from the date of operation to that of death or the last follow up, and tumor recurrence was monitored by AFP, ultrasonography, chest X-ray, and Emission computed tomography every 3 months for the first 2 years and every 6 months thereafter. Recurrence was diagnosed by imaging techniques, either intrahepatically or extrahepatically. The median follow up was 24 months.
Quantitative real-time reverse-transcription polymerase chain reaction (qPCR)
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA). RNA was purified using the RNeasy mini kit, RNeasy micro kit and RNase-Free DNase Set(Qiagen Sciences Inc, Germantown, MD) and its quality was assessed with an Agilent Bioanalyzer Nano Chip 2100 (Agilent, Foster City, CA).
The messenger RNA (mRNA) expression levels of MACC1, MET and focal adhesion kinase (FAK) in the HCC tissues and cell lines were determined by qPCR, and the primer sequences were listed in Table 2. qPCR reactions were performed by the ABI7500 system (Applied Biosystems, CA) according to the manufacturer's instructions. β-actin and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as internal control. Expression levels of MACC1, MET and FAK were calculated using the 2exp(-ΔΔCt) formula, and then normalized to the internal control.
Statistical analysis
Results were expressed as mean ± standard deviation (SD), as appropriate. Comparisons of continuous data were analyzed by the Student t test between two groups, whereas categorical data were analyzed assessed by the Chi-square test. Overall survival and cumulative recurrence rates were analyzed by the Kaplan-Meier method and the differences between groups were estimated by the log-rank test. Independent prognostic indicators were assessed in the univariate and multivariate analysis using Cox's proportional hazard model. Statistical analyses were performed using SPSS for Windows v.16.0 (SPSS, Chicago, IL) and GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
Results
Expression of MACC1 and combined expression of MACC1 and FAK are correlated with poor prognosis in HCC patients after LT
To explore whether MACC1 and its downstream genes could be candidate biomarkers in predicting clinical outcome of HCC patients following LT, we examined the expression of MACC1, MET and FAK in 160 HCC samples. Patients were segregated into high/low expression groups based on Receiver Operating Characteristics analysis. Upon clinico-pathological correlation analysis, clinical characteristics, including age, gender, tumor differentiation, vascular invasion, preoperative alphafetoprotein (AFP) level, tumor number, tumor size, Milan criteria or UCSF criteria were not directly related to the expression of MACC1 and MET, while high expression of FAK was significantly correlated with vascular invasion (P = 0.039) (Table 1).
