3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(7):1493-1503. doi:10.7150/ijms.106518 This issue Cite
Research Paper
Impact of Chronic Kidney Disease on Aortic Dissection in Patients with Polycystic Kidney Disease: A Fifteen-year Nationwide Population-based Cohort Study in Taiwan
1. Department of Surgery, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
2. Department of Surgery, National Defense Medical Center, Division of Cardiovascular Surgery, Tri-Service General Hospital, Taipei, Taiwan.
3. Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
4. School of Public Health, National Defense Medical Center, Taipei, Taiwan.
5. Department of Microbiology & Immunology, National Defense Medical Center, Taipei, Taiwan.
6. Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
7. Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan City, Taiwan.
8. Department of Biomedical Sciences and Engineering, Institute of Systems Biology and Bioinformatics, National Central University, Taoyuan, Taiwan.
9. Department of Life Sciences, National Central University, Taoyuan City, Taiwan.
Received 2024-11-7; Accepted 2025-2-15; Published 2025-2-26
Abstract
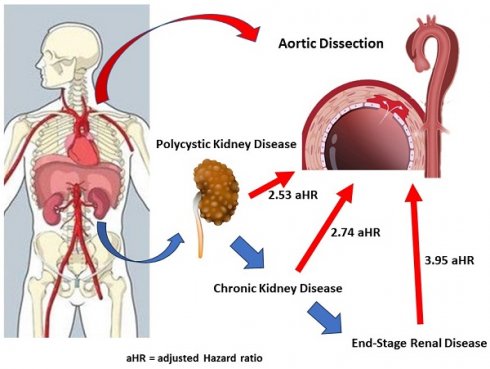
Background: Aortic dissection is a life-threatening condition associated with polycystic kidney disease (PKD). Additionally, PKD often progresses to chronic kidney disease (CKD), a known risk factor for cardiovascular disease. However, the impact of CKD on aortic dissection, particularly in patients with PKD, remains unclear. This study aims to investigate the effects of both CKD and PKD on aortic dissection.
Materials and methods: This nationwide, population-based, retrospective cohort study used data from the National Health Insurance Research Database (NHIRD) in Taiwan. The primary outcome evaluated in this study was the cumulative incidence of aortic dissection, compared between PKD patients and a control group without PKD over a 15-year follow-up period. CKD subgroup analyses were performed to further assess the impact of CKD progression on the development of aortic dissection.
Results: From 2000 to 2015, this study included 9,192 PKD patients and 36,768 matched controls without PKD from the NHIRD. Our findings demonstrated that PKD patients who developed aortic dissection had a higher incidence of comorbidities, including hypertension and coronary artery disease. Aortic dissection was more prevalent among male patients, individuals over 45 years of age, and those in the lowest insured premium group. PKD patients had a 2.53-fold higher adjusted hazard ratio (HR) for developing aortic dissection compared to the control group (95% CI: 1.74 to 3.66, p < 0.001). Notably, PKD patients with concurrent hypertension had a 7.77-fold increased risk of aortic dissection (95% CI: 4.97 to 12.13, p < 0.001). In CKD subgroup analyses, PKD patients without CKD and those with CKD had adjusted HRs of 1.74 and 3.38, respectively (p < 0.001). Among PKD patients with CKD, those who initiated hemodialysis (HD) and those who did not showed adjusted HRs of 3.95 and 2.74, respectively, for aortic dissection (p < 0.001).
Conclusion: These findings indicate that the risk of aortic dissection in PKD patients significantly increases with CKD progression. Additionally, hypertension is an independent risk factor for aortic dissection in PKD patients. Careful management of blood pressure and strategies to prevent CKD progression may reduce the incidence of aortic dissection in this population.
Keywords: polycystic kidney disease, aortic dissection, chronic kidney disease, end-stage renal disease, hemodialysis, cardiovascular risk
Introduction
Acute aortic dissection is a life-threatening condition within the spectrum of acute aortic syndromes and has often been misdiagnosed and underestimated in past decades [1, 2]. The incidence of aortic dissection is estimated to be 3-30 cases per million people per year, with most data derived from autopsy studies. Identifying and assessing the risk factors contributing to aortic dissection is essential. Previous population-based cohort studies have provided data on the incidence and traditional risk factors for aortic dissection, including cardiovascular diseases, uncontrolled hypertension, and atherosclerosis [3-5].
PKD, the most common hereditary kidney disease, affecting an estimated 1 in 1,000 to 1 in 2,500 individuals worldwide. PKD leads to a progressive decline in renal function, often culminating in end-stage renal disease, and is a multisystem disorder with numerous clinical manifestations, which may result in early mortality for some patients [6, 7]. Major cardiovascular complications reported in PKD patients include acute coronary syndrome, stroke, and congestive heart failure [8, 9]. Aortic dissection, though potentially catastrophic in PKD patients, has only been reported in a few studies [10, 11]. Unlike cerebral aneurysms, aortic dissections are more challenging to screen for before severe complication arise, except in cases of pre-existing aneurysmal change. Current literature suggests an association between PKD and aortic dissection [11, 12]. CKD itself also increases the risk of AD-related mortality, suggesting that its development may be a target for prevention and early identification of high-risk individuals in the general population [13]. However, the mechanism linking CKD to aortic dissection remains unclear. This study investigates the association between aortic dissection, PKD, and CKD in PKD patients, drawing on data from Taiwan's NHIRD, which includes records for the majority of Taiwan's residents and is widely used for epidemiological research.
Materials and methods
Data source
The NHIRD contains outpatient and inpatient claims for all enrollees in Taiwan's mandatory National Health Insurance (NHI) program, representing more than 99% of the Taiwanese population (over 23 million people). The NHIRD includes patient identifiers, birthdates, sex, dates of admission and discharge, ICD-9-CM (International Classification of Disease, 9th Revision, Clinical Modification) diagnostic codes (up to five per case), and outcomes. This study utilized data from the Longitudinal Health Insurance Database (LHID), a randomly selected subset of the NHIRD containing information on approximately one million beneficiaries, representing around 5% of Taiwan's population. Data were randomly selected from the NHIRD between 2000 and 2015. Previous studies have validated the accuracy of major diseases diagnoses in the NHIRD, including polycystic kidney disease (PKD) and aortic dissection [9, 14]. This study was approved by the Institutional Review Board of Tri-Service General Hospital at the National Defense Medical Center in Taipei, Taiwan (TSGH IRB E202416015). The requirement for informed patient consent was waived.
Sampled patients
In this Taiwan's NHI research, all diagnoses were defined using the ICD-9-CM. The study included a PKD cohort and a comparison cohort. Patients aged ≥20 years newly diagnosed with cystic kidney disease (ICD-9-CM 753.12-753.14: polycystic kidney, adult type, autosomal dominant, autosomal recessive) were followed from 2000 to 2015. Patients were excluded if they had a history of PKD before the inclusion date, were under 20 years of age, had unknown sex, lacked follow-up, or had an initial diagnosis of aortic dissection (ICD-9-CM 441.0). The PKD diagnosis date was set as the inclusion date. Control patients were randomly selected from individuals in the LHID based on age, sex, comorbidities and insured premium level, excluding those with a history of PKD. CKD is defined by ICD-9-CM diagnostic codes system including from ICD-9-CM codes 580 to 589 including glomerulonephritis, nephrotic syndrome and renal failure.
