3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(7):1720-1735. doi:10.7150/ijms.103806 This issue Cite
Research Paper
The Role of Mesenchymal Stem Cells in Treating Diabetic Kidney Disease: Immunomodulatory Effects and Kidney Regeneration
1. Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
2. Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defence Medical Centre, Taipei, Taiwan.
3. Department of Life Sciences, National Central University, Taoyuan, Taiwan.
4. Department of Biochemical Science and Technology, National Taiwan University, Taipei, Taiwan.
5. Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
6. Division of Cardiology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
7. Taipei Medical University-Research Centre of Urology and Kidney, Taipei Medical University, Taipei, Taiwan.
8. Division of Cardiology, Department of Internal Medicine, Tri-Service General Hospital, Taipei, Taiwan.
9. Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan.
10. Nephrology Division, Department of Medicine, Mount Sinai Hospital, New York, NY, USA.
11. Biochemistry, Department of Chemistry, Hofstra University, Hempstead, New York, USA.
12. Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
13. Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
14. Division of Nephrology, Department of Internal Medicine, Hsin Kuo Min Hospital, Taipei Medical University, Taoyuan City, Taiwan.
Po-Jen Hsiao, Wen-Yi Kao, and Li-Chin Sung contributed equally.
Received 2024-9-17; Accepted 2025-2-21; Published 2025-3-3
Abstract
Background: Diabetic kidney disease (DKD), also known as diabetic nephropathy (DN), is characterized by progressive glomerulosclerosis and chronic inflammation. The potential of mesenchymal stem cells (MSCs) in treating DKD could be explored.
Methods: In this study, a streptozotocin (STZ)-induced type 1 diabetes mellitus (T1DM) DKD mouse model was utilized to investigate the renoprotective potential of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) through immunohistochemical, histopathological, and biochemical analyses. Two separate experiments were conducted to assess the therapeutic efficacy of hUC-MSCs in a DN mouse model. The first experiment determined the optimal dose by assigning the body weight and food intake alterations, serum cytokines and kidney function changes post hUC-MSCs treatment. STZ-induced DKD mice were divided to four groups: DKD control and other three hUC-MSCs treatment groups (low-dose: 3x106, intermediate (middle)-dose: 1x107, and high-dose: 3x107 cells/kg), with intravenous administration at weeks 8, 10, and 12 over 14 weeks. The second experiment evaluated treatment frequency, with mice assigned to hUC-MSCs x1, x2, and x3 groups (3x107 cells/kg) administered at weeks 5, 6, and 7 across 12 weeks, assessing the kidney histology and morphometry changes.
Results: In the first experiment, the body weight and food intake showed no significant alterations among the DN and other 3 hUC-MSCs treatment groups. Compared to the DKD control group, only high-dose hUC-MSCs (3x107 cells/kg) treatment group significantly reduced the levels of inflammatory cytokines (IL-1β, and TNF-α) (p <0.05). Additionally, the urine albumin-to-creatinine ratio (UACR) levels in the high-dose hUC-MSCs (3×10⁷ cells/kg) treatment group showed a decreasing trend compared to those in the DN control group (p = 0.06). In the second experiment, the hUC-MSCs x3 treatment group (3×10⁷ cells/kg) significantly alleviated kidney histopathology compared to the DKD group (p <0.05).
Conclusion: hUC-MSCs treatment may present a potential avenue for reversing glomerulosclerosis and mitigating inflammation in DKD mice. The long-term therapeutic benefits of MSCs-based treatments in patients with DKD and other kidney diseases could be further investigated.
Keywords: mesenchymal stem cells, diabetic nephropathy, diabetic kidney disease, immunomodulation, immunomodulatory effects, kidney regeneration
Introduction
The increasing incidence of end-stage renal disease (ESRD) is a global phenomenon. Diabetic kidney disease (DKD), also known as diabetic nephropathy (DN), is a severe complication of diabetes mellitus (DM) and the leading cause of ESRD worldwide [1]. Compared with patients with other types of diabetes, DKD patients have a higher mortality rate, and most of the deaths are due to cardiovascular events [2]. DKD usually manifests as albuminuria, a progressive decline in renal function, and glomerulosclerosis and increases the risk for cardiovascular disease [3-5]. Until now, however, specific therapies to restore kidney function or slow the progression of DKD remain unavailable. Despite the present therapeutic options for controlling blood glucose and blood pressure, including angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), direct renin inhibitors (DRIs) and sodium-glucose cotransporter-2 (SGLT2) inhibitors, kidney damage and kidney function deterioration still inevitably occur in a majority of patients with DN and chronic kidney disease (CKD), which consequently progresses to ESRD [5-9]. Globally, finding a new and safe strategy for treatment is of utmost importance for diabetic patients and for decreasing medical costs.
DKD is triggered by mesangial proliferation and the accumulation of extracellular matrix (ECM) proteins in the mesangial region, resulting in nodular or diffuse glomerulosclerosis [10]. Concerning the pathological mechanisms underlying diabetic nephropathy, transforming growth factor-β (TGF-β) has been recognized as a critical regulator cytokine that mediates this pathophysiology [11, 12]. The intracellular SMAD pathway transduces TGF-β signals and is responsible for collagen type 1 transcription and integrity [13]. Nevertheless, the involvement of other pathways supporting TGF-β/SMAD3 signalling might change the outcome of fibrosis. For example, the PI3K/Akt pathway has been implicated in a crucial pathway in TGF-β-mediated collagen type 1 accumulation [14]. Moreover, there is evidence that high glucose levels induce collagen type 1 accumulation in mesangial cells and increase PI3K/Akt activity [15, 16]. These findings suggest essential cross-talk between the various pathways resulting in diabetic nephropathy.
Mesenchymal stem cells (MSCs) are used systemically or locally to treat many diseases because they have high potential for self-regeneration and differentiation [17, 18]. Stem cells are self-regenerating pluripotent cells that can be classified as embryonic stem cells, adult stem cells, or induced pluripotent stem cells. Among these, adult stem cells can be isolated from umbilical cord blood, bone marrow, fat tissue, and deciduous teeth. MSCs have been used for the treatment of inflammatory diseases [19], tissue regeneration and repair [20], the prevention of transplant rejection [21], and other clinical applications.
The use of MSCs has garnered considerable attention due to the regenerative and immunomodulatory properties of these cells. MSCs are used systemically or locally to treat DN because they have high potential for self-regeneration and differentiation [17, 18]. Compared with MSCs from other sources, MSCs collected from the umbilical cord are considered to have greater proliferative and differentiation abilities because of their greater ability to express paracrine factors [22]. A previous study reported the successful differentiation of MSCs into insulin-producing cells for diabetes cell therapy [23]. In addition, MSCs are considered immunomodulators that modulate key inflammatory cell types and interact with cells involved in innate and adaptive immunity such as T cells, natural killer cells, B cells, and dendritic cells [24]. MSCs and cell-secreted extracellular vesicles (EVs) have also been proposed to mediate the downregulation of inflammatory markers, including interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), while increasing the levels of protective cytokines such as interleukin-10 (IL-10), prostaglandin E2, and indoleamine 2,3-dioxygenase [25]. Bone degeneration studies have shown that MSCs can decrease the secretion of macrophage inflammatory protein and monocyte chemoattractant protein [26]. In rodent models of acute lung injury (ALI), Gupta and coworkers demonstrated that MSCs increase the expression levels of the anti-inflammatory cytokine interleukin-10 [27].
Human umbilical cord-derived MSCs (hUC-MSCs) have a more regular doubling time, rarely produce teratomas, and have a greater differentiation capability than bone marrow-derived MSCs. The expression level of major histocompatibility complex classes I and II is reduced in hUC-MSCs, leading to decreased immunogenicity [28]. The antifibrotic and immunomodulatory properties of hUC-MSCs have been observed through their effects on prostaglandin E2 [29]. hUC-MSCs decreased reactive oxygen species levels and inflammatory processes in ALI in vivo [30, 31]. Additionally, hUC-MSCs alleviated inflammation and augmented the percentage of regulatory T cells in ovalbumin-induced murine models [32]. To date, some data indicate that MSCs might alleviate the symptoms of comorbidities of diabetes mellitus (DM) [33-35]. However, the potential therapeutic effects of hUC-MSCs on streptozotocin (STZ)-induced type 1 diabetes mellitus (T1DM) DKD remain to be elucidated. The aim of this research was to investigate the effects of hUC-MSCs on inflammation and glomerulosclerosis in DKD mice, with a focused review on animal models and the clinical implications of MSCs-based/EVs therapy.
