3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(7):1544-1554. doi:10.7150/ijms.103141 This issue Cite
Research Paper
Targeted inhibition of integrin αVβ3 induces cytotoxicity and suppresses migration ability in ovarian cancer cells and tumor spheroids
1. Institute of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
2. Department of Nursing, National Taichung University of Science and Technology, Taichung, 40640, Taiwan.
3. Institute and Department of Food Science, Central Taiwan University of Science and Technology, Taichung, 40601, Taiwan.
4. School of Medicine, Chung Shan Medical University, Taichung 40201, Taiwan.
5. Division of Medical Oncology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, 40201, Taiwan.
6. Department of Obstetrics and Gynecology, Chung Shan Medical University Hospital, Taichung, 40201, Taiwan.
7. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, 40201, Taiwan.
Received 2024-9-2; Accepted 2025-2-9; Published 2025-2-28
Abstract
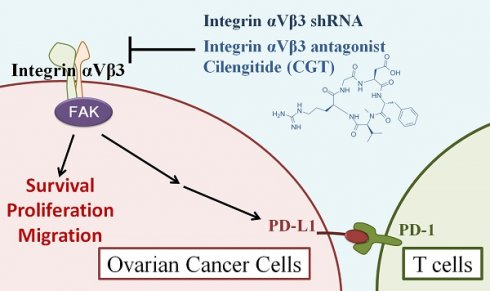
Ovarian cancer is a gynecological malignancy that has poor prognosis and high lethality. Integrin αVβ3 is highly expressed in solid cancer cells, including ovarian cancer, and is important in proliferation and cell migration. In this study, we performed two-dimensional (2D) and three‑dimensional (3D) cell culture systems to investigate the potential of integrin αVβ3 as a therapeutic target for ovarian cancer. Inhibition of integrin αVβ3 by antagonist cilengitide (CGT) and shRNA significantly reduce the cell viability of ovarian cancer cells. Co-treatment of CGT and cisplatin induced synergistic cytotoxicity in SKOV3 cells. CGT reduced the protein expressions of phospho-FAK, CD44, and PD-L1. CGT reduced mitochondrial membrane potential and induced apoptotic cell death. To mimic the tumor growth in the extracellular matrix, a tumor spheroid formation assay was performed with Matrigel and epidermal growth factor (EGF). CGT reduced the size of spheroids that grew in 50% Matrigel with or without EGF induction. CGT also enhanced the inhibiting effect of T cells on tumor spheroids. The cell migration ability of SKOV3 cells was blunted by CGT by tumor spheroid-based migration assay. This study used 2D and 3D cell models to provide novel insight into ovarian cancer therapy by targeting integrin αVβ3 and suitable cell models for searching integrin αVβ3-targeting drugs.
Keywords: 3D cell culture, Cell migration, extracellular matrix, Integrin αVβ3, ovarian cancer
1. Introduction
Ovarian cancer is a gynecological malignancy that has poor prognosis and high lethality [1]. Surgery and chemotherapy are the common treatments for ovarian cancer. Bevacizumab and olaparib are the few targeted therapy drugs that approved to treat ovarian cancer [2-4]. Therefore, it is important to develop new targeted therapy drugs for ovarian cancer therapy.
Integrins are critical proteins that act as receptor of extracellular matrix (ECM) and induce several signals for cell survival, proliferation and motility [5, 6]. Integrin αVβ3 is highly expressed in cancer cells from different solid cancer, including ovarian cancer [7]. In recent years, integrin αVβ3 is considered a potential target for cancer therapy [7, 8]. Integrin αVβ3 promotes the proliferation of ovarian cancer cells via crosstalk with thyroid hormones and estrogen receptor alpha [9, 10]. Anoikis resistance of ovarian cancer cells is triggered by activation of integrin αVβ3 [11]. Interaction of integrin αVβ3 and vitronectin enhances the cell migration ability of ovarian cancer cells [12, 13]. These evidences hint that integrin αVβ3 is a potential therapeutic target to inhibit ovarian cancer progression.
In this study, two-dimensional and three‑dimensional cell culture systems were performed to investigate the potential of integrin αVβ3 as a therapeutic target for ovarian cancer. Inhibition of integrin αVβ3 significantly inhibited cell viability and reduced the migration ability of ovarian cancer cells. This study provided insight into integrin αVβ3-targeting therapy in ovarian cancer and the cell models used to investigate the integrin αVβ3-targeting drugs.
2. Materials and methods
2.1 Cell culture and chemicals
SKOV3 cells (ATCC, HTB-77) and TOV-21G cells (ATCC, CRL-3577) were obtained from the American Type Culture Collection. SKOV3 cells were cultured in Minimum Essential Medium (MEM) (GIBCO, 41500-034) supplemented with 10% fetal bovine serum and 4.5 g Glucose per L. TOV-21G cells were cultured in 1:1 mixture of MCDB 105 medium (Sigma-Aldrich, M6395) and Medium 199 (GIBCO, 31100035), and supplemented with 15% fetal bovine serum. Cilengitide (22289) was purchased from Cayman Chemical (Ann Arbor, MI). Cisplatin (P4394) was purchased from Sigma (St. Louis, MO, USA).
2.2 MTT assay
After treatment with indicated drugs for 48 h, the medium with drugs was removed, and 100 μL of fresh medium containing 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-25-diphenyltetrazolium bromide (MTT; Sigma, M 2128) was added to the wells and incubated for another 3 h. The blue water-insoluble MTT-formazan crystals are solubilized with 100 μl dimethyl sulfoxide (DMSO) (Merck, 1.02931.1000) and the intensity is measured colorimetrically at a wavelength of 570 nm.
