3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(9):2247-2256. doi:10.7150/ijms.107983 This issue Cite
Research Paper
Risk of Sleep Disorders among Patients with Tourette Syndrome: A Population-Based Cohort Study in Taiwan
1. School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
2. Department of Pharmacology, Chung Shan Medical University, Taichung 40201, Taiwan.
3. Department of Pharmacy, Chung Shan Medical University Hospital, Taichung 40201, Taiwan.
4. Department of Surgery, E-Da Hospital, I-Shou University, Kaohsiung 824, Taiwan.
5. Department and Graduate Institute of Business Administration, National Taiwan University, Taipei 106, Taiwan.
6. Department of Health Services Administration, China Medical University, Taichung 406040, Taiwan.
* These authors contributed equally to this work and share the first authorship.
† These authors contributed equally to this work and share the correspondence authorship.
Received 2024-12-1; Accepted 2025-3-28; Published 2025-4-22
Abstract
Background: Tourette syndrome (TS) is a complex neurodevelopmental disorder often linked with various neuropsychiatric comorbidities. This population-based cohort study examined the association between TS and sleep disorders.
Materials and methods: Utilizing data from a nationwide database, this retrospective cohort study assessed the risk of sleep disorders in patients with TS. We enrolled 13,646 patients with new-onset TS from 2002 to 2015, each matched with four controls by age, sex, insured salary, urbanization level, Charlson comorbidity index, and year of inclusion. Follow-up continued until the development of sleep disorders, death, or the end of 2018. The risk was evaluated using a Cox proportional hazards model, with sensitivity analyses at ≤1, 1-5, and >5 years.
Results: After adjusting for several variables, patients with TS had a higher risk of sleep disorders (adjusted hazard ratio [aHR] = 1.76, 95% confidence interval [CI] = 1.58-1.96). Those aged over 18 years had a higher risk than those under 7 years (aHR = 7.76, 95% CI = 6.32-9.53). Patients with comorbid attention deficit hyperactivity disorder (ADHD) and anxiety also showed increased risks (aHR = 1.35, 95% CI = 1.09-1.67 and aHR = 2.33, 95% CI = 1.88-2.88, respectively). Sensitivity analyses confirmed a higher risk of sleep disorders in TS patients at <1-, 1-5-, and >5-year follow-up periods.
Conclusion: TS is a significant risk factor for sleep disorders. Patients with comorbid ADHD and anxiety are particularly at higher risk for sleep disturbances.
Keywords: Tourette syndrome, sleep disorders, children, real-world evidence
Introduction
Tic disorders (TDs) are a class of neuropsychiatric disorders that develop in childhood and adolescence (before the age of 18 years); TDs are characterized by involuntary motor tics and vocal tics, and Tourette syndrome (TS) is the most severe form of TD [1, 2]. TS is characterized by fluctuating motor tics and vocal tics [3]. Epidemiological studies have revealed that motor tics typically begin at the age of 4-6 years, and the mean age at onset is 6 years [4, 5].
Patients with TS exhibit significantly decreased sleep quality as well as difficulties initiating and maintaining sleep [6]. Patients with TS often present with several comorbidities. Among these comorbidities, attention deficit hyperactivity disorder (ADHD) is the most common comorbidity, followed by obsessive compulsive disorder (OCD), with prevalence rates of 50%-60% and 36%-50%, respectively [1, 3].
Little knowledge is available regarding the risk of sleep disorder in patients with TS; a nationwide database-based epidemiological study should be conducted to investigate the risk of sleep disorders among patients with TS. Patients with TS comorbid with neuropsychiatric disorders may have an increased likelihood of sleep problems. In this study, by using data from the National Health Insurance Research Database (NHIRD) in Taiwan, we investigated neuropsychiatric comorbidities as risk factors for the risk of incident sleep disorders among patients with TS.
Materials and methods
Data source
This study conducted a secondary analysis of 2001-2018 NHIRD data. The NHIRD is a nationally representative database maintained by the Health and Welfare Data Science Center (HWDC) of the Ministry of Health and Welfare, Taiwan, and contains the detailed clinical records and the inpatient and outpatient claims of the beneficiaries of Taiwan's National Health Insurance (NHI) program. The HWDC provides scrambled random identification numbers for insured patients to ensure their privacy. The NHI program has provided coverage for up to 99% of the country's population since 1995. The NHIRD provides real-world evidence to support clinical decisions and health-care policy-making [7, 8]. Diagnostic data in the NHIRD before 2016 and after 2016 are, respectively, coded using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Ethics statements
This study was conducted in accordance with the Declaration of Helsinki. The data used in the present study are anonymous to ensure the privacy of beneficiaries. Informed consent from participants was not required given that the database in this study contains only de-identified data. The present study was reported in accordance with the STROCSS criteria [9]. The study protocol received ethical review and approval by the Institutional Review Board of Chung Shan Medical University Hospital, Taiwan (No. CSMUH CS1-22174).
Study participants
In this study, we enrolled patients with new-onset TS between 2002 and 2015. TS (ICD-9-CM code 307.23) was defined by the presence of three or more outpatient records of TS diagnoses in 1 year. Patients were excluded from this study if they had a sleep disorder diagnosis before TS. To minimize potential selection bias resulting from the use of unbalanced covariates in observational studies, we employed propensity score matching (PSM) to reduce selection bias due to unbalanced covariates in this observational study. Specifically, an optimized matching mode was used with a caliper width of 0.2 of the pooled standard deviation of the logit of the propensity score, matching each TS patient to four controls in a 1:4 ratio. To assess covariate balance, standardized mean differences (SMD) were calculated, with a threshold of SMD < 0.1 indicating adequate balance. PSM is a statistical matching technique that can be used to reduce potential confounding caused by unbalanced covariates in nonexperimental settings [10, 11]. A propensity score is a probability calculated using a logistic regression model. The score is a unit of a certain characteristic assigned to a patient with TS. These scores can reduce or eliminate selection bias in observational studies by accounting for the characteristics of controls. The variables used for matching were sex, age, insured salary, urbanization level, Charlson comorbidity index (CCI), and year of inclusion in the study. The CCI was used to estimate comorbidity severity in this study. The CCI classifies comorbidities into 17 types and converts them into weighted scores; the scores are then summed to calculate the corresponding CCI scores [12]. After matching, this study comprised 13 646 patients with TS and 54 584 matched controls from the general population as the comparison cohort. The patient selection process is presented in Figure 1.