Materials and methods
STZ-induced DKD animal model
Specific pathogen-free (SPF) male C57BL/6 mice (8 weeks old; National Laboratory Animal Centre, Taiwan) were used in this study. The animals were housed at 22 ± 2°C under a 12-h light/12-h dark cycle with free access to food and water. This study was approved by the Institutional Animal Care and Use Committee of Fu Jen Laboratory Animal Centre (Taiwan) (IACUC permission no. P11004) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol for STZ-induced T1DM DKD is briefly described as follows. C57BL/6 mice were intraperitoneally injected with 55 mg/kg/day STZ (Sigma‒Aldrich, St. Louis, MO) for 5 consecutive days [36, 37]. After 2-4 weeks, mice with a fasting blood glucose concentration >200 mg/dL were considered diabetic and were used in the DKD experiments. Blood glucose levels were measured using an Accu-Check Performa glucometer (Roche, Basel, Switzerland).
hUC-MSCs preparation
hUC-MSCs used in this study were obtained from Meridigen Biotech Co., Ltd. (Taipei, Taiwan), and their preparation followed the International Society for Cellular Therapy Guidelines. Briefly, umbilical cord tissue was digested with collagenase and incubated in α-minimal essential culture medium. hUC-MSCs were passaged upon reaching 80-90% confluence up to the sixth generation. For long-term storage, the cells were suspended in CryoStor CS10 and cryopreserved in a vapor-phase liquid nitrogen tank [38]. Prior to administration, hUC-MSCs were diluted in clinical-grade normal saline (NS) and 2% clinical-grade human serum albumin (HSA) and intravenously administered via the tail vein.
hUC-MSCs treatment of STZ-induced DKD animal model
To evaluate the in vivo efficacy of hUC-MSCs, 2 different experiments were performed. The first experiment determined the optimal dose by evaluating changes in body weight, food intake, serum cytokines, and kidney function following hUC-MSCs treatment. The animals were grouped as follows: (i) normal group (without STZ; iv NS with 2% HSA); (ii) DKD control group (NS with 2% HSA) and (iii) low-dose hUC-MSCs (3x106 cells/kg) treatment group, (iv) intermediate (middle)-dose hUC-MSCs (1x107 cells/kg) treatment group, and (v) high-dose hUC-MSCs (3x107 cells/kg) treatment group. Two hundred microlitre hUC-MSCs suspensions (from batches RDWP19001U163006060, RDWP19001U163006061, and RDWP19001U163006062) were administered. All animals were injected intravenously through the tail vein 3 times at 2-week intervals.
The second experiment evaluated treatment frequency by assigning mice to hUC-MSCs X1, X2, and X3 groups (3×10⁷ cells/kg), with treatments administered at weeks 5, 6, and 7 over a 12-week period, and assessing changes in kidney histology and morphology. The animals were divided into (i) normal (without STZ; NS with 2% HSA) and diabetic groups, where the diabetic group was further randomly divided into four groups: (ii) DKD group (NS with 2% HSA), (iii) MSC X1 group, (iv) MSC X2 group, and (v) MSC X3 group, which received 3x107 cells/kg of hUC-MSCs (batch RDWP19001U163006058, RDWP 19001U163006057, and RDWP19001U163006056) via 200-µl iv tail vein injection 1, 2, and 3 times, respectively, with a 1-week interval between administrations. Twenty-four hours before sacrifice, the mice were transferred to a metabolic cage for the collection of urine and the measurement of 24-h urine volume. Access to food and water was not limited.
Serum cytokine/chemokine biomarkers
Serum was collected by centrifugation at 1500 × g for 30 min at 4°C and stored at -20°C until analysis. To determine the presence of systemic inflammation, the serum concentrations of IL-6, IL-10, IL-1β, and TNF-α were measured using a Mouse Cytokine/Chemokine Magnetic Bead Panel (MHSTCMAG-70K-04; Milliplex, St. Louis, MO) on the Luminex-MAGPIX multiplex immunoassay system according to the manufacturer's instructions. The data were analysed using Milliplex Analyst 5.1 software (EMD Millipore, Billerica, MA, United States).
Biochemical and urine parameter measurement
Whole-blood samples from treated mice were collected by intracardiac puncture and centrifuged at 2000×g for 20 min to separate the serum. The biochemical parameters analysed included serum (blood urea nitrogen [BUN] and creatinine) and urine (albumin and creatinine) levels, which were measured with a Beckman Coulter AU480. The 24-h creatinine clearance rate (CCR) was calculated based on the 24-h urine volume, serum creatinine concentration and urine creatinine concentration. The urine albumin-to-creatinine ratio (UACR) was the ratio of albumin to creatinine.
Kidney histopathological staining, Masson's trichrome staining and analysis
All mice were anaesthetized, and their kidneys were removed and preserved in 10% formalin. The kidneys were trimmed, embedded in paraffin, sectioned in 4- to 5-μm sections, and stained with haematoxylin-eosin (HE) and Masson's trichrome (MT). Subsequently, the sections were examined microscopically using an optical microscope (Leica DM2700M, USA). Elbe H, et al. demonstrated the amelioration of streptozotocin-induced DN by melatonin, quercetin, and resveratrol in rats. In our study, the semiquantitative scoring of renal histopathological lesions was conducted following the previous report [39]. Kidney injury was graded as follows: 0, normal; 1, mild injury; 2, moderate injury; and 3, severe injury. Tubular changes, including hydropic degeneration (swelling/vacuolization), desquamation, brush border loss, and peritubular infiltration, were graded as follows, with a maximum score of 12: 0, normal; 1, mild changes; 2, moderate changes; and 3, severe changes. Glomerulosclerotic injury in MT-stained sections was graded as follows: 0, normal; 1, injury in ≤25% of the glomerular area (mild sclerosis); 2, injury in 25-50% of the glomerular area (moderate sclerosis); and 3, injury in ≥75% of the glomerular area (severe sclerosis) [40].
Statistical analysis
Differences in the frequency distribution (percentage) of categorical variables between groups were evaluated using the chi-square test, and differences in continuous variables (presented as the mean ± standard deviation [SD]) were evaluated using two-sample t tests or one-way analysis of variance with Dunnett's test. All the statistical analyses were performed using the Statistical Analysis Software SPSS 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Changes in body weight and food intake
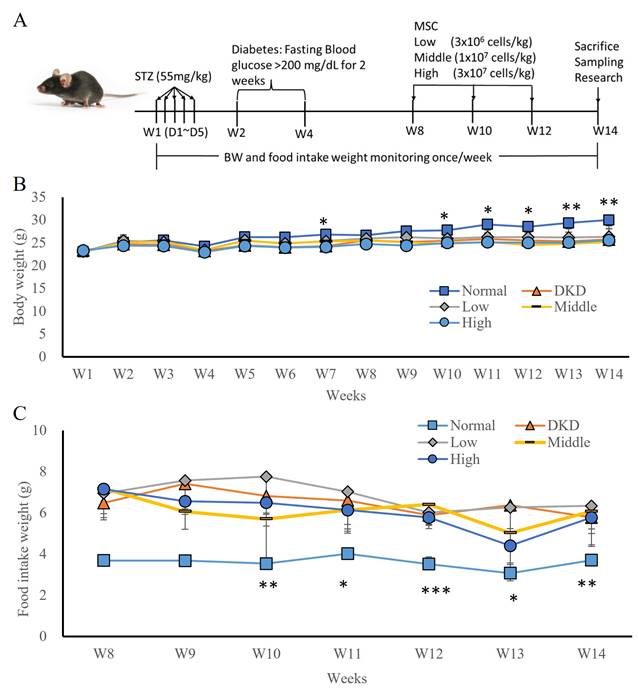
In the first experiment, a STZ-induced T1DM hyperglycaemia animal model was established (Fig. 1A) by randomly grouping the animals into 4 groups: DKD, low-dose hUC-MSCs (3x106 cells/kg), intermediate-dose hUC-MSCs (1x107 cells/kg), and high-dose hUC-MSCs (3×107 cells/kg) treatment groups. hUC-MSCs were intravenously administered at weeks 8, 10, and 12 (Fig. 1B). The body weights of the DKD and hUC-MSCs treatment groups were not significantly different, but the body weights of the normal group were significantly different from those of the DKD group at weeks 7, 10, 11, 12, 13, and 14 (Fig. 1B, p <0.05). The weight of food intake during the hUC-MSCs treatment period did not significantly differ between the DKD and hUC-MSCs (low-dose, intermediate-dose, and high-dose) groups, but the food intake of the normal group was significantly different from that of the DKD group (Fig. 1C, p <0.05). Moreover, fasting blood glucose levels did not differ between the DKD and hUC-MSCs groups before or after hUC-MSCs treatment.
Immunomodulatory effects
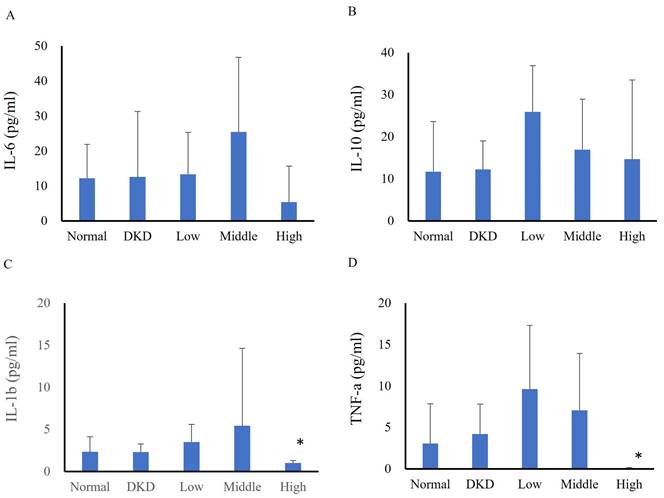
In the first experiment, serum cytokine analysis revealed no differences among groups in terms of IL-6 (normal: 12.15±9.73 pg/ml, DKD: 12.56±18.75 pg/ml, low-dose: 13.32±12.01 pg/ml, intermediate-dose: 25.43±21.27 pg/ml, high-dose: 5.34±10.34 pg/ml) (Fig. 2A) or IL-10 levels (normal: 11.70±6.77 pg/ml, DKD: 12.21±6.77 pg/ml, low-dose: 25.90±10.94 pg/ml, intermediate-dose: 16.94±12.01 pg/ml, high-dose: 14.68±18.80 pg/ml) (Fig. 2B). The IL-1β (normal: 2.33±1.81 pg/ml, DKD: 2.30±0.99 pg/ml, low-dose: 3.50±2.07 pg/ml, intermediate-dose: 5.42±9.21 pg/ml, high-dose: 1.00±0.31 pg/ml) (Fig. 2C) and TNF-α (normal: 3.06±4.78 pg/ml, DKD: 4.19±3.64 pg/ml, low-dose: 9.63±7.69 pg/ml, intermediate-dose: 7.08±6.84 pg/ml, high-dose: 0.05±0.09 pg/ml) (Fig. 2D) levels significantly differed between the high-dose group and the DKD group. Changes in serum cytokines of DKD mice following hUM-MSCs treatments were summarized in Table 1.
