3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(9):2132-2138. doi:10.7150/ijms.109689 This issue Cite
Research Paper
Reduced risk of diabetic retinopathy in osteoarthritis patients undergoing joint replacement surgery
1. School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Department of Ophthalmology, Chung Shan Medical University Hospital, Taichung, Taiwan.
3. Department of Orthopedics, Chung Shan Medical University Hospital, Taichung, Taiwan.
4. Department of Ophthalmology, Cathay General Hospital, Taipei, Taiwan.
5. Departments of Ophthalmology, Sijhih Cathay General Hospital, New Taipei City, Taiwan.
6. School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan.
7. Nobel Eye Institute, Taipei, Taiwan.
8. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
9. School of Medicine, National Tsing Hua University, Hsinchu, Taiwan.
Received 2024-12-31; Accepted 2025-3-24; Published 2025-4-9
Abstract
Osteoarthritis (OA) is a progressive joint disorder frequently associated with multiple comorbidities. Emerging research suggests a potential link between OA and diabetic retinopathy, a microvascular complication of diabetes mellitus. This study investigates whether joint replacement surgery influences the risk of developing diabetic retinopathy in individuals with OA. Using data from the TriNetX database, we conducted a retrospective cohort study, categorizing OA patients into two groups based on whether they had undergone joint replacement surgery, with each group comprising 164,653 individuals. The primary outcome was the incidence of diabetic retinopathy, analyzed using Cox proportional hazards regression. Among patients who underwent joint replacement surgery, 844 developed diabetic retinopathy, compared to 1,336 cases in the non-surgery group. The incidence of diabetic retinopathy was significantly lower in the surgery group (P < 0.001). Additionally, cumulative incidence analysis confirmed a reduced risk in the surgery group (P < 0.001). Subgroup analyses further demonstrated a consistently lower risk across most demographic subgroups. In conclusion, our findings suggest that joint replacement surgery in OA patients is associated with a reduced risk of developing diabetic retinopathy. Further research is warranted to explore the underlying mechanisms and potential clinical implications.
Keywords: osteoarthritis, epidemiology, database, joint replacement surgery, diabetic retinopathy
Introduction
Osteoarthritis (OA) is a degenerative joint disease that predominantly affects the knees and hips [1]. Clinically, OA is characterized by joint pain, transient morning stiffness, and physical disability [2, 3]. In severe cases, OA significantly restricts mobility, often necessitating total knee arthroplasty (TKA) or other joint replacement procedures [4, 5]. Although surgical outcomes are generally favorable, postoperative pain and the need for analgesic use can influence overall health and recovery [6-8].
Previous studies have established associations between OA and various comorbidities [9]. Notably, diabetes mellitus has been linked to an increased incidence of OA compared to non-diabetic populations [10, 11]. Additionally, OA is correlated with a higher prevalence of cardiovascular disease and an elevated risk of premature mortality [12]. Obesity, particularly in individuals with knee and hip OA, is a well-documented risk factor for disease progression and related complications [13]. Moreover, beyond the direct impact of OA, patients undergoing TKA or hip replacement surgery may have an increased risk of postoperative cardiovascular events [14].
Diabetes mellitus affects the vascular system throughout the body, and its microvascular complications include diabetic retinopathy [15-18]. Previous research has demonstrated that reducing serum glucose concentrations can lower the incidence of diabetic microvascular complications [19]. However, the potential impact of joint replacement surgery on the incidence of diabetic retinopathy in OA patients remains unexplored. Given that joint replacement surgery has been associated with improved life expectancy in OA patients [20], it is plausible that surgical intervention may also influence the progression of diabetic retinopathy, necessitating further investigation.
Therefore, the objective of the present study is to evaluate the incidence of diabetic retinopathy among OA patients who undergo joint replacement surgery compared to those who do not. Additionally, this study aims to assess the correlation between joint replacement surgery and diabetic retinopathy across different patient subgroups.
