3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(9):2103-2118. doi:10.7150/ijms.108299 This issue Cite
Review
Heterogeneity of Renal Endothelial Cells, Interact with Neighboring Cells, and Endothelial Injury in Chronic Kidney Disease: Mechanisms and Therapeutic Implications
1. Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing 100010, China.
2. Beijing University of Chinese Medicine, Beijing 100029, China.
3. Shunyi Branch, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing 100310, China.
4. Laboratory for Clinical Medicine, Capital Medical University, Beijing 100069, China.
5. Beijing Research Institute of Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China.
Received 2024-12-6; Accepted 2025-3-4; Published 2025-4-9
Abstract
Chronic kidney disease (CKD) is closely associated with endothelial dysfunction, leading to symptoms such as albuminuria, edema, and coagulopathy. Recent advancements in single-cell sequencing have deepened our understanding of the heterogeneity of renal endothelial cells, which is significantly influenced by their microenvironment. Understanding the influence of neighboring cells on endothelial heterogeneity is essential for elucidating the mechanisms underlying vascular dysfunction and CKD progression. This review explores the latest research on renal endothelial cell heterogeneity and their interactions with neighboring cells. We further discuss the mechanisms of endothelial injury in CKD, including alterations to the endothelial glycocalyx, inflammation, oxidative stress, and dysfunction of the glomerular filtration barrier. Renal endothelial injury contributes to complications, including cardiovascular disease, diabetic nephropathy, and impaired vascular function. Therapeutic strategies encompass antihypertensive, hypoglycemic, and lipid-lowering treatments, supplemented by emerging approaches such as anti-inflammatory therapies, gene therapy, and lifestyle modifications. Through reviewing the relationship between endothelial injury and CKD progression, we emphasize potential strategies to enhance prognosis and mitigate disease progression.
Keywords: Chronic Kidney Disease, Endothelial Heterogeneity, Endothelial dysfunction, Endothelial injury
1. Introduction
Endothelial cells (ECs), also known as vascular endothelial cells, form a monolayer lining the inner surfaces of arteries, veins, and capillaries. These cells have a luminal membrane exposed to blood and circulating cells, while their basolateral surface is supported by a glycoprotein basement membrane that they produce1. ECs play critical roles in barrier functions, filtration, angiogenesis, and the regulation of vascular tone2. Additionally, they are involved in immune responses, inflammation, and maintaining the balance between coagulation and fibrinolysis3,4. Recent research has provided detailed insights into the roles of vascular endothelium in mechanotransduction, metabolism, guidance signaling, and aging5. Therefore, studying ECs is essential for understanding various pathological conditions.
Recent studies have identified distinct subsets of ECs, including glomerular ECs and those forming the blood-brain barrier6. Advances in technology, particularly single-cell RNA sequencing (scRNA-seq), have significantly deepened our understanding of these subsets. Compared to traditional methods such as serial analysis of gene expression, microarrays, or bulk RNA sequencing, scRNA-seq enables a more comprehensive analysis by integrating transcriptomic profiles across diverse cell types7-9.
CKD affects approximately 10% of the global adult population and is becoming increasingly prevalent10. CKD arises from a variety of pathological conditions, influenced by factors such as blood glucose, lipid levels, homocysteine, and bilirubin, which impact tissues through the circulatory system. These changes alter the microenvironment within tissues. Clinically, CKD is characterized by symptoms such as edema, proteinuria, coagulation abnormalities, and a reduced glomerular filtration rate (GFR), all of which are associated with endothelial cell damage11,12.
Recent research has delved into these complexities. Sedrakyan et al. classified renal endothelial cells based on transcriptomic differences and reviewed several scRNA-seq studies that explored the effects of CKD and acute kidney injury (AKI) on the renal endothelium13. Despite these advancements, the underlying causes of endothelial heterogeneity and the mechanisms of injury remain poorly understood. This study seeks to unravel the origins of renal endothelial cell heterogeneity by examining intra-tissue cell interactions. Furthermore, it consolidates current insights into the mechanisms and clinical manifestations of endothelial cell damage in CKD, offering new perspectives for identifying therapeutic targets related to the interplay between endothelial cells and CKD.
2. Heterogeneity of Renal Endothelial Cells
The renal vascular system exhibits remarkable diversity, with blood entering the kidney via the renal artery, passing through the glomerular capillaries, and exiting through the efferent arterioles. The renal vasculature adapts to distinct microenvironments, demonstrating specialization in endothelial cell structure, blood flow dynamics, and fenestration. Early studies employing electron microscopy and microarray analysis highlighted significant heterogeneity in the morphology and function of renal endothelial cells across various vascular sites. Techniques such as immunostaining and FACS analysis have identified renal endothelial markers, including Erg, VE-cadherin, Meca32, Thrombomodulin, and vWF1, yet further classification of renal endothelium remains necessary14.
2.1 Endothelial Cell Subtypes and Characteristics
Advances in scRNA-seq technology have significantly enhanced our understanding of individual cell types, uncovering organ-specific gene expression signatures9. For instance, Jihwan Park et al. constructed a single-cell transcriptome atlas of mouse kidneys, producing the most comprehensive map of kidney transcriptomes to date15. Their findings suggest that these data can facilitate the inference of cell type-specific functions and link numerous genetic kidney diseases to specific cell types.
Dumas et al. employed scRNA-seq to comprehensively characterize the transcriptional landscape of kidney endothelial cells in adult mice, identifying 24 transcriptionally distinct subtypes, including 5 glomerular, 9 cortical, and 10 medullary EC subtypes7. Within the cortical subtypes, specific populations such as large artery endothelial cells, afferent arterioles, efferent arterioles, four distinct capillary subtypes, and large vein endothelial cells were delineated. Furthermore, Dumas et al. highlighted gene-specific markers associated with endothelial cell function and their adaptation to the local microenvironment. For example, in glomerular endothelial subtypes, genes such as Edn1, Alox12, and S1pr1, which are implicated in the regulation of angiotensin signaling, were found to be selectively expressed in afferent arterioles. Notably, the S1P-S1PR1 signaling pathway was shown to regulate angiotensin levels by activating the nitric oxide synthase (eNOS) system, a critical mechanism for maintaining glomerular blood flow and preserving GFR16.
Several genes remain under-characterized. For example, the ELN gene, expressed in large artery endothelial cells, encodes elastin, a protein crucial for vascular elasticity and associated with renal cyst progression and diabetic nephropathy17,18. The Calca gene, expressed in efferent arterioles, encodes calcitonin, a hormone that influences G protein-coupled receptor signaling and NMDA receptor function, regulating vascular tone by modulating calcium influx19. Col4a1 and Col4a2, expressed in capillary endothelial cells, encode collagen type IV proteins in the basement membrane and are linked to vascular diseases. Studies have shown that the Col4a1 G498V mutation can delay glomerular development and podocyte differentiation, underscoring its role in kidney vascular and podocyte development20,21.
Furthermore, gene expressions are also connected to renal tubular function. For instance, the Jup gene, encoding Aquaporin-2 and expressed in the post-capillary venule subtype, interacts with the transcription regulator β-catenin and contributes to the renal anti-diuretic response22.
2.2 Involvement of Endothelial Cell Subtypes in Vascular Dysfunction and CKD Pathogenesis
It is well-established that different endothelial cell subtypes play critical roles in the progression of CKD by regulating vascular permeability, inflammatory responses, vascular tone, fibrotic signaling, and microvascular integrity. These subtypes exhibit unique characteristics and gene expression profiles, which collectively determine their central functions in disease mechanisms23.
Regulation of vascular permeability is a key function of endothelial cell subtypes. Capillary endothelial subtypes express crucial genes involved in permeability regulation, including VEGF and Plvap24,25. Aberrant expression of VEGF in glomerular collapse leads to rapid loss of glomerular endothelial cells (gRECs) and proteinuria, while PV1, the protein product of Plvap, facilitates water, ion, and solute exchange by covering endothelial fenestrae26. In the medullary capillary plexus, Plvap works in concert with VEGF receptor genes such as Kdr, Flt1, and Nrp1 to maintain vascular barrier function. However, in CKD, dysfunction of these genes disrupts the barrier, increasing inflammatory cell infiltration and tissue damage, thereby exacerbating inflammation, fibrosis, and renal dysfunction16.
Inflammatory responses are central to CKD progression, with specific endothelial cell subtypes exhibiting significant pro-inflammatory properties. For example, glomerular endothelial cells derived from efferent arterioles express Klf2, Klf4, and their target gene Thbd, which are suppressed under conditions of low shear stress, triggering pro-inflammatory signaling that worsens both local and systemic inflammation27. Studies have shown that activation of KLF2 protects gRECs from CKD-related injury28. Additionally, certain endothelial subtypes, such as capillary endothelial cells of the interferon (IFN) response phenotype, express Isg15 and Ifit gene families, which are involved in antigen processing and presentation, suggesting their potential role in CKD-associated inflammatory responses29.
Regulation of vascular tone depends on the secretion of vasoactive substances such as nitric oxide (NO) and endothelin-1 (ET-1) by endothelial subtypes. For instance, capillary endothelial cells express NOSTRIN, whose protein product interacts with eNOS to regulate NO production30,31. In CKD, dysfunction of NOSTRIN leads to reduced NO production, enhanced vasoconstriction, and decreased blood flow, exacerbating renal ischemia and injury32. Furthermore, eNOS uncoupling induces endothelial surface remodeling, promoting receptor expression and facilitating interactions with platelets and immune cells, thereby aggravating coagulopathy and disease progression33,34.
Fibrotic signaling and microvascular integrity are further disrupted during CKD progression. Certain capillary endothelial subtypes express genes such as Apln, Aplnr, Col4a1, Col4a2, Esm1, and Fscn1, which play essential roles in angiogenesis and fibrotic signaling. Additionally, venous endothelial cells of the IFN response phenotype express Isg15 and Ifit gene families, potentially contributing to immune regulation in fibrosis. The loss of microvascular barrier function creates a feedback loop that exacerbates inflammation and fibrosis11,35.
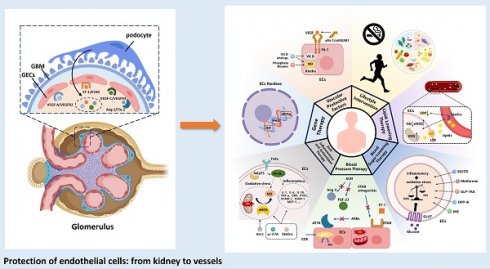
3. Interaction of Glomerular Endothelial Cells (GECs) with Neighboring Cells
Recent research has shown that the microenvironment significantly influences the development of endothelial cells in various tissues. In the kidney, the gene expression of renal endothelial cells is closely linked to signals from neighboring cells. GECs, key components of blood vessel walls, engage in complex communication with tubular epithelial cells, interstitial cells, and immune cells. This signaling network plays a critical role in regulating glomerular filtration, maintaining vascular tone, and modulating the inflammatory response in the glomerulus36 (Table 1).
