3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(3):240-245. doi:10.7150/ijms.7489 This issue Cite
Short Research Communication
Stromal Cell-derived Factor-1 and its Receptor CXCR4 are Upregulated Expression in Degenerated Intervertebral Discs
1. Department of Orthopaedic Surgery, 2nd Affiliated Hospital, School of Medicine, Zhejiang University, No.88 Jiefang Road, Hangzhou, China
2. Department of Clinical Laboratory, the Red Cross Hospital, Zhejiang University of Traditional Chinese Medicine, No. 208 East Road, Hangzhou Ring, Hangzhou, China
* Hua Zhang and Li Zhang have contributed equally to this study.
Received 2013-8-25; Accepted 2013-12-23; Published 2014-1-11
Abstract
Background: Although chemokine stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 induce degradation of articular cartilage in rheumatoid arthritis (RA) and osteoarthritis (OA), the association between the SDF-1/CXCR4 pathway and degradation of the cartilaginous endplate and nucleus pulposus has not been thoroughly clarified. We investigated the expression of SDF-1 and CXCR4 in intervertebral discs (IVDs).
Methods: SDF-1 and CXCR4 levels in human IVDs and the rat L5/6 motion segment were quantified by enzyme-linked immunosorbent assay. SDF-1 staining was quantified using a microscope and Image-Pro Plus software. Integrated optical density (IOD) served as the measurement parameter. The number of CXCR4 immunoreactive cells was expressed as a percentage of the total number of cells.
Results: SDF-1 and CXCR4 were both expressed in IVDs, and the levels of SDF-1 and CXCR4 were both significantly higher in the degeneration group than in the normal group of human (or rat) discs. Both nucleus pulposus cells and cartilaginous endplate cells expressed the CXCR4 protein. Furthermore, a positive correlation was observed between the SDF-1 IOD value and the percentage of CXCR4-positive disc cells in the nucleus pulposus and cartilaginous endplate. The SDF-1 IOD values were significantly higher in the outer annular fibrosus and bone/endplate junction region than in the nucleus pulposus and cartilaginous endplate in the rat specimens.
Conclusions: Our findings suggest upregulated expression of SDF-1 and its receptor CXCR4 in degenerated IVD.
Keywords: SDF-1, CXCR4, intervertebral disc, nucleus pulposus, endplate
Introduction
Recent advances in cell and tissue engineering offer potential methods for inhibiting or reversing intervertebral disc (IVD) degeneration. However, a greater understanding of the pathobiology of cartilaginous endplate and nucleus pulposus degeneration is needed to ensure their success.[1]
Both the cartilaginous endplate and articular cartilage are hyaline cartilage tissues. The main components of the extracellular matrix of the nucleus pulposus are collagen type II and proteoglycans. Mainly chondrocyte-like nucleus pulposus cells are observed in the adult nucleus pulposus, and the expression of chondrocyte marker SOX-9 has been detected.[2,3] Other studies have suggested that nucleus pulposus cells were migration and differentiation of cartilaginous endplate cells.[4,5]. Cui et al.[6] confirmed that the types and expression of matrix metalloproteinases (MMPs) from bovine nucleus pulposus cells were similar to those of articular chondrocytes.
Synovial cells induce degradation of the cartilage matrix via the SDF-1/CXCR4 pathway in patients with rheumatoid arthritis (RA) and osteoarthritis (OA).[7-9] SDF-1 is synthesized by synovial fibroblasts and bone marrow stromal cells in the articular cartilage/bone junction region, but not by chondrocytes. Conversely, CXCR4 is expressed by chondrocytes rather than synovial fibroblasts. The combination of SDF-1 and CXCR4 induces expression of MMP-3, -9, and -13 by cartilage cells in patients with OA and RA.[7,8] Furthermore, removal of the synovium in vivo or cartilage cells cultured in vitro that are transfected with a mutant CXCR4 gene to block the SDF-1/CXCR4 pathway effectively reduce MMP-9 and -13 expression by chondrocytes.[9] These results suggest that MMPs targeting the SDF-1/CXCR4 signaling pathway play an important role in the degeneration of articular cartilage. Jia et al.[10] found that SDF-1 expression was higher in herniated discs than in normal discs, but they did not evaluate the distribution or expression of SDF-1 or CXCR4.