Correlation between MACC1, MET, or FAK expression and clinicopathological factors in 160 HCC tumors.
| MACC1 expression | MET expression | FAK expression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | low | high | P value* | low | high | P value* | low | high | P value* |
| Age, years | |||||||||
| <50 | 40 | 43 | 0.072 | 40 | 43 | 0.609 | 40 | 43 | 0.884 |
| ≥50 | 48 | 29 | 34 | 43 | 38 | 39 | |||
| Gender | |||||||||
| Female | 9 | 5 | 0.465 | 6 | 8 | 0.790 | 7 | 7 | 0.922 |
| Male | 79 | 67 | 68 | 78 | 71 | 75 | |||
| Preoperative tumor therapy | |||||||||
| No | 61 | 56 | 0.230 | 54 | 63 | 0.968 | 59 | 58 | 0.484 |
| Yes | 27 | 16 | 20 | 23 | 19 | 24 | |||
| Tumor size | |||||||||
| ≤5 cm | 49 | 43 | 0.607 | 43 | 49 | 0.885 | 47 | 45 | 0.492 |
| >5 cm | 39 | 29 | 31 | 37 | 31 | 37 | |||
| Tumor number | |||||||||
| Single | 40 | 30 | 0.631 | 32 | 38 | 0.905 | 37 | 33 | 0.359 |
| Multiple | 48 | 42 | 42 | 48 | 41 | 49 | |||
| Tumor differentiation | |||||||||
| Well+moderate | 49 | 39 | 0.848 | 42 | 46 | 0.679 | 38 | 50 | 0.119 |
| Poor | 39 | 33 | 32 | 40 | 40 | 32 | |||
| Preoperative AFP | |||||||||
| ≤400 ng/ml | 40 | 32 | 0.898 | 32 | 40 | 0.679 | 41 | 31 | 0.061 |
| >400 ng/ml | 48 | 40 | 42 | 46 | 37 | 51 | |||
| Vascular invasion | |||||||||
| None | 59 | 43 | 0.338 | 50 | 52 | 0.351 | 56 | 46 | 0.039 |
| Yes | 29 | 29 | 24 | 34 | 22 | 36 | |||
| Milan criteria | |||||||||
| Within | 32 | 23 | 0.558 | 27 | 28 | 0.602 | 30 | 25 | 0.288 |
| Beyond | 56 | 49 | 47 | 58 | 48 | 57 | |||
| UCSF criteria | |||||||||
| Within | 39 | 27 | 0.383 | 31 | 35 | 0.878 | 37 | 29 | 0.121 |
| Beyond | 49 | 45 | 43 | 51 | 41 | 53 | |||
Patients with HCC who underwent LT were segregated into MACC1-low/high expression groups, MET-low/high expression groups, and FAK-low/high expression groups based on receiver operating characteristics analysis (MACC1 cut-off point 1.45, MET cut-off point 2.67, FAK cut-off point 3.28).
Apart from the presence of macrovascular invasion, the Milan criteria are matched if a single tumor is ≤ 5 cm in diameter or if there are ≤ 3 tumor nodules, each of which is 3 cm or less in diameter. The UCSF criteria are matched if a single tumor is ≤ 6.5 cm or if 2-3 lesions are each ≤ 4.5 cm with a total tumor diameter of ≤ 8 cm[18].
Abbreviations: MACC1, metastasis-associated in colon cancer-1; FAK, focal adhesion kinase; AFP, alpha-fetoprotein.
*Statistical analyses were performed with the Chi-square test.
Oligonucleotides Used for qRT-PCR
| Genes | Sequence(5'-3') | |
|---|---|---|
| MACC1 | Forward | TCGGTCAGGAAGAATTGCAC |
| Reverse | TTGTGAAGCAAGTCTGGGTCC | |