3.1 GECs Interact with Podocytes
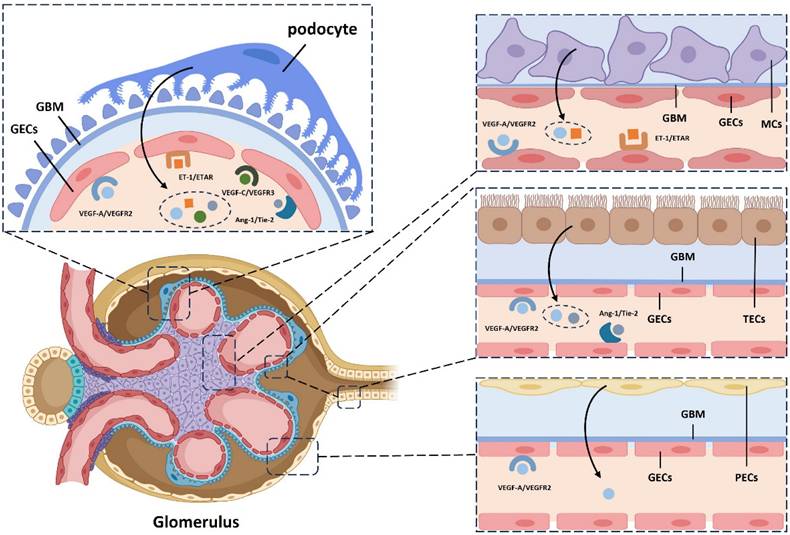
GECs are connected to podocytes through the glomerular basement membrane (GBM) within the glomerular filtration barrier. Their differentiation is regulated by key signaling molecules, including vascular endothelial growth factor-A (VEGF-A), angiopoietin (Ang), and ET-1, which are secreted by podocytes.
In the kidney, podocyte-derived VEGF-A is essential for maintaining the structure and function of glomerular capillaries37. VEGF-A also protects podocytes from apoptosis by promoting nephrin phosphorylation and enhancing the podocin-CD2-associated protein (CD2AP) interaction55. Additionally, VEGF-C increases endothelial fenestration density, reduces albumin permeability, and lowers microalbuminuria in patients with diabetic kidney disease36,38. The specific knockout of the VEGF gene in mouse podocytes leads to endothelial abnormalities and thrombotic microangiopathy, highlighting the importance of VEGF in these processes37.
Ang, a key vascular growth factor involved in vascular remodeling and stability, is widely expressed in the kidney. Podocyte-derived Ang-1 interacts with the Tie-2 receptor on GECs, promoting endothelial survival. Tie-2 activation triggers Akt-dependent phosphorylation, inactivating forkhead box protein O1 (FOXO1) and suppressing gene expression linked to endothelial instability and apoptosis. This pathway supports vascular integrity, enhances cell survival, and promotes vascular stability. Ang-1 also exerts anti-inflammatory effects by inhibiting tumor necrosis factor-alpha (TNF-α)-induced leukocyte migration, suppressing damage-induced angiogenesis and fibrosis, and protecting glomerular capillaries from high blood sugar and other harmful factors39.
ET-1, a potent vasoconstrictor peptide secreted by endothelial cells, mediates communication between podocytes and GECs. Podocyte-derived ET-1 induces calcium influx in GECs, regulating vascular tone and glomerular hemodynamics. ET-1 also stimulates endothelial proliferation and cytokine production, influencing inflammation and fibrosis in GECs40. Additionally, endothelial-derived ET-1 regulates podocyte function and differentiation. By binding to podocyte receptors, ET-1 affects podocyte morphology, function, and proliferation, and regulates extracellular matrix synthesis and secretion. Activation of the ETAR on podocytes triggers the mitogen-activated protein kinase (MAPK), p21waf/cip1, and nuclear factor-kappa B (NF-κB) pathways, disrupting the F-actin cytoskeleton and impairing slit diaphragm function via Rho kinase and phosphoinositide 3-kinase (PI3 kinase) activation41.
Summary of interaction of glomerular endothelial cell (GECs) with surrounding cells under physiological conditions
| Interaction with GECs | Mediators | Related Pathways or Mechanisms | Physiological effects | Reference(s) |
|---|---|---|---|---|
| Podocyte → GECs | VEGF-A | VEGF-A/VEGFR2 signaling pathway | Promoting endothelial cell differentiation and development, maintaining endothelial cell structure and function | 37 |
| VEGF-C | VEGF-C/VEGFR3 signaling pathway | Increasing fenestration density in endothelial cells, reducing albumin permeability | 36,38 | |
| Ang-1 | Ang-1/Tie-2 signaling pathway | Maintaining vascular integrity, enhances cell survival, promotes vascular stability, and facilitating angiogenesis; Inhibiting injury-induced angiogenesis and fibrosis | 39 | |
| ET-1 | ET-1 signaling pathway | Promoting endothelial cell proliferation, enhances cytokine production, and affecting the regulation of inflammatory responses and fibrosis in glomerular endothelial cells | 40 | |
| GECs→ podocyte | VEGF-A | VEGF-A/VEGFR1 signaling pathway | Protecting podocytes from apoptosis by promoting nephrin phosphorylation and enhancing podocin-CD2AP interaction | 36,37 |
| ET-1 | ET-1/ETAR signaling pathway | Influencing the morphology, function, and proliferation of podocytes; Regulating the synthesis and secretion of extracellular matrix proteins by podocytes, influencing podocyte adhesion, migration, and invasion | 41 | |
| TECs→ GECs | Ang-(1-7) | Ang-(1-7)/Mas signaling pathway | Leading to sustained activation of the klotho and Nrf2/HO-1 signaling pathways, collectively inhibiting the aging process of GECs | 42,43 |
| VEGF-A | VEGF-A/ VEGFR2 signaling pathway | Promoting endothelial cell differentiation and development, maintaining endothelial cell structure and function | 37,44 | |
| VEGF-C | VEGF-C/VEGFR3 signaling pathway | Increasing fenestration density in endothelial cells, reducing albumin permeability | 36,38 | |
| GECs→ TECs | IGFBPs | IGF signaling pathway | Producing IGFBP4, IGFBP-2, and IGFBP-3, and express mRNA for IGFBP-2 to IGFBP-5, regulating IGF signaling in TECs and influencing renal tubular function | 45 |
| MCs→ GECs | Ang-2 | Ang-2/Tie-2 signaling pathway | Regulating endothelial cell proliferation | 43,46 |
| VEGF-A | VEGF-A/VEGFR2 signaling pathway | Inhibiting Tie2 phosphorylation and promoting endothelial cell proliferation | 47,48 | |
| GECs→ MCs | PDGF-B | PDGF-B/PDGFR-β signaling pathways | Promoting the differentiation and development of MCs | 49 |
| NO | Nitric oxide-mediated signaling pathways | Stimulating cGMP production in MCs through a NO-dependent pathway | 50 | |
| Exosome containing TGF-β1 mRNA | TGFβ1/Smad3 signaling pathways | Promoting cellular proliferation and extra cellular matrix production | 46,51 | |
| PECs→ GECs | VEGF-A | VEGF-A/VEGFR2 | Inhibiting Tie2 phosphorylation and promoting endothelial cell proliferation | 52 |
| GECs→ PECs | EGF | EGF/EGFR | Regulating cell survival, proliferation and apoptosis | 53,54 |
GECs: Glomerular Endothelial Cells; TECs: Tubular Epithelial Cells; MCs: Mesangial Cells; PECs: Parietal Epithelial Cells; VEGF: Vascular Endothelial Growth Factor; VEGFR: Vascular Endothelial Growth Factor Receptor; Ang-1: Angiopoietin-1; ET-1: Endothelin-1; ETAR: Endothelin A Receptor; IGFBPs: Insulin-like Growth Factor Binding Proteins; NO: Nitric Oxide; PDGF-B: platelet-derived growth factor-B; TGF-β1: Transforming growth factor Beta 1; EGF: Epidermal Growth Factor; EGFR: Epidermal Growth Factor Receptor; cGMP: cyclic guanosine monophosphate
3.2 GECs Interact with Renal Tubular Epithelium
The balance between glomerular-tubular interactions and feedback mechanisms is essential for maintaining renal metabolic function. GECs are closely linked with tubular epithelial cells, forming a complex network within the renal microenvironment.
Research has shown the significant role of the klotho protein in the kidney. Studies on gene-deficient mice reveal endothelial dysfunction, highlighting klotho's importance in renal homeostasis. Angiotensin-(1-7), a bioactive peptide produced by tubular epithelial cells, binds to the Mas receptor, activating the klotho and Nrf2/HO-1 pathways42,43. This mechanism helps inhibit GEC aging and preserves renal function. Additionally, tubular epithelial cells secrete VEGF, which binds to VEGFR on GECs, promoting endothelial differentiation and supporting renal microvasculature integrity44.
Endothelial cells also influence tubular epithelial cells by releasing NO and various growth factors and regulatory proteins. For instance, insulin-like growth factors (IGFs) expressed by GECs regulate renal cell growth and function, with IGFBPs modulating IGF signaling in tubular epithelial cells45.
3.3 GECs Interact with Glomerular Mesangial Cells (MCs)
The strategic location of MCs within the glomerulus positions them as a hub for intercellular communication46. Ang-1 and Ang-2 are thought to competitively regulate GEC proliferation and differentiation via the Tie-2 receptor43. In models of mesangial proliferative glomerulonephritis (MPGN), co-culture studies have shown that mesangial cell-derived VEGF-A induces the expression of VEGF receptor 2 and Ang-2 in GECs, inhibiting Tie-2 phosphorylation and modulating GEC proliferation47. Mesangial cells also influence GEC secretion of ET-1, as demonstrated in co-culture experiments, which show decreased mRNA and protein levels of endothelin-converting enzyme-1 (ECE-1)48.
Research indicates that MC development relies on endothelial cell-derived PDGF-B49. Endothelial cells also release NO, altering cGMP levels in MCs, thereby impacting their structure and function50. In vitro studies further reveal that extracellular vesicles from endothelial cells are internalized by MCs, promoting proliferation and matrix production via the TGF-β1/Smad3 pathway46,51.
3.4 GECs Interact with Glomerular Parietal Epithelium
While the influence of the glomerular parietal epithelium on GECs is less prominent than that of other cell types, it still plays a role in modulating endothelial function. Glomerular epithelial cells, derived from mesenchymal cells, are located near GECs along the vascular lumen. Studies have shown that VEGF release by glomerular epithelial cells promotes fenestration formation and GEC differentiation52. Additionally, the glomerular parietal epithelium secretes various cytokines, hormones, and bioactive substances, such as aldosterone, vasopressin, and prostaglandins, which can influence GEC function56. Simultaneously, studies have also found that GECs can regulate the survival, proliferation, and apoptosis of glomerular epithelial cells in the glomerular wall layer through the epidermal growth factor (EGF)/epidermal growth factor receptor (EGFR) signaling pathway53,54 (Figure 1).