We used immunohistochemical staining to investigate SDF-1 and CXCR4 expression in IVDs and to determine whether an association exists between their expression and degeneration of the cartilaginous endplate and nucleus pulposus.
Materials and methods
Patients
Human lumbar IVDs were obtained from patients following surgical discectomy for treatment of various diseases. Each patient provided signed informed consent for their participation. This experiment followed the Tenets of the 1964 Declaration of Helsinki and was approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China. Forty-two human lumbar IVD specimens were obtained from 38 patients (21 men and 17 women; age, 19-65 years; mean, 41.2 ± 12.5 years). The degeneration group (disc hernia and spondylolisthesis) consisted of 22 specimens from 15 patients with a disc herniation and 5 patients with lumbar spondylolisthesis. The magnetic resonance imaging (MRI) of the pathological IVDs were all Pfirrmann grade IV-V. The normal group consisted of 20 specimens collected from 18 patients with spine-fresh burst fractures, and the MRIs of the IVDs showed Pfirrmann grade I-II.
Study animals
All animal procedures were approved by the Animal Care and Use Committee of Zhejiang Provincial Medical Institute. Thirty Sprague-Dawley rats (8 weeks old) from the Medical Institute Animal Center (Zhejiang University, China) were used, including 15 rats for the lumbar disc degeneration model and 15 rats for the normal group. This experiment followed the principles of laboratory animal care. Because rats achieve most of their skeletal maturity before 3 months of age, there is likely to be less interference from growth from induced disc degeneration. All rats were of similar weight, about 450 g, to ensure that the discs at the chosen position for the experiment were of similar size, so that when they were punctured with a needle of a defined gauge, similar injuries would be produced. The rats were given several days to adapt to the new housing and husbandry environment before each experiment.
Animal surgical procedures for the degeneration model
The animals were aseptically washed and an anterior midline transperitoneal approach was used under general anesthesia. After separating the hind peritoneum and psoas major muscles, the L5 and L6 vertebral bodies were identified. The L5-L6 disc was punctured with a 30-gauge needle for the degeneration model study, and the puncture was a half penetration (approximately the vertical distance from the annulus fibrosus to the center of the nucleus pulposus), according to the method of Liang et al.[11] The needle was inserted parallel to the endplate to avoid injuring them. The needle was rotated 360° in the disc and held in position for 30 s before extraction, which resulted in more rapid degeneration. After the operation, the incision was closed and the rats were put back in their cages to recover for 12 h on an absolute diet to prevent enteroparalysis, before being permitted to consume food and water.
Grouping of specimens and tissue preparation
All patients underwent radiography and MRI of the lumbar spine. The IVDs specimens in the degeneration group were from patients with lumbar IVD hernia or spondylolisthesis. Disc specimens for the control group came from patients with adolescent idiopathic scoliosis or lumbar vertebral burst fractures. The IVD specimens were dissected into two parts, including nucleus pulposus and cartilaginous endplate. All specimens were covered immediately with dry ice in the operating room and kept at -80°C until the experiments were performed.
All rats were sacrificed by an intraperitoneal injection of 10% chloral hydrate 4 weeks after the operation. The lumbar specimens were decalcified en bloc with a rapid decalcifier solution (RapidCal·Immuno, BBC Biochemical, Seattle, WA, USA). The L5-L6 motion segment, including the IVD and its adjacent half vertebrae, was sectioned mid-sagittally parallel to the direction of puncture with a scalpel, dehydrated in a graded ethanol series, and embedded in paraffin.