Materials and Methods
Data source
This study adheres to the principles outlined in the Declaration of Helsinki (1964) and its subsequent amendments. Furthermore, it has been approved by the Institutional Review Board of Chung Shan Medical University Hospital (Project Code: CS2-23208). The TriNetX database consolidates de-identified claims data from multiple health insurance providers across the United States, encompassing a diverse population with an estimated coverage exceeding 200 million individuals. This database includes a comprehensive range of medical records, such as International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes; demographic information (including age, sex, employment status, and geographic region); socioeconomic data; details of medical services received; hospitalization duration; imaging and laboratory test codes; laboratory results; surgical and procedural codes; and Anatomical Therapeutic Chemical (ATC) classification codes for medications.
Subject selection
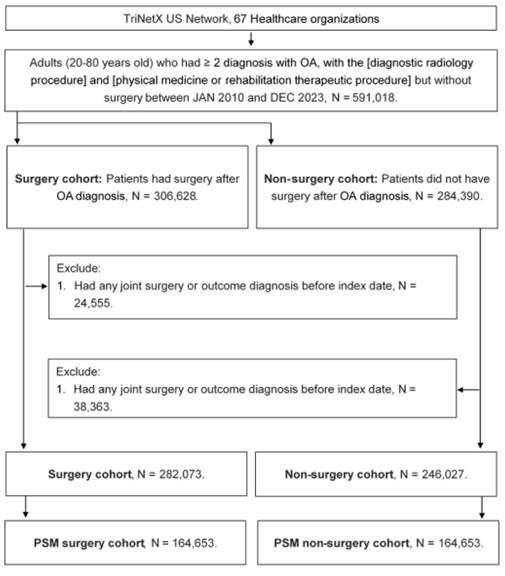
A retrospective cohort study was conducted, and patients meeting the following criteria were identified as having osteoarthritis (OA): (1) age below 20 or above 80 years, (2) receipt of an OA diagnosis based on the corresponding ICD-10-CM codes, (3) completion of a complete blood cell count, white blood cell differential count, and X-ray examination prior to the OA diagnosis, and (4) OA diagnosis confirmed by an orthopedist. The index date was defined as six months after the initial OA diagnosis. To enhance the homogeneity of the study population, the following exclusion criteria were applied: (1) prior history of joint surgery before the index date and (2) occurrence of the study outcome (as defined in the subsequent section) before the index date. Next, patients with OA who underwent joint replacement surgery were matched to OA patients who did not receive joint replacement surgery using the propensity score matching (PSM) method. The PSM approach accounts for demographic characteristics, systemic disorders, and medication history to generate a propensity score, ensuring comparability between matched individuals. Ultimately, 164,653 patients were included in both the surgery and non-surgery groups. The subject selection process is illustrated in Figure 1.
Main outcome
The primary outcome of this study was the development of diabetic retinopathy. Diabetic retinopathy was defined based on the following criteria: (1) a documented diagnosis of diabetic retinopathy according to the corresponding ICD-10-CM codes and (2) confirmation of the diagnosis by an ophthalmologist. Only diabetic retinopathy events recorded after the index date were considered for outcome assessment. Patients were followed until the occurrence of the outcome, withdrawal from the available health insurance program, or the end of data availability in the TriNetX database (December 31, 2022).
Confounder adjustment
To comprehensively assess the association between OA-related surgery and subsequent diabetic retinopathy, the following potential confounders were included in multivariable analyses: age, sex, ethnicity, medical encounter frequency, hypertension, nicotine dependence, cerebrovascular disease, alcohol-related disorders, hyperlipidemia, ischemic heart disease, peripheral vascular disease, cerebrovascular disease, chronic lower respiratory disease, body mass index (BMI), estimated glomerular filtration rate (eGFR), serum leukocyte count, serum cholesterol levels, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and glycated hemoglobin (HbA1c). To ensure that systemic disorders had a sufficiently prolonged duration to influence the incidence of diabetic retinopathy, only conditions persisting for more than two years were included in the multivariable analysis.
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Baseline characteristics were summarized using descriptive statistics, and standardized mean differences (SMD > 0.1) were used to identify significant differences between groups. Cox proportional hazards regression models were employed to estimate the incidence of diabetic retinopathy, with adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) calculated to account for potential confounders, including demographics, systemic conditions, substance dependence, and laboratory findings. A Kaplan-Meier curve was used to illustrate cumulative incidence, and group comparisons were conducted using the log-rank test. Subgroup analyses were performed by stratifying patients based on age, race, sex, eGFR, and HbA1c, followed by Cox regression to assess subgroup-specific outcomes. Statistical significance was set at P < 0.05, with P < 0.001 considered highly significant.