hUC-MSCs treatment STZ-induced DKD animal models and changes in body weight (BW) and food intake following hUM-MSCs treatment. (A): Experimental timeline. (B): body weight. (C): food intake weight. Normal: normal mice; DKD: diabetic mice; Low: diabetic mice treated with MSCs (3 x 106 cells/kg); Middle: diabetic mice treated with MSCs (1 x 107 cells/kg); High: diabetic mice treated with MSCs (3 x 107 cells/kg). Values are expressed as mean ± SD (n = 5~8). * p<0.05; ** p < 0.01; *** p < 0.001 versus DKD group.
Immunomodulatory effects in serum cytokines following hUC-MSCs treatments.
| Cytokines | Group | ||||
|---|---|---|---|---|---|
| (pg/ml) | Normal | DKD | Low | Intermediate | High |
| IL-6 | 12.15 ± 9.73 | 12.56 ± 18.75 | 13.32 ± 12.01 | 25.43 ± 21.27 | 5.34 ± 10.34 |
| IL-10 | 11.70 ± 6.77 | 12.21 ± 6.77 | 25.90 ± 10.94 | 16.94 ± 12.01 | 14.68 ± 18.80 |
| IL-1β | 2.33 ± 1.81 | 2.30 ± 0.99 | 3.50 ± 2.07 | 5.42 ± 9.21 | 1.00 ± 0.31* |
| TNF-α | 3.06 ± 4.78 | 4.19 ± 3.64 | 9.63 ± 7.69 | 7.08 ± 6.84 | 0.05 ± 0.09* |
Abbreviations: Normal: normal mice; DKD: diabetic mice; Low: diabetic mice treated with hUC-MSCs (3 x 106 cells/kg); Intermediate: diabetic mice treated with hUC-MSCs (1 x 107 cells/kg); High: diabetic mice treated with hUC-MSCs (3 x 107 cells/kg); IL-6: interleukin-6; IL-10: interleukin-10; IL-1β: interleukin-1β; TNF-α: tumor necrosis factor-alpha. Values are expressed as mean ± SD (n = 5~8). * p<0.05 versus DKD group.
Kidney function changes
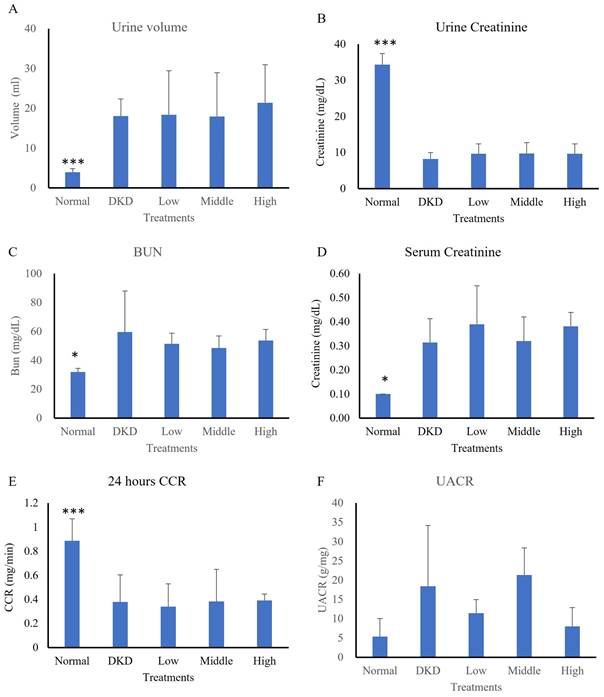
In the first experiment model, an analysis of 24-h urine collection, the urine volume of the normal group (3.93±0.89 ml) significantly differed from that of the DKD group (18.04±4.28 ml) (p<0.001), while that of the hUC-MSCs treatment groups was not significantly different (low-dose: 18.37±11.05 ml, intermediate-dose: 17.92±11.00 ml, high-dose: 21.38 ±9.55 ml) (Fig. 3A). The average urine creatinine level of the normal group (34.30±3.07 mg/dL) was also significantly different from that of the DKD group (8.21±1.78 mg/dL) (p<0.001), while those of the hUC-MSCs treatment groups were not significantly different (low-dose: 9.64±2.72 mg/dL, intermediate-dose: 9.68±3.02 mg/dL, high-dose: 9.64±2.72 mg/dL) (Fig. 3B). The average level of albumin in urine did not differ among the groups (normal: 0.18±0.15 mg/dL, DKD: 0.14±0.11 mg/dL, low-dose: 0.11±0.03 mg/dL, intermediate-dose: 0.21±0.11 mg/dL, high-dose: 0.09±0.03 mg/dL) (Fig. 3C). The average serum creatinine level in the normal group (0.10±0.00 mg/dL) significantly differed from that in the DKD group (0.31±0.10 mg/dL) (p<0.05), while the serum creatinine levels in hUC-MSCs treatment groups did not significantly differ (low-dose: 0.39±0.16 mg/dL, intermediate-dose: 0.32±0.10 mg/dL, high-dose: 0.38±0.06 mg/dL) (Fig. 3D). The average 24-h CCR of the normal group (0.89±0.18 ml/min) significantly differed from that of the DKD group (0.38±0.23 ml/min) (p<0.001), while the CCRs of the hUC-MSCs groups did not significantly differ (low-dose: 0.34±0.19 ml/min, intermediate-dose: 0.28±0.27 ml/min, high-dose: 0.39±0.05 ml/min) (Fig. 3E). The UACR levels showed a difference among the 5 groups (normal: 5.33±4.68 mg/g, DKD: 18.45±4.68 mg/g, low-dose: 11.45±3.54 mg/g, intermediate-dose: 21.31±7.06 mg/g, high-dose: 8.01±4.89 mg/g) (Fig. 3F). Results showed a decreasing trend in UACR levels in the high-dose hUC-MSC treatment group compared to the DKD control group (p = 0.063). Changes in kidney function of DKD mice following hUC-MSCs treatments were summarized in Table 2.
Kidney function changes following hUC-MSCs treatment.
| Biochemistry | Group | ||||
|---|---|---|---|---|---|
| Normal | DKD | Low | Intermediate | High | |
| Urine volume (ml) | 3.93 ± 0.89* | 18.04 ± 4.28 | 18.37 ± 11.05 | 17.92 ± 11.00 | 21.38 ± 9.55 |
| Urine creatinine (mg/dL) | 34.30 ± 3.07* | 8.21 ± 1.78 | 9.64 ± 2.72 | 9.68 ± 3.02 | 9.64 ± 2.72 |
| Albuminuria (mg/dL) | 0.18 ± 0.15 | 0.14 ± 0.11 | 0.11 ± 0.03 | 0.21 ± 0.11 | 0.09 ± 0.03 |
| Serum creatinine (mg/dL) | 0.10 ± 0.00* | 0.31 ± 0.10 | 0.39 ± 0.16 | 0.32 ± 0.10 | 0.38 ± 0.06 |
Abbreviations: Normal: normal mice; DKD: diabetic mice; Low: diabetic mice treated with hUC-MSCs (3 x 106 cells/kg); Intermediate: diabetic mice treated with hUC-MSCs (1 x 107 cells/kg); High: diabetic mice treated with hUC-MSCs (3 x 107 cells/kg). Values are expressed as mean ± SD (n = 5~8). * p<0.05 versus DKD group.
Changes in mouse serum cytokines following hUC-MSCs treatment (N=5-8). (A): IL-6, (B): IL-10, (C): IL-1β, (D): TNF-α. Normal: normal mice; DKD: diabetic mice; Low: diabetic mice treated with hUC-MSCs (3 x 106 cells/kg); Middle: diabetic mice treated with hUC-MSCs (1 x 107 cells/kg); High: diabetic mice treated with hUC-MSCs (3 x 107 cells/kg). Values are expressed as mean ± SD (n = 5~8). * p<0.05 versus DKD group.