Immunohistochemical examination
All specimens were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (3 μm) were prepared and collected on positively charged glass slides. The sections were dried at 60°C for 2 h to increase adherence to the slides. The sections were deparaffinized and rehydrated using conventional methods. To block endogenous peroxidase activity, the slides were treated with 3% hydrogen peroxide in methanol at room temperature for 30 min, then washed with PBS three times (3 min each) and immersed in boiling 0.01 M sodium citrate buffer (pH 6.0) for 10 min. Next, the sections were incubated with goat serum for 30 min at room temperature. Subsequently, the sections were incubated with anti-SDF-1 (Abcam, Cambridge, UK) and anti-CXCR4 antibodies (Abcam) at 4°C overnight. After washing in PBS for 5 min, the sections were incubated in biotin-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology, CA, USA) as the secondary antibody at 37°C for 30 min. Finally, the specimens were reacted with 3,3′- diaminobenzidine (Santa Cruz Biotechnology, CA, USA). Hematoxylin was used as the counterstain.
Microscopic examination
SDF-1 staining of human IVDs (nucleus pulposus and cartilaginous endplate, respectively) and the rat motion segment was quantified using a microscope and Image-Pro Plus ver. 6.0 software (Media Cybernetics, Silver Spring, MD, USA), according to the method developed by Xavie et al.[12] Briefly, an area of interest in each section was first selected at 40× magnification, and then 10 digital images at 1360 × 1024 pixel resolution and 400× magnification were captured with a DP 70 CCD camera (Olympus, Tokyo, Japan) coupled with an AX-70 microscope (Olympus). The measurement parameter was integrated optical density (IOD). Optical density was calibrated and the area of interest was set as follows: hue, 0-30; saturation, 0-255; intensity, 0-255. Then the values were counted.
The number of CXCR4 immunoreactive cells in human and rat specimens was expressed as a percentage of the total number of cells, which were randomly counted in 10 fields at ×400 magnification.
Statistical analysis
The IOD was log10 transformed. The nonparametric Mann-Whitney U-test and Friedman's test were used to determine intergroup differences. Spearman's rank correlation coefficient was used to determine correlations between SDF-1 and CXCR4 expression. All statistical analyses were performed using SPSS ver. 11.5 software (SPSS, Inc., Chicago, IL, USA). Tests were two-sided, and p < 0.05 was considered significant.
Results
CXCR4-positive cells in human IVD
CXCR4-positive cells were observed in both groups (Figure 1A-D), and the percentage frequencies of the total number of disc cells in the normal group were 23.3 ± 8.7% (nucleus pulposus) and 28.3 ± 10.7% (cartilaginous endplate). In the degeneration group these were 72.3 ± 7.6% (P < 0.01) and 80.3 ± 9.6% (P < 0.01). Spearman's rank correlation coefficients were 0.68 and 0.77 in the nucleus pulposus and cartilaginous endplate, respectively.
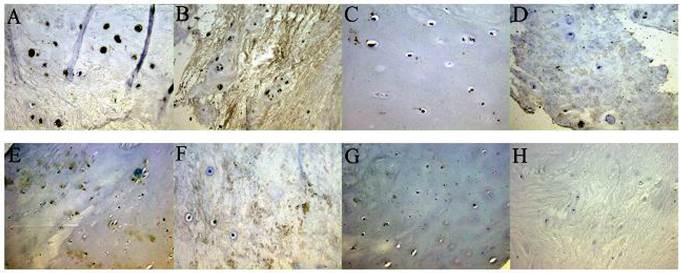
Under a light microscope, both CXCR4 (A-D) and SDF-1 (E-H) appear light brown by in situ immunohistochemical staining of human intervertebral disc specimens. CXCR4 was expressed by the nucleus pulposus and endplate cartilage cells, while SDF-1 was found mainly in the extracellular matrix. Patients with lumbar disc herniation (Pfirrmann grade V) had high CXCR4 expression in the (A) cartilage endplate (×40) and (B) nucleus pulposus (×40), and high SDF-1 expression in the (E) cartilage endplate (×20) and (F) nucleus pulposus (×40). Patients with lumbar fractures (Pfirrmann grade I) had low CXCR4 expression in the (C) cartilage endplate (×40) and (D) nucleus pulposus (×40) and low SDF-1 expression in the (G) cartilage endplate (×20) and (H) nucleus pulposus (×20)
SDF-1 IOD in human IVDs
SDF-1 was detected in both the degeneration and normal groups (Figure 1E-H). The SDF-1 IOD values in the human nucleus pulposus and cartilaginous endplate were as follows: degeneration group, 3.64 ± 0.48 and 3.53 ± 0.52; normal group, 2.98 ± 0.52 and 3.05 ± 0.76. SDF-1 expression was significantly higher in the degeneration group than in the normal group (both in the nucleus pulposus and endplate) (P < 0.01). A positive correlation was observed between the SDF-1 IOD value and the percentage of CXCR4-positive disc cells (P < 0.01).