Results
The baseline characteristics of the surgery and non-surgery groups are presented in Table 1. The mean age was 64.8 ± 10.5 years in the surgery group and 64.7 ± 11.9 years in the non-surgery group, with no significant difference between the groups (SMD = 0.0019). Similarly, the distributions of sex and ethnicity did not differ significantly between the two groups (both SMD < 0.1). Additionally, the prevalence of systemic comorbidities and substance dependence was statistically comparable between the surgery and non-surgery groups (all SMD < 0.1). Regarding laboratory data, the values were well balanced between the two groups due to the application of the PSM approach (all SMD < 0.1) (Table 1).
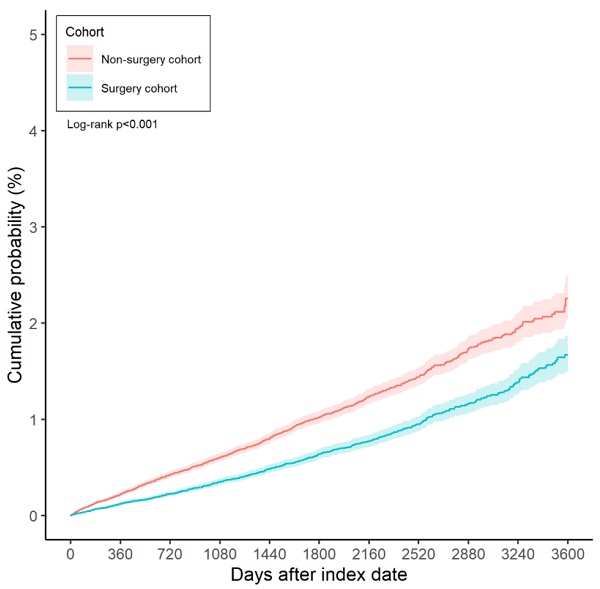
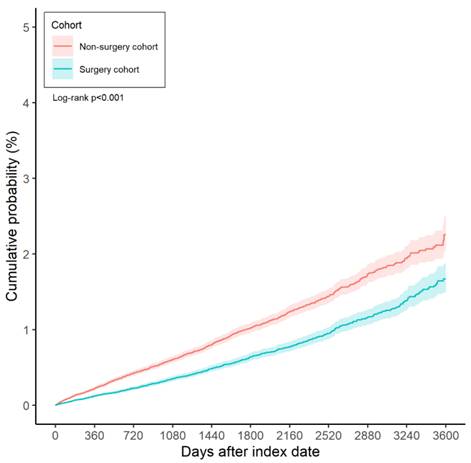
Over the entire study period, 844 cases of diabetic retinopathy were recorded in the surgery group, compared to 1,336 cases in the non-surgery group. According to the results of the multivariable analysis, the incidence of diabetic retinopathy was significantly lower in the surgery group than in the non-surgery group (aHR: 0.633, 95% CI: 0.581-0.690, P < 0.001) (Table 2). Furthermore, the cumulative incidence of diabetic retinopathy was also significantly lower in the surgery group (P < 0.001) (Figure 2).