Kidney histopathological evaluations
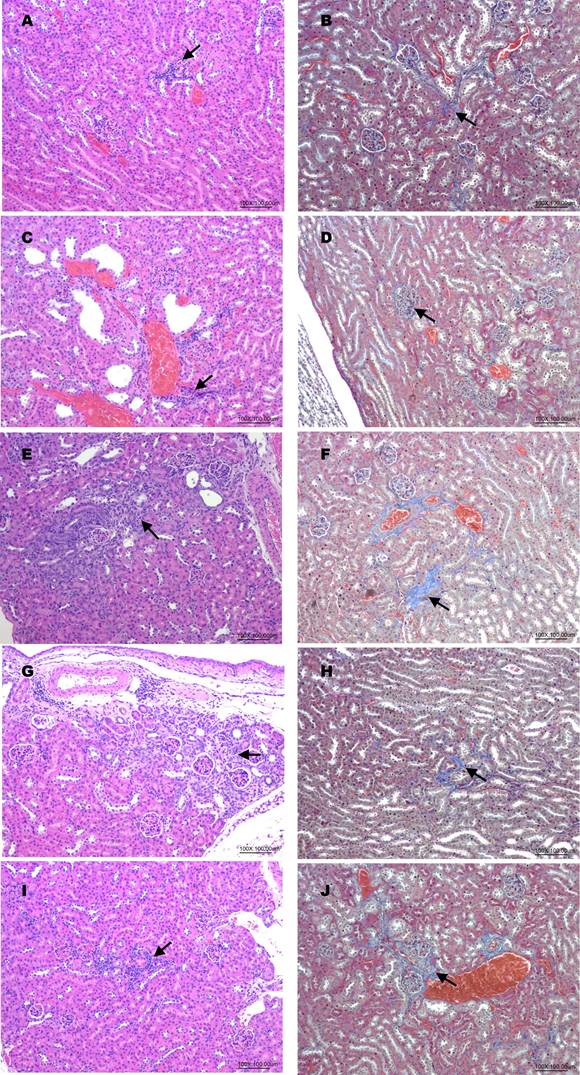
To determine the potential renal protective effects of hUC-MSCs in treating DKD mice, the second experimental animal model evaluated treatment frequency. Mice were assigned to hUC-MSCs X1, X2, and X3 groups (3×10⁷ cells/kg), with administrations at weeks 5, 6, and 7 over a 12-week period (Fig. 4A). HE staining analysis of kidney tissues indicated inflammation, dilation, degeneration, congestion, basophilia, hyaline casts, and fibrosis. The semiquantitative scores of renal lesions (Table 3) in the normal and DKD groups were significantly different in terms of dilation, degeneration, congestion, and fibrosis. After hUC-MSCs treatment, the congestion scores of the X2 and X3 groups were significantly different from that of the DKD group, and the fibrosis score of the X3 group was also significantly different from that of the DKD group. One-way ANOVA showed that the DKD group was significantly different from the normal group (Fig. 4B). MT staining analysis revealed a significant difference in glomerulosclerosis injury scores between the normal and DKD groups, with all 3 hUC-MSCs treatment groups being significantly different from the DKD group (Table 4, and Fig. 4C). Representative kidneys sections in mice were shown in Fig. 5.
Histopathological damage as indicated by semiquantitative scores of renal lesions.
| Measurementa | Group | ||||
|---|---|---|---|---|---|
| Normal | DKD | MSCs X1 | MSCs X2 | MSCs X3 | |
| Inflammation | 0.63 ± 0.52 | 1.00 ± 0.00 | 0.89 ± 0.60 | 0.56 ± 0.53 | 0.43 ± 0.53 |
| Dilation | 0.25 ± 0.46 | 1.75 ± 0.71# | 1.33 ± 1.22 | 1.78 ± 0.67 | 2.00 ± 1.00 |
| Degeneration | 0.50 ± 0.76 | 1.63 ± 0.52# | 1.67 ± 0.71 | 1.78 ± 0.67 | 1.43 ± 0.98 |
| Congestion | 0.13 ± 0.35 | 1.25 ± 0.46# | 0.67 ± 0.50 | 0.44 ± 0.53* | 0.57 ± 0.53* |
| Basophilia | 0.63 ± 0.52 | 1.00 ± 0.00 | 1.33 ± 0.50 | 0.89 ± 0.60 | 0.86 ± 0.38 |
| Hyaline casts | 0.13 ± 0.35 | 0.25 ± 0.71 | 0.11 ± 0.33 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Fibrosis | 0.38 ± 0.52 | 1.38 ± 0.52# | 1.33 ± 0.50 | 1.11 ± 0.60 | 0.43 ± 0.53* |
| Total scores | 2.63 ± 1.60 | 8.25 ± 1.98# | 7.33 ± 3.08 | 6.56 ± 2.46 | 5.71 ± 1.98 |
Abbreviations: Normal: normal mice; DKD: diabetic mice; MSCs: diabetic mice treated with hUC-MSCs (3 x 107 cells/kg).
ANOVA: analysis of variance; SD: standard deviation.
a Values represent the mean ± SD.
# p < 0.05, DKD (Group 2) vs. Control (Group 1); unpaired Student's t test.
* p < 0.05, treated vs. DKD (Group 2); one-way ANOVA followed by Dunnett's test.
Renal function of streptozotocin-induced DKD animal treated with hUC-MSCs (N=5-8). (A): 24 hours urine volume. (B): Urine creatinine. (C): Blood urea nitrogen (BUN). (D): serum creatinine. (E): 24 hours creatinine clearance rate (CCR). (F): Urine Albumin to Creatinine Ratio (UACR). Normal: normal mice; DKD: diabetic mice; Low: diabetic mice treated with hUC-MSCs (3 x 106 cells/kg); Middle: diabetic mice treated with hUC-MSCs (1 x 107 cells/kg); High: diabetic mice treated with hUM-MSCs (3 x 107 cells/kg). Values are expressed as mean ± SD. * p<0.05; *** p < 0.001 versus DKD group.
Histopathological changes following hUC-MSCs treatment. (A): Experimental timeline. (B): Histopathological damage scores DKD group showed significant different with normal group (#: unpaired student's test, p < 0.05). (C): Glomerulosclerosis score DKD group showed significant different with normal group (#: unpaired student's test, p < 0.05), in treatment groups, hUC-MSCs (3 x 107 cells/kg) x 3 group showed significant different with DKD group (*: one-way ANOVA, P<0.05). Control: normal mice; DKD: diabetic mice; Values are expressed as mean ± SD (n = 5~8).
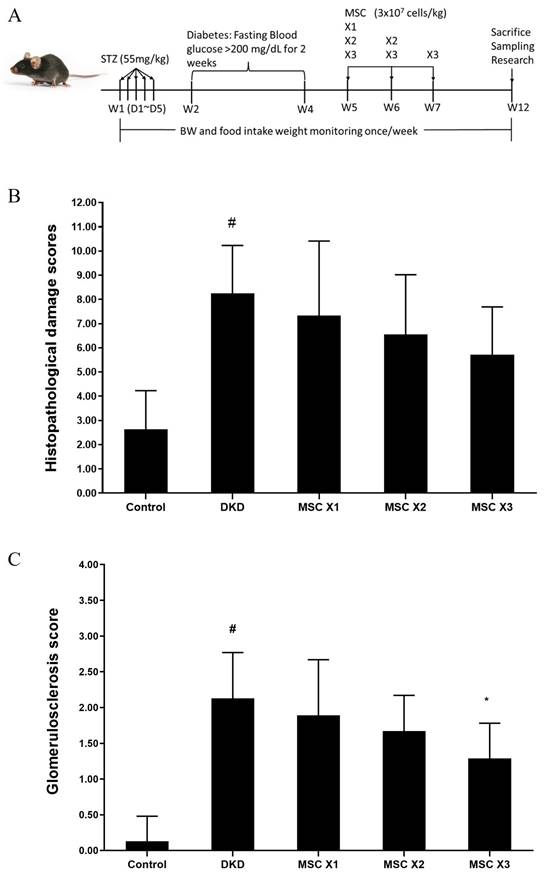
Semiquantitative scores of glomerulosclerotic injury.
| Measurementa | Group | ||||
|---|---|---|---|---|---|
| Normal | DKD | MSCs X1 | MSCs X2 | MSCs X3 | |
| Glomerulosclerosis score | 0.13 ± 0.35 | 2.13 ± 0.64# | 1.89 ± 0.78 | 1.67 ± 0.50 | 1.29 ± 0.49* |
Abbreviations: Normal: normal mice; DKD: diabetic mice; MSCs: diabetic mice treated with hUC-MSCs (3 x 107 cells/kg).
ANOVA: analysis of variance; SD: standard deviation.
a Values represent the mean ± SD.
# p < 0.05, DKD group (Group 2) vs. control group (Group 1); unpaired Student's t test.
* p < 0.05, treated vs. DKD (Group 2); one-way ANOVA followed by Dunnett's test.
Histopathological changes in haematoxylin-eosin (HE) and Masson's trichrome (MT)-stained kidneys sections in mice. (A, B): Normal (HE, MT); (C, D): DKD (HE, MT); (E, F): hUM-MSCs (3x107 cells/kg) X1 (HE, MT); (G, H): hUM-MSCs (3x107 cells/kg) X2 (HE, MT); (I, J): hUM-MSCs (3x107 cells/kg) X3 (HE, MT). Inflammatory cell infiltrations (arrow) were observed in HE stained sessions. Fibrosis (arrow) was observed in MT-stained sessions.