| MET | Forward | GCTAAAATGCTGGCACCCTAA |
| Reverse | ATAGTGCTCCCCAATGAAAGTAGAGA | |
| FAK | Forward | TCCCTATGGTGAAGGAAGT |
| Reverse | TTCTGTGCCATCTCAATCT | |
| GAPDH | Forward | GCTGAGAACGGGAAGCTTGT |
| Reverse | GCCAGGGGTGCTAAGCAG |
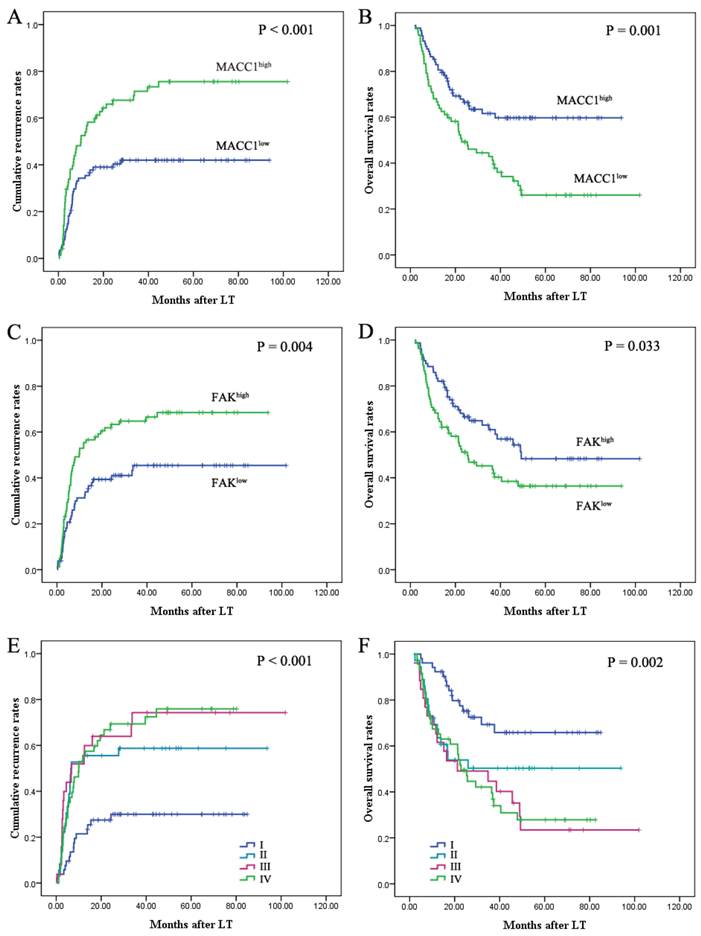
Univariate analysis revealed that vascular invasion, preoperative serum AFP level (>400 ng/ml), multiple tumors and tumor size (>5cm) were predictors for overall survival (OS) and cumulative recurrence (Table 3). Patients with MACC1-high HCC had significantly worse prognosis than those with MACC1-low (Table 3). The 1-year, 3-year, and 5-year cumulative recurrence rates of MACC1-high HCC were much higher than those of MACC1-low HCC (P < 0.001; Fig. 1A). The 1-year, 3-year, and 5-year overall survival rates of patients with MACC1-high HCC were significantly lower than those of patients with MACC1-low HCC (P = 0.001; Fig. 1B). When the patients were stratified according to the criteria whether matched UCSF criteria or exceeded the criteria, those with increased MACC1 expression revealed a significantly shorter overall survival and higher cumulative recurrence rates (Fig. 2). In addition, expression of FAK was also found to be correlated with cumulative recurrence rates and OS (Table 3; Fig. 1C, D), while MET expression had no prognostic significance. When evaluating the combined effect of MACC1 and FAK on prognosis of HCC, we found that the 1-year, 3-year, and 5-year cumulative recurrence rates in the MACC1low/FAKlow patients were significantly lower than that in the MACC1high/FAKhigh patients. The 1-year, 3-year, and 5-year OS in the MACC1low/FAKlow patients were significantly higher than those in the MACC1high/FAKhigh patients (Table 3; Fig. 1E, F).
MACC1 expression is an independent prognostic factor for HCC patients following LT.
| Cumulative recurrence | Overall survival | |||
|---|---|---|---|---|
| Variable | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Univariate analysis† | ||||
| Age, year (≥50 versus <50) | 0.546 (0.355-0.841) | 0.006 | 0.808 (0.521-1.253) | 0.341 |
| Gender (male versus female) | 2.161 (0.834-5.095) | 0.117 | 2.654 (0.970-7.261) | 0.057 |
| Preoperative treatment (yes versus no) | 1.196 (0.747-1.916) | 0.457 | 1.025 (0.617-1.703) | 0.924 |
| Tumor size (>5 cm versus ≤5 cm) | 3.934 (2.527-6.123) | < 0.001 | 3.070 (1.960-4.809) | < 0.001 |
| Tumor number (multiple versus single) | 2.595 (1.637-4.114) | < 0.001 | 2.356 (1.468-3.779) | < 0.001 |
| Tumor differentiation (poor versus well+moderate) | 1.485 (0.947-2.265) | 0.066 | 1.043 (0.672-1.620) | 0.851 |
| Preoperative AFP, ng/ml (>400 versus ≤400) | 2.261 (1.440-3.548) | < 0.001 | 1.820 (1.151-2.878) | 0.010 |