2.3 Western blot assay
Anti-integrin αV (#4711, Cell Signaling, Danvers, MA, USA), anti-integrin β3 (#13166, Cell Signaling, Danvers, MA, USA), anti-PD-L1 (NBP1-76769, Novus Biologicals, Centennial, CO, USA), anti-phospho-FAK Tyr397 (#8556, Cell Signaling, Danvers, MA, USA), anti-CD44 (#3570, Cell Signaling, Danvers, MA, USA), anti-cleaved PARP (#5625, Cell Signaling, Danvers, MA, USA), anti-cleaved caspase-7 (#9491, Cell Signaling, Danvers, MA, USA), and anti-β-actin (AC-40, Sigma, St. Louis, Missouri) were used to detect the protein expression levels of integrin αV, integrin β3, PD-L1, phospho-FAK Tyr397, CD44, cleaved PARP, cleaved caspase-7, and β-actin. The complete protocol for Western blot assay has been described in a previous publication [14].
2.4 Integrin αVβ3 detection by immunocytochemistry and flow cytometry
SKOV3 and TOV-21G cells were seeded onto coverslips in 60 mm plates and then incubated for 16 h. The complete protocol for fixation, permeabilization, and blocking was described in a previous publication [15]. The FITC conjugated anti-integrin αVβ3 antibody (MAB1976F, Merck, Darmstadt, Germany) was used to detect the integrin αVβ3 in cells. After overnight hybridization at 4 °C, the cells were DAPI (4',6-diamidino-2-phenylindole) for 30 min at room temperature and observed under a fluorescence microscope.
To investigate the surface integrin αVβ3 expression in SKOV3 and TOV-21G cells, the cells were stained and analyzed by flow cytometry. SKOV3 and TOV-21G cells were seeded onto 60 mm dish and then incubated for 16 h. Both cells were collected by Accutase cell detachment solution (SCR005, Merck, Darmstadt, Germany). Cells were stained by FITC conjugated anti-integrin αVβ3 antibody. The non-fluorescent anti-integrin αVβ3 antibody was used as competing antibody. After staining, the cells were analyzed by flow cytometry.
2.5 Apoptosis and mitochondrial membrane potential assay
After treatment of CGT for 48h, the SKOV3 cells were used to investigate apoptosis and mitochondrial membrane potential. Annexin V-FITC Apoptosis Detection Kit (556 547, BD Biosciences, CA, USA) and 5,5,6,6'-tetrachloro-1,1,3,3'-tetraethylbenzimi-dazolylcarbocy-anine iodide dye (JC-1, T3168, Invitrogen, Carlsbad, CA, USA; Thermo Fisher Scientific, Inc., San Jose, CA, USA) were used to analyze the apoptosis and mitochondrial membrane potential. The complete protocols for these analyses are described elsewhere [14, 16].
2.6 Tumor spheroid formation and migration assay
SKOV3 cells (1 × 103 cells) were seeded into a well of ultra-low attachment 96-well plate (Corning Inc., Corning, NY, USA) containing 100 μL of culture medium (Minimum Essential Medium supplemented with 10% fetal bovine serum and 4.5 g Glucose per L). After incubation for 96 h, 200 μL of fresh medium containing CGT was added to the well. To examine the effect of ECM on spheroid formation, after seeding for 96h, the Matrigel (BD Biosciences, 354234) was added to the well to lead to spheroid growth in the 50% Matrigel. After the Matrigel form the gel, 100 μL culture medium containing CGT with or without epidermal growth factor (EGF) was added to the well. The spheroids were incubated for another 7 days and observed under inverted light microscopy. The volume of the spheroids was calculated by the formula 0.5 × larger diameter (mm) × small diameter (mm)2.
To analyze the effect of CGT on T cells induced cytotoxicity on SKOV3 tumor spheroid, Jurkat T-cells were used to perform the assay. The method of Jurkat T-cells activation by T Cell Stimulation Cocktail (eBioscience) was based on the previous study [17]. After 96 h incubation for SKOV3-EGFP tumor spheroid formation (1 × 103 cells/well of ultra-low attachment 96-well plate), the Jurkat T-cells (1 × 104 cells/well of ultra-low attachment 96-well plate) in 200 μL of culture medium in the presence of CGT and 1× T Cell Stimulation Cocktail were added to the well. The fluorescence intensity was analyzed by ImageJ software.
To investigate the cell surface PD-L1 expression, the spheroids with or without treating with CGT for 7 days were removed to eppendorf tube. After washing with PBS, the spheroids were stained by Alexa Fluor 488 conjucated PD-L1 antibody (#25048, Cell Signaling, Danvers, MA, USA) that specific recognizes extracellular domain of PD-L1. After staining, the spheroids were removed to 60 mm dish and observed under inverted fluorescence microscope. The fluorescence intensity was analyzed by ImageJ software.
To investigate the cell migration, the spheroids after treating with CGT for 7 days were removed to the 6-well plate for attachment. The spreading area of SKOV3 cells migrated from the tumor spheroid was measured by ImageJ software at indicated time.
