3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(7):1555-1561. doi:10.7150/ijms.109616 This issue Cite
Research Paper
Metabolic syndrome is associated with gastroesophageal reflux disease in a large Taiwanese population study
1. Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 812, Taiwan.
2. Division of Gastroenterology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan.
3. Department of Urology, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
4. Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
5. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung 807, Taiwan.
6. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan.
Received 2024-12-30; Accepted 2025-2-19; Published 2025-2-28
Abstract
Gastroesophageal reflux disease (GERD) and metabolic syndrome (MetS) have emerged as prominent health issues in past decades. This study aimed to evaluate whether the presence of MetS and its constituent factors was associated with a higher likelihood of GERD in a large cohort of Taiwanese adults. MetS and its components were defined according to the modified National Cholesterol Education Program Expert Panel and Adult Treatment Panel III for Asians, and the presence of GERD was assessed using standardized interviews and questionnaires. Of 121,583 participants from the Taiwan biobank, 16,664 (13.7%) were diagnosed with GERD and 27,441 (22.6%) were diagnosed with MetS. After multivariable analysis, the participants with MetS (odds ratio [OR] = 1.079), abdominal obesity (OR = 1.094), hypertriglyceridemia (OR = 1.085), and low high-density lipoprotein cholesterol (OR = 1.073) (all p < 0.001) were significantly associated with GERD, but high blood pressure and hyperglycemia were not. Furthermore, there was a trend of a stepwise increase in the rate of GERD in accordance with the number of MetS components. Participants with 3 components (vs. 0; OR = 1.093; p = 0.002), 4 components (vs. 0; OR = 1.108; p = 0.005), and 5 components (vs. 0; OR = 1.137; p = 0.029) were significantly associated with GERD. Our results suggest that MetS may be associated with the development of GERD in the Taiwanese population.
Keywords: metabolic syndrome, gastroesophageal reflux disease, Taiwan Biobank
Introduction
Gastroesophageal reflux disease (GERD) is an increasingly significant health issue in Asian countries, resulting in significant morbidity and substantial burden on medical expenditure [1]. The adoption of western lifestyle habits in Asian countries has led to an increasing prevalence of both GERD and metabolic syndrome (MetS) [2, 3]. Reports indicate that in Taiwan, the prevalence of reflux esophagitis has increased from 9% to 25% in the past decades [4-6]. Risk factors for reflux esophagitis include older age, sex, obesity, smoking, and the consumption of alcohol and fatty foods [7-12], of which obesity is the most significant [13, 14]. While obesity is a major risk factor for reflux esophagitis, it is noteworthy that GERD may have multiple pathogenic mechanisms, and not all of them may be linked to or influenced by obesity [15]. The precise pathophysiological mechanisms underpinning this association have yet to be completely elucidated. Further evidence suggests that abdominal obesity, a fundamental component of MetS, may also be an important risk factor for reflux esophagitis [16, 17]. MetS is defined by the presence of metabolic abnormalities including high triglyceride level, abdominal obesity, hyperglycemia, hypertension, and low level of high-density lipoprotein cholesterol (HDL-C), and it has emerged as a prominent health issue in recent decades [18, 19]. MetS is closely tied to environmental conditions and diet behavior [20]. The dramatic increase in both MetS and GERD has sparked research into the underlying pathophysiology. Given the increasing importance of these conditions and their effect on public health, we evaluated the associations between MetS and its five components with GERD in this study of enrollees in the Taiwan Biobank (TWB). Our aim was to evaluate whether MetS and its components were associated with an increased likelihood of GERD.
Materials and methods
The TWB was established by the Ministry of Health and Welfare and approved by the Institutional Review Board on Biomedical Science Research, Academia Sinica, Taiwan, and the Ethics and Governance Council of the TWB. Established in 2008 as a large-scale community-based re-search program, the TWB database includes the data from Taiwanese adults aged 30 to 70 years and has been used in previous studies to explore the connections between clinical, personal health factors and various diseases [21-26]. Eligible participants were required to be of Taiwanese ethnicity, provide written informed consent, and have no history of severe chronic diseases such as cancer at enrollment. Individuals with incomplete baseline data were excluded. Participants were selected based on their willingness to provide biospecimens and completed questionnaires regarding lifestyle, medical history, and other health-related factors.