4. The factors contributing to endothelial injury in chronic kidney disease
4.1 Inflammation and oxidative stress
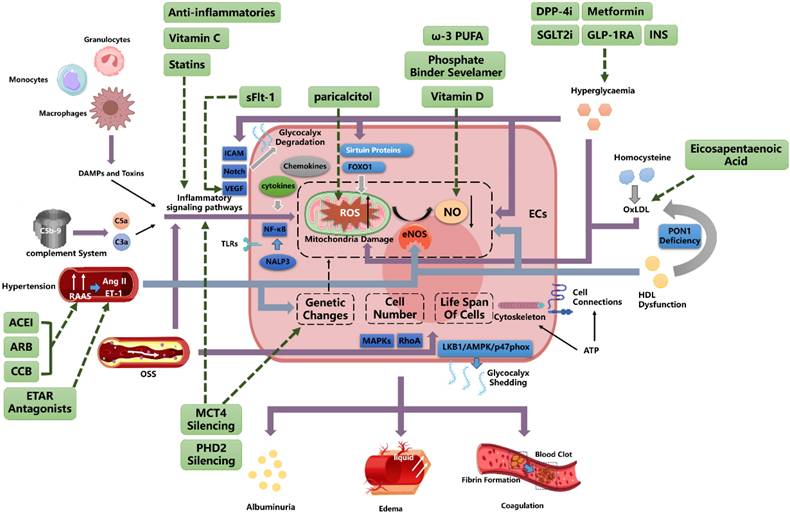
Chronic low-grade inflammation is common in CKD, triggered by unresolved kidney damage57. This involves activation of the innate immune system, including monocytes, macrophages, and granulocytes, leading to persistent inflammation and endothelial cell damage58,59. Damaged cells release damage-associated molecular patterns (DAMPs) and toxins, increasing toll-like receptor (TLR) and NALP3 inflammasome expression in endothelial cells, which activates NF-κB60. This amplifies inflammation and increases ROS production while reducing NO bioavailability61,62, further damaging endothelial cells. Key inflammatory markers include elevated cytokines such as interleukin (IL)-1, IL-6, IL-18, TNF-α, C-reactive protein (CRP), and pentraxin-3 (PTX3), as well as adhesion molecules like vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and monocyte chemoattractant protein-1 (MCP-1), all of which promote endothelial damage63.
Chemokines also contribute to this damage. For instance, in kidney diseases such as crescentic glomerulonephritis and diabetic kidney disease, CX3CL1, produced by renal endothelial cells, interacts with CX3CR1 to mediate inflammation64. TNF-α, interleukin-1 beta (IL-1β), and lipopolysaccharides (LPS) stimulate CX3CL1 expression, though its role in CKD requires further research65. CCR6 is constitutively expressed in glomerular endothelial cells but decreases during glomerular inflammation, suggesting its level can indicate endothelial damage66.
Complement activation also plays a role in kidney diseases, with anaphylatoxins from complement activation contributing to CKD by activating neutrophil inflammation, indirectly damaging glomerular endothelial cells67,68.
4.2 Factors Associated with Hemodynamics
4.2.1 Blood Pressure
Hypertension often affects the kidneys and can lead to CKD, exacerbated by the overactivation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system, causing sustained high blood flow and pressure59. Hypertension also contributes to cardiovascular disease by damaging endothelial cells69.
Studies in hypertensive rats have shown impaired vasodilation, with increased sensitivity to vasoconstrictors such as angiotensin II and endothelin, and reduced NO levels, leading to endothelial damage70-72. Circulating endothelial microparticles (EMPs) are elevated in hypertensive patients, impairing vascular function and serving as early biomarkers of endothelial dysfunction73. Hypertension also alters endothelial progenitor cell numbers, gene expression, and lifespan, contributing to oxidative stress74.
Research suggests a mutual influence between blood pressure and endothelial cells, potentially creating a "vicious cycle"75. For example, inhibiting nitric oxide synthase increases arterial pressure, indicating that endothelial damage affects blood pressure regulation76.
4.2.2 Shear Stress
Vascular endothelial cells respond to shear stress, which regulates their function. Laminar shear stress (LSS) in straight arteries supports endothelial cell growth and prevents apoptosis77,78. In contrast, oscillatory shear stress (OSS) in artery branches and curves promotes endothelial dysfunction, increases oxidative stress, and triggers inflammation, thereby raising the risk of cardiovascular disease in CKD patients79.
Enhanced schematic illustrating the influence of diverse cellular signaling pathways on the differentiation and development of glomerular endothelium. GBM, Glomerular Basement Membrane; GECs, Glomerular Endothelial Cells; TECs, Tubular Epithelial Cells; MCs, Mesangial Cells; PECs, Parietal Epithelial Cells; VEGF-A, Vascular Endothelial Growth Factor A; VEGFR2, Vascular Endothelial Growth Factor 2 Receptor; Ang-1, Angiopoietin-1; ET-1, Endothelin-1; ETAR, Endothelin A Receptor.
OSS induces oxidative stress via NADPH oxidase, activating pro-inflammatory signals such as NF-κB and disrupting endothelial eNOS function77. Integrins interact with extracellular matrix proteins, activating RhoA and mitogen-activated protein kinases (MAPKs), which regulate endothelial cell proliferation, migration, and morphological changes80. This process leads to high cell turnover and replicative senescence, particularly at arterial bifurcations, contributing to atherosclerosis81,82.
Low shear stress may induce endothelial dysfunction through the liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK)/p47phox pathway. Studies have shown that glycocalyx shedding under OSS is associated with increased endothelial injury markers, indicating a potential pathway for endothelial damage83.
4.3 Factors Related to Metabolism
4.3.1 Glycometabolism
Chronic hyperglycemia is the leading cause of diabetes-related renal microvascular complications. Metabolic dysregulation, increased ROS, activation of the polyol pathway, and the formation of advanced glycation end products (AGEs) contribute to early endothelial dysfunction84,85. Elevated glucose promotes oxidative stress in endothelial cells, reduces NO bioavailability, and inhibits sirtuin proteins and histone acetyltransferases, which suppress forkhead box O1 (FOXO1) activity and induce ROS generation86.
Studies have shown that endothelial cells rely heavily on anaerobic glycolysis for energy87. However, diabetes-induced endothelial dysfunction involves mitochondrial defects, leading to elevated ROS levels and further damage88. Hyperglycemia also reduces telomerase activity and endothelial eNOS phosphorylation, thereby lowering NO production89,90.
Hyperglycemia promotes vascular dysfunction by thinning the glycocalyx, thereby reducing its protective role91. Increased glycocalyx shedding and oxidative stress markers indicate impaired endothelial function92. Additionally, hyperglycemia induces an inflammatory environment, affecting ICAM, VEGF, and Notch signaling, ultimately leading to endothelial cell apoptosis and glycocalyx degradation93.
4.3.2 Amino Acid Metabolism
Homocysteine, a methionine metabolite, is associated with endothelial damage94-96, particularly in advanced CKD patients with hyperhomocysteinemia97. Elevated homocysteine levels stimulate hydroxyl radical production, reduce NO activity, and increase oxidative stress, leading to endothelial dysfunction98,99. Studies have shown higher homocysteine levels in patients with coronary artery disease and endothelial dysfunction100. Homocysteine-mediated low-density lipoprotein (LDL) oxidation further damages the endothelium by altering mitochondrial gene expression and promoting oxidative stress98,101.
4.3.3 Lipid Metabolism
CKD patients often experience lipoprotein metabolism disorders, characterized by abnormal lipid profiles and the accumulation of atherogenic particles102,103, which contribute to endothelial damage via oxidative stress and inflammation104,105.
High-density lipoprotein (HDL) normally protects against LDL oxidation by ROS; however, in CKD, HDL's protective functions are impaired due to decreased apolipoproteins and abnormal post-translational modifications106-108. CKD-related HDL dysfunction reduces eNOS activation and impairs endothelial repair. Moreover, paraoxonase 1 (PON1) deficiency in CKD further diminishes HDL's antioxidant capacity, exacerbating LDL oxidation and endothelial damage34,103,109,110.
ATP and Energy Uptake
Endothelial cell stability depends on energy metabolism, particularly ATP production. ATP generated by endothelial cell mitochondria regulates vascular tone by controlling calcium-dependent nitric oxide (NO)-mediated relaxation111. ATP deficiency or disruption of calcium influx can lead to endothelial dysfunction and proteinuria112-114. Studies have shown that ATP influences endothelial fenestrae stability, cytoskeleton maintenance, and cell connections. In CKD patients, decreased ATP levels result in impaired vascular tension control and endothelial barrier damage caused by prolonged ischemia and hypoxia115-118.
5. Outcomes of Endothelial Injury in CKD
5.1 Albuminuria
Patients with cardiovascular conditions, such as hypertension and heart failure, often exhibit trace albuminuria, which signals endothelial barrier damage, including glycocalyx injury and endothelial dysfunction119. The presence of albuminuria in cardiovascular diseases indicates shared pathophysiological processes, such as endothelial dysfunction, chronic inflammation, and increased vascular leakage120. A study by Stephen L. Seliger and colleagues confirmed a close association between microvascular endothelial dysfunction, significant albuminuria, and CKD, underscoring the systemic cardiovascular risk in these patients121.
Early research identified a correlation between the prevalence of microalbuminuria and the severity of hypertension122,123. Sparving and colleagues first described the association between primary hypertension and microalbuminuria in 1974, noting that urinary albumin excretion increased with blood pressure but decreased when blood pressure was controlled124. Microalbuminuria is also associated with glomerular endothelial glycocalyx damage. Studies on rat kidneys demonstrated that albumin remains confined to the glomerular capillary lumen, indicating that the endothelial surface regulates albumin leakage125. In vitro studies further revealed that removing the glycocalyx reduces endothelial resistance and increases albumin flux126.
In the early stages of diabetes, GEC dysfunction serves as an early marker of diabetic nephropathy. Elevated glucose levels induce mitochondrial dysfunction and increase ROS, which damage endothelial cells and the glomerular filtration barrier (GFB), leading to albuminuria85. In diabetic patients, increased endothelial cell surface adhesion molecules and selectins exacerbate injury. Research has shown that platelet activation via the mTORC1 pathway contributes to GEC damage127.
The GFB also functions as an electrical charge barrier that repels negatively charged proteins, preventing albumin leakage. Studies have demonstrated that glycocalyx thinning reduces the charge selectivity of the GFB, resulting in albuminuria128,129. Increased expression of proteinases, such as MMP9, hyaluronidase, and heparanase, in diabetic patients degrades the endothelial glycocalyx, compromising the charge barrier and exacerbating albuminuria130,131.
5.2 Edema
Edema, the accumulation of excess fluid in tissues, is traditionally attributed to inadequate blood volume and activation of the renin-angiotensin-aldosterone system. However, changes in the endothelial filtration barrier also contribute to edema development132. The low-filling theory proposes that proteinuria and hypoalbuminemia reduce serum osmotic pressure, resulting in edema. Research has shown that, in some patients, a primary renal defect in sodium and water excretion increases plasma volume, leading to overflow edema. Clinical studies have identified an increased capillary filtration coefficient (CFC) and elevated capillary permeability as key factors in peripheral edema133. Tight junctions between endothelial cells regulate hydraulic conductivity, and hypoalbuminemia may enhance capillary permeability by promoting intracellular calcium influx134.