3. Results
3.1 Integrin αVβ3 inhibition decreases cell viability and increases cisplatin cytotoxicity in ovarian cancer cells
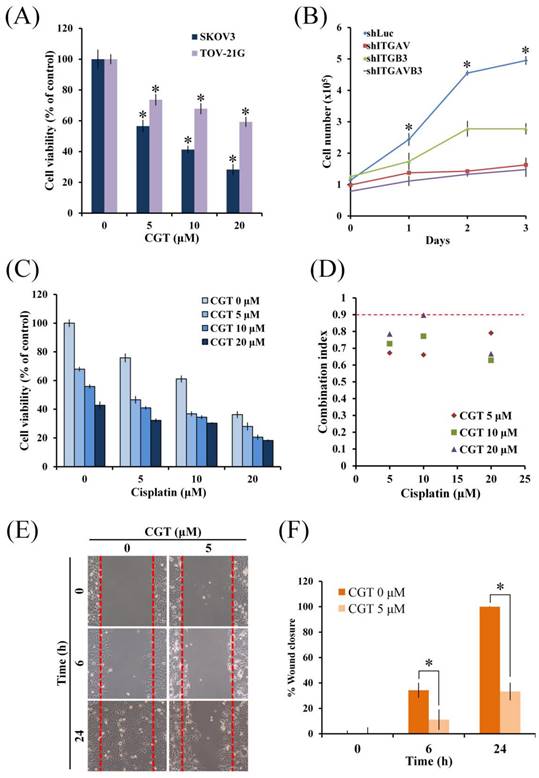
To investigate the cytotoxic effect of cilengitide (CGT), an antagonist of integrin αVβ3 and αVβ5, MTT assay was performed using two ovarian cancer cell lines. As shown in Figure 1A, CGT significantly reduced the cell viability of SKOV3 and TOV-21G cells in a dose-dependent manner. Knockdown of integrin αV (ITGAV) and β3 (ITGB3) by shRNA was used to further evaluate the role of integrin αV and β3 in cell growth of SKOV3 cells. The proliferation of SKOV3 shITGAV and shITGB3 cells was significantly lower than SKOV3 shLuc cells (Figure 1B). The rate of proliferation of SKOV3 shITGAVB3 cells, double knockdown of integrin αV and β3, was only slightly lower than SKOV3 shITGAV cells (Figure 1B). Furthermore, the combinational effect of CGT and cisplatin on cell viability of ovarian cancer cells was performed. As shown in Figure 1C, CGT increased cisplatin-induced cytotoxicity in SKOV3 cells. Co-treatment of CGT and cisplatin induced synergistic cytotoxic effects (Figure 1D). To investigate the effect of CGT on cell migration, wound healing assay was performed by Culture-Insert. The migration ability of SKOV3 cells was significantly inhibited by CGT (Figures 1E, F). These results demonstrated that CGT inhibits the cell survival and migration, and enhances cisplatin-mediated cytotoxicity.
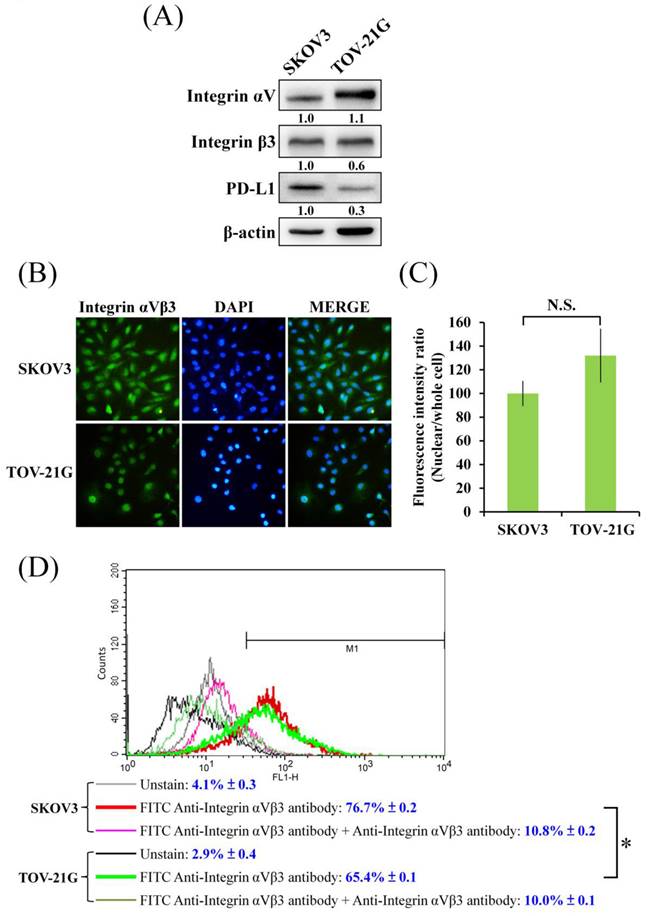
In Figure 1A, different level of cell viability inhibition by CGT between SKOV3 and TOV-21G cells was found. Because integrin αVβ3 is one of the major targets of CGT; therefore, the protein expressions of integrin αV and β3 were detected to elucidate the difference. As shown in Figure 2A, without standardized by the β-actin, the protein level of integrin αV in SKOV3 cells was lower than that in TOV-21G cells, and there was no marked difference in the expression of integrin β3 between SKOV3 and TOV-21G cells. However, after standardized by the β-actin, the protein level of integrin αV is no marked difference between SKOV3 and TOV-21G cells, but the expression of integrin β3 in TOV-21G cells was lower than it in SKOV3 cells. Immunocytochemistry was performed to further investigate the expression and distribution of integrin αVβ3 in SKOV3 and TOV-21G cells. The integrin αVβ3 heterodimer was differently localized in cells between SKOV3 and TOV-21G cells (Figure 2B). Integrin αVβ3 heterodimer was evenly distributed in SKOV3 cells (Figure 2B). A tendential increase of nuclear-localized integrin αVβ3 was shown in TOV-21G cells compared to SKOV3 cells (Figures 2B, C). Furthermore, the expressions of cell surface integrin αVβ3 in SKOV3 and TOV-21G cells were analyzed by flow cytometry. As shown in Figure 2D, it showed that SKOV3 cells have 10% more expression of surface integrin αVβ3 than TOV-21G cells. These results demonstrated that different expression levels and distribution of integrin αVβ3 heterodimer may make different cytotoxic effects of CGT between SKOV3 and TOV-21G cells.