Study participants
The study participants between 2008 to 2016 were enrolled from the TWB. According to the inclusion criteria of the TWB, none of the participants had a history of cancer, and they were all aged from 30 to 70 years. Information included in the TWB covered lifestyle factors, genetic data and medical history [26, 27]. All of the participants were interviewed and underwent physical examinations, during which fasting blood samples were obtained. The baseline study included 121,583 participants.
Study variables
Blood pressure (BP: systolic and diastolic), weight, height, waist and hip circumference were recorded. The presence of hypertension and diabetes mellitus was also recorded, along with exercise habits, sex and age. In this study, we considered regular exercise as playing a sport or engaging in a physical activity other than work-related on at least 3 occasions in 1 week with each session lasting 30 minutes or more.
Laboratory tests were conducted using the fasting blood specimens to obtain data on uric acid, glucose, hemoglobin, triglycerides, total cholesterol, HDL-C, low-density lipoprotein cholesterol (LDL-C), and estimated glomerular filtration rate (eGFR: calculated according to the method proposed by Lewy et al [28]).
Definitions of GERD and MetS
The participants were asked, “Have you been diagnosed with GERD?”, and those who replied “Yes” were considered to have GERD. The participants were defined as having MetS if they had ≥ 3 of these criteria following the modified criteria for Asians [4] and National Cholesterol Education Program Expert Panel and Adult Treatment Panel III (NCEP-ATP III) guidelines [4]: (1) hypertension, defined as systolic/diastolic BP ≥ 130/85 mmHg or antihypertensive agent prescriptions; (2) triglycerides ≥ 150 mg/dL; (3) HDL-C in women/men of < 50/40 mg/dL; (4) abdominal obesity, defined as a waist circumference in women/men of ≥ 80/90 cm, respectively; and (5) hyperglycemia, defined as a fasting glucose concentration ≥ 100 mg/dL or a diagnosis of diabetes mellitus.
Statistical analysis
Continuous data are given as mean (SD) and compared with the independent t test. Categorical data are given as number (%) and compared with the chi-square test. Univariable analysis was performed, and significant variables were then entered into multivariable logistic regression analysis to evaluate associations among MetS and its components with GERD. SPSS version 19.0 (IBM Inc., Armonk, New York, USA) was used for the analysis, and two-tailed p-values < 0.05 were considered significant.
Results
The 121,583 enrolled participants had a mean age of 49.9 ± 11.0 years, 43,698 were men and 77,885 were women. In addition, 16,664 (13.7%) had GERD and 104,919 (86.3%) did not.
Clinical characteristics of the GERD groups
A comparison of the clinical characteristics between the participants without or with GERD is shown in Table 1. Compared to the participants without GERD (n = 104,919), those with GERD (n = 16,664) were older (49.6 vs. 51.9 years, p < 0.001), predominantly female (65.8 vs. 63.8%, p < 0.001), had higher prevalence rates of diabetes mellitus (6.3 vs. 5.0%, p < 0.001) and hypertension (15.3 vs. 11.7%, p < 0.001), higher prevalence rates of smoking (29.3 vs. 26.9%, p < 0.001) and alcohol history (9.8 vs. 8.3%, p < 0.001), higher prevalence of regular exercise habit (43.0 vs. 40.2%, p < 0.001), lower height (161.5 vs. 162.0 cms, p < 0.001), lower weight (63.5 vs. 63.8 kg, p = 0.010), higher waist circumference (83.7 vs. 83.2 cm, p < 0.001), lower hip circumference (95.9 vs. 96.0 cm, p = 0.011), higher fasting glucose (96.4 vs. 95.9 mg/dL, p = 0.002), higher triglyceride (119.0 vs. 115.1 mg/dL, p < 0.001), higher total cholesterol (197.4 vs. 195.4 mg/dL, p < 0.001), higher LDL-C (121.8 vs. 120.8 mg/dL, p < 0.001), lower eGFR (102.1 vs. 103.5 mL/min/1.73m2, p < 0.001) and lower uric acid (5.39 vs. 5.43 mg/dL, p = 0.002). Regarding MetS, the participants with GERD had higher prevalence of MetS (24.9 vs. 22.2%, p < 0.001), higher numbers of MetS components (1.6 vs. 1.5, p < 0.001), higher prevalence of five components, including abdominal obesity (49.4 vs. 46.0%, p < 0.001), hypertriglyceridemia (22.4 vs. 20.7%, p < 0.001), low HDL-cholesterol (26.6 vs. 25.4%, p = 0.001), hyperglycemia (22.7 vs. 20.5%, p < 0.001) and high BP (37.1 vs. 34.9%, p < 0.001).