Baseline characteristics between the two cohorts after propensity score matching
| Characteristics | Surgery cohort | Non-Surgery cohort | SMD |
|---|---|---|---|
| N | 164,653 | 164,653 | |
| Age at Index | 64.8±10.5 | 64.7±11.9 | 0.0019 |
| Sex | |||
| Female | 101362(61.6%) | 101626(61.7%) | 0.0033 |
| Male | 63274(38.4%) | 63013(38.3%) | 0.0033 |
| Race | |||
| Caucasian | 128630(78.1%) | 128266(77.9%) | 0.0053 |
| African | 17103(10.4%) | 17667(10.7%) | 0.0111 |
| Asian | 3883(2.4%) | 3740(2.3%) | 0.0058 |
| Medical encounter | |||
| Preventive medicine services | 11540(7.0%) | 11536(7.0%) | 0.0001 |
| Inpatient encounter | 87083(52.9%) | 45424(27.6%) | 0.5340 |
| Emergency | 24386(14.8%) | 26032(15.8%) | 0.0278 |
| Critical care services | 1643(1.0%) | 7620(4.6%) | 0.2209 |
| Comorbidities | |||
| Nicotine dependence | 14423(8.8%) | 14657(8.9%) | 0.0050 |
| Alcohol related disorders | 3882(2.4%) | 4069(2.5%) | 0.0074 |
| Hypertensive diseases | 91357(55.5%) | 91137(55.4%) | 0.0027 |
| Hyperlipidemia | 72534(44.1%) | 71950(43.7%) | 0.0071 |
| Ischemic heart diseases | 21198(12.9%) | 21253(12.9%) | 0.0010 |
| Peripheral vascular disorders | 12316(7.5%) | 12718(7.7%) | 0.0092 |
| Chronic lower respiratory diseases | 28377(17.2%) | 28685(17.4%) | 0.0049 |
| Cerebrovascular diseases | 7651(4.6%) | 8176(5.0%) | 0.0149 |
| Lab data | |||
| BMI | 30.7±6.4 | 30.9±7.8 | 0.0369 |
| eGFR | 77.9±21.9 | 78.2±25.5 | 0.0121 |
| Serum leukocytes | 7.5±19.7 | 8.6±37.9 | 0.0336 |
| Serum cholesterol | 181.6±45.9 | 178.6±49.5 | 0.0646 |
| HDL | 53.9±19.8 | 52.1±19.1 | 0.0911 |
| LDL | 101.9±37.1 | 101.1±38.7 | 0.0199 |
| HbA1c | 5.9±0.9 | 6.1±1.4 | 0.1932 |
BMI: body mass index, eGFR: estimated glomerular filtration rate, HbA1c: glycated hemoglobin, N: number, SMD: standard mean difference.
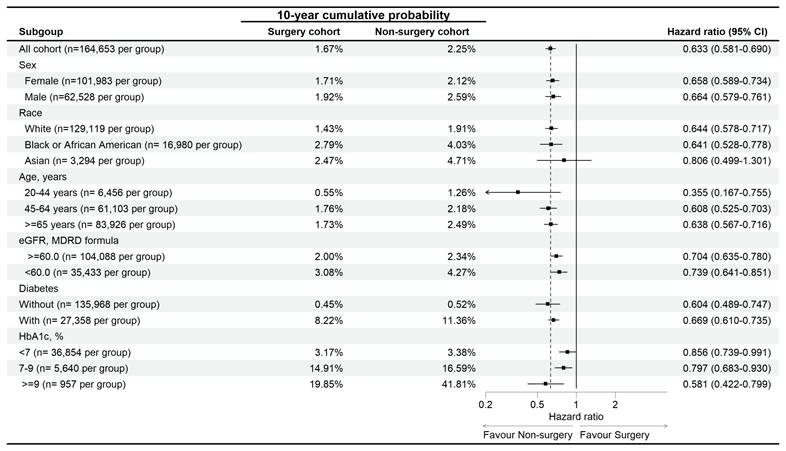
In the subgroup analysis, the reduced risk of diabetic retinopathy associated with surgery was observed across most subgroups, except for the Asian population, where the association was not statistically significant (aHR: 0.806, 95% CI: 0.499-1.301) (Figure 3).
Discussion
The present study demonstrated that the incidence of diabetic retinopathy was significantly lower in OA patients who underwent joint replacement surgery compared to those who did not. Furthermore, the cumulative incidence of diabetic retinopathy was also reduced in the surgery group. Subgroup analysis revealed a consistent protective effect of surgery across different patient characteristics, except in the Asian population, where no significant association was observed.
OA has been linked to multiple pathological mechanisms and comorbidities, as reported in previous literature [9, 10, 21, 22]. Mechanical stress and subsequent inflammation play central roles in OA development, with low-grade chronic inflammation persisting in affected joint spaces [23, 24]. Recurrent inflammation and neutrophil aggregation contribute to joint damage and OA progression [25, 26]. Additionally, individuals with OA exhibit elevated levels of inflammatory biomarkers, such as interleukins and prostaglandins [21, 27]. Apart from inflammation, OA pathophysiology involves increased subchondral bone remodeling, oxidative stress, and osteophyte formation [23, 28]. Beyond its pathological mechanisms, OA is associated with metabolic disorders, including glucose intolerance and diabetes mellitus [9, 29]. Notably, the therapeutic outcomes of TKA in OA patients are significantly influenced by the presence of diabetes mellitus [11].