Pre-existing comorbidities were assessed for each participant, including diabetes mellitus (ICD-9-CM codes 250), hypertension (401-405), hyperlipidemia (272), coronary artery disease (410-414), stroke (430-438), congestive heart failure (428), peripheral arterial occlusive disease (443.9), atrial fibrillation (427.31), chronic obstructive pulmonary disease (490-496), chest trauma (338.11, 338.21, 862, 901.0, 959.1), and cancer (140-239). Insured premiums (in New Taiwan dollars, NTD) were stratified into three categories according to enrollee's monthly salary.
Statistical analysis
This population-based, retrospective cohort study used SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA) for all analyses. Standardized difference and standardized mean difference were presented for categorical and continuous variable distributions, respectively. In this study, the Kolmogorov-Smirnov test was used to assess whether the dataset followed a normal distribution. Chi-square (χ²) tests and t-tests were conducted, with t-tests used for continuous variables. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI) and Wald test statistics. The AD-free survival rate between PKD and non-PKD group was analyzed using Kaplan-Meier method. Multivariable cox proportional hazards regression analysis determined the risk of aortic dissection in patients with and without PKD, clarifying the impact of each comorbidity. Subgroup analyses were performed to evaluate the risk of aortic dissection in PKD patients with and without CKD, as well as in those with end-stage renal disease (ESRD) undergoing hemodialysis.
Results
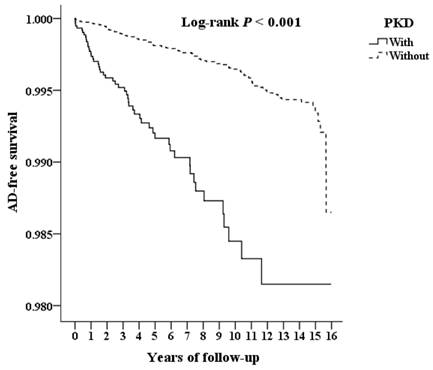
The flowchart of patient enrollment from 2000 to 2015 is shown in Figure 1. Among the 1,936,512 patients in the LHID from the NHIRD, 9,192 PKD patients were identified. The cumulative incidence of aortic dissection during the 15-year follow-up was recorded for PKD patients and the control group and further analyzed according to CKD status. Kaplan-Meier analysis (Figure 2) shows a significantly lower AD-free survival rate in PKD patients compared to the non-PKD group (p < 0.001) in the log-rank test over the 15-year follow-up. Baseline characteristics of the study subjects and controls, including sex, age, income, and comorbidities, are presented in Table 1. Compared with controls (non-PKD group), PKD patients had higher rate of hypertension (p < 0.001), coronary artery disease (p < 0.001), congestive heart failure (p < 0.001) and chronic kidney disease (p < 0.001); but lower rates of diabetes mellitus (p < 0.001), hyperlipidemia (p = 0.011), stroke (p < 0.001), chronic obstructive pulmonary disease (p < 0.001), and chest trauma (p = 0.048).
Flowchart of the study. Abbreviations: AD = aortic dissection, CKD = chronic kidney disease, HD = hemodialysis, PKD = polycystic kidney disease.
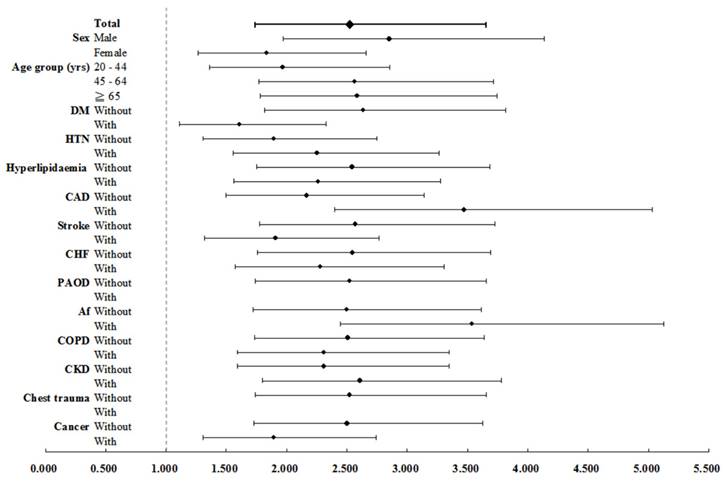
In Table 2, the incidence rate ratio of aortic dissection in PKD versus non-PKD was 4.05 (95% CI: 2.87 to 5.71, p < 0.001). After adjusting for age, sex, and comorbidities using multivariate Cox regression, PKD patients had a 2.53-fold higher risk of developing aortic dissection than non-PKD patients (95% CI: 1.74 to 3.66, p < 0.001). Additionally, male sex and hypertension were identified as independent risk factors for aortic dissection (both p < 0.001), while cancer was associated with a lower risk (adjust HR 0.33, 95% CI: 0.15 to 0.71, p < 0.005). Table 3 shows the incidence rates of aortic dissection in PKD patients and non-PKD patients were 60.44 and 27.44 per 100000 person-years, respectively. PKD patients had a 2.53-fold increased risk of aortic dissection as compared with controls. Adjust HR for aortic dissection was significantly elevated across subgroups of both sexes, all ages, and patients with or without atherosclerotic risk factors or comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, chronic obstructive pulmonary disease and CKD (all p < 0.001) (Figure 3). Subgroup analysis (Table 4) examined the impact of CKD on aortic dissection. PKD patients without CKD and those with CKD had adjusted HRs of 1.74 and 3.38, respectively, after adjusting for age, sex, and comorbidities (p < 0.001). Among PKD patients with CKD on hemodialysis and those without hemodialysis showed adjusted HRs of 3.95 and 2.74, respectively (p < 0.001). In the non-PKD group, CKD patients on hemodialysis had an adjusted HR of 1.58 (p = 0.001).