3.2 Integrin αVβ3 inhibition decreases the expressions of downstream protein
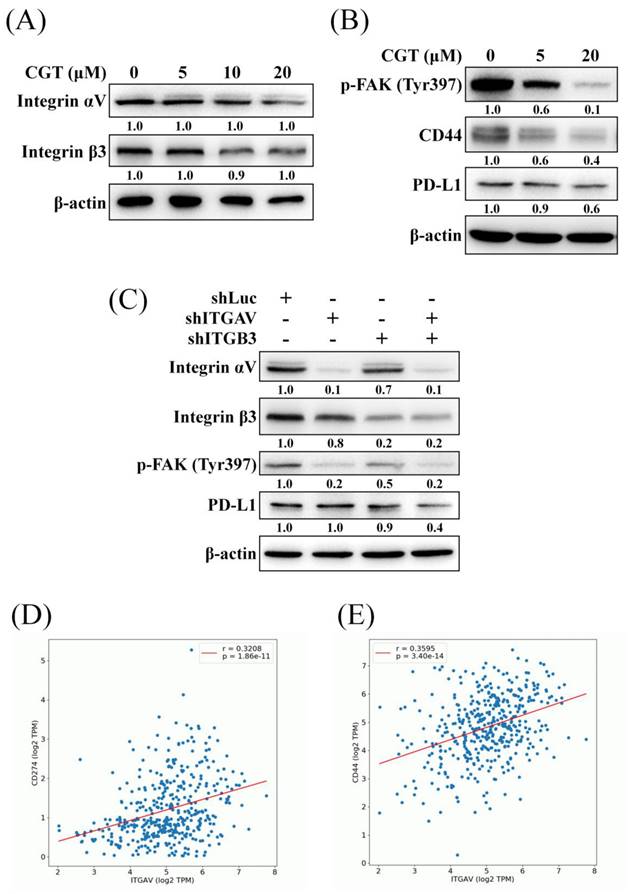
To investigate the effect of CGT on the expressions of integrin αV and β3 and the downstream protein, the western blot assay was performed. As shown in Figure 3A, CGT did not alter the protein expressions of integrin αV and β3 in SKOV3 cells. CGT inhibited the activity of FAK, the important downstream mediator of integrins, in a dose-dependent manner (Figure 3B). Integrin αVβ3 and CD44 are the receptors of osteopontin (OPN) and crosstalk via regulating signaling pathways [18]. The expression of CD44 was reduced by CGT (Figure 3B). In addition, PD-L1, an inhibitory ligand of PD-1 to protect cancer cells from direct attack by cytotoxic T cells, is also regulated by the integrin αVβ3 signaling pathway [19]. The result showed that CGT decreases PD-L1 expression in SKOV3 cells (Figure 3B). However, CGT did not reduce but slightly increased the mRNA expressions of CD44 and PD-L1 in SKOV3 cells (Supplementary Figure 1). The above effects on protein expression induced by integrin αVβ3 inhibition were confirmed by knockdown of integrin αV and β3. The efficiency of alone- or double-knockdown of integrin αV and β3 was detected by western blot analysis (Figure 3C). Alone-knockdown of integrin αV or β3 obviously inhibited the phosphorylation of FAK, and double-knockdown did not further decrease the activity of FAK (Figure 3C). Moreover, only double-knockdown of integrin αV and β3 could reduce the expression of PD-L1 (Figure 3C). Positive correlations have been found between the gene expression of integrin αV (ITGAV) and PD-L1 (CD274) or CD44 in ovarian serous cystadenocarcinoma from the OncoDB online database (Figures 3D, E). These results demonstrated that inhibition of integrin αVβ3 decreases the activity of FAK and the expressions of CD44 and PD-L1.
Effect of CGT and knockdown of integrin αVβ3 on cell viability in ovarian cancer cells. (A) SKOV3 and TOV-21G (4 × 103 cells/well of 96-well plate) were treated with various concentrations of CGT (0, 5, 10, and 20 μM) for 48h. Cell viability was analyzed by MTT assay. (B) The indicated cells were harvested by trypsin, stained with trypan blue, and counted under an inverted microscope at indicated times. (C) After combined treatment with CGT and cisplatin for 48h, MTT assay was performed to analyze the cell viability of SKOV3 cells. (D) Combination index of cotreatment of CGT and cisplatin for 48 on SKOV3 cells was calculated by software Compusyn 1.0. The definitions of combination index are synergistic effect (combination index < 0.9), additive effect (0.9 < combination index < 1.1), and antagonism (combination index > 1). (E) Representative images of wound healing of SKOV3 cells with or without CGT treatment. (F) Values of percentage wound closure. The areas lacking cells were analyzed by ImageJ. The symbol '*' indicates P < 0.05.
Expression and localization of integrin αVβ3 in ovarian cancer cells. (A) Total cell lysates of SKOV3 and TOV-21G cells (4 × 105 cells of 60 mm dish) were used to investigate the indicated protein expression Western blot assay. (B) SKOV3 and TOV-21G cells were stained with FITC-conjugated integrin αVβ3 antibody and DAPI, and investigate the distribution of integrin αVβ3 in both ovarian cancer cells. (C) Fluorescence intensity of nuclear or whole cell was analyzed by ImageJ. (D) The cells were stained with FITC-conjugated anti-integrin αVβ3 antibody. Non-fluorescent anti-integrin αVβ3 antibody was performed as competing antibody. The unstained and stained cells were analyzed by flow cytometry. The symbol '*' indicates P < 0.05. N.S., not significant.