Main outcomes between the surgery and non-surgery groups
| Study event | N | Cumulative probability | Cumulative probability | Cumulative probability | Cumulative probability | aHR (95% CI) | P |
|---|---|---|---|---|---|---|---|
| Study event | N | 1-year | 3-year | 5-years | 10-years | aHR (95% CI) | P |
| Diabetic retinopathy | |||||||
| Non-surgery cohort | 1,336 | 0.22% | 0.60% | 1.02% | 2.25% | Reference | |
| Surgery cohort | 844 | 0.12% | 0.35% | 0.64% | 1.67% | 0.633(0.581-0.690) | <0.001* |
aHR: adjusted hazard ratio, CI: confidence interval, N: number.
* denotes significant difference between groups.
The flowchart of participant selection. N: number, OA: osteoarthritis, PSM: propensity score-matching.
The Kaplan-Meier curve and cumulative incidence of diabetic retinopathy between the surgery and non-surgery groups.
Sensitive analysis of diabetic retinopathy risk in osteoarthritis. eGFR: estimated glomerular filtration rate, HbA1c: glycated hemoglobin, N: number.
Moreover, hyperlipidemia is more prevalent in individuals with OA, and the use of lipid-lowering agents has been linked to reduced structural damage in OA patients [30]. OA is also correlated with ischemic heart disease, both as a risk factor for its development and as a condition that worsens cardiovascular symptoms [31, 32]. Diabetic microvascular complications, including diabetic retinopathy, are characterized by heightened inflammatory responses [33]. Patients with diabetic microvascular complications exhibit elevated inflammatory cytokines, such as C-reactive protein [34]. Given that both OA and diabetic microvascular complications share an inflammatory etiology and that joint replacement surgery primarily induces localized inflammation [24, 33, 35], we hypothesize that OA patients undergoing surgery may experience reduced systemic inflammation, thereby lowering their risk of developing diabetic retinopathy. This hypothesis is supported by the findings of the present study.
Our results align with previous research indicating that OA patients undergoing joint replacement surgery experience improved long-term health outcomes. A prior study reported that TKA in knee OA patients was associated with a lower mortality rate compared to the general population over a 10-year follow-up period [36]. Additionally, joint replacement surgery has been linked to a reduced risk of cardiovascular diseases in OA patients [20]. However, limited research has specifically examined the impact of joint replacement surgery on the development of diabetic retinopathy. To our knowledge, this study provides preliminary evidence suggesting a negative correlation between joint replacement surgery and the incidence of diabetic retinopathy in OA patients. To ensure the temporal sequence between surgery and diabetic retinopathy, we excluded participants with a prior diagnosis of diabetic retinopathy before the index date. Additionally, we accounted for key confounding factors related to diabetes mellitus, including age, sex, hypertension, and hyperlipidemia, in the Cox proportional hazards regression model. As a result, joint replacement surgery may serve as an independent protective factor against diabetic retinopathy in OA patients. Beyond its anti-inflammatory effects, surgery may also facilitate increased physical activity by restoring joint function, thereby improving glycemic control, and reducing the risk of diabetic microvascular complications [19, 37]. The lower cumulative probability of diabetic retinopathy in the surgery group further supports the notion that persistent OA-related inflammation should be managed to mitigate the risk of diabetic complications.
In the subgroup analysis, joint replacement surgery was associated with a lower risk of diabetic microvascular complications across most patient subgroups, except in the Asian population. Previous studies have shown that TKA reduces the risk of cerebrovascular disease in patients with various characteristics [38]. These findings, in combination with the present study, further highlight the potential benefits of joint replacement surgery in reducing comorbidities in OA patients. However, there is limited research addressing the similar incidence of diabetic retinopathy between Asian OA patients with and without surgery. Prior studies suggest that Asian ethnicity is neither a strong predisposing nor a protective factor for diabetic retinopathy [16]. A possible explanation for our findings is the small sample size of the Asian population in the TriNetX database, which may have led to statistical bias. Asian participants accounted for approximately 2% of the study population, with a total of only around 3,000 cases--substantially fewer than the African American and Caucasian subgroups. Furthermore, the number of diabetic retinopathy cases in the Asian population was relatively low, with only about 100 recorded cases. The limited sample size may have affected the statistical power of the analysis. Additionally, laboratory data did not significantly influence the incidence of diabetic retinopathy, suggesting that joint replacement surgery may exert a stronger protective effect than other predisposing factors for diabetic retinopathy [16].