Study design
This retrospective cohort study investigated the risk of sleep disorders (ICD-9-CM code 307.4; ICD-10-CM codes G47.8 and G47.9) among patients with TS. The comorbidities included in the analysis were ADHD (ICD-9-CM code 314.0), OCD, (ICD-9-CM code 300.3), anxiety (ICD-9-CM code 300.0), depression (ICD-9-CM codes 296.2 and 296.3), obesity (ICD-9-CM code 287.0), atopic dermatitis (ICD-9-CM code 691), allergic rhinitis (ICD-9-CM code 477), and chronic urticarial (ICD-9-CM code 708). The date of TS diagnosis was the observation start date for patients with TS, and after matching, the same date was assigned as the observation start date for controls. All patients were followed up from the observation start date until death, diagnosis of sleep disorders, or the end date of 2018.
Statistical analysis
All statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA), and statistical significance was indicated by p < 0.05. Chi-square tests and SMD were used to evaluate the distributions of baseline characteristics. A Cox proportional hazards model was employed to investigate the association between TS and sleep disorders, adjusting for the following relevant variables: sex, age, insured salary, urbanization level, CCI, ADHD, OCD, anxiety, depression, obesity, atopic dermatitis, allergic rhinitis, and chronic urticaria. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). Besides, we used the variance inflation factor (VIF) to assess multicollinearity among variables and confirmed that all VIF of variables were below 10, suggesting no substantial multicollinearity in the model. Sensitivity analyses were conducted to examine the risk of sleep disorders among patients with TS at <1-, 1-5-, and >5-year follow-up periods.
Results
Table 1 provides the baseline characteristics of patients with TS and matched controls. Of the 68 230 study individuals, 56 620 were male patients, and 11 610 were female patients. Of these patients, 13 646 patients had TS, and 54 584 were from the general population. The average ages of patients with TS and controls were 11.32 ± 7.82 and 14.29 ± 11.91 years, respectively. Sex, age, insured salary, urbanization, and CCI did not differ significantly between the groups (p > 0.05). Among patients with TS, 2 260 (16.56%) had ADHD, 188 (1.38%) had OCD, 526 (3.85%) had anxiety, 79 (0.58%) had depression, 437 (3.20%) had atopic dermatitis, 4 324 (31.69%) had allergic rhinitis, and 525 (3.85%) had chronic urticarial. Post-matching results confirmed that all SMD values were below 0.1 and p-values exceeded 0.05, indicating no significant differences between the TS and control groups on each matching variable.
The flowchart of participant selection.
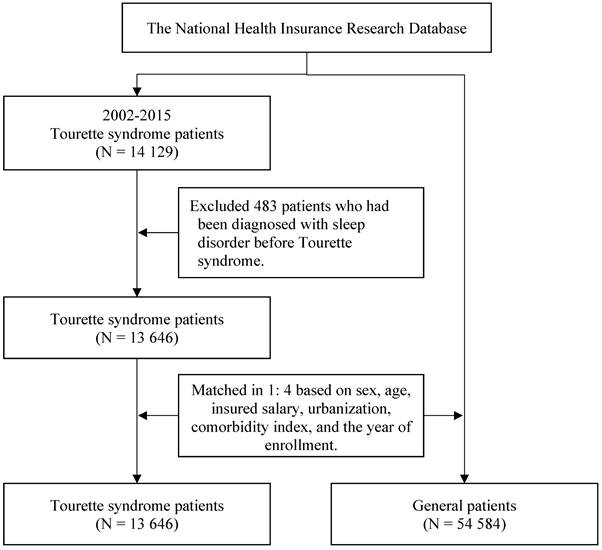
Table 2 presents the incidence rate of sleep disorders in patients with TS and controls. In total, 1 706 patients (2.50%) developed sleep disorders, of whom 1 158 (4.02%) had TS. The incidence rates of sleep disorders were 4.61 and 2.40 per 1 000 person-years in patients with TS and controls, respectively. In total, 406 female (3.50%) and 1 300 male (2.30%) patients developed sleep disorders; the incidence rates were 3.95 and 2.61 per 1 000 person-years, respectively. Patients with ADHD, OCD, anxiety, or depression were more likely to develop sleep disorders than were those without comorbidities.
Baseline characteristics of study subjects after matching.