Baseline characteristics of patients with aortic dissection
| PKD | Total | With | Without | P | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 45,960 | 9,192 | 20.00 | 36,768 | 80.00 | ||
| Sex | 0.999 | ||||||
| Male | 26,035 | 56.65 | 5,207 | 56.65 | 20,828 | 56.65 | |
| Female | 19,925 | 43.35 | 3,985 | 43.35 | 15,940 | 43.35 | |
| Age (years) | 56.65 ± 16.13 | 56.79 ± 15.39 | 56.62 ± 16.31 | 0.366 | |||
| Age groups (years) | 0.999 | ||||||
| 20 - 44 | 10,055 | 21.88 | 2,011 | 21.88 | 8,044 | 21.88 | |
| 45 - 64 | 19,435 | 42.29 | 3,887 | 42.29 | 15,548 | 42.29 | |
| ≧ 65 | 16,470 | 35.84 | 3,294 | 35.84 | 13,176 | 35.84 | |
| Insured premium (NT$) | 0.083 | ||||||
| < 18,000 | 45,268 | 98.49 | 9,031 | 98.25 | 36,237 | 98.56 | |
| 18,000 - 34,999 | 522 | 1.14 | 119 | 1.29 | 403 | 1.10 | |
| ≧ 35,000 | 170 | 0.37 | 42 | 0.46 | 128 | 0.35 | |
| DM | < 0.001 | ||||||
| Without | 40,568 | 88.27 | 8,383 | 91.20 | 32,185 | 87.54 | |
| With | 5,392 | 11.73 | 809 | 8.80 | 4,583 | 12.46 | |
| HTN | < 0.001 | ||||||
| Without | 36,993 | 80.49 | 5,708 | 62.10 | 31,285 | 85.09 | |
| With | 8,967 | 19.51 | 3,484 | 37.90 | 5,483 | 14.91 | |
| Hyperlipidemia | 0.011 | ||||||
| Without | 44,727 | 97.32 | 8,981 | 97.70 | 35,746 | 97.22 | |
| With | 1,233 | 2.68 | 211 | 2.30 | 1,022 | 2.78 | |
| CAD | < 0.001 | ||||||
| Without | 42,217 | 91.86 | 8,350 | 90.84 | 33,867 | 92.11 | |
| With | 3,743 | 8.14 | 842 | 9.16 | 2,901 | 7.89 | |
| Stroke | < 0.001 | ||||||
| Without | 42,679 | 92.86 | 8,652 | 94.13 | 34,027 | 92.55 | |
| With | 3,281 | 7.14 | 540 | 5.87 | 2,741 | 7.45 | |
| CHF | < 0.001 | ||||||
| Without | 44,897 | 97.69 | 8,927 | 97.12 | 35,970 | 97.83 | |
| With | 1,063 | 2.31 | 265 | 2.88 | 798 | 2.17 | |
| PAOD | 0.224 | ||||||
| Without | 45,943 | 99.96 | 9,191 | 99.99 | 36,752 | 99.96 | |
| With | 17 | 0.04 | 1 | 0.01 | 16 | 0.04 | |
| Af | 0.403 | ||||||
| Without | 45,451 | 98.89 | 9,098 | 98.98 | 36,353 | 98.87 | |
| With | 509 | 1.11 | 94 | 1.02 | 415 | 1.13 | |
| COPD | < 0.001 | ||||||
| Without | 42,796 | 93.12 | 8,781 | 95.53 | 34,015 | 92.51 | |
| With | 3,164 | 6.88 | 411 | 4.47 | 2,753 | 7.49 | |
| CKD | < 0.001 | ||||||
| Without | 42,050 | 91.49 | 6,721 | 73.12 | 35,329 | 96.09 | |
| With | 3,910 | 8.51 | 2,471 | 26.88 | 1,439 | 3.91 | |
| Chest trauma | 0.048 | ||||||
| Without | 45,927 | 99.93 | 9,190 | 99.98 | 36,737 | 99.92 | |
| With | 33 | 0.07 | 2 | 0.02 | 31 | 0.08 | |
| Cancer | 0.852 | ||||||
| Without | 42,833 | 93.20 | 8,571 | 93.24 | 34,262 | 93.18 | |
| With | 3,127 | 6.80 | 621 | 6.76 | 2,506 | 6.82 | |
| CCI_R | 0.14 ± 0.40 | 0.16 ± 0.45 | 0.13 ± 0.39 | < 0.001 | |||
| Season | 0.999 | ||||||
| Spring (Mar - May) | 12,375 | 26.93 | 2,475 | 26.93 | 9,900 | 26.93 | |
| Summer (Jun - Aug) | 11,425 | 24.86 | 2,285 | 24.86 | 9,140 | 24.86 | |
| Autumn (Sep - Nov) | 11,215 | 24.40 | 2,243 | 24.40 | 8,972 | 24.40 | |
| Winter (Dec - Feb) | 10,945 | 23.81 | 2,189 | 23.81 | 8,756 | 23.81 | |
| Location | < 0.001 | ||||||
| Northern Taiwan | 18,599 | 40.47 | 4,145 | 45.09 | 14,454 | 39.31 | |
| Middle Taiwan | 12,555 | 27.32 | 2,242 | 24.39 | 10,313 | 28.05 | |
| Southern Taiwan | 11,787 | 25.65 | 2,268 | 24.67 | 9,519 | 25.89 | |
| Eastern Taiwan | 2,805 | 6.10 | 509 | 5.54 | 2,296 | 6.24 | |
| Outlets islands | 214 | 0.47 | 28 | 0.30 | 186 | 0.51 | |
| Urbanization level | < 0.001 | ||||||
| 1 (The highest) | 15,878 | 34.55 | 3,563 | 38.76 | 12,315 | 33.49 | |
| 2 | 19,675 | 42.81 | 4,042 | 43.97 | 15,633 | 42.52 | |
| 3 | 3,221 | 7.01 | 477 | 5.19 | 2,744 | 7.46 | |
| 4 (The lowest) | 7,186 | 15.64 | 1,110 | 12.08 | 6,076 | 16.53 | |
| Level of care | < 0.001 | ||||||
| Hospital center | 15,539 | 33.81 | 4,120 | 44.82 | 11,419 | 31.06 | |
| Regional hospital | 14,845 | 32.30 | 3,662 | 39.84 | 11,183 | 30.42 | |
| Local hospital | 15,576 | 33.89 | 1,410 | 15.34 | 14,166 | 38.53 | |
P: Chi-square / Fisher exact test on category variables and t-test on continue variables
Abbreviations: PKD = polycystic kidney disease, NT$ = new Taiwan dollar, DM = diabetes mellitus, HTN = hypertension, CAD = coronary artery disease, CHF = congestive heart failure, PAOD = peripheral arterial occlusive disease, Af = atrial fibrillation, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease
In Table S1 and Figure S1, after adjusting for age, sex, and comorbidities, hypertension and PKD were associated with adjusted HRs of 2.83 and 2.18 for aortic dissection (p < 0.001 and p = 0.014, respectively). PKD patients with hypertension had a 7.77-fold increased risk of aortic dissection (p < 0.001). These results suggest that hypertension significantly increases the risk of aortic dissection in PKD patients.
Discussion
This study is the first to investigate the relationship between CKD and aortic dissection in patients with PKD. In PKD patients, the risk of aortic dissection is notably increased by the presence of hypertension and the progressive decline in renal function, characteristic of CKD. We purpose this increased risk may result from vascular remodeling and heightened hemodynamic stress associated with PKD, which can weaken the aortic wall over time.
Kaplan-Meier for cumulative survival of AD aged 20 and over stratified by PKD with log-rank test. Abbreviations: AD = aortic dissection, PKD = polycystic kidney disease.
Forest plot for factors of AD stratified by sex, age groups, and comorbidities. Abbreviations: AD = aortic dissection, DM = diabetes mellitus, HTN = hypertension, CAD = coronary artery disease, CHF = congestive heart failure, PAOD = peripheral arterial occlusive disease, Af = atrial fibrillation, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease.