Effect of CGT and knockdown of integrin αVβ3 on expressions of downstream protein. (A) The sample of Figure 3A and Figure 4C are the same. After treatment of various concentrations of CGT (0, 5, 10, and 20 μM) for 48h, total cell lysates of SKOV3 cells (4 × 105 cells of 60 mm dish) were analyzed by Western blot assay to detect the protein expressions of integrin αV and β3. (B) The protein expressions of phospho-FAK, CD44, and PD-L1 in SKOV3 cells after treating with CGT for 48h were analyzed by Western blot assay. (C) Western blot assay was performed to detect the expressions of the indicated protein in SKOV3 shLuc, shITGAV, shITGB3, and shITGAVB3 cells. β-actin served as a loading control. The results showed correlations between the gene expression of integrin αV and (D) CD274 (PD-L1) or (E) CD44 by the OncoDB database.
3.3 CGT induces apoptosis in SKOV3 cells
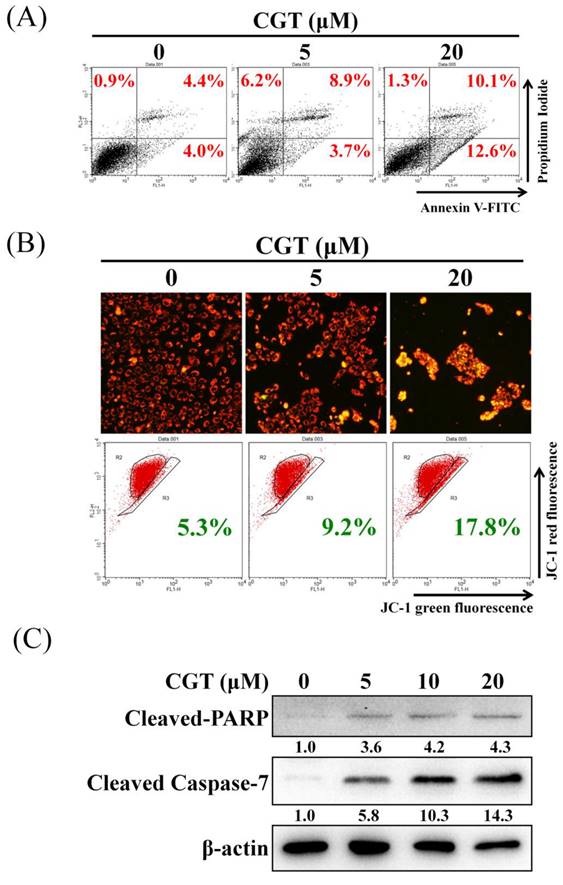
To elucidate the mechanism of cell viability inhibition by CGT, the annexin V/propidium iodide staining assay was performed to investigate cell death. As shown in Figure 4A, CGT induced cell death in SKOV3 cells. The mitochondrial membrane potential was suppressed by CGT in a dose-dependent manner (Figure 4B). The expressions of cleaved PARP and cleaved caspase-7 were increased by CGT (Figure 4C). These results demonstrated that CGT induces apoptotic cell death in SKOV3 cells.
Effect of CGT on apoptosis induction. (A) After treatment with CGT (0, 5, and 20 μM) for 48 h, SKOV3 cells (4 × 105 cells of 60 mm dish) were stained with annexin V-FITC/PI and analyzed by flow cytometry. (B) Mitochondrial membrane potential in SKOV3 cells (4 × 105 cells of 60 mm dish) treated with CGT (0, 5, and 20 μM) for 48 h was analyzed by fluorescence microscope and flow cytometry. (C) The sample of Figure 3A and Figure 4C are the same. The protein expressions of cleaved PARP and cleaved caspase-7 in SKOV3 cells after treating with CGT for 48h were analyzed by Western blot assay. β-actin served as a loading control.
3.4 CGT inhibits SKOV3 tumor spheroid formation in extracellular matrix
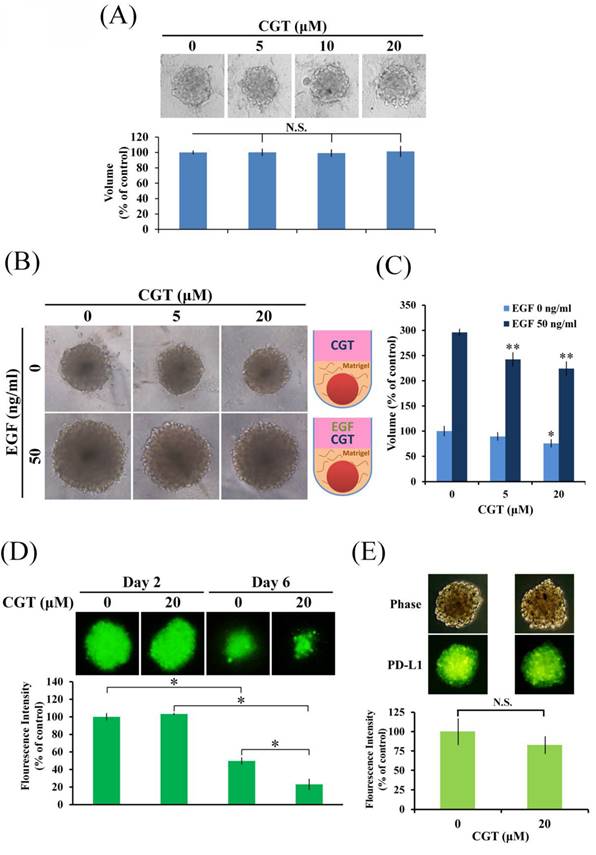
CGT has been investigated in in vitro models not in vivo. To correlate the findings with tumor suppression, 3D tumor spheroid formation assay was performed to investigate drug effects in vitro. As shown in Figure 5A, CGT did not alter the size of tumor spheroid of SKOV3 cells. There are also no differences in the size of tumor spheroid formation between SKOV3 shLuc and SKOV3 shITGAVB3 cells (Supplementary Figure 2). Integrins are the receptor of ECM; therefore, we further examine the effect of CGT on tumor spheroid formation in ECM. The volume of SKOV3 tumor spheroids was inhibited by CGT (Figures 5B, C). Furthermore, epidermal growth factor (EGF), an important protein in stimulating tumor growth, was used to promote the growth of SKOV3 tumor spheroids. The size of the spheroids was markedly increased after EGF stimulation, and the EGF-induced spheroid growth was significantly reduced by CGT (Figures 5B, C). In addition, the tumor spheroid model using SKOV3-EGFP cells was performed to investigate the effect of CGT on T cell-mediated cytotoxicity. As shown in Figure 5D, CGT significantly enhanced the inhibiting effect of T cells on SKOV3-EGFP tumor spheroid. Cell surface PD-L1 expression was detected by Alexa Fluor 488 conjucated PD-L1 antibody that specific recognizes extracellular domain of PD-L1. A tendential decrease in spheroid surface PD-L1 expression was shown after CGT treatment (Figure 5E). These results demonstrated that CGT inhibits ECM-mediated tumor spheroid growth and enhances the inhibiting function of T cells on the spheroid.