| Variables | Total | General patients | Tourette syndrome | p-value | SMD 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Total | 68,230 | 100.00 | 54,584 | 100.00 | 13,646 | 100.00 | |||
| Sex 1 | 1.000 | 0 | |||||||
| Female | 11,610 | 17.02 | 9,288 | 17.02 | 2,322 | 17.02 | |||
| Male | 56,620 | 82.98 | 45,296 | 82.98 | 11,324 | 82.98 | |||
| Age (year) 1 | 0.998 | 0.003 | |||||||
| <7 | 11,560 | 16.94 | 9,246 | 16.94 | 2,314 | 16.96 | |||
| 7-18 | 49,952 | 73.21 | 39,962 | 73.21 | 9,990 | 73.21 | |||
| >18 | 6,718 | 9.85 | 5,376 | 9.85 | 1,342 | 9.83 | |||
| Mean ± SD | 13.69 ± 11.27 | 14.29 ± 11.91 | 11.32 ± 7.82 | ||||||
| Insured salary (NTD) 1,2 | 0.998 | 0.003 | |||||||
| ≤19,200 | 21,863 | 32.04 | 17,487 | 32.04 | 4,376 | 32.07 | |||
| 19,201-21,900 | 14,075 | 20.63 | 11,254 | 20.62 | 2,821 | 20.67 | |||
| 21,900-36,300 | 15,155 | 22.21 | 12,128 | 22.22 | 3,027 | 22.18 | |||
| ≥36,301 | 17,137 | 25.12 | 13,715 | 25.13 | 3,422 | 25.08 | |||
| Urbanization 1,2 | 1.000 | 0 | |||||||
| Level 1 | 21,896 | 32.09 | 17,515 | 32.09 | 4,381 | 32.10 | |||
| Level 2 | 21,405 | 31.37 | 17,120 | 31.36 | 4,285 | 31.40 | |||
| Level 3 | 11,656 | 17.08 | 9,324 | 17.08 | 2,332 | 17.09 | |||
| Level 4 | 8,272 | 12.12 | 6,616 | 12.12 | 1,656 | 12.14 | |||
| Level 5 | 947 | 1.39 | 758 | 1.39 | 189 | 1.39 | |||
| Level 6 | 1,813 | 2.66 | 1,459 | 2.67 | 354 | 2.59 | |||
| Level 7 | 2,241 | 3.28 | 1,792 | 3.28 | 449 | 3.29 | |||
| CCI score 1,3 | 0.998 | 0.002 | |||||||
| 0 | 47,384 | 69.45 | 37,904 | 69.44 | 9,480 | 69.47 | |||
| 1 | 19,353 | 28.36 | 15,485 | 28.37 | 3,868 | 28.35 | |||
| ≥2 | 1,493 | 2.19 | 1,195 | 2.19 | 298 | 2.18 | |||
| ADHD 3 | <0.001 | 0.069 | |||||||
| No | 64,872 | 95.08 | 53,486 | 97.99 | 11,386 | 83.44 | |||
| Yes | 3,358 | 4.92 | 1,098 | 2.01 | 2,260 | 16.56 | |||
| OCD 3 | <0.001 | 0.073 | |||||||
| No | 68,009 | 99.68 | 54,551 | 99.94 | 13,458 | 98.62 | |||
| Yes | 221 | 0.32 | 33 | 0.06 | 188 | 1.38 | |||
| Anxiety | <0.001 | 0.104 | |||||||
| No | 67,314 | 98.66 | 54,194 | 99.29 | 13,120 | 96.15 | |||
| Yes | 916 | 1.34 | 390 | 0.71 | 526 | 3.85 | |||
| Depression | <0.001 | 0.533 | |||||||
| No | 68,113 | 99.83 | 54,546 | 99.93 | 13,567 | 99.42 | |||
| Yes | 117 | 0.17 | 38 | 0.07 | 79 | 0.58 | |||
| Obesity | 0.711 | 0.070 | |||||||
| No | 68,113 | 99.83 | 54,492 | 99.83 | 13,621 | 99.82 | |||
| Yes | 117 | 0.17 | 92 | 0.17 | 25 | 0.18 | |||
| Atopic dermatitis | 0.009 | 0.034 | |||||||
| No | 66,273 | 97.13 | 53,064 | 97.22 | 13,209 | 96.80 | |||
| Yes | 1,957 | 2.87 | 1,520 | 2.78 | 437 | 3.20 | |||
| Allergic rhinitis | <0.001 | 0.848 | |||||||
| No | 55,542 | 81.40 | 46,220 | 84.68 | 9,322 | 68.31 | |||
| Yes | 12,688 | 18.60 | 8,364 | 15.32 | 4,324 | 31.69 | |||
| Chronic urticarial | <0.001 | 0.221 | |||||||
| No | 66,039 | 96.79 | 52,918 | 96.95 | 13,121 | 96.15 | |||
| Yes | 2,191 | 3.21 | 1,666 | 3.05 | 525 | 3.85 | |||
1 Variables for propensity score matching. 2 NTD is New Taiwan Dollar (NTD 1 ≈ USD 0.034); Urbanization Level 1 denoted the highest degree of urbanization, whereas level 7 denoted the lowest degree of urbanization.3 Abbreviations: CCI, Charlson comorbidity index; ADHD, attention deficit hyperactivity disorder; OCD, obsessive-compulsive disorder. SMD; standardized mean differences.
The incident rate of sleep disorder.
| Variables | Sleep disorder | |||||
|---|---|---|---|---|---|---|
| No | Yes | IR 1 | p-value | |||
| N | % | N | % | |||
| Total | 66,524 | 97.50 | 1,706 | 2.50 | 2.84 | |
| Patient group | <0.001 | |||||
| General | 53,426 | 97.88 | 1,158 | 2.12 | 2.40 | |
| Tourette syndrome | 13,098 | 95.98 | 548 | 4.02 | 4.61 | |
| Sex | <0.001 | |||||
| Female | 11,204 | 96.50 | 406 | 3.50 | 3.95 | |
| Male | 55,320 | 97.70 | 1,300 | 2.30 | 2.61 | |
| Age (year) | <0.001 | |||||
| <7 | 11,438 | 98.94 | 122 | 1.06 | 1.17 | |
| 7-18 | 48,897 | 97.89 | 1,055 | 2.11 | 2.39 | |
| >18 | 6,189 | 92.13 | 529 | 7.87 | 9.43 | |
| Mean ± SD | 13.49 ± 11.00 | 21.64 ± 17.35 | ||||
| Insured salary (NTD) 2 | <0.001 | |||||
| ≤19,200 | 21,126 | 96.63 | 737 | 3.37 | 3.33 | |
| 19,201-21,900 | 13,797 | 98.02 | 278 | 1.98 | 2.47 | |
| 21,900-36,300 | 14,831 | 97.86 | 324 | 2.14 | 2.79 | |
| ≥36,301 | 16,770 | 97.86 | 367 | 2.14 | 2.43 | |
| Urbanization 2 | 0.051 | |||||
| Level 1 | 21,285 | 97.21 | 611 | 2.79 | 3.08 | |
| Level 2 | 20,894 | 97.61 | 511 | 2.39 | 2.74 | |
| Level 3 | 11,390 | 97.72 | 266 | 2.28 | 2.64 | |
| Level 4 | 8,068 | 97.53 | 204 | 2.47 | 2.90 | |
| Level 5 | 926 | 97.78 | 21 | 2.22 | 2.56 | |
| Level 6 | 1,775 | 97.90 | 38 | 2.10 | 2.17 | |
| Level 7 | 2,186 | 97.55 | 55 | 2.45 | 2.80 | |