Association of MetS and GERD
The factors associated with GERD in the study participants in multivariable logistic regression analysis are shown in Table 2. The correlation matrix in the logistic regression model reveals a strong correlation (|r| = 0.87) between LDL-cholesterol and total cholesterol (supplementary Table 1), indicating potential multicollinearity. In the linear regression model, the Variance Inflation Factor (VIF) analysis showed that total cholesterol and LDL-cholesterol had a relatively high VIF of approximately 4.39 (supplementary Table 2). Therefore, we do not put LDL-cholesterol in the further multivariable analysis.
After adjusting for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, eGFR, uric acid and MetS (significant variables in univariable analysis of Table 1), MetS (odds ratio [OR] = 1.079; 95% confidence interval [CI] = 1.037-1.124; p < 0.001), old age (p < 0.001), female (p < 0.001), smoking history (p < 0.001), alcohol history (p < 0.001), high total cholesterol (p = 0.021), low eGFR (p = 0.025), and low uric acid (p < 0.001) remained significant factors for GERD in the multivariable logistic regression.
Association of number of MetS components and GERD
The associations among the number of MetS components and GERD in multivariable logistic regression analysis are shown in Table 3. After multivariable analysis adjusting for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, LDL-cholesterol, eGFR and uric acid, participants with 3 MetS components (vs. 0; OR = 1.093; 95% CI = 1.033-1.157; p = 0.002), 4 MetS components (vs. 0; OR = 1.108; 95% CI = 1.031-1.190; p = 0.005), and 5 MetS components (vs. 0; OR = 1.137; 95% CI = 1.073-1.275; p = 0.029) were significantly associated with GERD.
Comparison of clinical characteristics among participants according to GERD in study participants
| Characteristics | GERD (-) (n = 104,919) | GERD (+) (n = 16,664) | p |
|---|---|---|---|
| Age (year) | 49.6 ± 11.0 | 51.9 ± 10.4 | < 0.001 |
| Male sex (%) | 36.2 | 34.2 | < 0.001 |
| DM (%) | 5.0 | 6.3 | < 0.001 |
| Hypertension (%) | 11.7 | 15.3 | < 0.001 |
| Smoking history (%) | 26.9 | 29.3 | < 0.001 |
| Alcohol history (%) | 8.3 | 9.8 | < 0.001 |
| Regular exercise habits (%) | 40.2 | 43.0 | < 0.001 |
| Systolic BP (mmHg) | 120.4 ± 18.8 | 120.7 ± 17.8 | 0.102 |
| Diastolic BP (mmHg) | 73.8 ± 11.5 | 73.8 ± 10.9 | 0.562 |
| Body height (cm) | 162.0 ± 8.3 | 161.5 ± 8.2 | < 0.001 |
| Body weight (kg) | 63.8 ± 12.7 | 63.5 ± 12.7 | 0.010 |
| Waist circumference (cm) | 83.2 ± 10.2 | 83.7 ± 10.3 | < 0.001 |
| Hip circumference (cm) | 96.0 ± 7.1 | 95.9 ± 7.2 | 0.011 |
| Laboratory parameters | |||
| Fasting glucose (mg/dL) | 95.9 ± 20.9 | 96.4 ± 19.7 | 0.002 |
| Hemoglobin (g/dL) | 13.8 ± 1.6 | 13.8 ± 1.5 | 0.083 |
| Triglyceride (mg/dL) | 115.1 ± 94.3 | 119.0 ± 92.3 | < 0.001 |
| Total cholesterol (mg/dL) | 195.4 ± 35.9 | 197.4 ± 35.2 | < 0.001 |
| HDL-C (mg/dL) | 54.6 ± 13.4 | 54.6 ± 13.6 | 0.668 |
| LDL-C (mg/dL) | 120.8 ± 31.8 | 121.8 ± 31.3 | < 0.001 |
| eGFR (mL/min/1.73 m2) | 103.5 ± 23.9 | 102.1 ± 23.7 | < 0.001 |
| Uric acid (mg/dL) | 5.43 ± 1.43 | 5.39 ± 1.39 | 0.002 |
| MetS (%) | 22.2 | 24.9 | < 0.001 |
| Number of MetS components | 1.5 ± 1.3 | 1.6 ± 1.3 | < 0.001 |
| MetS component | |||
| Abdominal obesity (%) | 46.0 | 49.4 | < 0.001 |
| Hypertriglyceridemia (%) | 20.7 | 22.4 | < 0.001 |
| Low HDL-cholesterol (%) | 25.4 | 26.6 | 0.001 |
| Hyperglycemia (%) | 20.5 | 22.7 | < 0.001 |
| High blood pressure (%) | 34.9 | 37.1 | < 0.001 |
Abbreviations. GERD, gastroesophageal reflux disease; DM, diabetes mellitus; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; MetS, metabolic syndrome.
