3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(7):1672-1679. doi:10.7150/ijms.102404 This issue Cite
Research Paper
Association of lung immune prognostic index with overall survival in pancreatic ductal adenocarcinoma patients treated using chemotherapy
1. Medical School of Chinese PLA, Beijing 100853, China.
2. Department of Medical Oncology, the Fifth Medical Center, Chinese PLA General Hospital, Beijing 100071, China.
3. Department of Medical Oncology, the First Medical Center, Chinese PLA General Hospital, Beijing 100853, China.
* These authors contributed equally to this work.
Received 2024-8-15; Accepted 2025-2-11; Published 2025-3-3
Abstract
Background: The lung immune prognostic index (LIPI) has attracted considerable interest for its prognostic value in several malignancies. However, its prognostic value in pancreatic ductal adenocarcinoma (PDAC) has not yet been clarified.
Objective: This study aimed to assess the role of LIPI with regard to overall survival (OS) in locally advanced or metastatic PDAC patients undergoing chemotherapy.
Methods: Data from 256 patients with PDAC treated via chemotherapy at the Chinese PLA General Hospital between January 1, 2011 and July 1, 2018 were retrospectively reviewed. Their neutrophil-to-lymphocyte ratio (dNLR) with lactate dehydrogenase (LDH) values were used to calculate each one's LIPI. The Cox proportional hazard model was used to identify the association between LIPI and OS.
Results: Of the included patients, 154 were in the good LIPI group and 102 were in the intermediate/poor LIPI group. The OS in the two groups were 9.0 months (95% CI: 7.351-10.649) and 6.0 months (95% CI: 4.812-7.188), respectively. Patients in the good LIPI group had better OS compared to those in the intermediate/poor LIPI group (HR, 0.720; 95% CI: 0.554-0.935; P = 0.014).
Conclusion: This study revealed LIPI is significantly associated with OS in PDAC and could play a significant role in helping clinicians make appropriate decisions for PDAC patients undergoing chemotherapy.
Keywords: pancreatic ductal adenocarcinoma, Lung Immune Prognostic Index score, overall survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the deadliest and highly metastatic forms of cancer, with a projected 5-year survival rate of merely 3% [1, 2]. There are several factors that contribute to its invasiveness, such as the absence of early diagnostic markers, delayed detection caused by the lack of symptoms, intricate genetic characteristics, and early spread of metastasis [3, 4]. Complete surgical removal is currently the sole potentially curative approach for patients diagnosed with metastatic PDAC, with the potential to increase the 5-year survival rate to around 20% [3, 5, 6]. However, > 80% of patients with PDAC have unresectable tumors at their time of diagnosis, most often due to vascular invasion and distant metastasis [7]. According to the ESMO guidelines, chemotherapy remains the primary treatment for pancreatic cancer [3]. With medical advancements, some patients with pancreatic cancer have achieved survival of more than one year. However, there are still patients with limited sensitivity to cytotoxic drugs who succumb to the disease due to ineffective treatment [8]. Therefore, it is particularly important to identify biomarkers that can effectively predict the prognosis of PDAC. Such biomarkers would help doctors to early identify patients who may not respond well to conventional chemotherapy. This, in turn, would allow for the optimization of treatment strategies and the exploration of additional treatment options, ultimately improving survival rates and quality of life [9].
The derived neutrophil-to-lymphocyte ratio (dNLR) reflects the composition of the tumor microenvironment, which determines the tumor's ability to evade the immune system [10]. Lactate dehydrogenase (LDH) plays a crucial role in the final step of glycolysis, providing both energy and biosynthesis precursors to tumor cells. Its impact on tumor survival is primarily through the inhibition of apoptosis, prevention of necrosis in hypoxic environments, and protection from damage caused by reactive oxygen species [11]. The lung immune prognostic index (LIPI) a compositional biomarker, was developed to reflect the association between dNLR and the blood LDH levels. LIPI was first reported by Mezquita et al., who found it to be significantly associated with the systemic inflammatory response and prognosis of non-small cell lung cancer following treatment with immune checkpoint inhibitors [12]. Increasing attention has been paid to LIPI in the field of extrapulmonary tumors [13, 14]. It has been shown to be significantly related to the prognosis of various cancers, including: osteosarcoma patients receiving standard treatment [15], esophageal squamous cell carcinoma patients undergoing radical surgery or chemoradiotherapy [13, 14], urothelial bladder cancer patients undergoing radical cystectomy [16], and advanced breast cancer patients receiving trastuzumab therapy [17]. This growing body of evidence highlights the potential of LIPI as a valuable prognostic tool across different types of cancer.