| CCI score 3 | <0.001 | |||||
| 0 | 46,324 | 97.76 | 1,060 | 2.24 | 2.54 | |
| 1 | 18,835 | 97.32 | 518 | 2.68 | 3.06 | |
| ≥2 | 1,365 | 91.43 | 128 | 8.57 | 9.30 | |
| ADHD 3 | 0.003 | |||||
| No | 63,276 | 97.54 | 1,596 | 2.46 | 2.77 | |
| Yes | 3,248 | 96.72 | 110 | 3.28 | 4.24 | |
| OCD 3 | <0.001 | |||||
| No | 66,322 | 97.52 | 1,687 | 2.48 | 2.82 | |
| Yes | 202 | 91.40 | 19 | 8.60 | 9.57 | |
| Anxiety | <0.001 | |||||
| No | 65,713 | 97.62 | 1,601 | 2.38 | 2.69 | |
| Yes | 811 | 88.54 | 105 | 11.46 | 14.70 | |
| Depression | <0.001 | |||||
| No | 66,431 | 97.53 | 1,682 | 2.47 | 2.80 | |
| Yes | 93 | 79.49 | 24 | 20.51 | 22.71 | |
| Obesity | ||||||
| No | 66,412 | 97.50 | 1,701 | 2.50 | 2.83 | 0.219 |
| Yes | 112 | 95.73 | 5 | 4.27 | 5.39 | |
| Atopic dermatitis | 0.080 | |||||
| No | 64,604 | 97.48 | 1,669 | 2.52 | 2.85 | |
| Yes | 1,920 | 98.11 | 37 | 1.89 | 2.23 | |
| Allergic rhinitis | 0.694 | |||||
| No | 54,147 | 97.49 | 1,395 | 2.51 | 2.82 | |
| Yes | 12,377 | 97.55 | 311 | 2.45 | 2.89 | |
| Chronic urticarial | 0.066 | |||||
| No | 64,401 | 97.52 | 1,638 | 2.48 | 2.81 | |
| Yes | 2,123 | 96.90 | 68 | 3.10 | 3.62 | |
1 IR, incidence rate per 1,000 person-year. 2 NTD is New Taiwan Dollar (NTD 1 ≈ USD 0.034); Urbanization Level 1 denoted the highest degree of urbanization, whereas level 7 denoted the lowest degree of urbanization. 3 Abbreviations: CCI, Charlson comorbidity index; ADHD, attention deficit hyperactivity disorder; OCD, obsessive-compulsive disorder.
The risk of sleep disorder between Tourette syndrome and general patients.
| Variables | Unadjusted model | Adjusted model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |||||
| Patient group | ||||||||||
| General (ref.) | 1 | 1 | ||||||||
| Tourette syndrome | 1.93 | 1.74 | - | 2.13 | <0.001 | 1.76 | 1.58 | - | 1.96 | <0.001 |
| Sex | ||||||||||
| Female (ref.) | 1 | 1 | ||||||||
| Male | 0.66 | 0.59 | - | 0.74 | <0.001 | 0.68 | 0.61 | - | 0.76 | <0.001 |
| Age (year) | ||||||||||
| <7 (ref.) | 1 | 1 | ||||||||
| 7-18 | 2.08 | 1.72 | - | 2.51 | <0.001 | 2.18 | 1.80 | - | 2.64 | <0.001 |
| >18 | 8.23 | 6.76 | - | 10.02 | <0.001 | 7.76 | 6.32 | - | 9.53 | <0.001 |
| Insured salary (NTD) 1 | ||||||||||
| ≤19,200 (ref.) | 1 | 1 | ||||||||
| 19,201-21,900 | 0.83 | 0.72 | - | 0.96 | 0.010 | 0.99 | 0.86 | - | 1.14 | 0.864 |
| 21,900-36,300 | 0.91 | 0.80 | - | 1.04 | 0.171 | 0.97 | 0.85 | - | 1.11 | 0.652 |
| ≥36,301 | 0.77 | 0.68 | - | 0.87 | <0.001 | 0.84 | 0.74 | - | 0.96 | 0.009 |
| Urbanization 1 | ||||||||||
| Level 1 (ref.) | 1 | 1 | ||||||||
| Level 2 | 0.91 | 0.81 | - | 1.02 | 0.099 | 0.88 | 0.78 | - | 0.99 | 0.034 |
| Level 3 | 0.88 | 0.76 | - | 1.01 | 0.072 | 0.91 | 0.78 | - | 1.05 | 0.176 |
| Level 4 | 0.96 | 0.82 | - | 1.13 | 0.646 | 0.97 | 0.83 | - | 1.14 | 0.741 |
| Level 5 | 0.85 | 0.55 | - | 1.31 | 0.459 | 0.79 | 0.51 | - | 1.22 | 0.282 |
| Level 6 | 0.69 | 0.50 | - | 0.96 | 0.028 | 0.68 | 0.49 | - | 0.95 | 0.023 |
| Level 7 | 0.93 | 0.70 | - | 1.22 | 0.592 | 0.91 | 0.69 | - | 1.20 | 0.512 |
| CCI score 2 | ||||||||||
| 0 (ref.) | 1 | 1 | ||||||||
| 1 | 1.21 | 1.09 | - | 1.34 | <0.001 | 1.28 | 1.15 | - | 1.43 | <0.001 |
| ≥2 | 3.57 | 2.97 | - | 4.28 | <0.001 | 1.78 | 1.47 | - | 2.17 | <0.001 |
| ADHD 2 | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 1.63 | 1.35 | - | 1.98 | <0.001 | 1.35 | 1.09 | - | 1.67 | 0.005 |
| OCD 2 | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 3.34 | 2.12 | - | 5.25 | <0.001 | 0.96 | 0.60 | - | 1.55 | 0.875 |
| Anxiety | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 5.67 | 4.65 | - | 6.90 | <0.001 | 2.33 | 1.88 | - | 2.88 | <0.001 |
| Depression | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 7.85 | 5.25 | - | 11.75 | <0.001 | 1.49 | 0.97 | - | 2.29 | 0.069 |
| Obesity | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 1.97 | 0.82 | - | 4.74 | 0.130 | 1.57 | 0.65 | - | 3.78 | 0.315 |
| Atopic dermatitis | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 0.79 | 0.57 | - | 1.10 | 0.164 | 0.94 | 0.67 | - | 1.31 | 0.709 |
| Allergic rhinitis | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 1.04 | 0.92 | - | 1.18 | 0.488 | 1.08 | 0.94 | - | 1.24 | 0.260 |
| Chronic urticarial | ||||||||||
| No (ref.) | 1 | 1 | ||||||||
| Yes | 1.30 | 1.02 | - | 1.66 | 0.034 | 1.24 | 0.97 | - | 1.58 | 0.083 |
1 NTD is New Taiwan Dollar (NTD 1 ≈ USD 0.034); Urbanization Level 1 denoted the highest degree of urbanization, whereas level 7 denoted the lowest degree of urbanization. 2 Abbreviations: CCI, Charlson comorbidity index; ADHD, attention deficit hyperactivity disorder; OCD, obsessive-compulsive disorder.