Association of MetS components and GERD
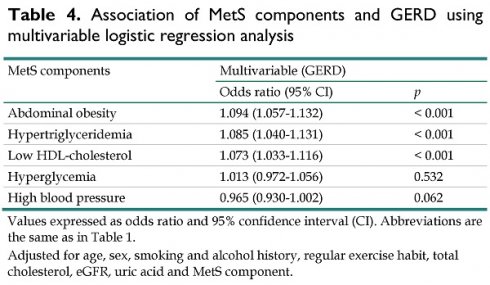
The associations among the MetS components and GERD in multivariable logistic regression analysis are shown in Table 4. After multivariable analysis adjusting for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, LDL-cholesterol, eGFR and uric acid, participants with abdominal obesity (OR = 1.094; 95% CI = 1.057-1.132; p < 0.001), hypertriglyceridemia (OR = 1.085; 95% CI = 1.040-1.131; p < 0.001), and low HDL-cholesterol (OR = 1.073; 95% CI = 1.033-1.116; p < 0.001), were significantly associated with GERD. However, hyperglycemia (p = 0.532), and high BP (p = 0.062) were not associated with GERD.
Risks of GERD using multivariable logistic regression analysis
| Variables | Multivariable (GERD) | |
|---|---|---|
| Odds ratio (95% CI) | p | |
| Age (per 1 year) | 1.019 (1.018-1.021) | < 0.001 |
| Male vs. female | 0.819 (0.782-0.859) | < 0.001 |
| Smoking history | 1.253 (1.199-1.310) | < 0.001 |
| Alcohol history | 1.176 (1.108-1.249) | < 0.001 |
| Regular exercise habits | 0.982 (0.948-1.018) | 0.323 |
| Total cholesterol (per 10 mg/dL) | 1.005 (1.001-1.010) | 0.021 |
| eGFR (per 1 mL/min/1.73 m2) | 0.999 (0.998-1.000) | 0.025 |
| Uric acid (per 1 mg/dL) | 0.968 (0.954-0.982) | < 0.001 |
| MetS | 1.079 (1.037-1.124) | < 0.001 |
Values expressed as odds ratio and 95% confidence interval (CI). Abbreviations are the same as in Table 1.
Adjusted for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, eGFR, uric acid and MetS.
Association of the number of MetS components and GERD using multivariable logistic regression analysis
| Number of MetS components | Multivariable (GERD) | |
|---|---|---|
| Odds ratio (95% CI) | p | |
| 0 MetS component | Reference | |
| 1 MetS component | 1.014 (0.968-1.061) | 0.558 |
| 2 MetS components | 1.042 (0.992-1.096) | 0.100 |
| 3 MetS components | 1.093 (1.033-1.157) | 0.002 |
| 4 MetS components | 1.108 (1.031-1.190) | 0.005 |
| 5 MetS components | 1.137 (1.073-1.275) | 0.029 |
Values expressed as odds ratio and 95% confidence interval (CI). Abbreviations are the same as in Table 1.
Adjusted for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, eGFR, uric acid and the number of MetS components.
Association of MetS components and GERD using multivariable logistic regression analysis
| MetS components | Multivariable (GERD) | |
|---|---|---|
| Odds ratio (95% CI) | p | |
| Abdominal obesity | 1.094 (1.057-1.132) | < 0.001 |
| Hypertriglyceridemia | 1.085 (1.040-1.131) | < 0.001 |
| Low HDL-cholesterol | 1.073 (1.033-1.116) | < 0.001 |
| Hyperglycemia | 1.013 (0.972-1.056) | 0.532 |
| High blood pressure | 0.965 (0.930-1.002) | 0.062 |
Values expressed as odds ratio and 95% confidence interval (CI). Abbreviations are the same as in Table 1.