The short overall survival (OS) of patients with PDAC is not only related to the disease stage, but also to the fact that the current treatment mainly relies on chemotherapy [18]. However, chemotherapeutic drugs face challenges when entering the internal environments of PDAC tumors [19]. The unique immunosuppressive microenvironment and dense stromal matrix of PDAC contribute to the low efficacy and short survival times associated with chemotherapy [20, 21]. To data, no studies have identified the potential roles that LIPI might play in predicting the prognosis of PDAC in patients undergoing chemotherapy. Thus, this study evaluated the prognostic role of LIPI regarding overall survival in patients with PDAC undergoing chemotherapy.
Participants and Methods
Study population
A retrospective review was conducted on a cohort of 256 patients with PDAC who received treatment at the Chinese PLA General Hospital from January 1, 2011 to July 1, 2018. Inclusion criteria for patients were as follows: (1) adults diagnosed with stage IV or locally advanced PDAC, (2) treated via chemotherapy, and (3) laboratory examinations were performed within one week before the initiation of treatment. Patients were excluded if: (1) laboratory examination results were not obtained; (2) patients underwent radical resection; and (3) patients with malignancies in other organs, inflammatory conditions, autoimmune disorders, or injuries. Informed consent was waived by the committee because of the retrospective nature of the study. This study was approved by the Ethics Committee of the PLA General Hospital (ethical approval number: S2014-031-01). Clinical data were electronically retrieved from the medical records of the PLA General Hospital Registry. All treatments were performed in accordance with the institution's guidelines and regulations.
Recorded variables
Demographic and clinical variables were obtained from the patients' electronic medical records. Which included age, sex, smoking status, alcohol status, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), LDH, platelets (PLT), albumin (ALB), diabetes, obstructive jaundice, chest/abdominal effusion, history of organ transplantations, liver metastases, and chemotherapy regimens. The chemotherapy regimens used included: (1) the GS regimen: S-1 (40-60 mg, twice daily, given orally after breakfast and dinner for 14 days, followed by 7 days off), and gemcitabine (1,000 mg/m2 intravenously given on the first and eighth days of each cycle); (2) the AS regimen: S-1 (40-60 mg, twice daily, given orally after breakfast and dinner for 14 days, followed by 7 days off), and paclitaxel (260 mg/m2 intravenously given on the first and eighth days of each cycle); and (3) the GEMOX regimen: oxaliplatin (130 mg/m2, given intravenously on the first day) combined with gemcitabine (1,000 mg/m2, given intravenously on the first day), 14 days per cycle. Treatment regimen selection was based on each patient's pathological stage, general health condition, and other considerations.
LIPI definition and grouping
LIPI is based on LDH and dNLR. We used dNLR before treatment as the variable, and a normal LDH level was defined as 0-250 U/L. Survival receiving operator characteristic (ROC) curves were used to calculate dNLR in order to predict OS at 2.3, using X-tile software (Version 3.6.1). Patients were divided into three groups based on their dNLR (above the optimal cutoff value) and LDH levels (above the upper limit of normal): good (total score of 0), intermediate (total score of 1), and poor (total score of 2).
Outcome definition
The outcome investigated was overall survival (OS), defined as the time from the beginning of chemotherapy until death.
Statistical analysis
The demographics and characteristics of the good and intermediate/poor LIPI groups were assigned as categorical and continuous data, respectively. Categorical data are presented as frequencies and percentages, while continuous data are reported as means (with standard deviations) or medians (with ranges) depending on the distribution of the data. Chi-squared or Fisher's exact tests were used to compare the differences between groups. The optimal cutoff value of dNLR was evaluated using ROC curves, and the survival analysis was performed using the survival curve and Kaplan-Meier analysis. Cox proportional hazards regression analysis was employed to evaluate the prognostic significance of the LIPI for OS, and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were computed as the measure of effect. Stratified analyses were performed to assess the prognostic role of LIPI for OS according to patients' characteristics. All P values reported in this study are two-tailed, and the significance level was set at 0.05. Statistical analyses were conducted using SPSS 26.0 software (Chicago, IL, United States).
Results
Baseline patient and group characteristics
A total of 256 patients (154 male and 102 female) were enrolled, with a mean age of 55.46 years. Forty-three had locally advanced PDAC with no chance for surgical treatment, and the remaining 213 had metastatic PDAC. Of those with metastatic PDAC, 194 had liver metastasis and 54 had ≥ 2 metastases. Each patient's treatment plan was based on their ECOG score, tumor stage, and general condition.