Polycystic kidney disease and associated complications
PKD is a genetic disorder characterized by mutations in the PKD1 and PKD2 genes, which disrupt the integrity of polycystin1 and polycystin2 in vascular smooth muscle [15]. In addition to impaired renal function, PKD patients frequently experience other systemic complications, including hypertension, liver or pancreatic cysts, intracranial aneurysms, diverticular disease, and cardiac valve abnormalities [16-18]. Mortality in PKD patients frequently results from cardiac complications, infection and central nervous system disorders, often due to ruptured intracranial aneurysms [19]. Clinical guidelines recommend antihypertensive treatment and intracranial aneurysm screening to improve the quality of life and prognosis for PKD patients [20]. Our findings indicate that PKD patients have a higher incidence of comorbidities such as hypertension, coronary artery disease, congestive heart failure, and CKD. The incidence rate of aortic dissection in PKD patients is 2.52 times higher than in non-PKD patients. Vascular disease, such as aneurysms and arterial dissection of large arteries (e.g., the aorta and coronary arteries), are significant causes of mortality in PKD [12]. However, the incidence of abdominal aortic aneurysms does not appear to be elevated in PKD patients [21, 22].
Factors of aortic dissection by using Cox regression
| Variables | Crude HR | 95% CI | 95% CI | P | aHR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| PKD | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 4.054 | 2.877 | 5.713 | < 0.001 | 2.525 | 1.742 | 3.658 | < 0.001 |
| Sex | ||||||||
| Male | 2.063 | 1.451 | 2.934 | < 0.001 | 2.178 | 1.528 | 3.104 | < 0.001 |
| Female | 1.000 | 1.000 | ||||||
| Age groups (years) | ||||||||
| 20 - 44 | 1.000 | 1.000 | ||||||
| 45 - 64 | 2.669 | 1.212 | 5.879 | 0.015 | 1.786 | 0.803 | 3.972 | 0.155 |
| ≥ 65 | 2.279 | 1.056 | 4.916 | 0.036 | 1.805 | 0.821 | 3.968 | 0.142 |
| Insured premium (NT$) | ||||||||
| < 18,000 | 1.000 | 1.000 | ||||||
| 18,000 - 34,999 | 0.484 | 0.068 | 3.457 | 0.469 | 0.494 | 0.069 | 3.530 | 0.482 |
| ≥ 35,000 | 0.000 | - | - | 0.943 | 0.000 | - | - | 0.939 |
| DM | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.755 | 0.492 | 1.160 | 0.200 | 0.660 | 0.425 | 1.025 | 0.064 |
| HTN | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 3.640 | 2.635 | 5.030 | < 0.001 | 3.102 | 2.182 | 4.409 | < 0.001 |
| Hyperlipidaemia | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 1.173 | 0.518 | 2.655 | 0.702 | 0.751 | 0.324 | 1.742 | 0.505 |
| CAD | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 1.790 | 1.189 | 2.697 | 0.005 | 1.204 | 0.784 | 1.848 | 0.396 |
| Stroke | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.883 | 0.500 | 1.558 | 0.666 | 0.715 | 0.402 | 1.272 | 0.254 |
| CHF | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 1.107 | 0.583 | 2.101 | 0.756 | 0.955 | 0.493 | 1.853 | 0.893 |
| PAOD | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.000 | - | - | 0.857 | 0.000 | - | - | 0.976 |
| Af | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 1.276 | 0.523 | 3.110 | 0.593 | 1.086 | 0.437 | 2.699 | 0.860 |
| COPD | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.686 | 0.361 | 1.303 | 0.249 | 0.793 | 0.412 | 1.526 | 0.487 |
| CKD | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 1.563 | 1.010 | 2.419 | 0.045 | 1.423 | 0.894 | 2.266 | 0.137 |
| Chest trauma | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.000 | - | - | 0.879 | 0.000 | - | - | 0.988 |
| Cancer | ||||||||
| Without | 1.000 | 1.000 | ||||||
| With | 0.313 | 0.147 | 0.668 | 0.003 | 0.311 | 0.144 | 0.671 | 0.003 |
| CCI_R | 1.044 | 0.557 | 1.278 | 0.423 | 1.042 | 0.623 | 1.425 | 0.778 |
| Season | ||||||||
| Spring | 1.000 | 1.000 | ||||||
| Summer | 1.196 | 0.762 | 1.875 | 0.436 | 1.186 | 0.756 | 1.862 | 0.457 |
| Autumn | 0.827 | 0.514 | 1.329 | 0.432 | 0.806 | 0.501 | 1.297 | 0.375 |
| Winter | 1.181 | 0.750 | 1.859 | 0.472 | 1.172 | 0.744 | 1.846 | 0.493 |
| Location | Multicollinearity with urbanization level | |||||||
| Northern Taiwan | 1.000 | Multicollinearity with urbanization level | ||||||
| Middle Taiwan | 0.894 | 0.606 | 1.319 | 0.572 | Multicollinearity with urbanization level | |||
| Southern Taiwan | 0.931 | 0.625 | 1.386 | 0.724 | Multicollinearity with urbanization level | |||
| Eastern Taiwan | 0.784 | 0.390 | 1.576 | 0.495 | Multicollinearity with urbanization level | |||
| Outlets islands | 0.000 | - | - | 0.935 | Multicollinearity with urbanization level | |||
| Urbanization level | ||||||||
| 1 (The highest) | 3.592 | 1.843 | 7.000 | < 0.001 | 1.952 | 0.947 | 4.024 | 0.070 |
| 2 | 2.738 | 1.415 | 5.300 | 0.003 | 1.725 | 0.863 | 3.447 | 0.123 |
| 3 | 1.591 | 0.606 | 4.179 | 0.346 | 1.541 | 0.586 | 4.050 | 0.381 |
| 4 (The lowest) | 1.000 | 1.000 | ||||||
| Level of care | ||||||||
| Hospital center | 4.368 | 2.439 | 7.824 | < 0.001 | 2.799 | 1.489 | 5.262 | 0.001 |
| Regional hospital | 2.050 | 1.119 | 3.757 | 0.020 | 1.641 | 0.888 | 3.033 | 0.114 |
| Local hospital | 1.000 | 1.000 | ||||||
aHR = Adjusted hazard ratio: Adjusted variables listed in the table, CI = confidence interval
Abbreviations: PKD = polycystic kidney disease, NT$ = new Taiwan dollar, DM = diabetes mellitus, HTN = hypertension, CAD = coronary artery disease, CHF = congestive heart failure, PAOD = peripheral arterial occlusive disease, Af = atrial fibrillation, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease
Factors of aortic dissection by using Cox regression
| PKD | With | Without (Reference) | With vs. Without (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Events | PYs | Rate | Events | PYs | Rate | aHR | 95% CI | 95% CI | P |
| Total | 53 | 87,686.41 | 60.44 | 101 | 368,082.64 | 27.44 | 2.525 | 1.742 | 3.658 | < 0.001 |
| Sex | ||||||||||
| Male | 41 | 48,152.67 | 85.15 | 70 | 204,665.22 | 34.20 | 2.853 | 1.969 | 4.135 | < 0.001 |
| Female | 12 | 39,533.74 | 30.35 | 31 | 163,417.43 | 18.97 | 1.834 | 1.266 | 2.657 | < 0.001 |
| Age groups (yrs) | ||||||||||
| 20 - 44 | 5 | 9,034.30 | 55.34 | 12 | 37,246.23 | 32.22 | 1.969 | 1.359 | 2.853 | < 0.001 |
| 45 - 64 | 22 | 34,961.79 | 62.93 | 28 | 99,577.15 | 28.12 | 2.565 | 1.770 | 3.717 | < 0.001 |
| ≥ 65 | 26 | 43,690.33 | 59.51 | 61 | 231,259.26 | 26.38 | 2.586 | 1.784 | 3.747 | < 0.001 |
| Insured premium (NT$) | ||||||||||
| < 18,000 | 52 | 86,180.19 | 60.34 | 101 | 361,883.48 | 27.91 | 2.478 | 1.710 | 3.591 | < 0.001 |
| 18,000 - 34,999 | 1 | 1,139.05 | 87.79 | 0 | 5,014.88 | 0.00 | ∞ | - | - | 0.999 |
| ≥ 35,000 | 0 | 367.16 | 0.00 | 0 | 1,184.28 | 0.00 | - | - | - | - |
| DM | ||||||||||
| Without | 49 | 76,928.06 | 63.70 | 80 | 288,824.80 | 27.70 | 2.636 | 1.819 | 3.819 | < 0.001 |
| With | 4 | 10,758.35 | 37.18 | 21 | 79,257.84 | 26.50 | 1.608 | 1.110 | 2.331 | < 0.001 |
| HTN | ||||||||||
| Without | 15 | 51,472.01 | 29.14 | 47 | 266,892.02 | 17.61 | 1.897 | 1.309 | 2.748 | < 0.001 |
| With | 38 | 36,214.40 | 104.93 | 54 | 101,190.62 | 53.36 | 2.254 | 1.555 | 3.266 | < 0.001 |
| Hyperlipidemia | ||||||||||
| Without | 51 | 85,163.30 | 59.88 | 96 | 355,641.00 | 26.99 | 2.543 | 1.755 | 3.685 | < 0.001 |
| With | 2 | 2,523.11 | 79.27 | 5 | 12,441.64 | 40.19 | 2.261 | 1.560 | 3.276 | < 0.001 |
| CAD | ||||||||||
| Without | 35 | 76,901.21 | 45.51 | 79 | 328,144.93 | 24.07 | 2.167 | 1.495 | 3.140 | < 0.001 |
| With | 18 | 10,785.20 | 166.90 | 22 | 39,937.71 | 55.09 | 3.472 | 2.396 | 5.032 | < 0.001 |
| Stroke | ||||||||||
| Without | 50 | 80,573.71 | 62.05 | 92 | 332,545.44 | 27.67 | 2.571 | 1.774 | 3.725 | < 0.001 |
| With | 3 | 7,112.70 | 42.18 | 9 | 35,537.20 | 25.33 | 1.909 | 1.317 | 2.766 | < 0.001 |
| CHF | ||||||||||
| Without | 48 | 82,263.05 | 58.35 | 91 | 346,494.67 | 26.26 | 2.546 | 1.757 | 3.690 | < 0.001 |
| With | 5 | 5,423.36 | 92.19 | 10 | 21,587.97 | 46.32 | 2.281 | 1.574 | 3.305 | < 0.001 |
| PAOD | ||||||||||
| Without | 53 | 87,640.68 | 60.47 | 101 | 367,925.17 | 27.45 | 2.525 | 1.742 | 3.658 | < 0.001 |
| With | 0 | 45.73 | 0.00 | 0 | 157.47 | 0.00 | - | - | - | - |
| Af | ||||||||||
| Without | 51 | 85,621.47 | 59.56 | 98 | 358,518.06 | 27.33 | 2.497 | 1.723 | 3.619 | < 0.001 |
| With | 2 | 2,064.94 | 96.85 | 3 | 9,564.58 | 31.37 | 3.539 | 2.442 | 5.128 | < 0.001 |
| COPD | ||||||||||
| Without | 51 | 83,268.60 | 61.25 | 93 | 332,486.75 | 27.97 | 2.510 | 1.732 | 3.637 | < 0.001 |
| With | 2 | 4,417.81 | 45.27 | 8 | 35,595.89 | 22.47 | 2.309 | 1.593 | 3.345 | < 0.001 |
| CKD | ||||||||||
| Without | 34 | 62,642.24 | 54.28 | 91 | 338,065.87 | 26.92 | 2.311 | 1.595 | 3.349 | < 0.001 |
| With | 19 | 25,044.17 | 75.87 | 10 | 30,016.77 | 33.31 | 2.610 | 1.801 | 3.782 | < 0.001 |
| Chest trauma | ||||||||||
| Without | 53 | 87,686.41 | 60.44 | 101 | 367,959.13 | 27.45 | 2.525 | 1.742 | 3.658 | < 0.001 |
| With | 0 | 0.00 | - | 0 | 123.51 | 0.00 | - | - | - | - |
| Cancer | ||||||||||
| Without | 51 | 78,996.95 | 64.56 | 94 | 317,845.17 | 29.57 | 2.502 | 1.727 | 3.626 | < 0.001 |
| With | 2 | 8,689.46 | 23.02 | 7 | 50,237.47 | 13.93 | 1.893 | 1.306 | 2.743 | < 0.001 |
| Season | ||||||||||
| Spring | 10 | 19,367.63 | 51.63 | 24 | 87,125.61 | 27.55 | 2.148 | 1.482 | 3.113 | < 0.001 |
| Summer | 16 | 20,902.42 | 76.55 | 24 | 91,328.79 | 26.28 | 3.338 | 2.304 | 4.838 | < 0.001 |
| Autumn | 10 | 26,263.26 | 38.08 | 25 | 102,001.30 | 24.51 | 1.780 | 1.229 | 2.580 | < 0.001 |
| Winter | 17 | 21,153.10 | 80.37 | 28 | 87,626.93 | 31.95 | 2.883 | 1.989 | 4.177 | < 0.001 |
| Urbanization level | ||||||||||
| 1 (The highest) | 24 | 31,046.48 | 77.30 | 35 | 106,638.51 | 32.82 | 2.699 | 1.863 | 3.912 | < 0.001 |
| 2 The 2nd | 21 | 40,157.87 | 52.29 | 41 | 168,804.41 | 24.29 | 2.468 | 1.703 | 3.576 | < 0.001 |
| 3 The 3rd | 5 | 4,768.95 | 104.84 | 15 | 28,516.79 | 52.60 | 2.284 | 1.576 | 3.310 | < 0.001 |
| 4 (The lowest) | 3 | 11,713.11 | 25.61 | 10 | 64,122.93 | 15.60 | 1.882 | 1.299 | 2.728 | < 0.001 |
| Level of care | ||||||||||
| Hospital center | 33 | 37,911.57 | 87.04 | 45 | 119,126.13 | 37.78 | 2.741 | 1.922 | 3.927 | < 0.001 |
| Regional hospital | 16 | 36,999.23 | 43.24 | 31 | 165,211.64 | 18.76 | 2.641 | 1.823 | 3.828 | < 0.001 |
| Local hospital | 4 | 12,775.61 | 31.31 | 25 | 83,744.88 | 29.85 | 1.202 | 0.830 | 1.742 | 0.279 |
| PYs = Person-years; Rate: per 100,000 PYs; aHR = Adjusted Hazard ratio: Adjusted for the variables listed in Table 2.; CI = confidence interval | ||||||||||
Abbreviations: PKD = polycystic kidney disease, NT$ = new Taiwan dollar, DM = diabetes mellitus, HTN = hypertension, CAD = coronary artery disease, CHF = congestive heart failure, PAOD = peripheral arterial occlusive disease, Af = atrial fibrillation, COPD = chronic obstructive pulmonary disease, CKD = chronic kidney disease
Factors of aortic dissection among patients with/without PKD in different CKD stages by using Cox regression
| PKD | Population | Events | PYs | Rate | aHR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| Without PKD | 36,768 | 101 | 368,082.64 | 27.44 | 1.000 | |||