Discussion
DKD is a major complication of DM and remains a significant challenge in clinical management. Various therapeutic strategies have been explored to slow the progression of DKD, including lifestyle modifications, pharmacological agents, and stem cell-based therapies. Among these approaches, the use of MSCs has garnered considerable attention due to their regenerative and immunomodulatory properties. Previous clinical studies on T1DM and T2DM reported that MSCs derived from human placenta (PL-MSCs), adipose tissue (AD-MSCs), and bone marrow (BM-MSCs) were able to reduce glycated haemoglobin (HbA1c) levels after 3-12 months of treatment [41-45]. Our study utilized an STZ-induced DKD mouse model representing T1DM to assess the renoprotective effects of hUC-MSCs through immunohistochemical, histopathological, and biochemical analyses. The results revealed hUC-MSCs treatment in DKD model animals did not significantly affect body weight, food intake, or blood glucose levels in either the DKD or hUC-MSCs treatment groups. However, the normal group showed significant differences in body weight and food intake compared to the DKD and hUC-MSCs treatment groups throughout the experiment, indicating that hUC-MSCs treatment did not have a significant effect on body weight or hyperglycaemia. Lee RH, et al. reported that after hUC-MSCs were infused intravenously into mice, 2x106 cells were cleared in 5 min (99±1.07%). Only a few hUC-MSCs differentiated into insulin-producing cells, but these differentiated cells were unable to affect T1DM symptoms [46].
Serum cytokine levels were analysed, and high-dose hUC-MSCs (3x107 cells/kg) treatment significantly decreased IL-1β and TNF-α levels compared to those in the DKD group. Additionally, low-dose hUC-MSCs (3x106 cells/kg) treatment resulted in significant differences in IL-10 levels compared to those in both the DKD and normal groups. These findings suggest that hUC-MSCs treatment may have some impact on inflammatory markers, particularly at higher doses. It has also been reported that UC-MSCs upregulate IL-10 and VEGF expression levels in macrophages through the secretion of PGE2; reduce the levels of proinflammatory factors such as IL-1b, TNF-α, and IL-6 and ameliorate renal interstitial fibrosis in the kidney [43, 47, 48].
In our study, to evaluate the ability of hUC-MSCs to reverse glomerulosclerosis and reduce the effects of proinflammatory factors, 3x107 hUC-MSCs/kg was administered 1, 2, and 3 times, and renal pathology was analysed. We performed HE and MT staining of kidney tissues to assess renal lesions and glomerulosclerosis injury, respectively. The results indicated that the DKD group exhibited inflammation, dilation, degeneration, congestion, basophilia, hyaline casts, and fibrosis. The semiquantitative scores of renal lesions were significantly different between the normal and DKD groups in terms of dilation, degeneration, congestion, and fibrosis. The congestion scores of the X2 and X3 groups were significantly different from those of the DKD group, and the fibrosis scores of the X3 group were also significantly different from those of the DKD group. Li et al. [48] reported that a single UC-MSCs treatment ameliorated glomerular abnormalities and interstitial fibrosis in an STZ-induced diabetic mouse model without affecting hyperglycaemia. Notably, He J and Zheng S, et al. explored the potential of UC-MSCs in the treatment of diabetic nephropathy [49, 50]. Zheng et al. also presented that UC-MSCs-derived miR-342-3p inhibited renal tubular epithelial cell pyroptosis by targeting the NLRP3/caspase1 pathway to effectively ameliorate kidney damage and reduce inflammation in a diabetic rat model [50]. These results are consistent with the current findings of reduced renal pathology scores following hUC-MSCs treatment, further supporting the potential therapeutic benefit of UC-MSCs in DKD.
A potential therapeutic role of different types of MSCs and EVs in treating CKD
The effectiveness of cell therapy for CKD depends on the quantity of MSCs reaching the targeted area. In addition, prolonged observation in hUC-MSCs transplantation for systemic lupus erythematosus (SLE) demonstrated the safety of administering a single dose or even a subsequent dose of hUC-MSCs [51-53]. Various types of MSCs and EVs are being explored for the treatment of CKD. Table 3 summarizes the utilization of MSCs and EVs from diverse sources in animal models of CKD [54-74]. Although BM-MSCs are prevalent in CKD treatment, their purification poses challenges in terms of speed and efficiency. Consequently, alternative options, such as UC-MSCs, PL-MSCs, and AD-MSCs, have emerged as promising alternatives. Compared with BM-MSCs, AD-MSCs offer various advantages, including simplified purification, accelerated proliferation, increased cell viability, easy availability, and stronger immunomodulatory effects. UC-MSCs multiply rapidly and can be mass produced without their efficacy being compromised. PL-MSCs possess a wide range of sources and minimal immunogenicity and are free from ethical concerns. Additionally, compared with BM-MSCs, PL-MSCs may exhibit superior proliferative potential. EVs could be alternatives to MSCs for treating CKD. In treating CKD, MSC-EVs would have the advantages of lower immunogenicity, greater tumorigenicity, and easier management than MSCs. EVs carry complex cargoes of biological molecules, including cytokines, chemokines, growth factors, and nucleic acids. The content of EV cargo is related to the state of the cell of origin and can reflect the phenotype of the releasing cell. Many preclinical animal studies (Table 5) and clinical trials have revealed that MSCs-EVs are effective in treating CKD (Table 6).
Samples of preclinical studies assessing the therapeutic efficacy of MSCs in models of CKD with different aetiologies.
| Aetiology | MSC number and source | Route | Main outcomes | Ref |
|---|---|---|---|---|
| Diabetes mellitus | ||||
| STZ-induced diabetic mice | 0.5 × 106 mice BM-MSCs | Tail vein injection | ↓ Blood glucose levels↑ β-pancreatic islet regeneration, prevented renal damage | [54] |
| STZ-induced diabetic mice | 2.5 × 106 human BM-MSCs | Left cardiac ventricle | ↑ Insulin secretion and perhaps ameliorating the renal lesions | [55] |
| STZ-induced diabetic mice | EVs from bone marrow or liver | IV | ↓ Collagen I, MMP3, TIMP1, FasL, serpina1a, SNAI1, CCL3, BUN, creatinine, fibrosis, EMT, recruitment of macrophages, T cells | [56] |
| STZ-induced diabetic rats | 2 × 106 UC-MSCs | Tail vein injection | ↓ Proteinuria, Scr, BUN, IL-6, IL-1β, TNF-α and TGF-β↑ FGFs, HGF, and VEGF | [57] |
| STZ-induced diabetic rats | 3 × 106 rat AD-MSCs | IV | Attenuated CKD↓ IL-6, IL-1β, TNF-α, iNOS(+) M1 macrophages↑ IL-10, CD163(+) M2 macrophages | [40] |
| Obstructive nephropathy | ||||
| UUO mice | 5 × 105 human BM-MSCs | Tail vein injection | ↓ CD68-positive macrophage, PTC loss, renal tubulointerstitial injury and fibrosis↑ Ki67, α-SMA | [58] |
| UUO mice | BM-MSC-EVs | IV | ↓ Fibrosis, collagen, MMP-9, α-SMA, TGF-βR1 | [59] |
| UUO mice | 1 × 106 human BM-MSCs | IV | ↓ InflammationNo antifibrotic effect | [60] |
| UUO rats | UC-MSC-CM | Left renal artery | ↓ MDA, ROS, expression of TGF-β1, α-SMA, TNF-α and collagen-I, RTE apoptosis | [61] |
| UUO rat | UC-MSC-EVs | left renal artery | ↓ Apoptosis of NRK-52 E cells, Scr, BUN, oxidative stress, renal tubular injury and tubulointerstitial fibrosis | [62] |
| Hypertension | ||||
| 2K1C-induced renovascular hypertension | 2 × 105 rat BM-MSCs | IV | Improved renal morphology and microvascular rarefaction; reduced fibrosis, proteinuria and inflammatory cytokines; suppressed intrarenal RAS | [63] |
| 2K1C induced renovascular hypertension | 1 × 106 rat BM-MSCs | IV | ↓ Inflammation and oxidative stress↓ Morphological and ultrastructural abnormalities↓ Serum urea and creatinine | [64] |
| 5/6 NPX rats | 0.5 × 106 AD-MSCs | IV | ↓Plasma creatinine, damage markers ED-1 and α-SMA↑Pax-2, BMP-7, and VEGF, Oct-4 | [65] |
| 5/6 NPX rats | 2 × 105 rat BM-MSCs | Subcapsular injection | ↓ SBP↑ Renal function (↓ Albuminuria, Scr) | [66] |
| 5/6 NPX rats | 2 × 105 rat BM-MSCs | IV | ↓ Fibrosis indices (collagen I, vimentin, TGF-β, α-SMA), Inflammation | [67] |
| 1K/DOCA/salt | 1 × 106 human BM-MSCs | IV | ↓ SBP, inflammation, fibrosis↑ Renal function and morphology | [68] |
| High-salt diet (8% NaCl) | 5 × 106 rat BM-MSCs | Intrarenal infusion | ↓ SBP, inflammasome activation, hypertensive kidney damage | [69] |
| MRL/lpr mice (Lupus nephritis) | 1 × 106 PD-MSCs | IV | ↓NF-κB mRNA levels, phosphor-NF-κB p65, TNF-α, PAI-1, and ICAM-1 expression | [70] |
| Cisplatin-induced CKD | 3 × 106 rat BM-MSCs | IV | ↓ Creatinine and urea↓ Inflammation and fibrosis↑ Hepatocyte growth factor | [71] |
| Nephrotoxicity | ||||
| Mouse model of AA induced nephropathy | BM-MSCs-EVs | IV | ↓α-SMA, Col1a1, profibrotic gene expression, blood creatinine and BUN, tubular necrosis, interstitial fibrosis, infiltration of CD45 positive immune cells, fibroblasts, and pericytes | [72] |
| CsA nephrotoxicity mouse model | BM-MSCs and EVs | Intraperitoneal injection | Improvement in renal outcomesEVs induced a partial recovery | [73] |
| CKD | ||||
| CKD cats | 2 × 106 cat AD-MSCs/kg | IV | No adverse effectsNo significant treatment effect on renal function in the 6 weeks after MSC treatment | [74] |
Abbreviations: 2K-1C: 2 kidney, 1 clip model; 5/6 NPX: nephrectomy; AAN: aristolochic acid nephropathy; AD-MSCs: adipose-derived mesenchymal stem cells; BM-MSCs: bone marrow mesenchymal stem cells; BMP-7: bone morphogenetic protein 7; BUN: blood urea nitrogen; CCR: creatinine clearance rate; CKD: chronic kidney disease; CsA: cyclosporine; FasL: Fas ligand; FGFs: fibroblast growth factor; HGF: hepatocyte growth factor; HIF-1α: hypoxia induction factor-1α; IL-10: interleukin 10; IL-1β: interleukin-1β; IL-6: interleukin 6; IV: intravenous; iNOS: inducible nitric oxide synthase; MDA: malondialdehyde; MSCs: mesenchymal stem cells; MVs: microvesicles; PAI-1: plasminogen activator-1; PL-MSCs: placenta-derived mesenchymal stem cells; PTC: peritubular capillary; RAS: renal artery stenosis; ROS: reactive oxygen species; SCr: serum creatinine; SLE: systemic lupus erythematosus; STZ: streptozotocin; TGF-β1: transforming growth factor-β1; TNF-α: tumour necrosis factor alpha; UC-MSC-Exo: exosomes from umbilical cord mesenchymal stem cells; UC-MSCs: umbilical cord blood mesenchymal stem cells; UUO: unilateral ureteral obstruction; VEGF: vascular endothelial-derived growth factor; α-SMA: α-smooth muscle actin.