Table 3 presents the risk of incident sleep disorder of patients with TS and the controls for comparison. With confounding variables adjusted for, the analysis revealed that patients with TS had a significantly higher risk of incident sleep disorders (adjusted HR [aHR] = 1.76, 95% CI = 1.58-1.96) than controls. Male patients had a lower risk of incident sleep disorders (aHR = 0.68, 95% CI = 0.61-0.76) than female patients. The risk of incident sleep disorders increased with age or CCI. Regarding comorbidities, patients with ADHD (aHR = 1.35, 95% CI = 1.09-1.67) or anxiety (aHR = 2.33, 95% CI = 1.88-2.88) had a high risk of incident sleep disorders after adjustment for relevant variables.
Furthermore, we estimated the risk of sleep disorders in different follow-up periods (Table 4). Compared with controls, patients with TS had the highest risk (aHR = 3.68, 95% CI = 2.62-5.17) of incident sleep disorders in <1 year. At the 1-5-year follow-up (aHR = 1.79, 95% CI = 1.47-2.17) and >5-year follow-up (aHR = 1.52, 95% CI = 1.31-1.76), patients with TS still had a high risk of sleep disorders.
Sensitivity analysis to investigate the risk of sleep disordor at different follow-up periods.
| Variables | General patients | Tourette syndrome | Adjusted model 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | IR | Events | IR | HR | 95% CI | p-value | |||
| Follow-up year | |||||||||
| <1 year | 72 | 1.32 | 82 | 6.03 | 3.68 | 2.62 | - | 5.17 | <0.001 |
| 1-5 year | 353 | 1.30 | 178 | 2.62 | 1.79 | 1.47 | - | 2.17 | <0.001 |
| >5 year | 733 | 1.52 | 288 | 2.42 | 1.52 | 1.31 | - | 1.76 | <0.001 |
1 All models were analyzed using the Cox proportional hazards model. Extraneous factors adjusted in the model were sex, age, insured salary, urbanization, CCI, and comorbidities.
Discussion
In this large-scale, nationwide, population-based cohort study, patients with TS had a higher risk of sleep disorders than controls from the general population. Our study confirms that TS is a risk factor for sleep disorders. We also found that patients comorbid with ADHD, anxiety, or depression had a high risk of sleep disturbance. The findings suggest that neuropsychiatric comorbidities increase the likelihood of sleep disorders in patients with TS. In our cohort, the risk of sleep disorders was significantly higher in patients with TS aged more than 18 years and 7-18 years than in those aged less than 7 years. The risk of sleep disorders was the highest in patients diagnosed with TS within less than 1 year.
In clinical settings, approximately half of the children and adolescents with TD and more than 80% of patients with TS have at least one comorbid developmental neuropsychiatric disorder, and approximately 60% of patients with TS have two or more comorbidities (ADHD, OCD, depression, anxiety, and sleep disorders) [1], [4, 13]. The most common and well characterized of these neuropsychiatric comorbidities are ADHD and OCD, with prevalence rates of 50% to 60% and of 36% to 50%, respectively [3, 14-16].
Several studies have indicated that sleep disorders and sleep problems are frequent in patients with TS, with a prevalence rate of 12% to 62% [17-19]. TD is associated with high rates of neuropsychiatric comorbidities, particularly ADHD and OCD [20]. Sleep disturbance, including difficulties with sleep initiation and maintenance, is common in children with TD, with rates as high as 65% in TD patients without neuropsychiatric comorbidities [21]. Although the cause of frequent sleep disturbances in patients with TS remains uncertain, several general theories have been proposed. A study indicated that tic behaviors may occur in various stages of sleep, contributing to overall increased movement during both rapid eye movement (REM) and non-REM sleep and therefore affecting normal sleep patterns [6]. Both dopamine (DA) and serotonin (5-HT) systems and their interaction are involved in the regulation of the circadian rhythm [22, 23]. Furthermore, decreased serotonin levels lead to increased phasic DA release as well as alterations in the expression of DA D2 receptor (D2R) and serotonin receptor 2A (5-HT2AR) in TS [24, 25]. D2R [26] and 5-HT2AR [27] are implicated in the physiological regulation of the circadian rhythm.
In our cohort study, patients with TS had a higher risk of sleep disorders than controls. The odds were 1.7 times higher in patients with TS than in controls from the general population. Increasing evidence has suggested that sleep disturbances are more common in children with TD [28, 29]; it is especially frequent in patients with severe and chronic symptoms [30]. Children with TD have a shorter sleep duration than children without TD, and sleep disturbances worsen with age [31]. Children with TD are at higher risk of sleep disturbances, and they have a higher prevalence of some sleep disorders, such as sleep-related movement disorders [31]. A previous study reported that the prevalence of sleep problems in patients with TS was approximately 60% [17]. A recent study also indicated that sleep disorders occur in 64% of patients with TS [32]. A systematic review revealed a high prevalence of 9.7% to 80.4% for sleep difficulties in children with TS [29]. Approximately one-third of patients with TS have insomnia. Compared with the general population, the period prevalence of insomnia translates into a 6.7-fold increased likelihood in the TS group [33].