Adjusted for age, sex, smoking and alcohol history, regular exercise habit, total cholesterol, eGFR, uric acid and MetS component.
Discussion
In this study of associations between MetS and its components with GERD in 121,583 participants from the TWB, we found the MetS group was associated with a high prevalence of GERD. Furthermore, of the MetS components, associations were found between hypertriglyceridemia, abdominal obesity, and low HDL-C with a high risk of GERD, but hyperglycemia and hypertension were not.
There are three key findings. First, the association between MetS and GERD, as well as the trend of a stepwise increase in the prevalence of GERD in accordance with the number of MetS components. MetS and its components abdominal obesity, hyperglycemia, hypertension and dyslipidemia predispose individuals to diabetes and cardiovascular disease [29, 30]. Its fundamental pathological mechanisms involve obesity and insulin resistance. The NCEP-ATP III guidelines reported that the overall prevalence of MetS is approximately 15% to 23%, escalating significantly with age [19, 31]. Previous studies based on endoscopic examination findings and classification of reflux esophagitis have delineated a close relationship between reflux esophagitis and MetS [32-34]. A cross-sectional study conducted in South Korean in 2008 included 1679 patients with erosive esophagitis and found that 21% of the patients had MetS, which was much higher than the control group (12%) [32]. In Taiwan, a retrospective study conducted in 2016 showed that 20% (19/95) of patients with Barrett's esophagus and 9.6% (214/1118) of those with erosive gastritis had MetS [33]. Similar findings were also reported in cross-sectional study of 4859 patients in 2019. Among 2916 patients with erosive esophagitis, 949 (32.5%) had MetS, which was much higher than the control group (22.5%; 446/1079) [34]. Most of these studies evaluated endoscopic erosive esophagitis, but excluded non-erosive esophagitis. Although the mechanism of the association between erosive esophagitis and MetS has yet to be elucidated, several possible factors have been proposed. An independent association between increased waist circumference, one of the most important components of MetS, and an elevated risk of erosive esophagitis has been reported. In addition, visceral fat accumulation can increase intra-abdominal pressure and promote gastroesophageal reflux [35]. Moreover, hypertriglyceridemia may be associated with a high-fat diet, which can result in delayed gastric emptying and further promote esophagogastric reflux [36]. Hypertension has also been considered to be a significant risk factor for erosive esophagitis due to the esophageal sphincter pressure-lowering effect of antihypertensive medications such as calcium channel blockers, and environmental factors such as life stress and high-salt diet have been shown to promote esophagogastric reflux [37, 38]. Hyperglycemia has also been associated with erosive esophagitis, possibly due to diabetic autonomic neuropathy and insulin resistance. The association between HDL-C and erosive esophagitis is controversial in previous studies. The present study of 121,583 participants is the largest population-based investigation to date. Of these participants, 16,664 (13.7%) were diagnosed with GERD and 27,441 (22.6%) were diagnosed with MetS. Notably, we found a correlation between the number of MetS components and the presence of reflux esophagitis, including erosive esophagitis and non-erosive esophagitis. A higher number of MetS components signifies not only a relationship between the two conditions, but also suggests a certain level of interaction.
The association between abdominal obesity and GERD is the second important finding of this study. Abdominal obesity has been hypothesized to promote gastroesophageal reflux through an intra-abdominal pressure-increasing effect and transient relaxation of the lower esophageal sphincter [19, 39]. Beyond mechanical causes, adipose tissue has been demonstrated to stimulate the overproduction of pro-inflammatory cytokines, leading to low esophageal sphincter relaxation and insulin resistance [40, 41].
The third key finding is the relationship between hypertriglyceridemia and low HDL-C with GERD. Hypertriglyceridemia has been associated with high dietary fat intake, and a diet overly high in fat diet can lead to delayed gastric emptying and further increase the risk of GERD [36, 41]. In this study, we found a weak association between low HDL-C and GERD. Associations between many diseases and a change in HDL-C level and composition have been reported. In addition, a decreased HDL-C level has been observed in patients with acute and chronic inflammatory diseases such as ankylosing spondylitis [42]. These findings suggest that a change in lipid metabolism may be the result of both MetS and GERD.