SDF-1 IOD in the rat lumbar motion segment
We examined intergroup differences in the SDF-1 IOD in the L5-L6 lumbar motion segment of rats. The IOD values were examined in the nucleus pulposus, cartilaginous endplate, outer annular fibrosus, and vertebral body (Figure 2A-F). Significant intergroup differences were observed in the normal group (P < 0.05), except for the nucleus pulposus vs. the cartilaginous endplate, and the highest SDF-1 expression was in the outer annular fibrosus (IOD, 4.61 ± 0.89), and the lowest was in the cartilaginous endplate (IOD, 3.57 ± 0.72). The results for the degeneration group were similar: outer annular fibrosus, 4.97 ± 0.63; vertebral body, 4.56 ± 1.15; nucleus pulposus, 4.13 ± 0.75; and cartilaginous endplate, 4.08 ± 0.88 (Table 1). The values were significantly higher in the degeneration group than in the normal group in the nucleus pulposus (P < 0.01), cartilaginous endplate (P < 0.01), and outer annular fibrosus (P < 0.05) but not in the vertebral body (P > 0.05).
IOD of SDF-1 in the rat lumbar motion segment
| nucleus pulposus | cartilaginous endplate | outer annular fibrosus | vertebral body | |
|---|---|---|---|---|
| normal group | 3.69 ± 0.68 | 3.57 ± 0.72 | 4.61 ± 0.89 *† | 4.37 ± 0.54*†‡ |
| degeneration group | 4.13 ± 0.75 | 4.08 ± 0.88 | 4.97 ± 0.63 *† | 4.56 ± 1.15 *†‡ |
Values are means ± standard deviation of IOD in several part of L5-L6 motion segment. Differences with each other were compared using nonparametric Friedman's test.
* p<0.05 vs nucleus pulposus, † p<0.05 vs cartilaginous endplate, ‡ p<0.05 vs outer annular fibrosus.
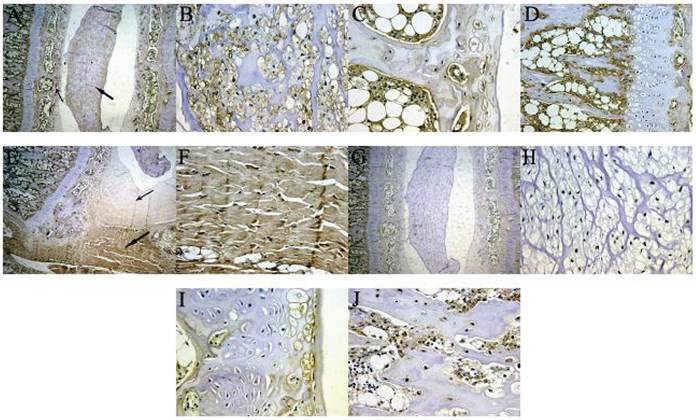
Under a light microscope, SDF-1 (A-F) and CXCR4 (G-J) both appear light brown by in situ immunohistochemical staining of rat vertebral-intervertebral disc-vertebral segment specimens. (A) The thick arrow indicates the nucleus; the thin arrow indicates the cartilage end plate (×5). (B) The nucleus pulposus expressed SDF-1 mainly in the extracellular matrix (×20). (C) SDF-1 expression was lower in cartilage endplate cells and the surrounding area and high in the capillary sinus (×40). (D) The vertebral body near the endplate was rich in SDF-1, suggesting that the bone marrow stromal cells in this area were the main source of SDF-1 for the disc (×20). (E) The thick arrow indicates the outer annular fibrosus; the thin arrow indicates the inner annular fibrosus (×5). (F) The outer annular fibrosus tissue showed strong positive staining, indicating that fibroblasts expressed SDF-1 (×40). (G) The distribution of CXCR4 expression in the vertebral body-intervertebral disc-vertebral segment (×5). (H-J) CXCR4 expression by nucleus pulposus cells, endplate chondrocytes, and osteoblasts, respectively.