5.3 Coagulation
CKD patients are at higher risk of coagulation disorders due to the loss of coagulation inhibitors through excretion and increased fibrinogen production135-137. Endothelial dysfunction contributes to venous thrombosis, with uremic toxins activating endothelial cells to exhibit procoagulant properties138. Elevated levels of the endothelial injury marker ProET-1 and depletion of platelet granules have been observed in end-stage CKD139. Inflammation-driven immune thrombosis further exacerbates fibrin formation and local clotting140.
Although CKD patients have an increased risk of venous thrombosis, they typically do not develop disseminated intravascular coagulation (DIC), as DIC is more commonly associated with acute illnesses, whereas CKD follows a chronic course141.
6. Improving CKD by Intervening in Endothelial Cells
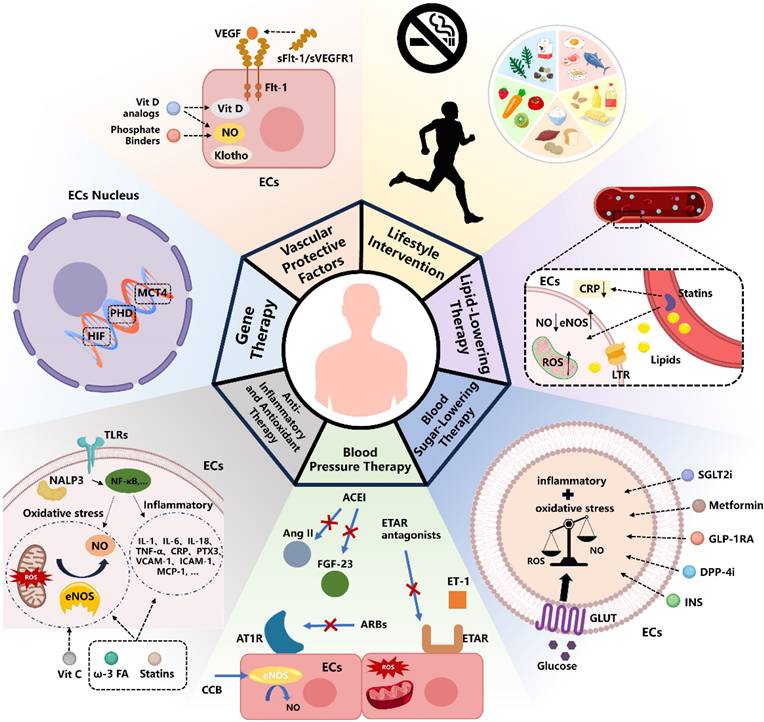
6.1 Vascular Protective Factors
Vascular protective factors are critical in managing CKD, as they enhance vascular function, regulate blood pressure through vasodilation, and reduce inflammation and oxidative stress, thereby protecting the endothelium and maintaining vascular health in CKD patients. Among these factors, nitric oxide (NO) plays a pivotal role. Reduced NO bioavailability is a hallmark of CKD progression, particularly in end-stage kidney disease (ESKD). This reduction is driven by various factors, including the accumulation of endogenous eNOS inhibitors, oxidative stress, inflammation, AGEs, disturbances in bone mineral metabolism (e.g., hyperphosphatemia), elevated FGF23 levels, and deficiencies in active vitamin D and Klotho. Collectively, these factors contribute to endothelial dysfunction142,143.
Interventions aimed at increasing NO bioavailability have shown potential in improving endothelial function. For instance, the phosphate binder sevelamer has been shown to lower serum phosphate levels and enhance endothelium-dependent vasodilation in CKD stage 4 patients144,145. Similarly, vitamin D analogs, such as paricalcitol, have demonstrated therapeutic efficacy in preserving endothelial integrity. Research by Amanda Lima Deluque et al. found that paricalcitol treatment in ARD rats increased eNOS/NO expression, reduced oxidative stress, and inhibited the TGF-β1/Smad2/3 pathway, thereby restoring endothelial structure and function146-148.
Furthermore, endothelial cell factors like soluble fms-like tyrosine kinase-1 (sFlt-1/sVEGFR1), a natural antagonist of VEGF, play a complex role in CKD. While sFlt-1 helps regulate VEGF activity to prevent excessive angiogenesis, elevated circulating sFlt-1 levels have been associated with endothelial dysfunction in CKD patients and post-kidney transplantation. Heparin administration during hemodialysis can further increase sFlt-1 secretion, exacerbating endothelial damage. However, clinical evidence regarding the benefits of targeting sFlt-1 levels to improve kidney and cardiovascular outcomes remains insufficient149.
Gene Therapy
Hypoxia-inducible factors (HIFs) regulate genes critical to the survival, metabolism, and angiogenic activity of vascular endothelial cells, playing a pivotal role in vascular development and diseases, including CKD150,151. Endothelial cell dysfunction is considered a key factor in the progression of AKI to CKD, with prolyl hydroxylases (PHD) 1-3 playing a crucial role in regulating kidney repair following ischemia152. Researchers developed a transgenic mouse model using Cdh5Cre (PAC)ER to induce the specific inactivation of PHD2 in endothelial cells, either alone or in combination with PHD1 and PHD3. Their findings highlight the multifaceted effects of the PHD/HIF pathway on vascular endothelial cells. Notably, metabolic alterations are associated with the upregulation of solute carrier family 16 member 3 (SLC16A3), which encodes monocarboxylate transporter 4 (MCT4). This regulation selectively impacts the endothelial cell hypoxia-driven glycolysis/MCT4 axis, effectively preventing the progression from AKI to CKD. Furthermore, the study demonstrated that MCT4 inhibition could attenuate the inflammatory activation of endothelial cells and reduce interactions between monocytes and endothelial cells. These findings suggest that both gene silencing and pharmacological inhibition of MCT4 hold potential as therapeutic strategies for reprogramming endothelial cell metabolism comprehensively153.
Despite the promising prospects of gene therapy, it faces several challenges. The high research and production costs, particularly for personalized gene editing technologies such as CRISPR-Cas9154, result in expensive treatments. Additionally, gene therapy requires customization based on patients' genetic characteristics, making the production process complex and difficult to scale up. Safety concerns are another significant issue, as gene editing may lead to off-target effects, causing unforeseen side effects such as cancer or other genetic disorders155. The use of viral vectors in gene therapy can also trigger immune reactions, leading to treatment failure or severe side effects156. Therefore, the long-term effects and potential risks of gene therapy require further investigation, particularly concerning possible complications following gene editing.
6.3 Anti-Inflammatory and Antioxidant Therapy
In CKD patients, inflammation markers such as C-reactive protein and cytokines play a pivotal role in endothelial dysfunction and serve as independent predictors of CKD prognosis157. Targeting inflammation presents a promising strategy for protecting endothelial cells. The interplay between inflammation and oxidative stress is profound, with NF-κB activation and Nrf2 imbalance contributing to endothelial dysfunction. Notably, IL-6, regulated via the NF-κB pathway, is a critical biomarker for CKD prognosis158.
Patients with CKD and concurrent cardiovascular disease often exhibit abnormal lipid profiles, which exacerbate oxidative stress and inflammation. Statins, such as rosuvastatin, have been shown to significantly reduce CRP levels and lower cardiovascular event rates in CKD patients159. Omega-3 fatty acids may enhance endothelial health by increasing NO bioavailability, though large-scale clinical trials are still needed to confirm their efficacy142,160. Similarly, vitamin C, recognized for its anti-inflammatory properties, has demonstrated benefits in small-scale studies, including improvements in carotid intima-media thickness and flow-mediated dilation in CKD patients161.
Nonetheless, prolonged use of anti-inflammatory agents in CKD patients can increase infection risks and potentially worsen renal function, as observed with NSAIDs162,163. The variability in CKD progression, influenced by genetic factors and disease stage, complicates treatment decisions. This underscores the importance of precision medicine approaches, such as genetic testing and biomarker analysis, to optimize therapeutic strategies164. While anti-inflammatory treatments show short-term benefits, their long-term impact on CKD progression remains uncertain, necessitating further investigation165,166.
6.4 Blood Pressure Therapy
Multiple antihypertensive drugs, including ARBs, CCBs, and ACE inhibitors, can reverse endothelial damage in primary hypertension by modulating redox states and Ang-II receptor signaling167. Recent studies have also highlighted the role of the vasoconstrictor ET-1 in CKD-related endothelial damage142.
Amlodipine, an LTCC blocker, has been shown to slightly improve renal function and reverse endothelial dysfunction, likely through enhanced kinin activity, NO generation, antioxidant effects, and free radical scavenging168-170. Elevated ET-1 levels in CKD patients contribute to kidney injury via ETAR activation, which reduces NO production, increases oxidative stress, and promotes inflammation171. ETAR antagonists, such as zibotentan, have demonstrated efficacy in improving renal blood flow, reducing proteinuria, and ameliorating NO-mediated endothelial function172. They may also improve coronary atherosclerosis, a common CKD complication, though more research is needed on their effects in this population173,174.
Angiotensin II, similar to ET-1, causes endothelial damage by activating inflammatory pathways such as NF-κB and oxidative stress. ACE inhibitors, such as ramipril, have been shown to improve endothelial function (e.g., increased FMD) and reduce FGF-23 levels, a key contributor to endothelial dysfunction in CKD175-177. Further studies are needed to explore the long-term benefits of these therapies in CKD patients.
Treatment Strategies for Endothelial Injury in CKD Currently. ECs, Endothelial Cells; Vit D, Vitamin D; Flt-1, Fms-like tyrosine kinase 1; sFlt-1, soluble Fms-like tyrosine kinase-1; VEGF, Vascular Endothelial Growth Factor; ROS, Reactive Oxygen Species; eNOS, Endothelial Nitric Oxide Synthase; ω-3 FA, omega-3 fatty acids; Vit C, Vitamin C; ACEI, Angiotensin-Converting Enzyme Inhibitors; ARB, Angiotensin II Receptor Blockers; CCB, Calcium Channel Blockers; AT1R, Angiotensin II Type 1 Receptor; ET-1, Endothelin-1; ETAR, Endothelin A Receptor; FGF-23, Fibroblast Growth Factor 23; GLUT, Glucose Transporter; INS, Insulin; DPP-4i, Dipeptidyl peptidase 4 inhibitors; GLP-1RA, Glucagon-like peptide-1 receptor agonists; SGLT2i, Sodium-Glucose Co-Transporter-2 Inhibitors; LTR, lipid transport receptor; CRP, C-reactive protein.