In the present study, the risk of sleep disorders was significantly higher in patients aged more than 18 years (7- to 8-fold) and 7-18 years (2- to 3-fold) than in those aged less than 7 years. In addition, female patients were at higher risk of sleep disorders than male patients. Our study also revealed that patients who had higher CCI scores were at higher risk, especially those with CCI scores of more than 2. A study demonstrated that older adolescents had lower sleep quality than younger children [31]. Consistently, our study result revealed that patients aged more than 18 years were at higher risk of sleep disorders than patients aged less than 7 years. The underlying mechanisms of sleep disturbances in children with TS are not fully understood, though several possible explanations have been proposed. Previous study indicated that a dysfunction in the dopaminergic system, likely involving increased dopaminergic activity, as indicated by symptom improvement with dopamine-depleting medications (such as tetrabenazine) [34]. It has been suggested that environmental influences on genetic predisposition may have an epigenetic basis. Findings from magnetic resonance spectroscopy and positron emission tomography studies indicate the involvement of other neurotransmitters, such as GABA and glutamate [35]. There is substantial evidence indicating the involvement of the cortical-basal ganglia-thalamocortical circuits, which play a key role in movement regulation and control [31]. Several neurotransmitters, particularly acetylcholine, norepinephrine, serotonin, histamine, and GABA, play a crucial role in regulating sleep and wakefulness, supporting a pathological basis for the heightened sleep disturbances observed in TS [31][36].
In this cohort study, patients with neuropsychiatric comorbidities exhibited an increased likelihood of sleep problems. Hence, we also investigated neuropsychiatric comorbidities as risk factors for and the risk of incident sleep disorder. The results revealed that patients comorbid with ADHD or anxiety had a higher risk for sleep disturbance. Most patients with TS have comorbid conditions, including neuropsychiatric disorders, which are more impairing than the tics themselves [37, 38]. A population-based case-control study revealed that TS is a risk factor for sleep disorders, and children with TS having comorbidities had an increased risk of sleep disorders [39]. A study indicated that insufficient sleep in young TD patients persists independently of comorbidity or psychiatric drug status [21]. Previous studies have also demonstrated sleep deficits in young TD patients without comorbid conditions [32]. Strong evidence suggests that sleep is negatively affected in patients with TD, particularly children with TD; a direct association between daytime tic severity and sleep disturbance has also been suggested [6]. Children with TDs had a higher frequency of sleep disorders than controls [19, 40]. The most severe tics were found in patients with both ADHD and OCD [41] [42]. Children with TS often present with several comorbidities. ADHD is the most common comorbidity [32]. A relationship of TD with ADHD has been found; ADHD has negative effects on sleep in terms of several indicators [31]. Previous studies have indicated that youth with TD and comorbid ADHD reported higher sleep disturbance relative to patients with TD without psychiatric comorbidity [32, 43]. Another study revealed that children with TS with or without comorbid ADHD exhibit sleep disorders [32]. A meta-analysis of actigraphy-derived parameters revealed that longer sleep onset latency and poorer sleep efficiency are the most common findings in patients with TS with or without comorbid ADHD [44]. However, patients with TS with comorbid ADHD and/or OCD have an increased likelihood of sleep problems [41, 43, 45]; this finding suggests that sleep disorders in patients with TS are attributed to comorbidities rather than TS itself. Several studies have shown that anxiety is a common comorbidity in patients with TS, with an incidence ranging from 11% to 70.3% [15, 46]. Patients with chronic TDs and anxiety disorders reported higher frequency of sleep-related problems than those without comorbid anxiety disorder [47]. The impact of ADHD, other comorbidities (such as anxiety), and medication effects on sleep disturbances in individuals with TS has been recognized [21][32][34][39]. A study found that neither medications nor comorbidities appeared to predict sufficient sleep duration in children with TS, suggesting that sleep disturbances are an inherent feature of TS rather than solely a consequence of comorbid conditions or medication effects [21]. A population-based study in Taiwan found that the prevalence of Tourette syndrome and chronic tic disorders was highest (57.13%) in patients younger than 10 years old, followed by a lower prevalence (23.53%) in those aged 10-19 years [48]. Another study from Taiwan reported that the prevalence of OCD peaked in males between 18-24 years and in females between 35-44 years, with the next highest prevalence occurring in males aged 25-34 years and females aged 25-34 years [49]. The peak prevalence ages for Tourette syndrome and OCD differed. Therefore, we included patients with Tourette syndrome comorbid OCD, which had a relatively low prevalence, potentially affecting the observed lack of association with sleep disorders compared to the control group.
In our cohort study, the risk of sleep disorders was the highest in patients diagnosed with TS within less than 1 year, with an aHR of 3.81, and the aHRs for sleep disorders were 1.84 and 1.50 at 1-5-and >5-year follow-up, respectively. Moreover, the risk of sleep disorders in patients with TS was identified most commonly in the earlier follow-up period. Our study result is similar to that of a population-based case-control study [39]. On average, the tics in patients with TS increase in severity and peak at the age of 10-12 years; they then tend to improve gradually during adolescence; however, symptom progression substantially differs across patients [3, 50]. Over the years, tic severity typically increases and reaches a peak between 8 and 12 years of age. Tic severity usually peaks during the second decade of life, and in the majority of children, tic severity improves by the late teens and early adulthood [16]. By the end of the second decade of life, many patients are mostly tic-free [51]. However, approximately 20% of patients with TS continue to experience clinically impairing tics into adulthood.
Our study has several strengths. In our study, patients were selected from the total population of a nationwide cohort in Taiwan; thus, the large sample size is representative, increasing statistical precision. We believe that the combination of the NHIRD with multiple data sources would be useful. The population-based design also minimized selection bias, which is common in observational studies. Although a prospective population-based cohort study is ideal for exploring risk factors, a retrospective population-based cohort study using insurance data is a suitable alternative.