| Vascular invasion (yes versus none) | 2.995 (1.959-4.580) | < 0.001 | 2.310 (1.491-3.579) | < 0.001 |
| MACC1high versus MACC1low | 2.193 (1.430-3.364) | < 0.001 | 2.127 (1.362-3.322) | 0.001 |
| METhigh versus METlow | 1.460 (0.951-2.242) | 0.084 | 1.369 (0.878-2.133) | 0.166 |
| FAKhigh versus FAKlow | 1.858 (1.204-2.867) | 0.005 | 1.613 (1.035-2.514) | 0.035 |
| Combination of MACC1 and FAK | < 0.001 | < 0.001 | ||
| II versus I | 2.722 (1.399-5.296) | 0.003 | 2.185 (1.088-4.387) | 0.028 |
| III versus I | 3.602 (1.809-7.174) | < 0.001 | 3.001 (1.509-5.970) | 0.002 |
| IV versus I | 3.478 (1.884-6.420) | < 0.001 | 2.904 (1.566-5.383) | 0.001 |
| Multivariate analysis† | ||||
| Age, year (≥50 versus <50) | - | 0.153 | - | - |
| Tumor size (>5 cm versus ≤5 cm) | 3.350 (2.123-5.288) | < 0.001 | 2.719 (1.725-4.286) | < 0.001 |
| Tumor number (multiple versus single) | - | 0.141 | - | 0.150 |
| Preoperative AFP, ng/ml (>400 versus ≤400) | 1.789 (1.130-2.833) | 0.013 | 1.624 (1.020-2.587) | 0.041 |
| Vascular invasion (yes versus none) | 2.267 (1.474-3.485) | < 0.001 | 1.853 (1.192-2.880) | 0.006 |
| MACC1high versus MACC1low | 2.495 (1.616-3.851) | < 0.001 | 2.280 (1.453-3.580) | < 0.001 |
| FAKhigh versus FAKlow | - | 0.127 | - | 0.136 |
| Combination of MACC1 and FAK | - | < 0.001 | - | < 0.001 |
| II versus I | 2.518 (1.286-4.929) | 0.007 | 2.116 (1.048-4.274) | 0.037 |
| III versus I | 3.444 (1.707-6.948) | 0.001 | 3.001 (1.509-5.970) | 0.002 |
| IV versus I | 3.902 (2.078-7.328) | < 0.001 | 3.381 (1.809-6.316) | < 0.001 |
Abbreviations: MACC1, metastasis-associated in colon cancer-1; FAK, focal adhesion kinase; AFP, alpha-fetoprotein; 95% CI, 95% confidence interval.
†Statistical analyses were performed by Cox proportional hazards regression (univariate and multivariate analysis).
I, MACC1low/FAKlow; II, MACC1low/FAKhigh; III, MACC1high/FAKlow; IV, MACC1high/FAKhigh.
MACC1 expression is correlated with poor prognosis in HCC patients following liver transplantation. Prognostic significance of MACC1 and FAK were assessed by Kaplan-Meier analysis and log-rank tests. The patients whose HCC tissue samples expressed high levels of MACC1 had a poor prognosis with respect to cumulative recurrence (A) and overall survival (B). HCC samples with high FAK expression presented with high cumulative recurrence rates (C) and poor overall survival (D). Upon evaluation of the combined expression of MACC1 and FAK in the prognosis of HCC after LT, MACC1low/FAKlow was associated with the most favorable prognosis with respect to cumulative recurrence rates (E) and overall survival rates (F) in the four subgroups. I, MACC1low/FAKlow; II, MACC1low/FAKhigh; III, MACC1high/FAKlow; and IV, MACC1high/FAKhigh.