Integrated Mechanistic Network: Endothelial Dysregulation Pathways Converging with Cellular Interactions to Inform Therapeutic Targeting AECI, Angiotensin-Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker; CCB, Calcium Channel Blockers; ETAR, Endothelin A Receptor; ET-1, Endothelin-1; OxLDL, oxidized low-density lipoprotein; GLUT, Glucose Transporter; INS, Insulin; DPP-4i, Dipeptidyl peptidase 4 inhibitors; GLP-1RA, Glucagon-like peptide-1 receptor agonists; SGLT2i, Sodium-Glucose Co-Transporter-2 Inhibitors; sFlt-1, soluble Fms-like tyrosine kinase-1; VEGF, Vascular Endothelial Growth Factor; ROS, Reactive Oxygen Species; eNOS, Endothelial Nitric Oxide Synthase; ω-3 PUFA, omega-3 polyunsaturated fatty acid; ECs, Endothelial Cells.
6.5 Blood Sugar-Lowering Therapy
Type 2 diabetes (T2D) often leads to microvascular complications, including CKD and ESRD178. Several antidiabetic medications, such as insulin, metformin, SGLT2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors, have shown protective effects on vascular endothelium by reducing oxidative stress and inflammation.
SGLT2 inhibitors consistently lower cardiovascular and renal event risks in T2D patients. For instance, empagliflozin improved endothelium-dependent vasodilation and reduced oxidative stress in diabetic mice after 8 weeks of treatment179,180. Similarly, the DEFENSE study demonstrated that dapagliflozin enhances endothelial function and glycemic control by reducing endothelial activation181.
DPP-4 inhibitors, especially when combined with insulin or metformin, also improve endothelial dysfunction in diabetic kidney disease (DKD). Linagliptin, for example, regulates endothelial markers like PECAM1, VEGF-A, and NOS3 by mitigating oxidative stress, as shown in a study by Hasan B Awal et al.182.
6.6 Lipid-Lowering Therapy
Statin-based lipid-lowering therapy has been shown to reduce proteinuria and slow renal function decline in CKD. The National Kidney Foundation recommends that CKD patients with LDL levels ≥100 mg/dL (2.59 mmol/L) should be managed with diet modifications or statins183. Statins can lower inflammatory markers, such as high-sensitivity C-reactive protein (HS-CRP), and improve endothelial function in high-risk cardiovascular populations184. Elevated total cholesterol and reduced HDL cholesterol are associated with an increased risk of CKD, and CKD patients face a higher risk of cardiovascular disease and mortality185,186.
Clinical studies indicate that atorvastatin improves endothelial function more effectively than ezetimibe, likely by reducing oxidative stress and upregulating eNOS187. Statins may also inhibit endothelial-to-mesenchymal transition (EndoMT). For example, lovastatin has been shown to protect endothelial cells in diabetic nephropathy by reducing oxidative stress and TGF-β1 signaling188.
However, statins may not mitigate all forms of endothelial injury. For instance, indoxyl sulfate (IS), a uremic toxin, increases endothelial activation markers (e.g., ICAM-1, VCAM-1), and atorvastatin does not significantly counteract IS-induced damage189. Thus, the role of statins in improving endothelial function in CKD requires further investigation.
6.7 Lifestyle Intervention
Controlling blood pressure and blood sugar, as well as lifestyle changes such as maintaining a healthy weight and quitting smoking, can significantly improve endothelial health in CKD patients. Moderate exercise and dietary adjustments also play a crucial role in slowing the progression of the disease (Figure 2).
7. Conclusion
Endothelial cell behavior in CKD is influenced by the internal environment, including inflammatory mediators and intercellular signaling pathways. The microenvironment regulates endothelial transcription factors and cell differentiation, leading to endothelial heterogeneity. This diversity contributes to the complex pathogenesis of CKD (Figure 3). Understanding the factors that drive endothelial dysfunction and heterogeneity is essential for developing new therapeutic strategies.
Supplementary Material
Supplementary table.
Acknowledgements
Funding
Capital's Funds for Health Improvement and Research (No. 2024-1-2231), National Natural Science Foundation of China (No. 82104784 to HX), Beijing Administration of Traditional Chinese Medicine Project (No. 2023BJSZDYNJBXTGG-008).
Author Contributions
Meiyu Zhang: Writing - review & editing, Writing - original draft, Conceptualization. Wu Liu: Writing - review & editing. Haoran Dai: Writing - review & editing. Hanxue Jiang: Writing - review & editing. Qihan Zhao: Writing - review & editing. WenBin Liu: Writing - review & editing, Conceptualization. Hongliang Rui: Writing - review & editing, Conceptualization. Baoli Liu: Writing - review & editing, Conceptualization.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Thorgeirsson G, Robertson AL. The vascular endothelium-pathobiologic significance. Am J Pathol. 1978Dec;93(3):803-48
2. Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci. 2019Sep7;20(18):4411
3. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G. et al. The Vascular Endothelium and Human Diseases. Int J Biol Sci. 2013Nov9;9(10):1057-69
4. Ricard N, Bailly S, Guignabert C, Simons M. The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy. Nat Rev Cardiol. 2021Aug;18(8):565-80
5. Augustin HG, Koh GY. A systems view of the vascular endothelium in health and disease. Cell. 2024Sep5;187(18):4833-58
6. Kalucka J, de Rooij LPMH, Goveia J, Rohlenova K, Dumas SJ, Meta E. et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell. 2020Feb20;180(4):764-779.e20
7. Dumas SJ, Meta E, Borri M, Goveia J, Rohlenova K, Conchinha NV. et al. Single-Cell RNA Sequencing Reveals Renal Endothelium Heterogeneity and Metabolic Adaptation to Water Deprivation. J Am Soc Nephrol. 2020Jan;31(1):118-38
8. Wakabayashi T, Naito H. Cellular heterogeneity and stem cells of vascular endothelial cells in blood vessel formation and homeostasis: Insights from single-cell RNA sequencing. Front Cell Dev Biol. 2023Mar21;11:1146399
9. Marx V. scRNA-seq: oh, the joys. Nat Methods. 2024May;21(5):750-3
10. Lohia S, Vlahou A, Zoidakis J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins (Basel). 2022Feb26;14(3):176
11. Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M. et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019Feb;15(2):87-108
12. Cardinal H, Dieudé M, Hébert MJ. Endothelial Dysfunction in Kidney Transplantation. Front Immunol. 2018;9:1130
13. Sedrakyan S. Kidney Endothelial Cell Biology in Health and Disease. Journal of the American Society of Nephrology. 2024May;35(5):522
14. Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E. et al. Endothelial Progenitors Exist within the Kidney and Lung Mesenchyme. PLoS One. 2013Jun18;8(6):e65993
15. Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018May18;360(6390):758-63
16. Dumas SJ, Meta E, Borri M, Luo Y, Li X, Rabelink TJ. et al. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat Rev Nephrol. 2021;17(7):441-64
17. Fu F, Chen F, Li R, Zhang Y, Pan M, Li D. et al. Prenatal diagnosis of fetal multicystic dysplastic kidney via high-resolution whole-genome array. Nephrology Dialysis Transplantation. 2016Oct1;31(10):1693-8
18. Feng S, Gao Y, Yin D, Lv L, Wen Y, Li Z. et al. Identification of Lumican and Fibromodulin as Hub Genes Associated with Accumulation of Extracellular Matrix in Diabetic Nephropathy. Kidney and Blood Pressure Research. 2021Apr22;46(3):275-85
19. Mathur Y, Shafie A, Alharbi B, Ashour AA, Al-Soud WA, Alhassan HH. et al. Genome-Wide Analysis of Kidney Renal Cell Carcinoma: Exploring Differentially Expressed Genes for Diagnostic and Therapeutic Targets. OMICS: A Journal of Integrative Biology. 2023Aug;27(8):393-401
20. Jeanne M, Gould DB. Genotype-Phenotype Correlations in Pathology Caused by Collagen Type IV alpha 1 and 2 Mutations. Matrix Biol. 2017Jan;57-58:29-44
21. Chen Z, Migeon T, Verpont MC, Zaidan M, Sado Y, Kerjaschki D. et al. HANAC Syndrome Col4a1 Mutation Causes Neonate Glomerular Hyperpermeability and Adult Glomerulocystic Kidney Disease. J Am Soc Nephrol. 2016Apr;27(4):1042-54
22. Hwang JR, Chou CL, Medvar B, Knepper MA, Jung HJ. Identification of β-catenin-interacting proteins in nuclear fractions of native rat collecting duct cells. Am J Physiol Renal Physiol. 2017Jul1;313(1):F30-46
23. Domingo-Gallego A, Pybus M, Bullich G, Furlano M, Ejarque-Vila L, Lorente-Grandoso L. et al. Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol Dial Transplant. 2022Mar25;37(4):687-96
24. Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003Mar;111(5):707-16
25. Wolf EE, Steglich A, Kessel F, Kröger H, Sradnick J, Reichelt-Wurm S. et al. PLVAP as an Early Marker of Glomerular Endothelial Damage in Mice with Diabetic Kidney Disease. Int J Mol Sci. 2023Jan6;24(2):1094
26. George M, Rainey MA, Naramura M, Foster KW, Holzapfel MS, Willoughby LL. et al. Renal thrombotic microangiopathy in mice with combined deletion of endocytic recycling regulators EHD3 and EHD4. PLoS One. 2011Mar9;6(3):e17838
27. Barry DM, McMillan EA, Kunar B, Lis R, Zhang T, Lu T. et al. Molecular determinants of nephron vascular specialization in the kidney. Nat Commun. 2019Dec13;10(1):5705
28. Neri T, Hiriart E, van Vliet PP, Faure E, Norris RA, Farhat B. et al. Human pre-valvular endocardial cells derived from pluripotent stem cells recapitulate cardiac pathophysiological valvulogenesis. Nat Commun. 2019Apr26;10:1929