Using dNLR as the independent variable, and based on the time-dependent ROC curve (Figure 1), the optimal cutoff value was determined to be 2.3. LDH levels within the range of 0-250 U/L were considered normal. The patients were divided into three groups based on their LIPI scores: the good group (total score of 0) consisted of 154 patients, the intermediate group (total score of 1) consisted of 89 patients, and the poor group (total score of 2) had 13 patients. Owing to the limited number of patients in the poor group, the intermediate group was merged with the poor group. A total of 154 (60.2%) patients had good LIPI scores, while 102 (40.9%) had intermediate/poor LIPI scores.
The demographic characteristics of the participants in both groups are presented in Table 1. There were notable disparities observed between the groups in relation to CEA (P = 0.006), LDH (P < 0.001), dNLR (P < 0.001), obstructive jaundice (P = 0.003), chest abdominal effusion (P = 0.041), and number of organ transplants (P = 0.019). We also observed no significant differences in age, sex, smoking status, alcohol consumption, PLT, ALB, diabetes, liver metastases, or chemotherapy regimens between the groups.
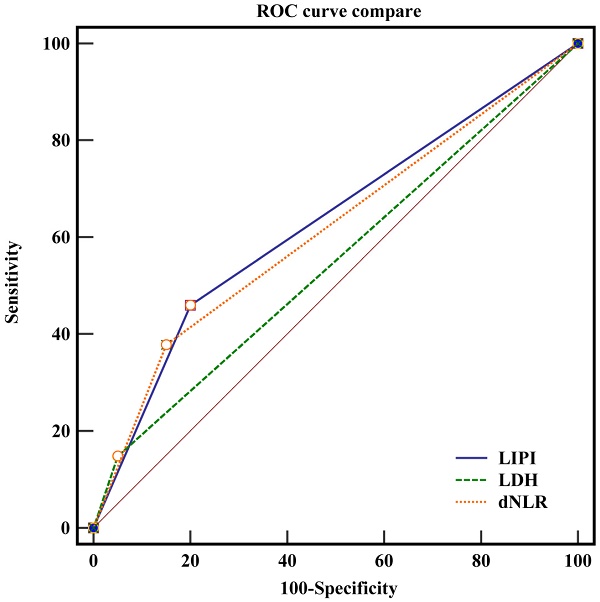
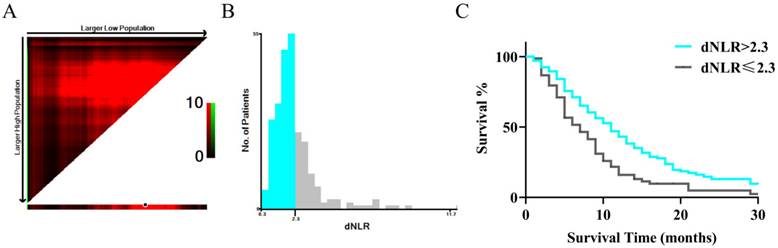
ROC curve of pretreatment dNLR in assessment of the overall survival at 2.3 through X-tile Software. In figure 1A, the X-axis represents all potential cut-points from low to high (left to right) that define a low subset, whereas the Y-axis represents cut-points from high to low (top to bottom), that define a high subset. The arrows represent the direction in which the low subset (X-axis) and the high subset (Y-axis) increase in size. Red coloration of cut-points indicates an inverse correlation with survival, whereas green coloration represents direct associations. The optimal cut-point occurs at the brightest pixel (green or red). The optimal cut-off point is shown on a histogram of the entire cohort in figure 1 B. In figure 1C, a Kaplan-Meier curve is plotted to show the correlation of dNLR with OS.