Effect of CGT on tumor spheroid formation. (A) SKOV3 cells (1 × 103 cells/well of 96-well dish) were seeded onto ultra-low attachment 96-well plates. After 96 h incubation for spheroid formation, CGT-containing medium was added to the well, and the spheroids were incubated for another 7 days. The volumes of spheroids were determined by the formula 0.5 × larger diameter × small diameter2. Data show the relative spheroid volume, and the volume of spheroid without treatment was set at 100%. (B) Effect of CGT on SKOV3 tumor spheroids growth in 50% Matrigel with or without EGF stimulation. (C) Volume of SKOV3 tumor spheroids in 50% Matrigel treating with CGT and EGF. (D) After 96 h incubation for spheroid formation, the spheroids were subsequently co-cultured with Jurkat T-cells for additional 48 hours in the presence of CGT and 1× T Cell Stimulation Cocktail. (E) Expression of surface PD-L1 in SKOV3 tumor spheroid with or without CGT treatment. The symbol '*' and '**' indicates P < 0.05 and P < 0.001, respectively. N.S., not significant.
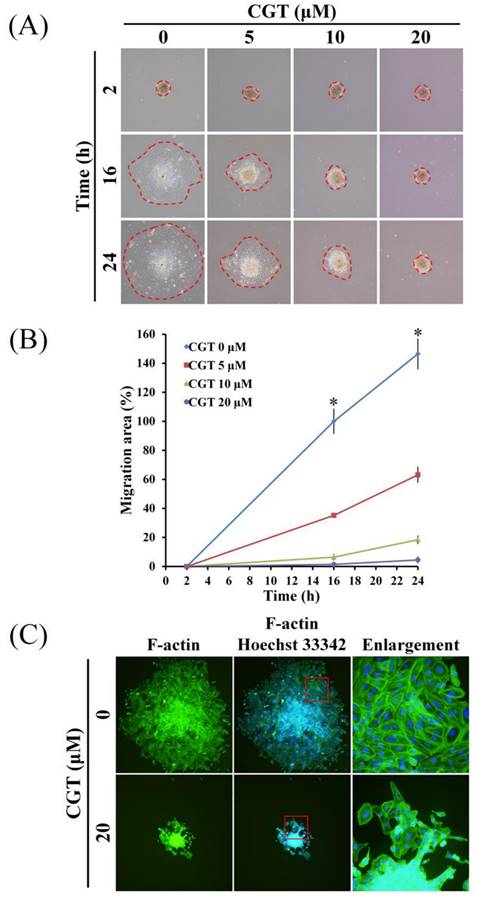
Effect of CGT on cell migration of tumor spheroid. (A) The spheroids with or without CGT treatment were removed to the 6-well plate for attachment and migration. (B) The spreading area of SKOV3 cells migrated from the tumor spheroid was measured by ImageJ software. (C) After attachment for 72h, the tumor spheroid and migrated cells was fixed and stained by phalloidin FITC reagent for detect the F-actin. The symbol '*' indicates P < 0.05.
3.5 CGT inhibits cell migration and F-actin formation of SKOV3 tumor spheroid
To investigate the effect of CGT on cell migration of ovarian cancer cells, SKOV3 tumor spheroid-based migration assay was performed. As shown in Figure 6A, CGT markedly reduced the cells migrated from the SKOV3 tumor spheroid. The spreading area of SKOV3 cells migrated from the tumor spheroid was significantly reduced by CGT in a dose-dependent manner (Figure 6B). Formation of F-actin cytoskeleton is important for cell migration; therefore, the F-actin staining assay was performed to investigate the effect of CGT on F-actin formation in SKOV3 cells migrated from the tumor spheroid. CGT decreased the expression of F-actin in the SKOV3 cells migrated from the tumor spheroid. These results demonstrated that CGT decreases the ability of migration of SKOV3 cells using tumor spheroid-based migration assay (Figure 6C).
4. Discussion
Integrins play roles in ovarian cancer progression and treatment resistance [20, 21]. Integrin αVβ5, but not αVβ3, is activated to protect the ovarian cancer cells from TRAIL-induced cell death [22]. MS-275, a HDAC inhibitor, inhibits the tumor spheroid formation of ovarian cancer cells and impacts on Talin‑1‑α5β1‑integrin‑mediated actin cytoskeleton and extracellular matrix protein remodeling [23]. CGT inhibits the cancer cell viability mainly via decreasing the cell attachment and inducing apoptosis and anoikis [24-26]. The nuclear-localized integrin αVβ3 has been found in high-grade serous ovarian cancer and can partially increase the cell proliferation [27]. The nuclear-localized integrin αVβ3 does not affect cell migration, suggesting that nuclear-localized integrin αVβ3 loses its function on cell adhesion [27]. The article demonstrated that nuclear-localized integrin αVβ3 performed moonlighting functions in ovarian cancer pathogenesis [27]. In this study, CGT decreased the cell viability in both ovarian cancer cell lines; however, the inhibiting effects of CGT on the cell viability of SKOV3 and TOV-21G cells were different (Figure 1A). The difference in inhibiting effect on ovarian cancer cells prompted us to investigate the expression and localization of integrin αV and β3. As shown in Figure 2A, the protein expression of integrin β3 in TOV-21G cells was lower than it was in SKOV3 cells. Furthermore, different from SKOV3 cells, the integrin αVβ3 showed a more nuclear localization and less membrane localization in TOV-21G cells (Figures 2B, C, D). Taken together, we suggested that the expression and localization of integrin αVβ3 may be factors that determine the effectiveness of CGT.