OA is a prevalent condition worldwide, with an estimated 300 million cases of hip and knee OA reported in previous studies [39]. Radiographic knee OA affects approximately 19% of adults [13], and OA, particularly knee OA, is a leading cause of disability globally [40]. Among patients with end-stage OA, joint replacement surgery is a commonly recommended intervention, albeit associated with substantial healthcare costs [41]. In the United Kingdom alone, over 60,000 TKA procedures are performed annually [6]. Similarly, diabetes mellitus is a widespread disease affecting approximately 500 million individuals worldwide [15]. Furthermore, diabetic microvascular complications impact more than 20% of individuals with diabetes mellitus [16], contributing to severe outcomes such as blindness and end-stage renal disease requiring dialysis--both of which impose significant socioeconomic burdens [19]. Given the substantial health impact of both OA and diabetic retinopathy, identifying strategies to reduce the risk of diabetic retinopathy in OA patients is of clinical and public health importance.
Despite its strengths, this study has several limitations. First, the TriNetX database is a claims-based dataset, meaning that only coded diagnoses are accessible, rather than complete medical records. Consequently, critical clinical details--such as radiographic findings, OA severity, affected joint sites, surgical techniques, postoperative alignment, range of motion, diabetic retinopathy severity, treatment response, and recurrence--could not be assessed. Second, the retrospective design may introduce heterogeneity in patient health status and disease severity, despite the application of PSM. Additionally, inflammatory biomarkers were not available in the dataset, limiting our ability to confirm the exact mechanisms underlying the reduced incidence of diabetic microvascular complications in the surgery group.
In conclusion, joint replacement surgery is associated with a lower risk of developing diabetic retinopathy in OA patients. Furthermore, the incidence of diabetic retinopathy is inversely correlated with the duration of OA in patients undergoing surgery compared to those who do not receive surgery. These findings suggest that early joint replacement surgery may be beneficial for OA patients with diabetes mellitus. Future large-scale prospective studies are warranted to further explore the relationship between joint replacement surgery and diabetic retinopathy outcomes in OA patients.
Acknowledgements
This study was supported by Chung Shan Medical University Hospital (CSH-2019-A-011).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H. et al. Osteoarthritis. Lancet. 2015;386:376-87
2. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB. et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072
3. Lu KH, Lu PW, Lin CW, Lu EW, Yang SF. Different molecular weights of hyaluronan research in knee osteoarthritis: A state-of-the-art review. Matrix Biol. 2023;117:46-71
4. Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152-62
5. Taruc-Uy RL, Lynch SA. Diagnosis and treatment of osteoarthritis. Prim Care. 2013;40:821-36 vii
6. Liddle AD, Pegg EC, Pandit H. Knee replacement for osteoarthritis. Maturitas. 2013;75:131-6
7. Canovas F, Dagneaux L. Quality of life after total knee arthroplasty. Orthop Traumatol Surg Res. 2018;104:S41-s6
8. Lu KH, Lu PW, Lu EW, Tang CH, Su SC, Lin CW. et al. The potential remedy of melatonin on osteoarthritis. J Pineal Res. 2021;71:e12762
9. Chan KW, Ngai HY, Ip KK, Lam KH, Lai WW. Co-morbidities of patients with knee osteoarthritis. Hong Kong Med J. 2009;15:168-72
10. Georgiev T, Angelov AK. Modifiable risk factors in knee osteoarthritis: treatment implications. Rheumatol Int. 2019;39:1145-57
11. Veronese N, Cooper C, Reginster JY, Hochberg M, Branco J, Bruyère O. et al. Type 2 diabetes mellitus and osteoarthritis. Semin Arthritis Rheum. 2019;49:9-19
12. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-8
13. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28:5-15