This study has several limitations. First, due to the nature of the National Health Insurance Research Database (NHIRD), which relies on claims data coded with ICD-9-CM and ICD-10-CM for sleep disorders, we were unable to differentiate specific sleep problems such as insomnia, excessive daytime somnolence, restless legs syndrome, enuresis, or REM sleep behavior disorder. The NHIRD does not provide detailed clinical information, such as symptom-specific assessments, sleep logs, or polysomnography results, which limits our ability to analyze the prevalence and impact of individual sleep disorders among TS patients. Future studies incorporating clinical evaluations or specialized sleep assessment tools could further elucidate the specific sleep disturbances associated with TS. Second, surveillance bias was unavoidable in this population-based cohort study, where data had been gathered in clinical settings. Children with TS are more likely to be diagnosed by pediatric neurologists or child psychiatrists, and the parents of TS patients may pay more attention to comorbidities, including sleep disorders and neuropsychiatric disorders.
Conclusion
TS is a risk factor for sleep disorders. Our study also indicated that patients with comorbid ADHD or anxiety had a higher risk of sleep disturbance, suggesting that the increased risk of sleep disorders is associated with comorbidities. In this cohort, the risk of sleep disorders was significantly higher in patients with TS aged more than 18 years than in those aged less than 7 years, and the risk of sleep disorders was the highest in patients diagnosed with TS within less than 1 year.
Acknowledgements
We are grateful to Health Data Science Center, China Medical University Hospital, for providing administrative, technical, and funding support. This study is based, in part, on data released by the Health and Welfare Data Science Center, Ministry of Health and Welfare. The interpretation and conclusions contained herein do not represent those of the Ministry of Health and Welfare.
Funding
This research was supported by the Chung Shan Medical University Hospital Taiwan (CSH-2023-C-002 and CSH-2024-C-001), China Medical University Taiwan (CMU113-MF-120 and CMU113-S-42), and the National Science and Technology Council Taiwan (MOST 111-2410-H-040-002).
Author contributions
All authors have participated in this study and have reviewed and agree with the final manuscript. Conceptualization, Ning-Jen Chung, Min-Ling Tsai, Kuang-Hua Huang, and Chien-Ying Lee; Data curation, Kuang-Hua Huang and Chien-Ying Lee; Formal analysis, Yih Yang, Shuo-Yan Gau, Shiang-Wen Huang, Tung-Han Tsai; Funding acquisition, Min-Ling Tsai, Kuang-Hua Huang, and Chien-Ying Lee; Methodology, Ning-Jen Chung, Min-Ling Tsai, Kuang-Hua Huang, and Chien-Ying Lee; Validation, Ning-Jen Chung, Kuang-Hua Huang, and Chien-Ying Lee; Writing - original draft, Ning-Jen Chung, Min-Ling Tsai, Kuang-Hua Huang, and Chien-Ying Lee; Writing - review & editing, Ning-Jen Chung, Min-Ling Tsai, Yih Yang, Shuo-Yan Gau, Shiang-Wen Huang, Tung-Han Tsai, Kuang-Hua Huang, Chien-Ying Lee.
Data availability statement
The database used to support the findings of this study was provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare (HWDC, MOHW) under license and so cannot be made freely available. Requests for access to these data should be made to HWDC (https://dep.mohw.gov.tw/dos/cp-5119-59201-113.html).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Liu ZS, Cui YH, Sun D, Lu Q, Jiang YW, Jiang L. et al. Current Status, Diagnosis, and Treatment Recommendation for Tic Disorders in China. Front Psychiatry. 2020;11:774
2. Deeb W, Malaty IA, Mathews CA. Tourette disorder and other tic disorders. Handb Clin Neurol. 2019;165:123-53
3. Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. 2009;67:497-501
4. Felling RJ, Singer HS. Neurobiology of tourette syndrome: current status and need for further investigation. J Neurosci. 2011;31:12387-95
5. Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42:436-47
6. Cohrs S, Rasch T, Altmeyer S, Kinkelbur J, Kostanecka T, Rothenberger A. et al. Decreased sleep quality and increased sleep related movements in patients with Tourette's syndrome. J Neurol Neurosurg Psychiatry. 2001;70:192-7
7. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH. et al. Taiwan's National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349-58
8. Lai SW, Liao KF, Lin CL, Lin CC, Lin CH. Longitudinal data of multimorbidity and polypharmacy in older adults in Taiwan from 2000 to 2013. Biomedicine (Taipei). 2020;10:1-4
9. Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P. et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165
10. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424
11. Austin PC, Xin Yu AY, Vyas MV, Kapral MK. Applying Propensity Score Methods in Clinical Research in Neurology. Neurology. 2021;97:856-63
12. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-9
13. Kurlan R. Clinical practice. Tourette's Syndrome. N Engl J Med. 2010;363:2332-8
14. Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2:68-87
15. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C. et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325-33
16. Swain JE, Leckman JF. Tourette syndrome and tic disorders: overview and practical guide to diagnosis and treatment. Psychiatry (Edgmont). 2005;2:26-36
17. Jankovic J, Rohaidy H. Motor, behavioral and pharmacologic findings in Tourette's syndrome. Can J Neurol Sci. 1987;14:541-6
18. Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JAG. Sleep Disorders and Sleep Problems in Patients With Tourette Syndrome and Other Tic Disorders: Current Perspectives. Nat Sci Sleep. 2022;14:1313-31