Tumor spheroid is a 3D cell culture model that is a good in vitro assay for analyzing drug effects [28-30]. A previous study found that CGT does not affect the growth of spheroids of malignant pleural mesothelioma cells [26]. Our result was consistent with the results in that article. We found that CGT and double-knockdown of integrin αV and β3 did not alter the size of SKOV3 spheroids (Figure 5A and Supplementary Figure 2). To mimic the ECM effect on integrins during tumor growth, the SKOV3 tumor spheroids were cultured in Matrigel. Interestingly, CGT significantly reduced the size of SKOV3 spheroids cultured in Matrigel with or without EGF stimulation (Figure 5B). Furthermore, we found that CGT slows down the speed of spheroids attachment in a dose-dependent manner during spheroids migration assay (data not shown), suggesting that integrin αVβ3 and αVβ5 expressed on the surface of the SKOV3 spheroids. Taken together, these results suggested that integrins, at least integrin αVβ3 and αVβ5, on the spheroids were not participating in the growth of the floating tumor spheroids. The floating spheroids may be an assay to investigate the compounds specifically targeting integrins.
PD-L1 is a critical protein in cancer cells to inhibit the function of cytotoxic T cells [31]. Integrin αVβ3 positively regulates the expression of PD-L1 via activating STAT1 [19]. CGT enhanced the inhibiting effect of anti-PD-L1 antibody on murine melanoma [32]. In the present study, we also found that CGT and double-knockdown of integrin αV and β3 inhibited the expression of PD-L1 in SKOV3 cells (Figures 3B, C). CGT promoted the cytotoxic effect of T cells on SKOV3 tumor spheroids (Figure 5D). The expressions of surface PD-L1 were partially decreased by CGT in the spheroids (Figure 5E). Taken together, we suggested that PD-L1 inhibition by CGT participate in CGT-enhanced T cells-mediated spheroid shrinkage.
5. Conclusion
In this study, CGT induced apoptosis and inhibited spheroid migration in ovarian cancer cells. This is the first study that uses 2D and 3D cell culture models to prove that integrin αVβ3 is a potential therapeutic target for ovarian cancer.
Abbreviations
2D: two-dimensional; 3D: three‑dimensional; EGF: epidermal growth factor; ECM: extracellular matrix.
Supplementary Material
Supplementary figures.
Acknowledgements
Funding
This research was funded by research grants from Taiwan National Science and Technology Council (112-2314-B-040 -008 -; 111-2320-B-040 -018 -MY3) and Chung Shan Medical University Hospital (CSH-2024-C-049).
Author contributions
I-Lun Hsin: Validation, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Visualization; Ling-Yen Chiu: Validation, Formal analysis, Investigation; Jiunn-Liang Ko: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration; Po-Hui Wang: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition. Pei-Ju Wu: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data availability statement
Data from the manuscript are available from the corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Veneziani AC, Gonzalez-Ochoa E, Alqaisi H, Madariaga A, Bhat G, Rouzbahman M. et al. Heterogeneity and treatment landscape of ovarian carcinoma. Nat Rev Clin Oncol. 2023;20:820-42
2. Zhu Y, Yang Q, Liu K, Cao H, Zhu H. Olaparib plus bevacizumab as a first-line maintenance treatment for patients with advanced ovarian cancer by molecular status: an updated PAOLA-1 based cost-effectiveness analysis. J Gynecol Oncol. 2024;35:e2
3. Ray-Coquard I, Leary A, Pignata S, Cropet C, Gonzalez-Martin A, Marth C. et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 2023;34:681-92
4. Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R. et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. 2019;381:2416-28
5. Yousefi H, Vatanmakanian M, Mahdiannasser M, Mashouri L, Alahari NV, Monjezi MR. et al. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene. 2021;40:1043-63
6. Kang YT, Chang HY, Hsieh YC, Chou CH, Hsin IL, Ko JL. Integrin alphaV Inhibition by GMI, a Ganoderma Microsporum Immunomodulatory Protein, Abolish Stemness and Migration in EGFR-Mutated Lung Cancer Cells Resistant to Osimertinib. Environ Toxicol. 2024;39:5238-49
7. Cheng TM, Chang WJ, Chu HY, De Luca R, Pedersen JZ, Incerpi S. et al. Nano-Strategies Targeting the Integrin alphavbeta3 Network for Cancer Therapy. Cells. 2021;10:1684
8. Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev Res. 2008;69:329-39
9. Shinderman-Maman E, Cohen K, Weingarten C, Nabriski D, Twito O, Baraf L. et al. The thyroid hormone-alphavbeta3 integrin axis in ovarian cancer: regulation of gene transcription and MAPK-dependent proliferation. Oncogene. 2016;35:1977-87
10. Hsieh MT, Wang LM, Changou CA, Chin YT, Yang YSH, Lai HY. et al. Crosstalk between integrin alphavbeta3 and ERalpha contributes to thyroid hormone-induced proliferation of ovarian cancer cells. Oncotarget. 2017;8:24237-49