We also found that hyperglycemia and high BP were not associated with GERD. Hyperglycemia is caused by changes in obesity-related hormones and insulin resistance, which have been associated with an increased prevalence of erosive esophagitis and GERD symptoms [43]. The association between hyperglycemia with reflux esophagitis remains controversial [32, 36]. While some studies have hypothesized that hyperglycemia may contribute to reflux esophagitis through diabetic autonomic neuropathy and insulin resistance [40, 41], this phenomenon was not found in our study. Delayed gastric emptying associated with diabetic autonomic neuropathy may represent a late stage of hyperglycemia, which was not evident in this study population. Hypertension has been associated with erosive esophagitis, but again we did not observe this in the present study. Since this is a cross-sectional study and due to the nature of the data stored in the TWB, we could not analyze environmental factors and drug history.
In our study, the results revealed that female sex is an independent risk factor for total GERD. TWB conducted wider spectrum epidemiologic study for reflux esophagitis including erosive esophagitis and non-erosive esophagitis. Although many studies showed male sex is a risk factor for erosive esophagitis [9, 10], several studies demonstrated female sex is associated with non-erosive esophagitis [11, 12]. TWB collects health-related data on healthy volunteers around Taiwan, women may be more willing or able to participate in research studies compared to men due to higher health awareness. As the presence of GERD was checked through the use of questionnaires without endoscopic verification, the severity and type of GERD could not be verified. Above explanations may partially explain the difference.
The key advantages of our research include the large Taiwanese adult cohort drawn from the community, and that we controlled for a wide range of confounding variables spanning lifestyle and health-related factors. Nevertheless, several limitations should also be mentioned. First, we lacked information about medications used to treat the various components of MetS. As these agents may have had an effect on the occurrence and prevention of MetS and its components, the associations between MetS and its components with GERD may be underestimated. Second, the cross-sectional design of the study meant that were unable to evaluate the duration of GERD, and hence we could not analyze causal relationships between MetS, its components and GERD was not possible. Longitudinal studies may be able to overcome this limitation. Third, as the presence of GERD was checked through the use of questionnaires without endoscopic verification, the severity and type of GERD could not be verified. However, a previous study in Taiwan showed moderate agreement between claims data and self-reported diseases [44]. The final limitation is that the study participants were of Chinese ethnicity, thus potentially limiting the conclusions to other ethnicities.
In conclusion, the results of this large cohort study of Taiwanese adults showed associations between MetS and its components abdominal obesity, hypertriglyceridemia, and low HDL-C with a high prevalence of GERD. Further, there was a trend of a stepwise increase in the prevalence of GERD in accordance with the number of MetS components. These results may indicate that MetS plays a role in the risk of GERD in the Taiwanese population. Further longitudinal studies are warranted to investigate the risk of incident GERD, and whether improving MetS can reduce the incidence of GERD.
Supplementary Material
Supplementary tables.
Acknowledgements
Ethical approval
The study was conducted according to the Declaration of Helsinki, and it was granted approval by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-E(I)-20210058), and the TWB was granted approval by the IRB on Biomedical Science Research, Academia Sinica, Taiwan and the Ethics and Governance Council of the TWB.