Summary of clinical trials on MSCs in the treatment of CKD.
| ClinicalTrials.gov identifier | Study type | Conditions | Interventions | Treatment effect |
|---|---|---|---|---|
| NCT01843387 [75] | A multicentre, randomized, double-blind, dose-escalating, sequential, placebo-controlled trial | Type 2 DM/advanced DN | BM-MSCs | No acute adverse events |
| NCT01539902 [76] | A randomized double-blind, placebo-controlled trial | WHO class III or IV LN | UC-MSCs | MSC infusion had no apparent additional effect over and beyond that of standard immunosuppression |
| NCT01741857 [53] | A long-term follow-up predesigned open-label phase II clinical trial | Severe and drug-refractory SLE | BM-MSCs UC-MSCs | Allogeneic MSC transplantation was safe and resulted in long-term clinical remission in SLE patients |
| NCT00698191 [77] | A pilot clinical study | Refractory SLE | BM-MSCs | MSC transplantation appeared beneficial in the treatment of patients with SLE refractory to conventional treatment options |
| NCT02166489 [78] | A single-arm phase I clinical trial | CKD due to polycystic kidney disease | BM-MSCs | An intravenous infusion of autologous MSCs was safe and well tolerated in ADPKD patients |
| NCT01741857 [79] | A pilot clinical study | Refractory SLE | UC-MSCs | UC-MSCs might suppress inflammation in lupus by upregulating tolerogenic DCs |
| NCT03174587 [80] | A phase I clinical trial | SLE with active renal disease | BM-MSCs | BM-MSCs were safe and tolerable |
| NCT02266394 [81] | A phase 1a escalating dose clinical trial | Atherosclerotic renovascular disease | AD-MSCs | An increase in cortical and whole kidney blood flows in the poststenotic kidney |
Abbreviations: ADPKD: autosomal dominant polycystic kidney disease; AD-MSCs: adipose tissue mesenchymal stem cells; BM-MSCs: bone marrow mesenchymal stem cells; DM: diabetes mellitus; DN: diabetic nephropathy; LN: lupus nephritis; SLE: systemic lupus erythematosus; UC-MSCs: umbilical cord blood mesenchymal stem cells; WHO: World Health Organization.
Molecular and biological mechanism of MSCs and EVs
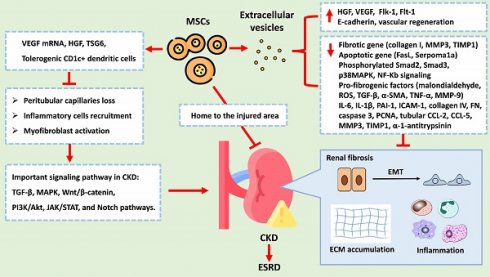
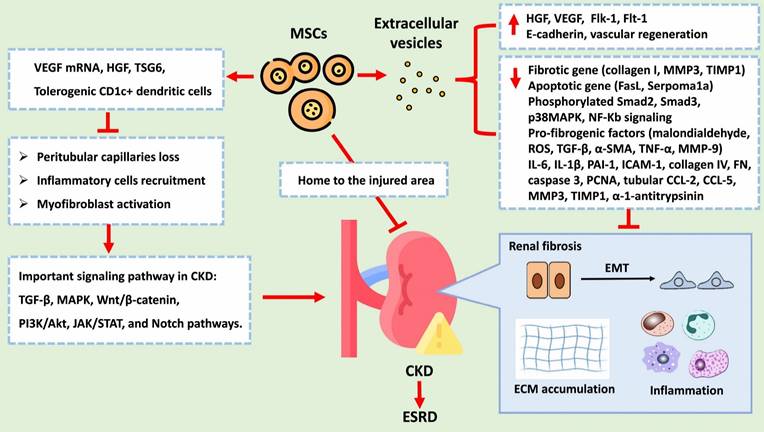
CKD is characterized by various pathological changes, with renal fibrosis being a defining feature. Pathological changes in CKD include a loss of peritubular capillaries, the recruitment of inflammatory cells, the activation of myofibroblasts, epithelial-to-mesenchymal transition (EMT), and ECM deposition. These processes are primarily induced and regulated by various signalling networks, such as the TGF-β, MAPK, Wnt/β-catenin, PI3K/Akt, JAK/STAT, and Notch pathways. EMT, which is notably triggered by TGF-β1, involves the transition of epithelial cells into mesenchymal cells and is marked by the overexpression of α-SMA and the loss of E-cadherin. ECM deposition is characterized by the overexpression of fibronectin, collagen I, and collagen IV. Despite multidrug treatment, CKD often progresses to ESRD. MSCs have emerged as a promising therapeutic approach for CKD treatment due to their ability to exert renoprotective effects. MSCs can mitigate CKD progression through various mechanisms: 1. Angiogenesis: MSCs can generate VEGF mRNA and other paracrine factors that promote angiogenesis, preventing the loss of peritubular capillaries. 2. Anti-inflammatory effects: MSCs reduce inflammation by promoting tolerogenic dendritic cells and inhibiting T-cell proliferation and differentiation. 3. Fibrosis reduction: MSCs decrease the levels of profibrotic factors such as IL-6, IL-1β, TNF-α, TGF-β, α-SMA, collagen I, and collagen IV while increasing the levels of antifibrotic factors such as FGFs, HGF, and VEGF, all of which inhibits EMT and reduces renal fibrosis. 4. Cellular protection: MSCs protect renal cells from damage by regulating apoptosis and inhibiting fibrogenic signalling pathways such as the p38 MAPK pathway. 5. EVs: MSC-derived EVs carry as cargo biological molecules that can decrease the expression levels of fibrotic and apoptotic genes, increase E-cadherin expression levels, and promote vascular regeneration. Figure 6 summarizes the potential therapeutic role of MSCs in treating CKD. Due to the therapeutic potential of MSCs, the feasibility of MSC-based clinical trials in various kidney diseases has been proposed [75-81]. These findings suggest that the therapeutic effects of hUC-MSCs, particularly at high doses and multiple doses, on DKD might involve multiple pathways beyond glycaemic control. Additionally, initial findings from clinical trials and research have demonstrated a potential from these interventions [77-82]. Shimasaki M, et al. also indicated that enhancing the resilience of MSCs to stress through preconditioning with dexamethasone or hypoxic conditions may promote accelerated osteogenic differentiation post-transplantation [83].
Strengths and limitations
Our report offers a concise overview of the potential renoprotective impacts of stem cell-based therapies on both acute and chronic renal dysfunction. In summary, MSCs have shown promise in treating renal fibrosis at various stages by addressing cellular damage, fibrogenic signalling activation, fibrogenic execution, and the destruction of fibrogenic tissue. A recent study also demonstrated that co-administration of a traditional Chinese herb, Alpinate Oxyphyllae Fructus (AOF) and ADMSCs confers renal protection against D-galactose-induced aging by attenuating cellular inflammation, reducing oxidative stress, and inhibiting apoptosis in renal cells [84]. The multifaceted mechanisms of MSCs make them a potential therapeutic option for kidney damage. However, further research and clinical trials are necessary to fully understand the therapeutic potential of MSCs and to optimize their application in the management of kidney dysfunction, including CKD and acute kidney injury. In our study, histopathological changes were evaluated in HE-stained and MT-stained kidney sections. However, quantitative morphometry could be more useful for assessing alterations in kidney histology. Recently, this approach has been applied to digitized images of histological preparations (Pannoramic MIDI) stained with hematoxylin and eosin or Masson's trichrome, using the freely available Pannoramic Viewer software [85]. Moreover, the manuscript delves into the mechanisms governing the process of kidney regeneration triggered by stem cells.