CXCR4-positive cells in the rat lumbar motion segment
CXCR4 expression in IVDs of rats was detected not only in nucleus pulposus cells but also in endplate chondrocytes (Figure 2G-J). The mean ± standard deviation frequencies of CXCR4-positive disc cells in the normal group were 61.2 ± 4.4% in the nucleus pulposus and 27.4 ± 3.1% in the cartilaginous endplate; however, the highest expression was observed in the endothecium of the endplate or mature chondrocytes. Significantly more CXCR4-positive disc cells were observed in the degeneration group than in normal discs: 72.2 ± 6.4% in the nucleus pulposus (P < 0.05) and 40.2 ± 5.3% in the cartilaginous endplate (P < 0.05). A positive correlation was observed between the SDF-1 IOD value and the percentage of CXCR4-positive disc cells in rat specimens (P < 0.01). Spearman's rank correlation coefficients were 0.33 and 0.54, respectively, in the nucleus pulposus and cartilaginous endplate.
Discussion
SDF-1 was expressed in the human nucleus pulposus and cartilaginous endplate, and were significantly higher in the degeneration group than in the normal group. In support of our data, a recent study reported that the frequency of SDF-1a and vascular endothelial growth factor (VEGF) expression was significantly higher in extruded and sequestrated herniated IVDs than in control discs, and there was a high correlation between SDF-1a and VEGF expression. They concluded that SDF-1a and VEGF are expressed in human IVDs and that their interaction may be important for accumulating endothelial progenitor cells during the neovascularization process in herniated IVDs.[10]
Furthermore, in the present study, the SDF-1 IOD values were significantly higher in the outer annular fibrosus and bone/endplate junction region in rat specimens than in the nucleus pulposus and cartilaginous endplate. Wei et al.[13] confirmed that bone marrow in the cartilage/bone junction area of the articulation was rich in SDF-1, and that SDF-1 could penetrate the cartilage in vivo, which induced a significant increase in MMP-13 expression in a dose-dependent manner. The SDF-1 concentration is greatly elevated in synovial fluid (SF) from patients with OA and RA, which is from synovial fibroblasts, as demonstrated by immunocytochemistry, protein chemistry, and reverse transcription-polymerase chain reaction analysis[7,8]. Thus, the above results suggest that bone marrow stromal cells in the bone/endplate junction region and fibroblasts in the annulus outer layer may be two sources of SDF-1 in IVDs.
This is the first study to show CXCR4 expression in IVDs. SDF-1 exerts its effects through its only physiologic cognate receptor, CXCR4, which mediates chemotaxis, hematopoiesis, vasculogenesis, and tumor spread and metastasis. Studies have suggested that CXCR4 was expressed by chondrocytes but not by synovial fibroblasts. Our results suggest that CXCR4 is expressed not only by endplate chondrocytes but also by nucleus pulposus cells. The level of CXCR4 was significantly higher in the nucleus pulposus than in cartilaginous endplate. Furthermore, there was a positive correlation between the SDF-1 IOD value in the nucleus pulposus and cartilaginous endplate and the percentage of CXCR4-positive disc cells in Spearman's rank correlation coefficient analyses. Our results suggest a complementary protein expression pattern, in which the SDF-1 ligand is expressed by bone marrow stromal cells in the bone/endplate junction region and fibroblasts in the annulus outer layer. The CXCR4 receptor is expressed by endplate chondrocytes and nucleus pulposus cells. This complementary expression pattern suggests a paracrine regulatory mechanism, in which SDF-1 produced by bone marrow stromal cells and fibroblasts signals nearby endplate chondrocytes and nucleus pulposus cells by binding to their receptors.