Authors contributions
Conceptualization, methodology, validation, formal analysis, writing—review and editing, and supervision: S-CC and C-HK. Software and investigation: S-CC. Resources, project administration, and funding acquisition: S-CC. Data curation: W-HH, J-HG, P-YW, J-CH, H-MH, S-CC and C-HK. Writing—original draft preparation: W-HH and S-CC. Visualization: J-HG and S-CC. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
The data underlying this study are from the Taiwan Biobank and Taiwan Air Quality Monitoring Database. Due to restrictions placed on the data by the Personal Information Protection Act of Taiwan, the minimal data set cannot be made publicly available. Data may be available upon request to interested researchers. Please send data requests to: Szu-Chia Chen, PhD, MD. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li N, Yang WL, Cai MH, Chen X, Zhao R, Li MT. et al. Burden of gastroesophageal reflux disease in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of disease study 2019. BMC Public Health. 2023;23:582
2. Ierardi E, Rosania R, Zotti M, Principe S, Laonigro G, Giorgio F. et al. Metabolic syndrome and gastro-esophageal reflux: A link towards a growing interest in developed countries. World J Gastrointest Pathophysiol. 2010;1:91-6
3. Ho KY, Cheung TK, Wong BC. Gastroesophageal reflux disease in Asian countries: disorder of nature or nurture? J Gastroenterol Hepatol. 2006;21:1362-5
4. Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004;27:1182-6
5. Hung CS, Lee CL, Yang JN, Liao PT, Tu TC, Chen TK. et al. Clinical application of Carlsson's questionnaire to predict erosive GERD among healthy Chinese. J Gastroenterol Hepatol. 2005;20:1900-5
6. Chen TS, Chang FY. The prevalence and risk factors of reflux esophagitis among adult Chinese population in Taiwan. J Clin Gastroenterol. 2007;41:819-22
7. Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:267-76
8. Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin Gastroenterol Hepatol. 2006;4:398-407
9. Lee YC, Wang HP, Chiu HM, Liao SC, Huang SP, Lai YP. et al. Comparative analysis between psychological and endoscopic profiles in patients with gastroesophageal reflux disease: a prospective study based on screening endoscopy. J Gastroenterol Hepatol. 2006;21:798-804
10. Du J, Liu J, Zhang H, Yu CH, Li YM. Risk factors for gastroesophageal reflux disease, reflux esophagitis and non-erosive reflux disease among Chinese patients undergoing upper gastrointestinal endoscopic examination. World J Gastroenterol. 2007;13:6009-15
11. Fujiwara Y, Higuchi K, Shiba M, Yamamori K, Watanabe Y, Sasaki E. et al. Differences in clinical characteristics between patients with endoscopy-negative reflux disease and erosive esophagitis in Japan. Am J Gastroenterol. 2005;100:754-8
12. Hung LJ, Hsu PI, Yang CY, Wang EM, Lai KH. Prevalence of gastroesophageal reflux disease in a general population in Taiwan. J Gastroenterol Hepatol. 2011;26:1164-8
13. Goh KL. Obesity and increasing gastroesophageal reflux disease in Asia. J Gastroenterol Hepatol. 2007;22:1557-8
14. Ze EY, Kim BJ, Kang H, Kim JG. Abdominal Visceral to Subcutaneous Adipose Tissue Ratio Is Associated with Increased Risk of Erosive Esophagitis. Dig Dis Sci. 2017;62:1265-71
15. Nam JH, Cho E, Kim JS, Park EC, Kim JH. The Influences of Visceral Fat Area on the Sites of Esophageal Mucosal Breaks in Subjects with Gastroesophageal Reflux Diseases. Gastroenterol Res Pract. 2019;2019:9672861
16. Lenglinger J, Riegler M, Schoppmann SF. Gastroesophageal reflux disease and metabolic syndrome. Intern Med. 2012;51:2993 author reply 5
17. Niigaki M, Adachi K, Hirakawa K, Furuta K, Kinoshita Y. Association between metabolic syndrome and prevalence of gastroesophageal reflux disease in a health screening facility in Japan. J Gastroenterol. 2013;48:463-72
18. Chuang SY, Chen CH, Chou P. Prevalence of metabolic syndrome in a large health check-up population in Taiwan. J Chin Med Assoc. 2004;67:611-20
19. Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc. 2006;105:626-35
20. Nestel P, Lyu R, Low LP, Sheu WH, Nitiyanant W, Saito I. et al. Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr. 2007;16:362-7
21. Li JY, Lee JI, Lu CC, Su YD, Chiu CT, Chen SC. et al. Hyperuricemia and Its Association with Osteoporosis in a Large Asian Cohort. Nutrients. 2022;14:2206