Conclusion
In summary, our study results demonstrate that hUC-MSCs may effectively inhibit inflammation, and ameliorate the progression of glomerulosclerosis. These findings provide a basis for the clinical use of hUC-MSCs as a new therapeutic approach for DKD. The results add to the growing body of evidence supporting the potential of MSCs-based therapies in CKD treatment. However, the exact mechanisms of action and optimal dosing regimens remain to be fully elucidated. Given the heterogeneity of DKD and CKD, along with the complex interplay of various cellular and molecular pathways, further investigation is needed to fully understand the therapeutic potential of MSCs and EVs.
Acknowledgements
Funding
This work is supported by funding from the Taoyuan Armed Forces General Hospital, Taiwan (Grant Nos. TYAFGH-D-113032 and TYAFGH-D-114032).
Author contributions
Study concept and design: CLC, KTL, WYK, LCS, and PJH. Acquisition, analysis, or interpretation of data: CLC, KTL, WYK, PJH. Drafting of the paper: LCS, PJH, and CYL. Critical revision of the paper for important intellectual content: LLT, PJH, YHK, and CLC. Study supervision: YHK, PJH, LLT, and KTL. Funding: PJH. All authors have read and approved the manuscript.
The potential therapeutic role of MSCs and EVs in treating CKD. Abbreviation: VEGF, Vascular Endothelial Growth Factor; HGF, Hepatocyte Growth Factor; TSG6, Tumor Necrosis Factor-Stimulated Gene 6; TGF-B, Transforming Growth Factor Beta (already listed in the previous response); MAPK, Mitogen-Activated Protein Kinases; PI3K/Akt, Phosphoinositide 3-Kinases/Protein Kinase B; JAK/STAT, Janus Kinase/Signal Transducer and Activator of Transcription; Notch, Notch Signaling Pathway. MMP3, Matrix Metallopeptidase 3; TIMP1, Tissue Inhibitor of Metalloproteinases 1; FasL, Fas Ligand; Smad2, Phosphorylated Smad2; Smad3, Phosphorylated Smad3; p38MAPK, Phosphorylated p38 Mitogen-Activated Protein Kinases; NF-Kb, Nuclear Factor Kappa-light-chain-enhancer of activated B cells; ROS, Reactive Oxygen Species; TGF-B, Transforming Growth Factor Beta; a-SMA, Alpha-Smooth Muscle Actin; TNF-a, Tumor Necrosis Factor Alpha; MMP-9, Matrix Metallopeptidase 9; IL-6, Interleukin 6; IL-18, Interleukin 18; PAI-1, Plasminogen Activator Inhibitor-1; ICAM-1, Intercellular Adhesion Molecule 1; PCNA, Proliferating Cell Nuclear Antigen; CCL-2, C-C Motif Chemokine Ligand 2; CCL-5, C-C Motif Chemokine Ligand 5.
Declaration of generative AI and AI-assisted technologies in the writing process
Nil.
Data availability
Data will be made available on request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A. et al. US renal data system 2010 annual data report. Am J Kidney Dis. 2011;57:A8 e1-526
2. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ. et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-73
3. Liu FX, Rutherford P, Smoyer-Tomic K, Prichard S, Laplante S. A global overview of renal registries: a systematic review. BMC Nephrol. 2015;16:31
4. Elliott MJ, Gil S, Hemmelgarn BR, Manns BJ, Tonelli M, Jun M. et al. A scoping review of adult chronic kidney disease clinical pathways for primary care. Nephrol Dial Transplant. 2017;32:838-46
5. Daios S, Kaiafa G, Pilalas D, Nakou I, Kanellos I, Kirdas K. et al. Endothelial dysfunction and platelet hyperaggregation in type 2 diabetes mellitus: the era of novel anti-diabetic agents. Curr Med Chem. 2021;28:3935-63
6. Ebaid H, Bashandy SAE, Abdel-Mageed AM, Al-Tamimi J, Hassan I, Alhazza IM. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr Metab (Lond). 2020;17:6
7. Lin W, Li HY, Yang Q, Chen G, Lin S, Liao C. et al. Administration of mesenchymal stem cells in diabetic kidney disease: a systematic review and meta-analysis. Stem Cell Res Ther. 2021;12:43
8. Shao YJ, Chen WT, Yu SM, Tsou LL, Hsu YH, Wu MS. et al. Investigation of cardiorenal outcomes and incidence of genitourinary tract infection after combined SGLT2 inhibitor and ACEI/ARB use in patients with chronic kidney disease stages 3-5: A real-world retrospective cohort study in Taiwan. Int J Med Sci. 2024;21:2109-18
9. Sardu C, Trotta MC, Sasso FC, Sacra C, Carpinella G, Mauro C. et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovasc Diabetol. 2023;22:80
10. Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18-26
11. Sharma K, McGowan TA. TGF-beta in diabetic kidney disease: role of novel signaling pathways. Cytokine Growth Factor Rev. 2000;11:115-23
12. Goldfarb S, Ziyadeh FN. TGF-beta: a crucial component of the pathogenesis of diabetic nephropathy. Trans Am Clin Climatol Assoc. 2001;112:27-32 discussion 3
13. Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int Suppl. 2000;77:S45-S52
14. Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279:2632-9
15. Wu D, Peng F, Zhang B, Ingram AJ, Gao B, Krepinsky JC. Collagen I induction by high glucose levels is mediated by epidermal growth factor receptor and phosphoinositide 3-kinase/Akt signalling in mesangial cells. Diabetologia. 2007;50:2008-18
16. Wu D, Peng F, Zhang B, Ingram AJ, Kelly DJ, Gilbert RE. et al. EGFR-PLCgamma1 signaling mediates high glucose-induced PKCbeta1-Akt activation and collagen I upregulation in mesangial cells. Am J Physiol Renal Physiol. 2009;297:F822-F34
17. Fazal N, Khawaja H, Naseer N, Khan AJ, Latief N. Daphne mucronata enhances cell proliferation and protects human adipose stem cells against monosodium iodoacetate induced oxidative stress in vitro. Adipocyte. 2020;9:495-508
18. Zhou T, Liao C, Lin S, Lin W, Zhong H, Huang S. The efficacy of mesenchymal stem cells in therapy of acute kidney injury induced by ischemia-reperfusion in animal models. Stem Cells Int. 2020;2020:1873921
19. Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC. et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with crohn's disease. Gastroenterology. 2018;154:1334-42.e4
20. Xuan K, Li B, Guo H, Sun W, Kou X, He X. et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10:eaaf3227
21. Jurado M, De La Mata C, Ruiz-Garcia A, Lopez-Fernandez E, Espinosa O, Remigia MJ. et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: a phase I/II study. Cytotherapy. 2017;19:927-36
22. Moreira A, Kahlenberg S, Hornsby P. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol. 2017;59:R109-R20
23. El-Demerdash RF, Hammad LN, Kamal MM, El Mesallamy HO. A comparison of Wharton's jelly and cord blood as a source of mesenchymal stem cells for diabetes cell therapy. Regen Med. 2015;10:841-55
24. Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91:1003-10
25. Pachler K, Lener T, Streif D, Dunai ZA, Desgeorges A, Feichtner M. et al. A good manufacturing practice-grade standard protocol for exclusively human mesenchymal stromal cell-derived extracellular vesicles. Cytotherapy. 2017;19:458-72
26. Pers YM, Ruiz M, Noel D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthr Cartil. 2015;23:2027-35
27. Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855-63
28. Li T, Xia M, Gao Y, Chen Y, Xu Y. Human umbilical cord mesenchymal stem cells: an overview of their potential in cell-based therapy. Expert Opin Biol Ther. 2015;15:1293-306
29. Wen YC, Du MK, Li MW, Hsuan YC, Su YC, Lin W. EphA2-positive human umbilical cord-derived mesenchymal stem cells exert anti-fibrosis and immunomodulatory activities via secretion of prostaglandin E2. Taiwan J Obstet Gynecol. 2018;57:722-5
30. Liu FB, Lin Q, Liu ZW. A study on the role of apoptotic human umbilical cord mesenchymal stem cells in bleomycin-induced acute lung injury in rat models. Eur Rev Med Pharmacol Sci. 2016;20:969-82
31. Curley GF, Jerkic M, Dixon S, Hogan G, Masterson C, O'Toole D. et al. Cryopreserved, xeno-free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent escherichia coli-induced acute respiratory distress syndrome. Crit Care Med. 2017;45:e202-e12
32. Kang SY, Park DE, Song WJ, Bae BR, Lee JW, Sohn KH. et al. Immunologic regulatory effects of human umbilical cord blood-derived mesenchymal stem cells in a murine ovalbumin asthma model. Clin Exp Allergy. 2017;47:937-45
33. Yu M, Liu W, Li J, Lu J, Lu H, Jia W. et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350
34. Chen S, Du K, Zou C. Current progress in stem cell therapy for type 1 diabetes mellitus. Stem Cell Res Ther. 2020;11:275
35. Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z. c-Jun overexpression accelerates wound healing in diabetic rats by human umbilical cord-derived mesenchymal stem cells. Stem Cells Int. 2020;2020:7430968
36. Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987-96
37. Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73-80
38. Chen XY, Chen YY, Lin W, Chien CW, Chen CH, Wen YC. et al. Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells on the Acute Cigarette Smoke-Induced Pulmonary Inflammation Model. Front Physiol. 2020;11:962