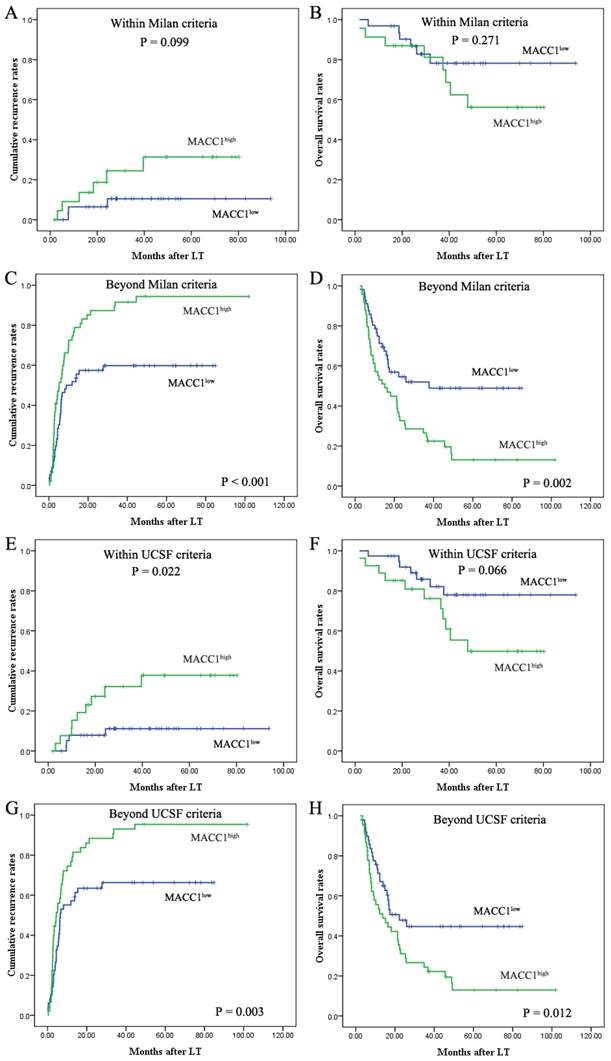
Prognostic significance of MACC1 in HCC patients stratified according to the Milan or UCSF criteria. Kaplan-Meier analysis of the cumulative recurrence rates (A) and overall survival (B) based on MACC1 expression in HCC patients within Milan criteria. The cumulative recurrence rates (C) and overall survival (D) based on MACC1 expression in HCC patients beyond Milan criteria. The cumulative recurrence rates (E) and overall survival (F) based on MACC1 expression in HCC patients within UCSF criteria. The cumulative recurrence rates (G) and overall survival (H) based on MACC1 expression in HCC patients beyond UCSF criteria.
Cox multivariate analysis revealed that, apart from vascular incasion, high preoperative AFP level (>400 ng/ml), and a larger tumor size (>5cm), MACC1 overexpression in HCC, rather than FAK, was an independent prognostic factor for predicting tumor recurrence (P < 0.001) and OS (P < 0.001) in HCC patients after LT. When MACC1 was combined with FAK, we found that MACC1/FAK was also an independent prognostic predictor for both OS (P < 0.001) and cumulative recurrence (P < 0.001) (Table 3).
Discussion
Hepatocellular carcinoma is a highly aggressive malignancy with a complex spectrum of molecular aberrations [15]. Liver transplantation offers a viable option for treating selected HCC patients. However, the clinical outcome remains challenging due to the tumor recurrence post-LT [16-18]. Recurrence is principally attributable to the presence of microscopic extrahepatic metastatic foci before LT [4]. Improved understanding of the underlying molecular mechanisms governing cancer metastasis is highly desirable.
Metastasis is a complicated process involving multiple steps, and multiple oncoproteins are documented to play important roles in this process [19, 20]. Recently, overexpression of MACC1 has been correlated with the metastasis of several malignancies [10-13, 21]. In the previous study, we identified for the first time that MACC1 promotes cell survival and metastasis in HCC, and overexpression of MACC1 is correlated with a more undifferentiated tumor phenotype. Our previous study reveals a novel molecular mechanism in HCC that MACC1 acts as a key factor for regulating both FAK and HGF/MET signaling network. In the present research, we identified for the first time that MACC1 alone or a combination with FAK could serve as a prognostic predictor for patients with HCC who have undergone LT therapy.
The gene encoding the hepatocyte growth factor (HGF) receptor, MET, is a transcriptional target of MACC1 [10]. In addition, HGF activates FAK in normal and cancer cells, suggesting a possible synergism between FAK and HGF signaling [22-24]. All of these suggest that there exist a regulatory network, in which MACC1 modulates and maintains the malignant phenotype of HCC cells. FAK was identified as a key signaling protein mediating the cross talk between adhesion-dependent signaling and growth factor receptors [25-27]. In addition, FAK activation is a key step to epithelial mesenchymal transition (EMT) [28-30]; as we known, progression toward malignancy is accompanied by loss of epithelial differentiation and a shift towards a mesenchymal phenotype, which referred to as EMT [31].