22. Huang YC, Geng JH, Wu PY, Huang JC, Chen SC, Chang JM. et al. Betel Nut Chewing Increases the Risk of Metabolic Syndrome and Its Components in a Large Taiwanese Population Follow-Up Study Category: Original Investigation. Nutrients. 2022;14:1018
23. Feng YA, Chen CY, Chen TT, Kuo PH, Hsu YH, Yang HI. et al. Taiwan Biobank: A rich biomedical research database of the Taiwanese population. Cell Genom. 2022;2:100197
24. Fan CT, Lin JC, Lee CH. Taiwan Biobank: a project aiming to aid Taiwan's transition into a biomedical island. Pharmacogenomics. 2008;9:235-46
25. Lin JC, Fan CT, Liao CC, Chen YS. Taiwan Biobank: making cross-database convergence possible in the Big Data era. Gigascience. 2018;7:1-4
26. Chen CH, Yang JH, Chiang CWK, Hsiung CN, Wu PE, Chang LC. et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum Mol Genet. 2016;25:5321-31
27. Fan CT, Hung TH, Yeh CK. Taiwan Regulation of Biobanks. J Law Med Ethics. 2015;43:816-26
28. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70
29. Lee MY, Hsiao PJ, Huang JC, Hsu WH, Chen SC, Shin SJ. Association Between Metabolic Syndrome and Microvascular and Macrovascular Disease in Type 2 Diabetic Mellitus. Am J Med Sci. 2018;355:342-9
30. Tsai SS, Lin YS, Chen ST, Chu PH. Metabolic syndrome positively correlates with the risks of atherosclerosis and diabetes in a Chinese population. Eur J Intern Med. 2018;54:40-5
31. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427-36
32. Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK. et al. Metabolic syndrome is associated with erosive esophagitis. World J Gastroenterol. 2008;14:5442-7
33. Lee SW, Lien HC, Chang CS, Lee TY, Peng YC, Yeh HZ. Association of metabolic syndrome with erosive esophagitis and Barrett's esophagus in a Chinese population. J Chin Med Assoc. 2017;80:15-8
34. Hsieh YH, Wu MF, Yang PY, Liao WC, Hsieh YH, Chang YJ. et al. What is the impact of metabolic syndrome and its components on reflux esophagitis? A cross-sectional study. BMC Gastroenterol. 2019;19:33
35. Chang P, Friedenberg F. Obesity and GERD. Gastroenterol Clin North Am. 2014;43:161-73
36. Chua CS, Lin YM, Yu FC, Hsu YH, Chen JH, Yang KC. et al. Metabolic risk factors associated with erosive esophagitis. J Gastroenterol Hepatol. 2009;24:1375-9
37. Suyu H, Liu Y, Jianyu X, Luo G, Cao L, Long X. Prevalence and Predictors of Silent Gastroesophageal Reflux Disease in Patients with Hypertension. Gastroenterol Res Pract. 2018;2018:7242917
38. Hughes J, Lockhart J, Joyce A. Do calcium antagonists contribute to gastro-oesophageal reflux disease and concomitant noncardiac chest pain? Br J Clin Pharmacol. 2007;64:83-9
39. Ohashi S, Maruno T, Fukuyama K, Kikuchi O, Sunami T, Kondo Y. et al. Visceral fat obesity is the key risk factor for the development of reflux erosive esophagitis in 40-69-years subjects. Esophagus. 2021;18:889-99
40. Moki F, Kusano M, Mizuide M, Shimoyama Y, Kawamura O, Takagi H. et al. Association between reflux oesophagitis and features of the metabolic syndrome in Japan. Aliment Pharmacol Ther. 2007;26:1069-75
41. Loke SS, Yang KD, Chen KD, Chen JF. Erosive esophagitis associated with metabolic syndrome, impaired liver function, and dyslipidemia. World J Gastroenterol. 2013;19:5883-8
42. Nazir S, Jankowski V, Bender G, Zewinger S, Rye KA, van der Vorst EPC. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration!. Adv Drug Deliv Rev. 2020;159:94-119
43. Hsu CS, Wang PC, Chen JH, Su WC, Tseng TC, Chen HD. et al. Increasing insulin resistance is associated with increased severity and prevalence of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011;34:994-1004
44. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9:e112257
Author contact
![]() Corresponding author: Szu-Chia Chen, M.D., Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, 482, Shan-Ming Rd., Siaogang Dist., 812 Kaohsiung, Taiwan; Tel.: 886-7-8036783-3441, Fax: 886- 7- 8063346; E-mail: scarchenonecom.tw.
Corresponding author: Szu-Chia Chen, M.D., Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, 482, Shan-Ming Rd., Siaogang Dist., 812 Kaohsiung, Taiwan; Tel.: 886-7-8036783-3441, Fax: 886- 7- 8063346; E-mail: scarchenonecom.tw.

 Global reach, higher impact
Global reach, higher impact