19. Saccomani L, Fabiana V, Manuela B, Giambattista R. Tourette syndrome and chronic tics in a sample of children and adolescents. Brain Dev. 2005;27:349-52
20. McNaught KS, Mink JW. Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol. 2011;7:667-76
21. Ricketts EJ, Rozenman M, Choy C, Goldberg HB, Kim JS, Colwell CS. et al. Sleep Sufficiency in Pediatric and Adolescent Tourette's Disorder: National Survey of Children's Health. J Dev Behav Pediatr. 2018;39:72-6
22. Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A. et al. Dopaminergic control of sleep-wake states. J Neurosci. 2006;26:10577-89
23. Monti JM, Jantos H. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625-46
24. Minzer K, Lee O, Hong JJ, Singer HS. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J Neurol Sci. 2004;219:55-61
25. Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y. et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239-51
26. Cade BE, Gottlieb DJ, Lauderdale DS, Bennett DA, Buchman AS, Buxbaum SG. et al. Common variants in DRD2 are associated with sleep duration: the CARe consortium. Hum Mol Genet. 2016;25:167-79
27. Monti JM. Serotonin 5-HT(2A) receptor antagonists in the treatment of insomnia: present status and future prospects. Drugs Today (Barc). 2010;46:183-93
28. Rodriguez-Blazquez C, Forjaz MJ, Kurtis MM, Balestrino R, Martinez-Martin P. Rating Scales for Movement Disorders With Sleep Disturbances: A Narrative Review. Front Neurol. 2018;9:435
29. Hibberd C, Charman T, Bhatoa RS, Tekes S, Hedderly T, Gringras P. et al. Sleep difficulties in children with Tourette syndrome and chronic tic disorders: a systematic review of characteristics and associated factors. Sleep. 2020;43:zsz308
30. Mi Y, Zhao R, Sun X, Yu P, Wang W, Li J. et al. Sleep disturbances and sleep patterns in children with tic disorder: A case-control study. Front Pediatr. 2022;10:911343
31. Blaty JL, DelRosso LM. Tourette disorder and sleep. Biomed J. 2022;45:240-9
32. Ghosh D, Rajan PV, Das D, Datta P, Rothner AD, Erenberg G. Sleep disorders in children with Tourette syndrome. Pediatr Neurol. 2014;51:31-5
33. Isomura K, Sidorchuk A, Sevilla-Cermeno L, Akerstedt T, Silverberg-Morse M, Larsson H. et al. Insomnia in Tourette Syndrome and Chronic Tic Disorder. Mov Disord. 2022;37:392-400
34. Jankovic J, Glaze DG, Frost JD Jr. Effect of tetrabenazine on tics and sleep of Gilles de la Tourette's syndrome. Neurology. 1984;34:688-92
35. Martino D, Ganos C, Worbe Y. Neuroimaging Applications in Tourette's Syndrome. Int Rev Neurobiol. 2018;143:65-108
36. Singer HS. Tics and Tourette Syndrome. Continuum (Minneap Minn). 2019;25:936-58
37. Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H. et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry. 2012;21:451-7
38. Martino D, Ganos C, Pringsheim TM. Tourette Syndrome and Chronic Tic Disorders: The Clinical Spectrum Beyond Tics. Int Rev Neurobiol. 2017;134:1461-90
39. Lee WT, Huang HL, Wong LC, Weng WC, Vasylenko T, Jong YJ. et al. Tourette Syndrome as an Independent Risk Factor for Subsequent Sleep Disorders in Children: A Nationwide Population-Based Case-Control Study. Sleep. 2017 40
40. Modafferi S, Stornelli M, Chiarotti F, Cardona F, Bruni O. Sleep, anxiety and psychiatric symptoms in children with Tourette syndrome and tic disorders. Eur J Paediatr Neurol. 2016;20:696-703
41. Mol Debes NM, Hjalgrim H, Skov L. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol. 2008;23:1017-27
42. Robertson MM. Mood disorders and Gilles de la Tourette's syndrome: An update on prevalence, etiology, comorbidity, clinical associations, and implications. J Psychosom Res. 2006;61:349-58
43. Kirov R, Kinkelbur J, Banaschewski T, Rothenberger A. Sleep patterns in children with attention-deficit/hyperactivity disorder, tic disorder, and comorbidity. J Child Psychol Psychiatry. 2007;48:561-70
44. Keenan L, Sherlock C, Bramham J, Downes M. Overlapping sleep disturbances in persistent tic disorders and attention-deficit hyperactivity disorder: A systematic review and meta-analysis of polysomnographic findings. Neurosci Biobehav Rev. 2021;126:194-212
45. Freeman RD, Tourette Syndrome International Database C. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):15-23
46. Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JAG. Sleep disorders in tourette syndrome. Sleep Med Rev. 2020;53:101335
47. Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK. et al. Quality of life in youth with Tourette's syndrome and chronic tic disorder. J Clin Child Adolesc Psychol. 2007;36:217-27
48. Chou IJ, Hung PC, Lin JJ, Hsieh MY, Wang YS, Kuo CY. et al. Incidence and prevalence of Tourette syndrome and chronic tic disorders in Taiwan: a nationwide population-based study. Soc Psychiatry Psychiatr Epidemiol. 2022;57:1711-21
49. Huang LC, Tsai KJ, Wang HK, Sung PS, Wu MH, Hung KW. et al. Prevalence, incidence, and comorbidity of clinically diagnosed obsessive-compulsive disorder in Taiwan: a national population-based study. Psychiatry Res. 2014;220:335-41
50. Groth C, Mol Debes N, Rask CU, Lange T, Skov L. Course of Tourette Syndrome and Comorbidities in a Large Prospective Clinical Study. J Am Acad Child Adolesc Psychiatry. 2017;56:304-12
51. Leckman JF, Bloch MH, Scahill L, King RA. Tourette syndrome: the self under siege. J Child Neurol. 2006;21:642-9
Author contact
![]() Corresponding author: Chien-Ying Lee, PhD, Department of Pharmacology, Chung Shan Medical University, No. 110, Sec.1, Jianguo N. Rd., Taichung City 40201, Taiwan. Tel.: 886-4-24730022 #11664; E-mail: cshd015edu.tw.
Corresponding author: Chien-Ying Lee, PhD, Department of Pharmacology, Chung Shan Medical University, No. 110, Sec.1, Jianguo N. Rd., Taichung City 40201, Taiwan. Tel.: 886-4-24730022 #11664; E-mail: cshd015edu.tw.

 Global reach, higher impact
Global reach, higher impact