The baseline characteristics of enrolled patients
| Variable | LIPI group | ||
|---|---|---|---|
| Good (n=154) | Intermediate/poor (n=102) | P value | |
| Age (years) | 56.11±9.02 | 54.31±9.11 | 0.122 |
| Sex | 0.867 | ||
| Male | 92 (59.7%) | 62 (60.8%) | |
| Female | 62 (40.3%) | 40 (39.2%) | |
| Smoking status | 0.664 | ||
| Yes | 60 (39.0%) | 37 (36.3%) | |
| No | 94 (61.0%) | 65 (63.7%) | |
| Alcohol intake | 0.236 | ||
| Yes | 58 (37.7%) | 46 (45.1%) | |
| No | 96 (62.3%) | 56 (54.9%) | |
| CEA | 0.006 | ||
| High (>5 μg/L) | 76 (49.4%) | 68 (66.7%) | |
| Normal (0-5 μg/L) | 78 (50.6%) | 34 (33.3%) | |
| LDH | < 0.001 | ||
| High (>250 U/L) | 0 (0.0%) | 32 (31.4%) | |
| Normal (0-250 U/L) | 154 (100.0%) | 70 (68.6%) | |
| dNLR | < 0.001 | ||
| > 2.3 | 0 (0.0%) | 83 (81.4%) | |
| ≤ 2.3 | 154 (100.0%) | 19 (18.6%) | |
| PLT (*109/L) | 197.5 (138.2-249.8) | 206.5 (151.7-266.2) | 0.430 |
| ALB (U/L) | 40.7 (38.5-43.8) | 40.2 (36.0-43.3) | 0.072 |
| Diabetes | 0.585 | ||
| Yes | 33 (21.4%) | 19 (18.6%) | |
| No | 121 (78.6%) | 83 (81.4%) | |
| Obstructive jaundice | 0.003 | ||
| Yes | 43 (28.3%) | 13 (12.7%) | |
| No | 109 (71.7%) | 89 (87.3%) | |
| Chest abdominal Effusion | 0.041 | ||
| Yes | 28 (18.4%) | 30 (29.4%) | |
| No | 124 (81.6%) | 72 (70.6%) | |
| Number of organs transferred | 0.019 | ||
| 2-3 | 23 (14.9%) | 31 (30.4%) | |
| 1 | 100 (64.9%) | 59 (57.8%) | |
| 0 | 31 (20.1%) | 12 (11.8%) | |
| Liver metastases | 0.343 | ||
| Yes | 114 (74.0%) | 80 (79.2%) | |
| No | 40 (26.0%) | 21 (20.8%) | |
| Chemotherapy regimens | 0.410 | ||
| Included G | 41 (26.6%) | 32 (31.4%) | |
| Others | 113 (73.4%) | 70 (68.6%) | |
The prognostic role of LIPI on OS
The median OSs in the good and intermediate/poor LIPI groups were 9.0 (95% CI: 7.351-10.649) and 6.0 months (95% CI: 4.812-7.188), respectively. Univariate analysis revealed that a good LIPI was associated with a higher OS compared to an intermediate/poor LIPI (HR: 0.734; 95% CI: 0.570-0.945; P = 0.003; Figure 2). It also revealed that other prognostic factors for OS included male sex (HR: 1.583; P < 0.001), smoking history (HR: 1.374; P = 0.010), high CEA (HR: 1.182; P = 0.005), high CA19-9 (HR: 1.189; P = 0.036), chest abdominal effusion (HR: 1.352; P = 0.034), and liver metastases (HR: 1.378; P = 0.022) (Table 2).
Univariate and multivariate analyses of prognostic factors for overall survival
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age | 1.007 (0.993-1.022) | 0.316 | ||
| Sex | ||||
| Male | 1.583 (1.226-2.046) | < 0.001 | 1.595 (1.143-2.225) | 0.006 |
| Female | Ref | Ref | ||
| Smoking status | ||||
| Yes | 1.374 (1.063-1.777) | 0.010 | 1.041 (0.752-1.442) | 0.807 |
| No | Ref | Ref | ||
| Alcohol intake | ||||
| Yes | 1.152 (0.896-1.482) | 0.243 | ||
| No | Ref | |||
| LIPI | ||||
| Good | 0.734 (0.570-0.945) | 0.003 | 0.720 (0.554-0.935) | 0.014 |
| Intermediate/poor | Ref | Ref | ||
| CEA | ||||
| High | 1.182 (1.043-1.341) | 0.005 | 1.150 (1.011-1.308) | 0.034 |
| Normal | Ref | Ref | ||
| CA19-9 | ||||
| High | 1.189 (1.001-1.411) | 0.036 | 1.215 (1.016-1.453) | 0.033 |
| Normal | Ref | Ref | ||
| PLT | 2.372 (0.288-19.569) | 0.422 | ||
| ALB | 0.851 (0.568-1.276) | 0.436 | ||
| Diabetes | ||||
| Yes | 0.966 (0.712-1.311) | 0.813 | ||
| No | Ref | |||
| Obstructive jaundice | ||||
| Yes | 1.118 (0.829-1.508) | 0.439 | ||
| No | Ref | |||
| Chest abdominal effusion | ||||
| Yes | 1.352 (1.004-1.821) | 0.034 | 1.147 (0.841-1.563) | 0.386 |
| No | Ref | Ref | ||
| Number of organs transferred | ||||
| 3 | 0.548 (0.246-1.220) | 0.141 | ||
| 2 | 0.694 (0.325-1.485) | 0.347 | ||
| 1 | 0.486 (0.217-1.088) | 0.079 | ||
| 0 | Ref | |||
| Liver metastases | ||||
| Yes | 1.378 (1.029-1.845) | 0.022 | 1.116 (0.821-1.518) | 0.482 |
| No | Ref | Ref | ||
| Chemotherapy regimens | ||||
| Included G | 0.914 (0.696-1.200) | 0.493 | ||
| Others | Ref | |||
After adjusting for potential confounding factors, patients in the good LIPI group showed higher OSs compared to those in the intermediate/poor LIPI group (HR, 0.720; 95% CI: 0.554-0.935; P = 0.014). Moreover, we noted that OS was also affected by male sex (HR: 1.595; 95% CI: 1.143-2.225; P = 0.006), high CEA (HR: 1.150; 95% CI: 1.011-1.308; P = 0.034), and high CA19-9 (HR: 1.215; 95% CI: 1.016-1.453; P = 0.033). Smoking status, chest and abdominal effusion, and liver metastases were not found to be associated with OS after adjusting for confounders (Table 2).