| Without CKD | 33,637 | 91 | 338,065.87 | 26.92 | 1.125 | 0.784 | 1.629 | 0.215 |
| With CKD | 3,131 | 10 | 30,016.77 | 33.31 | 1.394 | 0.965 | 2.018 | 0.074 |
| Without HD | 1,425 | 4 | 14,152.48 | 28.26 | 1.181 | 0.813 | 1.714 | 0.187 |
| With HD | 1,706 | 6 | 15,864.29 | 37.82 | 1.580 | 1.099 | 2.296 | 0.001 |
| With PKD | 9,192 | 53 | 87,686.41 | 60.44 | 2.525 | 1.742 | 3.658 | < 0.001 |
| Without CKD | 6,829 | 19 | 45,684.56 | 41.59 | 1.737 | 1.198 | 2.516 | < 0.001 |
| With CKD | 2,363 | 34 | 42,001.85 | 80.95 | 3.382 | 2.334 | 4.896 | < 0.001 |
| Without HD | 1,076 | 13 | 19,803.26 | 65.65 | 2.741 | 1.896 | 3.970 | < 0.001 |
| With HD | 1,287 | 21 | 22,198.60 | 94.60 | 3.950 | 2.723 | 5.724 | < 0.001 |
PYs = Person-years; Rate: per 100,000 PYs; aHR = Adjusted Hazard ratio: Adjusted for the variables listed in Table 2.; CI = confidence interval
Abbreviations: PKD = polycystic kidney disease, CKD = chronic kidney disease, HD = hemodialysis
The PKD1 and PKD2 genes play complex roles in mechano-transduction and intracellular calcium signaling, which are critical in the molecular basis of intracerebral aneurysm formation [23, 24]. Studies have shown that PKD patients exhibit endothelial dysfunction and hypertension, potentially due to reduced nitric oxide, oxidative stress, and antioxidant imbalance [25, 26]. There is also evidence that PKD patients have a higher incidence of aortic dilatation, a known risk factor for aortic dissection [27]. However, PKD patients with intracranial aneurysm do not always display the same tendency toward aortic and coronary aneurysms, aortic root dilatation, or aortic dissection, suggesting that the mechanism for intracranial aneurysm formation in PKD may differ from that of aortic aneurysm or dissection [28].
Hypertension is the most common risk factor for acute aortic dissection, contributing to 75% of cases. Other contributing factors include trauma, pharmacologic factors, vasculitis, infection, and genetic factors such as bicuspid aortic valve and connective tissue disorders [29]. Pathologically, acute aortic syndrome can be divided into few conditions such as classic dissection, intramural hematoma, penetrating aortic ulcer, symptomatic aneurysm or pseudoaneurysm and aortic rupture [30, 31]. Classic aortic dissection and intramural hematoma are more related to structural and genetic conditions, while penetrating aortic ulcer is more associated with atherosclerosis [32]. These risk factors can be categorized into conditions that increase aortic wall stress, aortic media abnormalities, and iatrogenic factors. Genetic syndromes and inflammatory vasculitis contribute to aortic media abnormalities [31]. Advances in molecular genetics and imaging have significantly enhanced the prediction and prevention of acute aortic dissection [3, 33-35]. Genetically related aortic syndromes, typically autosomal dominant, often affect younger individuals and are linked to connective tissue disorders in 20% of cases. Mutations affecting the contractile apparatus of vascular smooth muscle cells suggest that smooth muscle tone and function are crucial for aortic wall stress response [29]. Using the ClinGen framework, genes predisposing to heritable thoracic aortic aneurysms and dissection have been identified, though PKD1 and PKD2 mutations are considered risk alleles with limited evidence as a Mendelian cause of heritable thoracic aortic aneurysm and dissection [34]. Therefore, PKD1 and PKD2 gene abnormalities alone may not fully explain the increased risk of aortic dissection in PKD, warranting further investigation.
Approximately 60% of PKD patients develop early-onset hypertension before renal function declines, typically around age 29 [36, 37]. While the optimal blood pressure target for PKD patients is not well established, it is generally considered similar to that for CKD patients without PKD. For most PKD patients, the goal blood pressure should be 120-125/< 80 mmHg, with angiotensin-converting enzyme inhibitors (ACEIs) as the preferred treatment and angiotensin II receptor blockers (ARBs) as an alternative. Lower blood pressure targets may slow kidney volume increase and provide cardiovascular benefits in younger, healthier PKD patients [38, 39]. Literature indicates that PKD patients have a higher incidence of aortic dissection, with a younger average age and a higher prevalence of hypertension compared to non-PKD patients [11, 12]. Among PKD patients with hypertension, our study found a significantly elevated incidence of aortic dissection, with a 7.91-fold increase in risk, suggesting that hypertension significantly exacerbates the risk of aortic dissection in PKD patients. A previous NHIRD study with 2,076 PKD patients followed for 12 years identified hypertension as an aggravating factor in developing aortic dissection [14]. However, the role of CKD itself in developing aortic dissection in PKD patients was not identified in this study. CKD is a known risk factor for aortic dissection-related mortality in the general population [13]. Nevertheless, its impact on aortic dissection in PKD patients has not been widely studied in large datasets. In our study, Cox regression analysis of PKD-associated aortic dissection showed an elevated risk in patients with CKD, with adjusted hazard ratios of 3.38 (p < 0.001). The risk was even higher in PKD patients requiring hemodialysis, with an adjusted hazard ratio of 3.95 (p < 0.001). These findings suggest that aortic dissection risk in PKD patients rises with CKD.