The previous in vitro findings indicate that MACC1 has a crucial role in HCC progression and metastasis. We then examined the expression of MACC1 and its downstream genes MET and FAK using a large cohort of clinical HCC samples, and investigated their clinical implications. Multivariate analyses revealed that MACC1 expression was a powerful independent prognostic factor. Interestingly, in patients who matched UCSF criteria, those with increased MACC1 expression were prone to earlier tumor recurrence and shorter OS following LT. This indicates that MACC1 expression may provide additional prognostic values in HCC patients within UCSF criteria. For patients exceeding the Milan or UCSF criteria, measuring the expression of MACC1 could also be helpful to identify prognosis distinguishable subgroup in advanced HCC patients beyond the Milan or UCSF criteria. The qRT-PCR results revealed a positive correlation between MACC1 and its target gene FAK, in addition to the findings that MACC1high or FAKhigh patients had an increasing risk of tumor recurrence and shorter OS post-LT. Therefore, we made comparisons of prognosis among four subgroups (MACC1low/FAKlow, MACC1low/FAKhigh, MACC1high/FAKlow, and MACC1high/FAKhigh). The HCC patients with MACC1low/FAKlow had the most favorable prognosis. Although expression of MACC1 was an independent predictor for OS and cumulative recurrence, the predictive range of MACC1/FAK was more sensitive than that of MACC1 alone. Taken together, these clinical findings implicate that MACC1 alone or combination of MACC1/FAK could be a novel biomarker for predicting metastatic recurrence of HCC in patients following LT, which in turn may influence their overall survival.
Clinicopathological variables including tumor size, tumor grade, microscopic/macroscopic vascular invasion, and alpha-fetoprotein, were important prognostic factors for tumor recurrence in HCC patients post-LT [16-18]. However, the current predictive model based on clinicopathological characteristics is still unsatisfactory and molecular-based tumor staging is critical for individualized diagnosis and therapy [32, 33]. Our findings demonstrate MACC1 or combination of MACC1/FAK as a complement to predict the prognosis of HCC following LT. Despite an important role of MACC1 in tumor progression and its predictive implication, the study should therefore be viewed as hypothesis generating, to be followed by larger prospective studies to confirm our findings.
In conclusion, MACC1 alone or combination of MACC1 with FAK could serve as a novel biomarker in predicting the prognosis of HCC after liver transplantation and might be a promising new therapeutic target. HCC is a highly aggressive tumor of diverse etiology and complex molecular alterations, an in-depth understanding of the regulation and functions of key oncoproteins such as MACC1 in HCC could have a profound impact on both diagnosis and treatment of this disease.
Acknowledgements
The present study was supported in part by grants from the Major National S&T Program (No.2012ZX10002-017), the Innovative Research Group Project of the National Natural Science Foundation of China (No.81121002), the National Natural Science Foundation of China (No.81101840), and the National Natural Science Foundation of China (No.81302074).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Aravalli R.N, Steer C.J, Cressman E.N. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48(6):2047-63
2. Llovet J.M, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48(4):1312-27
3. Parkin D.M, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108
4. Befeler A.S, Hayashi P.H, Di Bisceglie A.M. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128(6):1752-64
5. El-Serag H.B, Marrero J.A, Rudolph L, Reddy K.R. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134(6):1752-63
6. Zimmerman M.A, Ghobrial R.M, Tong M.J, Hiatt J.R, Cameron A.M, Hong J. et al. Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg. 2008;143(2):182-8
7. Birchmeier C, Birchmeier W. et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915-25
8. Cecchi F, Rabe D.C, Bottaro D.P. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 2010;46(7):1260-70