29. Jacobs ME, de Vries DK, Engelse MA, Dumas SJ, Rabelink TJ. Endothelial to mesenchymal transition in kidney fibrosis. Nephrol Dial Transplant. 2024Apr26;39(5):752-60
30. Erfurt S, Lauxmann M, Asmus K, Oess S, Patschan D, Hoffmeister M. Serum Nostrin-A risk factor of death, kidney replacement therapy and acute kidney disease in acute kidney injury. PLoS One. 2024;19(4):e0299131
31. Brunskill EW, Potter SS. Gene expression programs of mouse endothelial cells in kidney development and disease. PLoS One. 2010Aug10;5(8):e12034
32. Guan Z, Gobé G, Willgoss D, Endre ZH. Renal endothelial dysfunction and impaired autoregulation after ischemia-reperfusion injury result from excess nitric oxide. Am J Physiol Renal Physiol. 2006Sep;291(3):F619-628
33. Kij A, Bar A, Czyzynska-Cichon I, Przyborowski K, Proniewski B, Mateuszuk L. et al. Vascular protein disulfide isomerase A1 mediates endothelial dysfunction induced by angiotensin II in mice. Acta Physiol (Oxf). 2024Apr;240(4):e14116
34. Speer T, Owala FO, Holy EW, Zewinger S, Frenzel FL, Stähli BE. et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. European Heart Journal. 2014Nov14;35(43):3021-32
35. Goligorsky MS. Permissive role of vascular endothelium in fibrosis: focus on the kidney. Am J Physiol Cell Physiol. 2024Mar1;326(3):C712-23
36. Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004Jun;65(6):2003-17
37. Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Current Opinion in Nephrology and Hypertension. 2004Jan;13(1):9
38. Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol. 2006Aug;291(2):F422-428
39. Li J, Li XL, Li CQ. Immunoregulation mechanism of VEGF signaling pathway inhibitors and its efficacy on the kidney. The American Journal of the Medical Sciences. 2023Dec1;366(6):404-12
40. Ff H, D Z, Q C, Y Z, L W, Zq L. et al. Angiopoietin-Tie signaling in kidney diseases: an updated review. FEBS letters [Internet]. 2019 Oct [cited. 2024 Apr 20];593(19). Available from: https://pubmed.ncbi.nlm.nih.gov/31380564/
41. Barton M, Sorokin A. Endothelin and the glomerulus in chronic kidney disease. Semin Nephrol. 2015Mar;35(2):156-67
42. Barton M, Tharaux PL. Endothelin and the podocyte. Clin Kidney J. 2012Feb;5(1):17-27
43. Chen S, Lv L, Liu B, Tang R. Crosstalk between tubular epithelial cells and glomerular endothelial cells in diabetic kidney disease. Cell Prolif. 2020Jan11;53(3):e12763
44. Romero A, San Hipólito-Luengo Á, Villalobos LA, Vallejo S, Valencia I, Michalska P. et al. The angiotensin-(1-7)/Mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell. 2019Jun;18(3):e12913
45. Wakelin SJ, Marson L, Howie SEM, Garden J, Lamb JR, Forsythe JLR. The Role of Vascular Endothelial Growth Factor in the Kidney in Health and Disease. Nephron Physiology. 2004Oct29;98(3):p73-9
46. Chin E, Bondy C. Insulin-like growth factor system gene expression in the human kidney. J Clin Endocrinol Metab. 1992Sep;75(3):962-8
47. Ebefors K, Bergwall L, Nyström J. The Glomerulus According to the Mesangium. Front Med (Lausanne). 2022Jan26;8:740527
48. Hu S, Hang X, Wei Y, Wang H, Zhang L, Zhao L. Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review. Cell Commun Signal. 2024Feb19;22:136
49. López-Ongil S, Díez-Marqués ML, Griera M, Rodríguez-Puyol M, Rodríguez-Puyol D. Crosstalk Between Mesangial and Endothelial Cells: Angiotensin II Down-Regulates Endothelin-Converting Enzyme 1. Cellular Physiology and Biochemistry. 2005Jan1;15(1-4):135-44
50. Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M. et al. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998Sep;125(17):3313-22
51. Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998Jul;78(3):723-44
52. Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016Apr15;5(4):484-91
53. Horita Y, Miyazaki M, Koji T, Kobayashi N, Shibuya M, Razzaque MS. et al. Expression of vascular endothelial growth factor and its receptors in rats with protein-overload nephrosis. Nephrol Dial Transplant. 1998Oct;13(10):2519-28
54. Ardaillou R. Biology of glomerular cells in culture. Cell Biol Toxicol. 1996Dec;12(4-6):257-61
55. Flamant M, Bollée G, Hénique C, Tharaux PL. Epidermal growth factor: a new therapeutic target in glomerular disease. Nephrology Dialysis Transplantation. 2012Apr1;27(4):1297-304
56. Bartlett CS, Jeansson M, Quaggin SE. Vascular Growth Factors and Glomerular Disease. Annu Rev Physiol. 2016;78:437-61
57. Yuan Q, Tang B, Zhang C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct Target Ther. 2022Jun9;7(1):182
58. Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017Jun;13(6):368-80
59. Vondenhoff S, Schunk SJ, Noels H. Increased cardiovascular risk in patients with chronic kidney disease. Herz. 2024;49(2):95-104
60. Martin-Rodriguez S, Caballo C, Gutierrez G, Vera M, Cruzado JM, Cases A. et al. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. European Journal of Clinical Investigation. 2015Feb1;45(2):160-9
61. Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Kränkel N. et al. Abnormal High-Density Lipoprotein Induces Endothelial Dysfunction via Activation of Toll-like Receptor-2. Immunity. 2013Apr18;38(4):754-68
62. Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate Treatment Improves Age-Associated Vascular Endothelial Dysfunction: Potential Role of Nuclear Factor κB and Forkhead Box O Phosphorylation. J Gerontol A Biol Sci Med Sci. 2011Apr;66A(4):409-18
63. Dri E, Lampas E, Lazaros G, Lazarou E, Theofilis P, Tsioufis C. et al. Inflammatory Mediators of Endothelial Dysfunction. Life (Basel). 2023Jun20;13(6):1420
64. Furuichi K, Wada T, Iwata Y, Sakai N, Yoshimoto K, Shimizu M. et al. Upregulation of fractalkine in human crescentic glomerulonephritis. Nephron. 2001Apr;87(4):314-20
65. von Vietinghoff S, Kurts C. Regulation and function of CX3CR1 and its ligand CX3CL1 in kidney disease. Cell Tissue Res. 2021;385(2):335-44
66. Welsh-Bacic D, Lindenmeyer M, Cohen CD, Draganovici D, Mandelbaum J, Edenhofer I. et al. Expression of the chemokine receptor CCR6 in human renal inflammation. Nephrology Dialysis Transplantation. 2011Apr1;26(4):1211-20
67. Brglez V, Boyer-Suavet S, Seitz-Polski B. Complement Pathways in Membranous Nephropathy: Complex and Multifactorial. Kidney Int Rep. 2020Mar6;5(5):572-4
68. H T, J L, C L, B R, A F, S P. et al. Natural antibody and complement activation characterize patients with idiopathic nephrotic syndrome. American journal of physiology Renal physiology [Internet]. 2021 Jan 10 [cited. 2024 Apr 20];321(4). Available from: https://pubmed.ncbi.nlm.nih.gov/34459222/
69. Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens. 2014Feb;28(2):74-9
70. Bernatova I, Conde MV, Kopincova J, González MC, Puzserova A, Arribas SM. Endothelial dysfunction in spontaneously hypertensive rats: focus on methodological aspects. J Hypertens Suppl. 2009Aug;27(6):S27-31
71. Lerman A, Holmes DR, Bell MR, Garratt KN, Nishimura RA, Burnett JC. Endothelin in Coronary Endothelial Dysfunction and Early Atherosclerosis in Humans. Circulation. 1995Nov;92(9):2426-31
72. Kumar G, Dey SK, Kundu S. Functional implications of vascular endothelium in regulation of endothelial nitric oxide synthesis to control blood pressure and cardiac functions. Life Sci. 2020Oct15;259:118377
73. Silambanan S, Hermes RS, Bhaskar E, Gayathri S. Endothelial Microparticle as an early Marker of Endothelial Dysfunction in Patients with Essential Hypertension: A Pilot Study. Indian J Clin Biochem. 2020Apr;35(2):245-50
74. Luo S, Xia W, Chen C, Robinson EA, Tao J. Endothelial progenitor cells and hypertension: current concepts and future implications. Clin Sci (Lond). 2016Nov1;130(22):2029-42
75. Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. Journal of Hypertension. 2016Aug;34(8):1464
76. Haynes WG, Noon JP, Walker BR, Webb DJ. Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens. 1993Dec;11(12):1375-80
77. He L, Zhang CL, Chen Q, Wang L, Huang Y. Endothelial shear stress signal transduction and atherogenesis: From mechanisms to therapeutics. Pharmacology & Therapeutics. 2022Jul1;235:108152
78. C H, Ma S. Mechanotransduction in vascular physiology and atherogenesis. Nature reviews Molecular cell biology [Internet]. 2009 Jan [cited. 2024 Apr 20];10(1). Available from: https://pubmed.ncbi.nlm.nih.gov/19197332/
79. Bloom SI, Islam MT, Lesniewski LA, Donato AJ. Mechanisms and consequences of endothelial cell senescence. Nat Rev Cardiol. 2023Jan;20(1):38-51
80. X W, Y S, M S, X L, Ll M. Endothelial mechanobiology in atherosclerosis. Cardiovascular research [Internet]. 2023 Jun 7 [cited. 2024 Apr 20];119(8). Available from: https://pubmed.ncbi.nlm.nih.gov/37163659/
81. Warboys CM, de Luca A, Amini N, Luong L, Duckles H, Hsiao S. et al. Disturbed Flow Promotes Endothelial Senescence via a p53-Dependent Pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014May;34(5):985-95
82. Kotla S, Vu HT, Ko KA, Wang Y, Imanishi M, Heo KS. et al. Endothelial senescence is induced by phosphorylation and nuclear export of telomeric repeat binding factor 2-interacting protein. JCI Insight. 2019May2;4(9):e124867 124867
83. Zhang L, Li J, Chen J, Lei J, Yuan Z, Zhang J. et al. Oscillatory shear stress-mediated aberrant O-GlcNAc SIRT3 accelerates glycocalyx inflammatory injury via LKB1/p47phox/Hyal2 signaling. Cellular Signalling. 2023Sep1;109:110790
84. Pi X, Xie L, Patterson C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ Res. 2018Aug3;123(4):477-94
85. Cl G, E H, B L. Diabetic Kidney Disease, Endothelial Damage, and Podocyte-Endothelial Crosstalk. Kidney medicine [Internet]. 2020 Jul 12 [cited. 2024 Apr 20];3(1). Available from: https://pubmed.ncbi.nlm.nih.gov/33604542/
86. Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004Jan14;23(1):212-20
87. Role of PFKFB3-Driven Glycolysis in Vessel Sprouting. Cell. 2013 Aug 1;154(3):651-63.
88. Santos JM, Mishra M, Kowluru RA. Posttranslational Modification of Mitochondrial Transcription Factor A in Impaired Mitochondria Biogenesis: Implications in Diabetic Retinopathy and Metabolic Memory Phenomenon. Exp Eye Res. 2014Apr;121:168-77
89. Hayashi T, Yano K, Matsui-Hirai H, Yokoo H, Hattori Y, Iguchi A. Nitric oxide and endothelial cellular senescence. Pharmacology & Therapeutics. 2008Dec1;120(3):333-9
90. Carracedo J, Buendía P, Merino A, Soriano S, Esquivias E, Martín-Malo A. et al. Cellular senescence determines endothelial cell damage induced by uremia. Exp Gerontol. 2013Aug;48(8):766-73
91. Nieuwdorp M, van Haeften TW, Gouverneur MCLG, Mooij HL, van Lieshout MHP, Levi M. et al. Loss of Endothelial Glycocalyx During Acute Hyperglycemia Coincides With Endothelial Dysfunction and Coagulation Activation In Vivo. Diabetes. 2006Feb1;55(2):480-6
92. Niu T, Zhao M, Jiang Y, Xing X, Shi X, Cheng L. et al. Endomucin restores depleted endothelial glycocalyx in the retinas of streptozotocin-induced diabetic rats. FASEB J. 2019Dec;33(12):13346-57
93. Swärd P, Rippe B. Acute and sustained actions of hyperglycaemia on endothelial and glomerular barrier permeability. Acta Physiol (Oxf). 2012Mar;204(3):294-307
94. Yuan D, Chu J, Lin H, Zhu G, Qian J, Yu Y. et al. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front Cardiovasc Med. 2022;9:1109445
95. Esse R, Barroso M, Tavares de Almeida I, Castro R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int J Mol Sci. 2019Feb17;20(4):867
96. O'Callaghan P, Meleady R, Fitzgerald T, Graham I, European COMAC group. Smoking and plasma homocysteine. European Heart Journal. 2002Oct1;23(20):1580-6
97. Li L, Hasegawa H, Inaba N, Yoshioka W, Chang D, Liu J. et al. Diet-induced hyperhomocysteinemia impairs vasodilation in 5/6-nephrectomized rats. Amino Acids. 2018Oct;50(10):1485-94
98. McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Review of Clinical Pharmacology. 2015Mar4;8(2):211-9
99. Bhalodia YS, Sheth NR, Vaghasiya JD, Jivani NP. Homocysteine-dependent endothelial dysfunction induced by renal ischemia/reperfusion injury. J Nephrol. 2011;24(5):631-5
100. Ahmad A, Corban MT, Toya T, Sara JD, Lerman B, Park JY. et al. Coronary Microvascular Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Artery Disease Is Associated With Elevated Serum Homocysteine Levels. J Am Heart Assoc. 2020Sep30;9(19):e017746
101. Yi F, Jin S, Zhang F, Xia M, Bao JX, Hu J. et al. Formation of lipid raft redox signalling platforms in glomerular endothelial cells: an early event of homocysteine-induced glomerular injury. J Cell Mol Med. 2009Sep;13(9b):3303-14
102. Kaseda R, Jabs K, Hunley TE, Jones D, Bian A, Allen RM. et al. Dysfunctional high-density lipoproteins in children with chronic kidney disease. Metabolism. 2015Feb;64(2):263-73
103. Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM. et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011Jul;121(7):2693-708
104. Rysz J, Gluba-Brzózka A, Rysz-Górzyńska M, Franczyk B. The Role and Function of HDL in Patients with Chronic Kidney Disease and the Risk of Cardiovascular Disease. Int J Mol Sci. 2020Jan17;21(2):601
105. K I, K Y, Y H, Y F, S T, S H. et al. Drug discovery for overcoming chronic kidney disease (CKD): development of drugs on endothelial cell protection for overcoming CKD. Journal of pharmacological sciences [Internet]. 2009 Jan [cited. 2024 Sep 1];109(1). Available from: https://pubmed.ncbi.nlm.nih.gov/19151535/
106. Riwanto M, Rohrer L, von Eckardstein A, Landmesser U. Dysfunctional HDL: from structure-function-relationships to biomarkers. Handb Exp Pharmacol. 2015;224:337-66
107. Gofman JW, Lindgren F. The role of lipids and lipoproteins in atherosclerosis. Science. 1950Feb17;111(2877):166-71
108. Kratzer A, Giral H, Landmesser U. High-density lipoproteins as modulators of endothelial cell functions: alterations in patients with coronary artery disease. Cardiovasc Res. 2014Aug1;103(3):350-61
109. Mineo C, Shaul PW. PON-dering differences in HDL function in coronary artery disease. J Clin Invest. 2011Jul;121(7):2545-8
110. Ujhelyi L, Balla G, Jeney V, Varga Z, Nagy E, Vercellotti GM. et al. Hemodialysis reduces inhibitory effect of plasma ultrafiltrate on LDL oxidation and subsequent endothelial reactions. Kidney International. 2006Jan1;69(1):144-51
111. Wilson C, Lee MD, Buckley C, Zhang X, McCarron JG. Mitochondrial ATP Production is Required for Endothelial Cell Control of Vascular Tone. Function (Oxf). 2023;4(2):zqac063
112. Berra-Romani R, Raqeeb A, Guzman-Silva A, Torres-Jácome J, Tanzi F, Moccia F. Na+-Ca2+ exchanger contributes to Ca2+extrusion in ATP-stimulated endothelium of intact rat aorta. Biochemical and Biophysical Research Communications. 2010Apr23;395(1):126-30
113. Extracellular ATP regulates glomerular endothelial cell function [Internet]. [cited 2024 Jul 28]. Available from: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1474-8673. 1996 tb00060.x
114. Briner VA, Kern F. ATP stimulates Ca2+ mobilization by a nucleotide receptor in glomerular endothelial cells. https://doi.org/101152/ajprenal19942662F210 [Internet]. 1994 Feb 1 [cited 2024 Jul 28]; Available from: https://journals.physiology.org/doi/10.1152/ajprenal. 1994 266.2.F210
115. DuBose DA, Haugland R. Comparisons of endothelial cell G- and F-actin distribution in situ and in vitro. Biotech Histochem. 1993Jan;68(1):8-16
116. Hinshaw DB, Burger JM, Miller MT, Adams JA, Beals TF, Omann GM. ATP depletion induces an increase in the assembly of a labile pool of polymerized actin in endothelial cells. Am J Physiol. 1993May;264(5 Pt 1):C1171-1179
117. Gruwel ML, Culíc O, Schrader J. A 133Cs nuclear magnetic resonance study of endothelial Na(+)-K(+)-ATPase activity: can actin regulate its activity? Biophys J. 1997Jun;72(6):2775-82
118. Braet F, Muller M, Vekemans K, Wisse E, Le Couteur DG. Antimycin A-induced defenestration in rat hepatic sinusoidal endothelial cells. Hepatology. 2003Aug;38(2):394-402
119. Weinstock Brown W, Keane WF. Proteinuria and cardiovascular disease. Am J Kidney Dis. 2001Oct;38(4 Suppl 1):S8-13
120. Koyoshi R, Hitaka-Yoshimine Y, Shiga Y, Kuwano T, Sugihara M, Ike A. et al. Associations between microalbuminuria and parameters of flow-mediated vasodilatation obtained by continuous measurement approaches. Clinical and Experimental Hypertension. 2018Nov17;40(8):715-20
121. Seliger SL, Salimi S, Pierre V, Giffuni J, Katzel L, Parsa A. Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol. 2016Jul13;17(1):82
122. Imamura S, Hirata K, Orii M, Shimamura K, Shiono Y, Ishibashi K. et al. Relation of albuminuria to coronary microvascular function in patients with chronic kidney disease. Am J Cardiol. 2014Mar1;113(5):779-85
123. Querfeld U, Mak RH, Pries AR. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond). 2020Jun26;134(12):1333-56
124. Parving HH, Mogensen CE, Jensen HÆ, Evrin PE. INCREASED URINARY ALBUMIN-EXCRETION RATE IN BENIGN ESSENTIAL HYPERTENSION. The Lancet. 1974Jun15;303(7868):1190-2
125. Ryan GB, Karnovsky MJ. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 1976Jan;9(1):36-45
126. Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular Endothelial Glycocalyx Constitutes a Barrier to Protein Permeability. Journal of the American Society of Nephrology. 2007Nov;18(11):2885
127. Zhang Y, Ma KL, Gong YX, Wang GH, Hu ZB, Liu L. et al. Platelet Microparticles Mediate Glomerular Endothelial Injury in Early Diabetic Nephropathy. J Am Soc Nephrol. 2018Nov;29(11):2671-95
128. Jeansson M, Björck K, Tenstad O, Haraldsson B. Adriamycin Alters Glomerular Endothelium to Induce Proteinuria. J Am Soc Nephrol. 2009Jan;20(1):114-22
129. Khramova A, Boi R, Fridén V, Granqvist AB, Nilsson U, Tenstad O. et al. Proteoglycans contribute to the functional integrity of the glomerular endothelial cell surface layer and are regulated in diabetic kidney disease. Scientific Reports [Internet]. 2021 [cited. 2024 Apr 20];11. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8055884/
130. Mahtal N, Lenoir O, Tharaux PL. Glomerular Endothelial Cell Crosstalk With Podocytes in Diabetic Kidney Disease. Front Med (Lausanne). 2021Mar24;8:659013
131. Thomas MC. Pathogenesis and progression of proteinuria. Contrib Nephrol. 2011;170:48-56
132. Hedin E, Bijelić V, Barrowman N, Geier P. Furosemide and albumin for the treatment of nephrotic edema: a systematic review. Pediatr Nephrol. 2022Aug1;37(8):1747-57
133. Lewis DM, Tooke JE, Beaman M, Gamble J, Shore AC. Peripheral microvascular parameters in the nephrotic syndrome. Kidney International. 1998Oct1;54(4):1261-6
134. Heo SH, Choi YJ, Ryoo HM, Cho JY. Expression profiling of ETS and MMP factors in VEGF-activated endothelial cells: Role of MMP-10 in VEGF-induced angiogenesis. Journal of Cellular Physiology. 2010;224(3):734-42
135. Jeele MOO, Adan AM. Nephrotic syndrome presented as a portal vein thrombosis: a case report. Ann Med Surg (Lond). 2023Apr6;85(5):2112-4
136. Wu T, Tang LV, Hu Y. Venous Thromboembolism in Kidney Diseases and Genetic Predisposition. Kidney Dis (Basel). 2022Apr11;8(3):181-9
137. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and Pathophysiology of Nephrotic Syndrome-Associated Thromboembolic Disease. Clin J Am Soc Nephrol. 2012Mar;7(3):513-20
138. Martin BJ, Anderson TJ. Risk prediction in cardiovascular disease: the prognostic significance of endothelial dysfunction. Can J Cardiol. 2009 Jun;25 Suppl A(Suppl A):15A-20A
139. Schoorl M, Schoorl M, Nubé MJ, Bartels PC. Coagulation activation, depletion of platelet granules and endothelial integrity in case of uraemia and haemodialysis treatment. BMC Nephrol. 2013Mar27;14:72
140. Jing H, Wu X, Xiang M, Liu L, Novakovic VA, Shi J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front Immunol. 2022;13:992384
141. Bhandari J, Rout P, Sedhai YR. Hemolytic Uremic Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited. 2024 Jul 30]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK556038/