Subgroup analysis
Subgroup analyses of the role of LIPI in OS were also performed (Table 3). We noted that good LIPI was associated with an improvement in OS compared to intermediate/poor LIPI in patients with no smoking history, no alcohol consumption history, high CEA, high CA19-9, obstructive jaundice, 2-3 organs transferred, liver metastases, and chemotherapy regimens that did not include G. LIPI was not significantly associated with OS in any other subgroups.
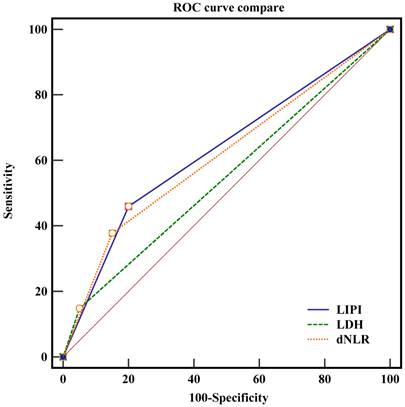
The ROC curve of LIPI, dNLR and LDH.
Discussion
The objective of this study was to evaluate the predictive significance of LIPI in relation to OS in patients with PDAC treated with chemotherapy. To ensure the reliability of the dNLR cutoff value, we used X-tile software to calculate it based on time-dependent ROC curves. This method allowed for an accurate assessment of LIPI's impact on PDAC prognosis. We recruited 256 patients with PDAC, and the median OS for the good LIPI group was 9.0 months, compared to 6.0 months for the intermediate/poor LIPI group. Patients in the good LIPI group had a significantly longer OS than those in the intermediate/poor LIPI group. After adjusting for potential confounding factors, other significant prognostic factors included sex, CEA levels, and CA19-9 levels. The prognostic role of LIPI for OS was statistically significant in the subgroups of patients with no smoking history, no alcohol consumption history, high CEA, high CA19-9, obstructive jaundice, 2-3 organ transplantations, liver metastases, and chemotherapy regimens that did not include G. These findings highlight the potential of LIPI as a valuable prognostic tool in predicting OS for PDAC patients undergoing chemotherapy.