9. Whittaker S, Marais R, Zhu A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989-5005
10. Stein U, Walther W, Arlt F, Schwabe H, Smith J, Fichtner I. et al. MACC1, a newly identified key regulator of HGF- signaling MET, predicts colon cancer metastasis. Nat Med. 2009;15(1):59-67
11. Shirahata A, Fan W, Sakuraba K, Yokomizo K, Goto T, Mizukami H. et al. MACC 1 as a marker for vascular invasive hepatocellular carcinoma. Anticancer Res. 2011;31(3):777-80
12. Qiu J, Huang P, Liu Q, Hong J, Li B, Lu C. et al. Identification of MACC1 as a novel prognostic marker in hepatocellular carcinoma. J Transl Med. 2011;9:166
13. Qu J.H, Chang X.J, Lu Y.Y, Bai W.L, Chen Y, Zhou L. et al. Overexpression of metastasis-associated in colon cancer 1 predicts a poor outcome of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2012;18(23):2995-3003
14. Wu L.M, Zhang F, Xie H.Y, Xu X, Chen Q.X, Yin S.Y. et al. MMP2 promoter polymorphism (C-1306T) and risk of recurrence in patients with hepatocellular carcinoma after transplantation. Clin Genet. 2008;73(3):273-8
15. Zender L, Villanueva A, Tovar V, Sia D, Chiang D.Y, Llovet J.M. Cancer gene discovery in hepatocellular carcinoma. J Hepatol. 2010;52(6):921-9
16. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-9
17. Yao F.Y, Ferrell L, Bass N.M, Watson J.J, Bacchetti P, Venook A. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-403
18. Zheng S.S, Xu X, Wu J, Chen J, Wang W.L, Zhang M. et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85(12):1726-32
19. Chiang A.C, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814-23
20. Nguyen D.X, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341-52
21. Shimokawa H, Uramoto H, Onitsuka T, Chundong G, Hanagiri T, Oyama T. et al. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg. 2011;141(4):895-8
22. Beviglia L, Kramer R.H. HGF induces FAK activation and integrin-mediated adhesion in MTLn3 breast carcinoma cells. Int J Cancer. 1999;83(5):640-9
23. Chen H.C, Chan P.C, Tang M.J, Cheng C.H, Chang T.J. Tyrosine phosphorylation of focal adhesion kinase stimulated by hepatocyte growth factor leads to mitogen-activated protein kinase activation. J Biol Chem. 1998;273(40):25777-82
24. Matsumoto K, Matsumoto K, Nakamura T, Kramer R.H. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269(50):31807-13
25. Kaposi-Novak P, Lee J.S, Gomez-Quiroz L, Coulouarn C, Factor V.M, Thorgeirsson S.S. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116(6):1582-95
26. Park W.S, Dong S.M, Kim S.Y, Na E.Y, Shin M.S, Pi J.H. et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59(2):307-10
27. Ueki T, Fujimoto J, Suzuki T, Yamamoto H, Okamoto E. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology. 1997;25(3):619-23
28. Avizienyte E, Frame M.C. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17(5):542-7
29. Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A. et al. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314(1):143-52
30. Kiefel H, Bondong S, Pfeifer M, Schirmer U, Erbe-Hoffmann N, Schafer H. et al. EMT-associated up-regulation of L1CAM provides insights into L1CAM-mediated integrin signalling and NF-kappaB activation. Carcinogenesis. 2012;33(10):1919-29
31. Christiansen J.J, Rajasekaran A.K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319-26
32. Schmidt C, Marsh J.W. Molecular signature for HCC: role in predicting outcomes after liver transplant and selection for potential adjuvant treatment. Curr Opin Organ Transplant. 2010;15(3):277-82
33. Schwartz M, Dvorchik I, Roayaie S, Fiel M.I, Finkelstein S, Marsh J.W. et al. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers. J Hepatol. 2008;49(4):581-8
Author contact
![]() Corresponding author: Shu-Sen Zheng, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, the First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou 310003, P.R. China. Phone: 86-571-87236570; Fax: 86-571-87236466. E-mail address: shusenzhengedu.cn
Corresponding author: Shu-Sen Zheng, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, the First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou 310003, P.R. China. Phone: 86-571-87236570; Fax: 86-571-87236466. E-mail address: shusenzhengedu.cn

 Global reach, higher impact
Global reach, higher impact