11. Dolinschek R, Hingerl J, Benge A, Zafiu C, Schuren E, Ehmoser EK. et al. Constitutive activation of integrin alphavbeta3 contributes to anoikis resistance of ovarian cancer cells. Mol Oncol. 2021;15:503-22
12. Hapke S, Kessler H, Arroyo de Prada N, Benge A, Schmitt M, Lengyel E. et al. Integrin alpha(v)beta(3)/vitronectin interaction affects expression of the urokinase system in human ovarian cancer cells. J Biol Chem. 2001;276:26340-8
13. Reuning U. Integrin alphavbeta3 promotes vitronectin gene expression in human ovarian cancer cells by implicating rel transcription factors. J Cell Biochem. 2011;112:1909-19
14. Wu PJ, Hsin IL, Hung WL, Lee MS, Wang PH, Ko JL. Combination treatment with cyclosporin A and arsenic trioxide induce synergistic cell death via non-apoptotic pathway in uterine cervical cancer cells. Chem Biol Interact. 2022;368:110177
15. Hsin IL, Chiu LY, Ou CC, Wu WJ, Sheu GT, Ko JL. CD133 inhibition via autophagic degradation in pemetrexed-resistant lung cancer cells by GMI, a fungal immunomodulatory protein from Ganoderma microsporum. Br J Cancer. 2020;123:449-58
16. Chiu LY, Hsin IL, Tsai JN, Chen CJ, Ou CC, Wu WJ. et al. Combination treatment of Src inhibitor Saracatinib with GMI, a Ganoderma microsporum immunomodulatory protein, induce synthetic lethality via autophagy and apoptosis in lung cancer cells. J Cell Physiol. 2021;236:1148-57
17. Lu CS, Lin CW, Chang YH, Chen HY, Chung WC, Lai WY. et al. Antimetabolite pemetrexed primes a favorable tumor microenvironment for immune checkpoint blockade therapy. J Immunother Cancer. 2020;8:e001392
18. Hao C, Lane J, Jiang WG. Osteopontin and Cancer: Insights into Its Role in Drug Resistance. Biomedicines. 2023;11:197
19. Vannini A, Leoni V, Barboni C, Sanapo M, Zaghini A, Malatesta P. et al. alphavbeta3-integrin regulates PD-L1 expression and is involved in cancer immune evasion. Proc Natl Acad Sci U S A. 2019;116:20141-50
20. Sawada K, Ohyagi-Hara C, Kimura T, Morishige K. Integrin inhibitors as a therapeutic agent for ovarian cancer. J Oncol. 2012;2012:915140
21. Kobayashi M, Sawada K, Kimura T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers (Basel). 2017;9:83
22. Lane D, Goncharenko-Khaider N, Rancourt C, Piche A. Ovarian cancer ascites protects from TRAIL-induced cell death through alphavbeta5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene. 2010;29:3519-31
23. Terri M, Sandoval P, Bontempi G, Montaldo C, Tomero-Sanz H, de Turris V. et al. HDAC1/2 control mesothelium/ovarian cancer adhesive interactions impacting on Talin-1-alpha5beta1-integrin-mediated actin cytoskeleton and extracellular matrix protein remodeling. J Exp Clin Cancer Res. 2024;43:27
24. Zhu Z, Fang C, Xu H, Yuan L, Du Y, Ni Y. et al. Anoikis resistance in diffuse glioma: The potential therapeutic targets in the future. Front Oncol. 2022;12:976557
25. Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I. et al. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer Res. 2008;27:86
26. Cheng NC, van Zandwijk N, Reid G. Cilengitide inhibits attachment and invasion of malignant pleural mesothelioma cells through antagonism of integrins alphavbeta3 and alphavbeta5. PLoS One. 2014;9:e90374
27. Seraya-Bareket C, Weisz A, Shinderman-Maman E, Teper-Roth S, Stamler D, Arbib N. et al. The identification of nuclear alphavbeta3 integrin in ovarian cancer: non-paradigmal localization with cancer promoting actions. Oncogenesis. 2020;9:69
28. Pinto B, Henriques AC, Silva PMA, Bousbaa H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics. 2020;12:1186
29. Hsin IL, Wu PJ, Tang SC, Ou CC, Chang HY, Shen HP. et al. beta-catenin inhibitor ICG-001 suppress cell cycle progression and induce autophagy in endometrial cancer cells. J Cell Physiol. 2023;238:2440-50
30. Hsin IL, Shen HP, Chang HY, Ko JL, Wang PH. Suppression of PI3K/Akt/mTOR/c-Myc/mtp53 Positive Feedback Loop Induces Cell Cycle Arrest by Dual PI3K/mTOR Inhibitor PQR309 in Endometrial Cancer Cell Lines. Cells. 2021;10:2916
31. Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19:287-305
32. Pan X, Yi M, Liu C, Jin Y, Liu B, Hu G. et al. Cilengitide, an alphavbeta3-integrin inhibitor, enhances the efficacy of anti-programmed cell death-1 therapy in a murine melanoma model. Bioengineered. 2022;13:4557-72
Author contact
![]() Corresponding author: Jiunn-Liang Ko, Po-Hui Wang and Pei-Ju Wu, Institute of Medicine, Chung Shan Medical University, 110, Sec. 1, Chien-Kuo N. Road, Taichung, Taiwan 40203; TEL.: 886-4-24730022ext11694; FAX: 886-4-24751101; E-mail: jlkoedu.tw.
Corresponding author: Jiunn-Liang Ko, Po-Hui Wang and Pei-Ju Wu, Institute of Medicine, Chung Shan Medical University, 110, Sec. 1, Chien-Kuo N. Road, Taichung, Taiwan 40203; TEL.: 886-4-24730022ext11694; FAX: 886-4-24751101; E-mail: jlkoedu.tw.

 Global reach, higher impact
Global reach, higher impact