Subgroup analyses for the role of LIPI on OS
| Variable | Subgroups | Multivariate | |
|---|---|---|---|
| HR (95% CI) | P value | ||
| Sex | Male | 0.682 (0.459-1.014) | 0.058 |
| Female | 0.666 (0.389-1.138) | 0.137 | |
| Smoking status | Yes | 0.788 (0.477-1.304) | 0.355 |
| No | 0.616 (0.418-0.906) | 0.014 | |
| Alcohol intake | Yes | 1.036 (0.627-1.709) | 0.891 |
| No | 0.572 (0.381-0.857) | 0.007 | |
| CEA | High | 0.655 (0.447-0.959) | 0.030 |
| Normal | 0.848 (0.517-1.392) | 0.515 | |
| CA19-9 | High | 0.588 (0.428-0.807) | 0.001 |
| Normal | 1.297 (0.547-3.073) | 0.555 | |
| Diabetes | Yes | 0.481 (0.231-1.003) | 0.051 |
| No | 0.805 (0.577-1.122) | 0.200 | |
| Obstructive jaundice | Yes | 0.399 (0.178-0.894) | 0.026 |
| No | 0.768 (0.556-1.061) | 0.110 | |
| Chest abdominal Effusion | Yes | 0.813 (0.401-1.651) | 0.567 |
| No | 0.724 (0.518-1.013) | 0.060 | |
| Number of organs transferred | 2-3 | 0.408 (0.200-0.829) | 0.013 |
| 0-1 | 0.798 (0.564-1.130) | 0.203 | |
| Liver metastases | Yes | 0.709 (0.506-0.995) | 0.046 |
| No | 0.662 (0.332-1.321) | 0.242 | |
| Chemotherapy regimens | Included G | 0.739 (0.419-1.302) | 0.295 |
| Others | 0.690 (0.477-0.998) | 0.049 | |
A previous study examined 205 patients with PDAC who were treated with radical resection, and assessed the value of preoperative LIPI in predicting OS and recurrence-free survival (RFS) [22]. They found that a preoperative intermediate/poor LIPI was associated with poor OS and RFS. Moreover, vascular invasion and chemotherapy were found to affect OS, while RFS was affected by CA-125 level and vascular invasion. However, this study focused on patients diagnosed with stage I-III PDAC, leaving the prognostic value of LIPI for stage IV or locally advanced PDAC unclear. To address this gap, the objective of the current study was to evaluate the prognostic significance of LIPI in terms of OS in patients with locally advanced or metastatic PDAC who are receiving chemotherapy.
We discovered a significant association between the LIPI and OS in PDAC patients undergoing chemotherapy. LIPI is computed based on two key factors: LDH and dNLR. Both LDH and dNLR have well - established links to patient prognosis across various solid cancers, as evidenced by previous studies [23, 24]. LDH is a crucial enzyme in tumor cell energy metabolism. In pancreatic cancer, it is highly expressed not only in cancer cells but also in peripheral blood and tissues. This elevated expression is associated with increased tumor invasiveness, which directly impacts the prognosis of pancreatic cancer patients [25, 26]. The high levels of LDH may be attributed to the enhanced glycolytic activity of pancreatic cancer cells, a characteristic known as the Warburg effect. This increased glycolysis provides the necessary energy for tumor growth, invasion, and metastasis. Moreover, dNLR is an indicator of the body's internal inflammatory state, which in turn reflects the tumor microenvironment. Neutrophils, a component of the dNLR calculation, can actively influence the tumor microenvironment. They secrete various cytokines and chemokines that promote tumor growth, angiogenesis, and immune evasion. For example, neutrophils can release vascular endothelial growth factor, which stimulates the formation of new blood vessels to supply nutrients to the growing tumor. Additionally, they can interact with tumor cells and other immune cells, modulating the inflammatory response in a way that is favorable for tumor progression [27]. Conversely, lymphocytes, the other component of dNLR, play a vital role in anti-tumor immunity. Lymphocyte infiltration into the tumor microenvironment is significantly associated with a better response to immunotherapy and improved prognosis. Cytotoxic T lymphocytes can directly recognize and kill tumor cells, while helper T cells can secrete cytokines that enhance the immune response. Regulatory T cells, although having an immunosuppressive function, can also be balanced by the presence of effector T cells and other immune cells in a healthy immune microenvironment [28]. Given the complexity of PDAC biology, a single biomarker may not comprehensively and accurately reflect the prognosis. By combining dNLR and LDH in the form of LIPI, we can capture multiple aspects of the tumor microenvironment, including inflammation, energy metabolism, and immune cell balance [29]. This integrated approach provides a more reliable and comprehensive prognostic tool for PDAC patients.
We also performed an exploratory analysis according to patients' characteristics, which revealed that the association between LIPI and OS in patients with PDAC could be affected by smoking status, alcohol intake, CEA level, CA19-9 level, obstructive jaundice, number of transplanted organs, liver metastases, and chemotherapy regimens. Cigarette smoking is significantly associated with the risk and prognosis of PDAC, which can be explained by the direct effect of cigarette smoke on the tumor cell microenvironment [30]. Moreover, the TGF-β pathway in patients with a history of alcohol consumption can lead to the formation of extensive stroma, which can affect the prognosis of PDAC [31]. CEA and CA19-9 levels reflect the severity of PDAC and can potentially influence its prognosis [32]. The disease status of PDAC is significantly related to obstructive jaundice, number of organ transplants, and liver metastases; whereas chemotherapy regimens are significantly related to OS in patients with locally advanced or metastatic PDAC. These findings suggest that while LIPI is a valuable prognostic tool, its predictive power for OS in PDAC patients can be modulated by these additional clinical and biological factors.