39. Elbe H, Vardi N, Esrefoglu M, Ates B, Yologlu S, Taskapan C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum Exp Toxicol. 2015;34:100-13
40. Yu S, Cheng Y, Zhang L, Yin Y, Xue J, Li B. et al. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res Ther. 2019;10:333
41. Madani S, Setudeh A, Aghayan HR, Alavi-Moghadam S, Rouhifard M, Rezaei N. et al. Placenta derived Mesenchymal Stem Cells transplantation in Type 1 diabetes: preliminary report of phase 1 clinical trial. J Diabetes Metab Disord. 2021;20:1179-89
42. Araujo DB, Dantas JR, Silva KR, Souto DL, Pereira MFC, Moreira JP. et al. Allogenic Adipose Tissue-Derived Stromal/Stem Cells and Vitamin D Supplementation in Patients With Recent-Onset Type 1 Diabetes Mellitus: A 3-Month Follow-Up Pilot Study. Front Immunol. 2020;11:993
43. Izadi M, Nejad ASH, Moazenchi M, Masoumi S, Rabbani A, Kompani F. et al. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13:264
44. Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N. et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407-16
45. Nguyen LT, Hoang DM, Nguyen KT, Bui DM, Nguyen HT, Le HTA. et al. Type 2 diabetes mellitus duration and obesity alter the efficacy of autologously transplanted bone marrow-derived mesenchymal stem/stromal cells. Stem Cells Transl Med. 2021;10:1266-78
46. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63
47. Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11:39
48. Li Y, Liu J, Liao G, Zhang J, Chen Y, Li L. et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int J Mol Med. 2018;41:2629-39
49. He J, Liu B, Du X, Wei Y, Kong D, Feng B. et al. Amelioration of diabetic nephropathy in mice by a single intravenous injection of human mesenchymal stromal cells at early and later disease stages is associated with restoration of autophagy. Stem Cell Res Ther. 2024;15:66
50. Zheng S, Zhang K, Zhang Y, He J, Ouyang Y, Lang R. et al. Human umbilical cord mesenchymal stem cells inhibit pyroptosis of renal tubular epithelial cells through miR-342-3p/Caspase1 signaling pathway in diabetic nephropathy. Stem Cells Int. 2023;2023:5584894
51. Peired AJ, Sisti A, Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016;2016:4798639
52. Wang J, Lin Y, Chen X, Liu Y, Zhou T. Mesenchymal stem cells: a new therapeutic tool for chronic kidney disease. Front Cell Dev Biol. 2022;10:910592
53. Wang D, Zhang H, Liang J, Wang H, Hua B, Feng X. et al. A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Rep. 2018;10:933-41
54. Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631-40
55. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD. et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438-43
56. Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G. et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468
57. Xiang E, Han B, Zhang Q, Rao W, Wang Z, Chang C. et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11:336
58. Xing L, Song E, Yu CY, Jia XB, Ma J, Sui MS. et al. Bone marrow-derived mesenchymal stem cells attenuate tubulointerstitial injury through multiple mechanisms in UUO model. J Cell Biochem. 2019;120:9737-46
59. Wang B, Yao K, Huuskes BM, Shen HH, Zhuang J, Godson C. et al. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via exosomes to attenuate renal fibrosis. Mol Ther. 2016;24:1290-301
60. Huuskes BM, Wise AF, Cox AJ, Lim EX, Payne NL, Kelly DJ. et al. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 2015;29:540-53
61. Liu B, Ding FX, Liu Y, Xiong G, Lin T, He DW. et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology (Carlton). 2018;23:728-36
62. Liu B, Hu D, Zhou Y, Yu Y, Shen L, Long C. et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am J Transl Res. 2020;12:4998-5014
63. Oliveira-Sales EB, Maquigussa E, Semedo P, Pereira LG, Ferreira VM, Camara NO. et al. Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One. 2013;8:e78464
64. Mohamed EM, Samak MA. Therapeutic potentials of mesenchymal stem cells on the renal cortex of experimentally induced hypertensive albino rats: relevant role of Nrf2. Tissue Cell. 2017;49:358-67
65. Villanueva S, Carreno JE, Salazar L, Vergara C, Strodthoff R, Fajre F. et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond). 2013;125:199-210
66. Cavaglieri RC, Martini D, Sogayar MC, Noronha IL. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc. 2009;41:947-51
67. Semedo P, Correa-Costa M, Cenedeze MA, Malheiros DMAC, dos Reis MA, Shimizu MH. et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063-73
68. Li Y, Shen M, Ferens D, Broughton BRS, Murthi P, Saini S. et al. Combining mesenchymal stem cells with serelaxin provides enhanced renoprotection against 1K/DOCA/salt-induced hypertension. Br J Pharmacol. 2021;178:1164-81
69. Zhu Q, Li XX, Wang W, Hu J, Li PL, Conley S. et al. Mesenchymal stem cell transplantation inhibited high salt-induced activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. Am J Physiol Renal Physiol. 2016;310:F621-F7
70. Liu J, Lu X, Lou Y, Cai Y, Cui W, Wang J. et al. Xenogeneic transplantation of human placenta-derived mesenchymal stem cells alleviates renal injury and reduces inflammation in a mouse model of lupus nephritis. Biomed Res Int. 2019;2019:9370919
71. Elseweidy MM, Askar ME, Elswefy SE, Shawky M. Nephrotoxicity induced by cisplatin intake in experimental rats and therapeutic approach of using mesenchymal stem cells and spironolactone. Appl Biochem Biotechnol. 2018;184:1390-403
72. Kholia S, Herrera Sanchez MB, Cedrino M, Papadimitriou E, Tapparo M, Deregibus MC. et al. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front Cell Dev Biol. 2020;8:188
73. Ramirez-Bajo MJ, Martin-Ramirez J, Bruno S, Pasquino C, Banon-Maneus E, Rovira J. et al. Nephroprotective potential of mesenchymal stromal cells and their extracellular vesicles in a murine model of chronic cyclosporine nephrotoxicity. Front Cell Dev Biol. 2020;8:296
74. Quimby JM, Webb TL, Randall E, Marolf A, Valdes-Martinez A, Dow SW. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: a randomized, placebo-controlled clinical trial in eight cats. J Feline Med Surg. 2016;18:165-71
75. Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263-9
76. Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436-9
77. Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X. et al. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9:312
78. Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N. et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116
79. Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X. et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79
80. Chun S, Choi CB, Kim MS, Nam JY, Lee TY, Lee YT. et al. Safety and tolerability of bone marrow-derived mesenchymal stem cells in lupus animal models and a phase I clinical trial in humans. Lupus. 2022;31:1245-53
81. Lerman LO. Cell-based regenerative medicine for renovascular disease. Trends Mol Med. 2021;27:882-94
82. Rajput S, Malviya R, Uniyal P. Advances in the treatment of kidney disorders using mesenchymal stem cells. Curr Pharm Des. 2024;30:825-840
83. Shimasaki M, Ichiseki T, Ueda S, Hirata H, Kawahara N, Ueda Y. Mesenchymal Stem Cells Preconditioned with Hypoxia and Dexamethasone Promote Osteoblast Differentiation Under Stress Conditions. Int J Med Sci. 2024;21:1511-7
84. Ho TJ, Shanmugam T, Liao PH, Shibu MA, Chen WS, Lin KH. et al. Renal protective effects of Alpinate Oxyphyllae Fructus and mesenchymal stem cells co-treatment against D- galactose induced renal deterioration. Int J Med Sci. 2024;21:1491-9
85. Yudintceva N, Bobkov D, Sulatsky M, Mikhailova N, Oganesyan E, Vinogradova T. et al. Mesenchymal stem cells-derived extracellular vesicles for therapeutics of renal tuberculosis. Sci Rep. 2024;14:4495
Author contact
![]() Corresponding authors: Kung-Ta Lee, PhD, Department of Biochemical Science and Technology, College of Life Science, National Taiwan University, Taipei, Taiwan, No. 1, Section 4, Roosevelt Rd, Da'an District, Taipei City, Taiwan 106; Tel.: 886-233-664-436; E-mail: ktleeedu.tw. Chu-Lin Chou, MD, PhD, Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, No. 250, Wuxing Street, Xinyi District, Taipei, Taiwan, 110; Tel.: 886-3-422-5180 Ext. 606; E-mail: chulin.chouedu.tw.
Corresponding authors: Kung-Ta Lee, PhD, Department of Biochemical Science and Technology, College of Life Science, National Taiwan University, Taipei, Taiwan, No. 1, Section 4, Roosevelt Rd, Da'an District, Taipei City, Taiwan 106; Tel.: 886-233-664-436; E-mail: ktleeedu.tw. Chu-Lin Chou, MD, PhD, Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan, No. 250, Wuxing Street, Xinyi District, Taipei, Taiwan, 110; Tel.: 886-3-422-5180 Ext. 606; E-mail: chulin.chouedu.tw.

 Global reach, higher impact
Global reach, higher impact