Therapeutic strategies to slow CKD progression in PKD patients
PKD is a genetic disorder characterized by the formation of cysts in the kidneys. These cysts cause the kidneys to enlarge and may lead to damage. Kidney cyst number increment and volume increase are indicators of PKD progression. The decline in kidney function in patients with PKD typically occurs at variable rates, usually depending on age and the kidney cystic burden or number of cysts. Rapid progression refers to these patients who reach kidney failure at an earlier age [40, 41]. The progressive enlargement of multiple cysts results in the deterioration of functional parenchyma, potentially leading to end-stage kidney disease. There are two main types of PKD: autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD). ADPKD is far more common than ARPKD in clinical practice. Both ADPKD and ARPKD results from abnormal genes that can be inherited from a parent or, in rare cases, arise due to spontaneous genetic mutations in individuals with no family history of the disease [40-42]. COX inhibition with Sulindac significantly reduced kidney size of the PKD2 mice, suggesting the COX pathway may be a therapeutic target for PKD. However, the long-term efficacy and side effects of non-steroidal anti-inflammatory drugs (NSAIDs) in managing cystic renal disease remain uncertain [40]. Tolvaptan, a vasopressin V2 receptor antagonist, is an approved treatment for PKD that helps to reduce kidney volume and slow renal function deterioration [41, 42]. Additionally, Tolvaptan may hold potential for treating aortic aneurysms and dissection by preserving aortic integrity, reducing inflammatory markers, and inhibiting vascular smooth muscle cell apoptosis [43]. Combined sodium-glucose cotransporter-2 (SGLT2) inhibitors and ACEIs/ARBs, known for their glycemic and cardiorenal benefits, showed therapeutic effects in patients with CKD [44]. Inducing glucosuria with the SGLT2-specific inhibitor dapagliflozin has been associated with improved renal function and reduced albuminuria in PKD rat models [45]. However, in examined patients, short-term dapagliflozin administration led to a decrease in eGFR and an increase in height-adjusted kidney volume (htTKV) [46]. SGLT2 inhibitors may offer additional benefits [47-49]; however, data on their safety and effects in patients with ADPKD are currently lacking, as these patients were excluded from SGLT2 inhibitors trials. Notably, there is speculation that SGLT2 inhibitors could promote cysts growth and accelerate kidney function decline in ADPKD [46, 50, 51]. Now, the ongoing EMPA-PKD trial is evaluating the safety of empagliflozin in patients with rapidly progressive ADPKD, both with and without concurrent tolvaptan use, by monitoring total kidney volume and kidney function decline [52]. Further studies, including clinical trials, are essential to evaluate the efficacy of SGLT2 inhibitors in patients with PKD. In summary, routine health management, especially blood pressure control and CKD prevention or early detection, may help reduce the likelihood of aortic dissection in PKD patients.
Limitation
Despite extensive adjustments through matching and multivariate logistic regression, our study has several limitations. The NHIRD database presents specific challenges: 1) as a study of a Taiwanese population, its generalizability to other countries is limited; 2) potential misclassification of disease diagnoses, as diagnosis codes may overestimate or underestimate actual conditions to qualify for NHIRD benefits; 3) limitations in result extrapolation due to the specific nature of NHIRD, its unique healthcare ecosystem, and the parent group's large sample size, which restricts over-interpretation based on statistically significant differences; and 4) the choice of control group could influence result interpretation.
Conclusion
The risk of aortic dissection in patients with PKD significantly increases with hypertension and CKD. Effective clinical management should focus on achieving optimal blood pressure control, utilizing antihypertensive agents such as ACEIs/ARBs, and employing renoprotective strategies among these patients. Regular cardiovascular and renal function monitoring is critical to mitigating the risk of life-threatening aortic events and managing PKD-related cardiovascular complications.
Supplementary Material
Supplementary figure.
Acknowledgements
Funding
This study was supported by grants TYAFGH-D-113032 and TYAFGH-D-114032 from the Taoyuan Armed Forces General Hospital, Taiwan.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Artificial Intelligence (AI) tools
The paper utilized ChatGPT for refining and enhancing the fluency and accuracy of the English language. No other AI tools were used for data generation or manuscript creation.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lovatt S, Wong CW, Schwarz K, Borovac JA, Lo T, Gunning M. et al. Misdiagnosis of aortic dissection: A systematic review of the literature. Am J Emerg Med. 2022;53:16-22
2. Zhan S, Hong S, Shan-shan L, Chen-ling Y, Lai W, Dong-wei S. et al. Misdiagnosis of aortic dissection: experience of 361 patients. The Journal of Clinical Hypertension. 2012;14:256-60
3. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE. et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: Executive Summary. Circulation. 2010;121:1544-79
4. Vilacosta I, Aragoncillo P, Canadas V, Roman JAS, Ferreiros J, Rodriguez E. Acute aortic syndrome: a new look at an old conundrum. Postgraduate Medical Journal. 2010;86:52-61
5. Vilacosta I, San Roman JA, di Bartolomeo R, Eagle K, Estrera AL, Ferrera C. et al. Acute Aortic Syndrome Revisited: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:2106-25
6. Kurschat CE, Müller RU, Franke M, Maintz D, Schermer B, Benzing T. An approach to cystic kidney diseases: the clinician's view. Nat Rev Nephrol. 2014;10:687-99
7. Lee LJ-H, Tw K, Chu T-S. Epidemiology of Polycystic Kidney Disease in Taiwan. Formosan J Med. 2017;21:427-33
8. Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nature Reviews Disease Primers. 2018;4:50
9. Chuang YW, Yu TM, Huang ST, Sun KT, Lo YC, Fu PK. et al. Young-Adult Polycystic Kidney Disease is Associated with Major Cardiovascular Complications. Int J Environ Res Public Health. 2018;15:903
10. Hydoub YM, Alnuaimi M, Nour S. Catastrophic extrarenal manifestation of autosomal dominant polycystic kidney disease: lessons learnt. BMJ Case Reports. 2019;12:e231944
11. Silverio A, Prota C, Di Maio M, Polito MV, Cogliani FM, Citro R. et al. Aortic dissection in patients with autosomal dominant polycystic kidney disease: A series of two cases and a review of the Literature. Nephrology. 2015;20:229-35
12. Nunes R, Gouveia e Melo R, Almeida AG, de Almeida E, Pinto FJ, Pedro LM. et al. Does autosomal dominant polycystic kidney disease increase the risk of aortic aneurysm or dissection: a point of view based on a systematic review and meta-analysis. Journal of Nephrology. 2022;35:1585-93
13. Otaki Y, Watanabe T, Konta T, Watanabe M, Asahi K, Yamagata K. et al. Impact of Chronic Kidney Disease on Aortic Disease-related Mortality: A Four-year Community-Based Cohort Study. Intern Med. 2021;60:689-97
14. Sung P-H, Yang Y-H, Chiang H-J, Chiang JY, Chen C-J, Liu C-T. et al. Risk of aortic aneurysm and dissection in patients with autosomal-dominant polycystic kidney disease: a nationwide population-based cohort study. Oncotarget. 2017;8:57594
15. Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC. et al. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280-7
16. Chapman AB, Stepniakowski K, Rahbari-Oskoui F. Hypertension in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis. 2010;17:153-63
17. Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019;393:919-35
18. Colbert GB, Elrggal ME, Gaur L, Lerma EV. Update and review of adult polycystic kidney disease. Disease-a-Month. 2020;66:100887
19. Rahman E, Niaz FA, Al-Suwaida A, Nahrir S, Bashir M, Rahman H. et al. Analysis of causes of mortality in patients with autosomal dominant polycystic kidney disease: a single center study. Saudi Journal of Kidney Diseases and Transplantation. 2009;20:806
20. Horie S, Mochizuki T, Muto S, Hanaoka K, Fukushima Y, Narita I. et al. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol. 2016;20:493-509
21. Gigante A, Perrotta AM, Tinti F, Assanto E, Muscaritoli M, Lai S. et al. Assessment of Cardiovascular Disease in Autosomal Dominant Polycystic Kidney Disease. Applied Sciences. 2023;13:7175
22. Torra R, Nicolau C, Badenas C, Brú C, Pérez L, Estivill X. et al. Abdominal aortic aneurysms and autosomal dominant polycystic kidney disease. Journal of the American Society of Nephrology. 1996;7:2483-6
23. Bichet D, Peters D, Patel AJ, Delmas P, Honore E. Cardiovascular polycystins: insights from autosomal dominant polycystic kidney disease and transgenic animal models. Trends Cardiovasc Med. 2006;16:292-8
24. Rossetti S, Chauveau D, Kubly V, Slezak JM, Saggar-Malik AK, Pei Y. et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. The Lancet. 2003;361:2196-201
25. Andries A, Daenen K, Jouret F, Bammens B, Mekahli D, Van Schepdael A. Oxidative stress in autosomal dominant polycystic kidney disease: player and/or early predictor for disease progression? Pediatric Nephrology. 2019;34:993-1008
26. Rahbari-Oskoui F, Williams O, Chapman A. Mechanisms and management of hypertension in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation. 2014;29:2194-201
27. Bouleti C, Flamant M, Escoubet B, Arnoult F, Milleron O, Vidal-Petiot E. et al. Risk of Ascending Aortic Aneurysm in Patients With Autosomal Dominant Polycystic Kidney Disease. The American Journal of Cardiology. 2019;123:482-8
28. Perrone RD, Malek AM, Watnick T. Vascular complications in autosomal dominant polycystic kidney disease. Nature Reviews Nephrology. 2015;11:589-98
29. Nienaber CA, Clough RE. Management of acute aortic dissection. The Lancet. 2015;385:800-11
30. Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. American Journal of Roentgenology. 2003;181:309-16
31. Bossone E, Eagle KA. Epidemiology and management of aortic disease: aortic aneurysms and acute aortic syndromes. Nat Rev Cardiol. 2021;18:331-48
32. Demartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A. et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circulation: Cardiovascular Quality and Outcomes. 2018;11:e004689
33. Cecchi AC, Boerio ML, Marin I, Pinard A, Milewicz DM. Preventing Acute Aortic Dissections: The Power of Familial Screening and Risk Assessment. Journal of the American Heart Association. 2022;11:e025441
34. Renard M, Francis C, Ghosh R, Scott AF, Witmer PD, Ades LC. et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J Am Coll Cardiol. 2018;72:605-15
35. Milewicz DM, Regalado E. Heritable thoracic aortic disease overview. 2017.
36. Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. Journal of the American Society of Nephrology. 2001;12:194-200
37. Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. Journal of the American Society of Nephrology. 2001;12:2427-33
38. Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G. et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. Journal of the American Society of Nephrology. 2002;13:1733-9
39. Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI. et al. Blood pressure in early autosomal dominant polycystic kidney disease. New England Journal of Medicine. 2014;371:2255-66
40. Zhang M, Srichai MB, Zhao M, Chen J, Davis LS, Wu G. et al. Nonselective Cyclooxygenase Inhibition Retards Cyst Progression in a Murine Model of Autosomal Dominant Polycystic Kidney Disease. Int J Med Sci. 2019;16:180-8
41. Muller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O. et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2022;37:825-39
42. Raina R, Houry A, Rath P, Mangat G, Pandher D, Islam M. et al. Clinical Utility and Tolerability of Tolvaptan in the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD). Drug Healthc Patient Saf. 2022;14:147-59
43. Wu TC, Chang WH, Lu HY, Shih CC. Tolvaptan reduces angiotensin II-induced experimental abdominal aortic aneurysm and dissection. Vascul Pharmacol. 2022;144:106973
44. Shao Y-HJ, Chen W-T, Yu SM-W, Tsou LL-A, Hsu Y-H, Wu M-S. et al. Investigation of cardiorenal outcomes and incidence of genitourinary tract infection after combined SGLT2 inhibitor and ACEI/ARB use in patients with chronic kidney disease stages 3-5: A real-world retrospective cohort study in Taiwan. International Journal of Medical Sciences. 2024;21:2109-18
45. Rodriguez D, Kapoor S, Edenhofer I, Segerer S, Riwanto M, Kipar A. et al. Inhibition of Sodium-Glucose Cotransporter 2 with Dapagliflozin in Han: SPRD Rats with Polycystic Kidney Disease. Kidney Blood Press Res. 2015;40:638-47
46. Morioka F, Nakatani S, Uedono H, Tsuda A, Mori K, Emoto M. Short-Term Dapagliflozin Administration in Autosomal Dominant Polycystic Kidney Disease-A Retrospective Single-Arm Case Series Study. J Clin Med. 2023;12:6341
47. Minatoguchi S, Hayashi H, Umeda R, Koide S, Hasegawa M, Tsuboi N. Additional renoprotective effect of the SGLT2 inhibitor dapagliflozin in a patient with ADPKD receiving tolvaptan treatment. CEN Case Rep. 2024;13:419-24
48. Tesař V. SGLT2 inhibitors in non-diabetic kidney disease. Adv Clin Exp Med. 2022Feb;31(2):105-107
49. Nowak KL, Hopp K. Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin J Am Soc Nephrol. 2020Apr7;15(4):577-584
50. Chebib FT, Torres VE. Assessing Risk of Rapid Progression in Autosomal Dominant Polycystic Kidney Disease and Special Considerations for Disease-Modifying Therapy. Am J Kidney Dis. 2021Aug;78(2):282-292
51. Kapoor S, Rodriguez D, Riwanto M, Edenhofer I, Segerer S, Mitchell K, Wüthrich RP. Effect of Sodium-Glucose Cotransport Inhibition on Polycystic Kidney Disease Progression in PCK Rats. PLoS One. 2015Apr30;10(4):e0125603
52. Bahlmann-Kroll E, Häckl S, Kramer S, Wulfmeyer VC, Glandorf J, Kaufeld J, Koch A, Hartung D, Schmidt BMW, Schmidt-Ott K, Schmitt R. Empagliflozin in patients with autosomal dominant polycystic kidney disease (EMPA-PKD): study protocol for a randomised controlled trial. BMJ Open. 2024Dec15;14(12):e088317
Author contact
![]() Corresponding author: Po-Jen Hsiao, MD, PhD, Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taiwan No. 168, Zhongxing Rd., Longtan Dist., Taoyuan City 325, Taiwan; Tel.: 886-3-4799595; E-mail: doc10510gov.tw or a2005a660820com.tw.
Corresponding author: Po-Jen Hsiao, MD, PhD, Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taiwan No. 168, Zhongxing Rd., Longtan Dist., Taoyuan City 325, Taiwan; Tel.: 886-3-4799595; E-mail: doc10510gov.tw or a2005a660820com.tw.

 Global reach, higher impact
Global reach, higher impact