142. Roumeliotis S, Mallamaci F, Zoccali C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J Clin Med. 2020Jul23;9(8):2359
143. Sun T, Yu X. FGF23 Actions in CKD-MBD and other Organs During CKD. Curr Med Chem. 2023;30(7):841-56
144. Zoccali C, Torino C, Curatola G, Panuccio V, Tripepi R, Pizzini P. et al. Serum phosphate modifies the vascular response to vitamin D receptor activation in chronic kidney disease (CKD) patients. Nutr Metab Cardiovasc Dis. 2016Jul;26(7):581-9
145. Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T. et al. Comparison of Calcium Acetate and Sevelamer on Vascular Function and Fibroblast Growth Factor 23 in CKD Patients: A Randomized Clinical Trial. American Journal of Kidney Diseases. 2012Feb1;59(2):177-85
146. Deluque AL, Oliveira BM, Souza CS, Maciel ALD, Francescato HDC, Giovanini C. et al. Paricalcitol Improves the Angiopoietin/Tie-2 and VEGF/VEGFR2 Signaling Pathways in Adriamycin-Induced Nephropathy. Nutrients. 2022Dec14;14(24):5316
147. Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M. et al. Paricalcitol and Endothelial Function in Chronic Kidney Disease Trial. Hypertension. 2014Nov;64(5):1005-11
148. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H. et al. Vitamin D Therapy and Cardiac Structure and Function in Patients With Chronic Kidney Disease: The PRIMO Randomized Controlled Trial. JAMA. 2012Feb15;307(7):674-84
149. Wewers TM, Schulz A, Nolte I, Pavenstädt H, Brand M, Di Marco GS. Circulating Soluble Fms-like Tyrosine Kinase in Renal Diseases Other than Preeclampsia. J Am Soc Nephrol. 2021Aug;32(8):1853-63
150. Sy P, Pz T, Yh C, Yt C, Fc C, Yl C. et al. Kidney pericyte hypoxia-inducible factor regulates erythropoiesis but not kidney fibrosis. Kidney international [Internet]. 2021 Jun [cited. 2024 Sep 18];99(6). Available from: https://pubmed.ncbi.nlm.nih.gov/33812664/
151. Yh C, Sy P, Hm S, Sl L. Update of pericytes function and their roles in kidney diseases. Journal of the Formosan Medical Association = Taiwan yi zhi [Internet]. 2024 Mar [cited. 2024 Sep 18];123(3). Available from: https://pubmed.ncbi.nlm.nih.gov/37586973/
152. Rajendran G, Schonfeld MP, Tiwari R, Huang S, Torosyan R, Fields T. et al. Inhibition of Endothelial PHD2 Suppresses Post-Ischemic Kidney Inflammation through Hypoxia-Inducible Factor-1. J Am Soc Nephrol. 2020Mar;31(3):501-16
153. Tiwari R, Sharma R, Rajendran G, Borkowski GS, An SY, Schonfeld M. et al. Post-ischemic inactivation of HIF prolyl hydroxylases in endothelium promotes maladaptive kidney repair by inducing glycolysis. bioRxiv. 2023 Oct 3. 2023 10.03.560700
154. Zhang X, Jin H, Huang X, Chaurasiya B, Dong D, Shanley TP. et al. Robust genome editing in adult vascular endothelium by nanoparticle delivery of CRISPR-Cas9 plasmid DNA. Cell Rep. 2022Jan4;38(1):110196
155. Sun S, Qin W, Tang X, Meng Y, Hu W, Zhang S. et al. Vascular endothelium-targeted Sirt7 gene therapy rejuvenates blood vessels and extends life span in a Hutchinson-Gilford progeria model. Sci Adv. 2020Feb;6(8):eaay5556
156. Thomas JW, Kuo MD, Chawla M, Waugh JM, Yuksel E, Wright KC. et al. Vascular gene therapy. Radiographics. 1998;18(6):1373-94
157. Machowska A, Carrero JJ, Lindholm B, Stenvinkel P. Therapeutics targeting persistent inflammation in chronic kidney disease. Translational Research. 2016Jan1;167(1):204-13
158. Adelibieke Y, Yisireyili M, Ng HY, Saito S, Nishijima F, Niwa T. Indoxyl sulfate induces IL-6 expression in vascular endothelial and smooth muscle cells through OAT3-mediated uptake and activation of AhR/NF-κB pathway. Nephron Exp Nephrol. 2014;128(1-2):1-8
159. Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of Rosuvastatin Among Men and Women With Moderate Chronic Kidney Disease and Elevated High-Sensitivity C-Reactive Protein: A Secondary Analysis From the JUPITER (Justification for the Use of Statins in Prevention-an Intervention Trial Evaluating Rosuvastatin) Trial. Journal of the American College of Cardiology. 2010Mar23;55(12):1266-73
160. Wu-Wong JR. Endothelial dysfunction and chronic kidney disease: treatment options. Curr Opin Investig Drugs. 2008Sep;9(9):970-82
161. Sabri MR, Tavana EN, Ahmadi A, Gheissari A. Effect of vitamin C on endothelial function of children with chronic renal failure: An experimental study. Adv Biomed Res. 2015Dec31;4:260
162. Soliman S, Ahmed RM, Ahmed MM, Attia A, Soliman AR. Non-Steroidal Anti-Inflammatory Drugs: What Is the Actual Risk of Chronic Kidney Disease? A Systematic Review and Meta-Analysis. Rom J Intern Med. 2024 Oct 16
163. Rocha-Rodrigues S, Santos-Alves E, Coxito PM, Marques-Aleixo I, Passos E, Guimarães JT. et al. Combined effects of aging and in vitro non-steroid anti-inflammatory drugs on kidney and liver mitochondrial physiology. Life Sci. 2013Sep3;93(8):329-37
164. Bozoglu T, Lee S, Ziegler T, Jurisch V, Maas S, Baehr A. et al. Endothelial Retargeting of AAV9 In Vivo. Adv Sci (Weinh). 2022Jan12;9(7):2103867
165. Gembillo G, Cernaro V, Salvo A, Siligato R, Laudani A, Buemi M. et al. Role of Vitamin D Status in Diabetic Patients with Renal Disease. Medicina (Kaunas). 2019Jun13;55(6):273
166. Czopek A, Moorhouse R, Gallacher PJ, Pugh D, Ivy JR, Farrah TE. et al. Endothelin blockade prevents the long-term cardiovascular and renal sequelae of acute kidney injury in mice. Sci Transl Med. 2022Dec14;14(675):eabf5074
167. Silva IVG, de Figueiredo RC, Rios DRA. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int J Mol Sci. 2019Jul14;20(14):3458
168. Xu B, Xiao-hong L, Lin G, Queen L, Ferro A. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. Br J Pharmacol. 2002Jun;136(3):375-82
169. Mak IT, Boehme P, Weglicki WB. Antioxidant effects of calcium channel blockers against free radical injury in endothelial cells. Correlation of protection with preservation of glutathione levels. Circ Res. 1992Jun;70(6):1099-103
170. Quek KJ, Ameer OZ, Phillips JK. Amlodipine Improves Vessel Function and Remodeling in the Lewis Polycystic Kidney Rat Mesenteric Artery. American Journal of Hypertension. 2020Jul18;33(7):634-43
171. Dhaun N, Goddard J, Webb D. The Endothelin System and Its Antagonism in Chronic Kidney Disease. Journal of the American Society of Nephrology. 2006Apr;17(4):943
172. Heerspink HJL, Kiyosue A, Wheeler DC, Lin M, Wijkmark E, Carlson G. et al. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet. 2023Nov25;402(10416):2004-17
173. Lin R, Junttila J, Piuhola J, Lepojärvi ES, Magga J, Kiviniemi AM. et al. Endothelin-1 is associated with mortality that can be attenuated with high intensity statin therapy in patients with stable coronary artery disease. Commun Med (Lond). 2023Jun22;3(1):87
174. Sahebkar A, Kotani K, Serban C, Ursoniu S, Mikhailidis DP, Jones SR. et al. Statin therapy reduces plasma endothelin-1 concentrations: A meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015Aug;241(2):433-42
175. Yilmaz MI, Sonmez A, Saglam M, Yaman H, Cayci T, Kilic S. et al. Reduced proteinuria using ramipril in diabetic CKD stage 1 decreases circulating cell death receptor activators concurrently with ADMA. A novel pathophysiological pathway? Nephrology Dialysis Transplantation. 2010Oct1;25(10):3250-6
176. Yilmaz MI, Sonmez A, Saglam M, Kurt YG, Unal HU, Karaman M. et al. Ramipril Lowers Plasma FGF-23 in Patients with Diabetic Nephropathy. American Journal of Nephrology. 2014Oct10;40(3):208-14
177. Haruna Y, Kashihara N, Satoh M, Tomita N, Namikoshi T, Sasaki T. et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc Natl Acad Sci U S A. 2007Feb13;104(7):2331-6
178. Tanaka A, Shimabukuro M, Okada Y, Sugimoto K, Kurozumi A, Torimoto K. et al. Rationale and design of an investigator-initiated, multicenter, prospective open-label, randomized trial to evaluate the effect of ipragliflozin on endothelial dysfunction in type 2 diabetes and chronic kidney disease: the PROCEED trial. Cardiovasc Diabetol. 2020Jun13;19:85
179. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X. et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Ma Q, editor. Pharmacol Rev. 2021Jul1;73(3):924-67
180. Rahadian A, Fukuda D, Salim HM, Yagi S, Kusunose K, Yamada H. et al. Canagliflozin Prevents Diabetes-Induced Vascular Dysfunction in ApoE-Deficient Mice. J Atheroscler Thromb. 2020Nov1;27(11):1141-51
181. Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H. et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017Jul6;16(1):84
182. Awal HB, Nandula SR, Domingues CC, Dore FJ, Kundu N, Brichacek B. et al. Linagliptin, when compared to placebo, improves CD34+ve endothelial progenitor cells in type 2 diabetes subjects with chronic kidney disease taking metformin and/or insulin: a randomized controlled trial. Cardiovasc Diabetol. 2020Jun3;19(1):72
183. Rysz J, Aronow WS, Stolarek RS, Hannam S, Mikhailidis DP, Banach M. Nephroprotective and clinical potential of statins in dialyzed patients. Expert Opinion on Therapeutic Targets. 2009May1;13(5):541-50
184. Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017Jan6;120(1):229-43
185. Schaeffner ES, Kurth T, Curhan GC, Glynn RJ, Rexrode KM, Baigent C. et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol. 2003Aug;14(8):2084-91
186. Ishimitsu T, Ohno E, Ueno Y, Onoda S, Nagase A, Ohira T. et al. Effects of atorvastatin and ezetimibe on endothelial function in dyslipidemic patients with chronic kidney disease. Clin Exp Nephrol. 2014Oct1;18(5):704-10
187. Zhang Z, Wang M, Xue SJ, Liu DH, Tang YB. Simvastatin ameliorates angiotensin II-induced endothelial dysfunction through restoration of Rho-BH4-eNOS-NO pathway. Cardiovasc Drugs Ther. 2012Feb;26(1):31-40
188. Ma Z, Zhu L, Liu Y, Wang Z, Yang Y, Chen L. et al. Lovastatin Alleviates Endothelial-to-Mesenchymal Transition in Glomeruli via Suppression of Oxidative Stress and TGF-β1 Signaling. Front Pharmacol. 2017;8:473
189. Lack of modulatory effect of simvastatin on indoxyl sulfate-induced activation of cultured endothelial cells. Life Sciences. 2012 Jan 2;90(1-2):47-53.
Author contact
![]() Corresponding author: Wenbin Liu; Hongliang Rui; Baoli Liu.
Corresponding author: Wenbin Liu; Hongliang Rui; Baoli Liu.

 Global reach, higher impact
Global reach, higher impact