14. Simon SJ, Patell R, Zwicker JI, Kazi DS, Hollenbeck BL. Venous Thromboembolism in Total Hip and Total Knee Arthroplasty. JAMA Netw Open. 2023;6:e2345883
15. Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400:1803-20
16. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:117-24
17. Ting KH, Yang PJ, Huang JY, Lee CY, Su SC, Yang SF. The severity of coronary heart disease and the incidence of later diabetic retinopathy in diabetic population: A retrospective cohort study. PLoS One. 2025;20:e0316112
18. Lee CY, Lin CW, Sun YH, Wang PH, Lee CY, Huang JY. et al. The association between endometrial cancer and subsequent diabetic retinopathy severity: A retrospective nationwide study. Int J Gynaecol Obstet. 2024;166:1313-22
19. Crasto W, Patel V, Davies MJ, Khunti K. Prevention of Microvascular Complications of Diabetes. Endocrinol Metab Clin North Am. 2021;50:431-55
20. Kim S, Won SJ, Lee NK, Chang CB. Life Expectancy of Patients Undergoing Total Knee Arthroplasty: Comparison with General Population. J Korean Med Sci. 2024;39:e106
21. Xia B, Di C, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95:495-505
22. Motta F, Barone E, Sica A, Selmi C. Inflammaging and Osteoarthritis. Clin Rev Allergy Immunol. 2023;64:222-38
23. Salman LA, Ahmed G, Dakin SG, Kendrick B, Price A. Osteoarthritis: a narrative review of molecular approaches to disease management. Arthritis Res Ther. 2023;25:27
24. Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-92
25. Knights AJ, Redding SJ, Maerz T. Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr Opin Rheumatol. 2023;35:128-34
26. Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750
27. Seow SR, Mat S, Ahmad Azam A, Rajab NF, Safinar Ismail I, Singh DKA. et al. Impact of diabetes mellitus on osteoarthritis: a scoping review on biomarkers. Expert Rev Mol Med. 2024;26:e8
28. Jiang Y. Osteoarthritis year in review 2021: biology. Osteoarthritis Cartilage. 2022;30:207-15
29. Xing X, Wang Y, Pan F, Cai G. Osteoarthritis and risk of type 2 diabetes: A two-sample Mendelian randomization analysis. J Diabetes. 2023;15:987-93
30. Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-7
31. Wang H, Bai J, He B, Hu X, Liu D. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep. 2016;6:39672
32. Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and patient outcomes. Eur J Clin Invest. 2015;45:405-14
33. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-88
34. Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L. et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PLoS Med. 2023;20:e1004135
35. Prince N, Penatzer JA, Dietz MJ, Boyd JW. Localized cytokine responses to total knee arthroplasty and total knee revision complications. Journal of Translational Medicine. 2020;18:330
36. Barahona M, Barrientos C, Martinez Á, Brañes J, Prieto JP, Hinzpeter J. Mortality after hip or knee arthroplasty for osteoarthritis in Chile: A survival analysis. Medwave. 2020;20:e8089
37. Aujla RS, Esler CN. Total Knee Arthroplasty for Osteoarthritis in Patients Less Than Fifty-Five Years of Age: A Systematic Review. J Arthroplasty. 2017;32:2598-603.e1
38. Lin WY, Lee CC, Hsu CW, Huang KY, Lyu SR. Patients with knee osteoarthritis undergoing total knee arthroplasty have a lower risk of subsequent severe cardiovascular events: propensity score and instrumental variable analysis. PLoS One. 2015;10:e0127454
39. Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthritis Cartilage. 2021;29:180-9
40. Courties A, Kouki I, Soliman N, Mathieu S, Sellam J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthritis Cartilage. 2024;32:1397-404
41. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-59
Author contact
![]() Corresponding authors: Shun-Fa Yang, PhD, or Hsiang-Wen Chien, MD, PhD. Email: ysfedu.tw (Shun-Fa Yang); bmw35chien1com (Hsiang-Wen Chien).
Corresponding authors: Shun-Fa Yang, PhD, or Hsiang-Wen Chien, MD, PhD. Email: ysfedu.tw (Shun-Fa Yang); bmw35chien1com (Hsiang-Wen Chien).

 Global reach, higher impact
Global reach, higher impact