There are several limitations to this study. First, the SDF-1 and CXCR4 expression levels were examined only by enzyme-linked immunosorbent assay. Second, the induction results of MMPs expressed by nucleus pulposus cells and cartilage endplate cells (CXCR4-positive) through SDF-1 stimulation were not investigated. Despite these limitations, this study revealed that SDF-1 and CXCR4 were expressed in IVDs through immunohistochemical staining, and that expression was upregulated to a greater level in the degeneration group than in the normal group. These results indicate a potential pathway for degeneration of the cartilaginous endplate and nucleus pulposus. A longitudinal study would further enhance knowledge on this degeneration mechanism.
Acknowledgements
This work is in financially supported by the National Natural Science Foundation of China (NSFC) (81301670) and Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP) (No. 20110101120122).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Freemont AJ, Watkins A, Le Maitre C. et al. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196:374-379
2. Pattappa G, Li Z, Peroglio M. et al. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221(6):480-496
3. Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J. 2006;15(Suppl 3):S333-337
4. Cui Y, Yu J, Urban JP. et al. Differential gene expression profiling of metalloproteinases and their inhibitors: a comparison between bovine intervertebral disc nucleus pulposus cells and articular chondrocytes. Spine (Phila Pa 1976). 2010;35(11):1101-1108
5. Kim KW, Lim TH, Kim JG. et al. The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine (Phila Pa 1976). 2003;28(10):982-990
6. Kim KW, Ha KY, Park JB. et al. Expressions of membrane-type I matrix metalloproteinase, Ki-67 protein, and type II collagen by chondrocytes migrating from cartilage endplate into nucleus pulposus in rat intervertebral discs: a cartilage endplate-fracture model using an intervertebral disc organ culture. Spine (Phila Pa 1976). 2005;30(12):1373-1378
7. Kanbe K, Takagishi K, Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002;46(1):130-137
8. Chiu YC, Yang RS, Hsieh KH. et al. Stromal cell-derived factor-1 induces matrix metalloprotease-13 expression in human chondrocytes. Mol Pharmacol. 2007;72(3):695-703
9. Kanbe K, Takemura T, Takeuchi K. et al. Synovectomy reduces stromal-cell-derived factor-1 (SDF-1) which is involved in the destruction of cartilage in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br. 2004;86(2):296-300
10. Jia CQ, Zhao JG, Zhang SF. et al. Stromal cell-derived factor-1 and vascular endothelial growth factor may play an important role in the process of neovascularization of herniated intervertebral discs. J Int Med Res. 2009;37(1):136-144
11. Liang HX, Ma SY, Feng G. et al. Therapeutic effects of adenovirus-mediated growth and differentiation factor-5 in a mice disc degeneration model induced by annulus needle puncture. The Spine J. 2010;10:32-41
12. Xavier LL, Viola GG, Ferraz AC. et al. A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Brain Res Protoc. 2005;16:58-64
13. Wei L, Kanbe K, Lee M, Wei X, Pei M, Sun X, Terek R, Chen Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol. 2010;341(1):236-245
Author contact
![]() Corresponding author: Hua Zhang. Tel: 0086+0571-87783550; Email: zhanghua068edu.cn
Corresponding author: Hua Zhang. Tel: 0086+0571-87783550; Email: zhanghua068edu.cn

 Global reach, higher impact
Global reach, higher impact