This study had several key limitations worth noting. First, the retrospective nature of this analysis is a significant constraint. Retrospective cohort studies inherently carry the risk of recall and selection biases. These biases can distort the results and may limit the generalizability of the findings. Given that our data was collected from past medical records, there could be missing or inaccurate information, and the selection of patients for the study may not be entirely representative of the broader PDAC patient population. Second, the relatively small sample size of 256 patients is another limitation. A larger sample size is generally required to increase the statistical power of the study and to ensure that the results are more robust and applicable to different patient populations. With a small sample, there is a higher chance of random errors and the inability to detect rare events or subtle associations accurately. Third, the effectiveness of currently available chemotherapies for advanced PDAC is modest, and the choice of chemotherapy regimen is often at the discretion of the treating physician. This variability in treatment selection may have introduced additional bias into the study. Fourth, the study primarily focused on the role of LIPI in predicting OS; other results regarding disease progression were not investigated. This narrow focus limits the comprehensive understanding of LIPI's prognostic value in PDAC.
Despite these limitations, we have demonstrated that a good LIPI is significantly related to longer OS in patients with locally advanced or metastatic PDAC undergoing chemotherapy, particularly in the subgroups of those with no smoking history, no alcohol consumption history, high CEA levels, high CA19-9 levels, obstructive jaundice, 2-3 organs transferred, liver metastases, and chemotherapy regimens that did not include G. Considering the data in this study were sourced from a single center, future studies involving multi - center data collection and analysis are essential to validate our results across different patient populations and healthcare settings. Future research should also aim to increase the sample size, reduce biases associated with treatment selection, and explore other aspects of disease progression in relation to LIPI. This will help to establish the true prognostic potential of LIPI in PDAC more comprehensively.
Abbreviations
CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; CIs: confidence intervals; dNLR: derived neutrophil-to-lymphocyte ratio; HRs: hazard ratios; LDH: lactate dehydrogenase; LIPI: lung immune prognostic index; OS: overall survival; PDAC: pancreatic ductal adenocarcinoma; RFS: recurrence-free survival; ROC: receiving operator characteristic.
Acknowledgements
Funding
This study was supported by the National Natural Science Foundation of China (No. B2272643) and Beijing Natural Science Foundation Project (No. 7232163).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the PLA General Hospital (ethical approval number: S2014-031-01). All treatments were performed in accordance with the institution's guidelines and regulations. Informed consent was waived by the committee because of the retrospective nature of the study.
Author contributions
GHD and QLH conducted the study conception and design. NZ, GCD and RJ contributed to data collection and analysis. The first draft of the manuscript was written by NZ and GCD. A thorough review and revisions were carried out by GHD and RJ. All authors read and approved the final manuscript. Nan Zhang and Guochao Deng contributed equally to this article.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10(1):10-27
2. National Institutes of Health Surveillance, National Cancer Institute Epidemiology and end results program: Cancer stat facts: pancreatic cancer. https://seer.cancer.gov/statfacts/html/pancreas.html
3. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T. et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-v68
4. Sarantis P, Koustas E, Papadimitropoulou A, Papavassiliou AG, Karamouzis MV. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J Gastrointest Oncol. 2020;12(2):173-181
5. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921
6. Taieb J, Abdallah R. How I treat pancreatic cancer. ESMO Open. 2020;4(Suppl 2):e000818
7. Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20(31):10740-10751
8. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33
9. Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013;14(11):e476-e485
10. Alessi JV, Ricciuti B, Alden SL, Bertram AA, Lin JJ, Sakhi M, Nishino M, Vaz VR, Lindsay J, Turner MM. et al. Low peripheral blood derived neutrophil-to-lymphocyte ratio (dNLR) is associated with increased tumor T-cell infiltration and favorable outcomes to first-line pembrolizumab in non-small cell lung cancer. J Immunother Cancer. 2021;9(11):e003536
11. Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703-707
12. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C. et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(3):351-357
13. Feng J-F, Zhao J-M, Chen S, Chen Q-X. Prognostic Significance of the Lung Immune Prognostic Index in Patients with Resected Esophageal Squamous Cell Carcinoma. Cancer Manag Res. 2021;13:2811-2819
14. Yu Y, Wu H, Qiu J, Ke D, Wu Y, Lin M, Zheng Q, Zheng H, Wang Z, Li H. et al. The novel pretreatment immune prognostic index discriminates survival outcomes in locally advanced non-operative esophageal squamous cell carcinoma patients treated with definitive chemoradiotherapy: a 6-year retrospective study. Transl Oncol. 2022;21:101430
15. He X, Wang Y, Ye Q, Wang Y, Min L, Luo Y, Zhou Y, Tu C. Lung Immune Prognostic Index Could Predict Metastasis in Patients With Osteosarcoma. Front Surg. 2022;9:923427
16. Obayashi K, Miki J, Fukuokaya W, Yanagisawa T, Kimura S, Tsuzuki S, Kimura T, Egawa S. The prognostic value of the preoperative lung immune prognostic index in patients with urothelial bladder cancer undergoing radical cystectomy. Int J Clin Oncol. 2022;27(2):396-402
17. Li L, Ai L, Jia L, Zhang L, Lei B, Zhang Q. High score of LDH plus dNLR predicts poor survival in patients with HER2-positive advanced breast cancer treated with trastuzumab emtansine. BMC Cancer. 2022;22(1):29
18. Springfeld C, Ferrone CR, Katz MHG, Philip PA, Hong TS, Hackert T, Büchler MW, Neoptolemos J. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20(5):318-337
19. Li K, Tandurella JA, Gai J, Zhu Q, Lim SJ, Thomas DL 2nd, Xia T, Mo G, Mitchell JT, Montagne J. et al. Multi-omic analyses of changes in the tumor microenvironment of pancreatic adenocarcinoma following neoadjuvant treatment with anti-PD-1 therapy. Cancer Cell. 2022;40(11):1374-1391.e7
20. Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezène P. et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun. 2017;8:16031
21. Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O, Lac S, Borge L, Roques J, Gayet O. et al. Vasseur S. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2015;112(8):2473-8
22. Zhou Q, Deng G, Wang Z, Dai G. Preoperative lung immune prognostic index predicts survival in patients with pancreatic cancer undergoing radical resection. Front Surg. 2022;9:1002075
23. Takada K, Takamori S, Matsubara T, Haratake N, Akamine T, Kinoshita F, Ono Y, Wakasu S, Tanaka K, Oku Y. et al. Clinical significance of preoperative inflammatory markers in non-small cell lung cancer patients: A multicenter retrospective study. PLoS One. 2020;15(11):e0241580
24. Liu C, Li L, Song K, Zhan Z-Y, Yao Y, Gong H, Chen Y, Wang Q, Dong X, Xie Z. et al. A nomogram for predicting mortality in patients with COVID-19 and solid tumors: a multicenter retrospective cohort study. J Immunother Cancer. 2020;8(2):e001314
25. Comandatore A, Franczak M, Smolenski RT, Morelli L, Peters GJ, Giovannetti E. Lactate Dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin Cancer Biol. 2022;86(Pt 2):93-100
26. Jahchan NS, Mujal AM, Pollack JL, Binnewies M, Sriram V, Reyno L, Krummel MF. Tuning the Tumor Myeloid Microenvironment to Fight Cancer. Front Immunol. 2019;10:1611
27. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124
28. Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93-103
29. Li Y, Zhang Z, Hu Y, Yan X, Song Q, Wang G, Chen R, Jiao S, Wang J. Pretreatment Neutrophil-to-Lymphocyte Ratio (NLR) May Predict the Outcomes of Advanced Non-small-cell Lung Cancer (NSCLC) Patients Treated With Immune Checkpoint Inhibitors (ICIs). Front Oncol. 2020;10:654
30. Weissman S, Takakura K, Eibl G, Pandol SJ, Saruta M. The Diverse Involvement of Cigarette Smoking in Pancreatic Cancer Development and Prognosis. Pancreas. 2020;49(5):612-620
31. Doronzo A, Porcelli L, Marziliano D, Inglese G, Argentiero A, Azzariti A, Solimando AG. Gene Expression Comparison between Alcohol-Exposed versus Not Exposed Pancreatic Ductal Adenocarcinoma Patients Reveals a Peculiar TGFβ-Related Phenotype: An Exploratory Analysis. Medicina (Kaunas). 2023;59(5):872
32. Gong Y, Song L, Ou L, Lu Y-Y, Huang X, Zeng Q. Diagnostic and Prognostic Performance of MicroRNA-25, Carbohydrate Antigen 19-9, Carcinoembryonic Antigen, and Carbohydrate Antigen 125 in Pancreatic Ductal Adenocarcinoma. Iran J Med Sci. 2023;48(4):401-413
Author contact
![]() Corresponding author: Guanghai Dai (E-mail: daiguanghaicom.cn).
Corresponding author: Guanghai Dai (E-mail: daiguanghaicom.cn).

 Global reach, higher impact
Global reach, higher impact