3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(6):1404-1414. doi:10.7150/ijms.103390 This issue Cite
Research Paper
Low-dose Spironolactone Combined with ACEIs/ARBs May Reduce Cardiovascular Events in Patients with CKD Stages 3b-5: A Nationwide Population-Based Cohort Study in Taiwan
1. Institute of Health and Welfare Policy, College of Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan.
2. Graduate Institute of Data Science, College of Management, Taipei Medical University, Taipei, Taiwan.
3. Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
4. Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
5. Department of Life Sciences, National Central University, Taoyuan, Taiwan.
6. Division of Infectious Disease, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
7. Division of Infectious Disease, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
8. Institute of molecular and cellular biology, National Tsing Hua University, Hsinchu, Taiwan.
9. Health and Clinical Data Research Center, Office of Data, Taipei Medical University, Taipei, Taiwan
10. Department of Internal Medicine, The University of Iowa Carver College of Medicine, Iowa City, USA.
11. Jericho High School, Jericho, Nassau County, New York, USA.
12. Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
13. Taipei Medical University-Research Center of Urology and Kidney, Taipei Medical University, Taipei, Taiwan.
14. Division of Nephrology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
15. Division of Nephrology, Department of Internal Medicine, Hsin Kuo Min Hospital, Taipei Medical University, Taoyuan City, Taiwan.
16. Graduate Institute of Clinical Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.
17. Division of Nephrology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan.
#Li-Nien Chien, Po-Jen Hsiao, and Chih-Chien Chiu contributed equally to this work (co-first authors).
*Chu-Lin Chou and Te-Chao Fang contributed equally to this work (co-corresponding authors).
Received 2024-9-7; Accepted 2025-2-11; Published 2025-2-24
Abstract
Background: ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are commonly prescribed for hypertension and chronic kidney disease (CKD) management, but they can increase the risk of renal function deterioration and hyperkalaemia. Spironolactone, known for reducing cardiovascular events in CKD patients, faces limited use due to the risk of hyperkalaemia. This study evaluates the potential efficacy and complications of low-dose spironolactone as an adjunct therapy in patients with CKD stages 3b to 5 who are maintained on ACEIs or ARBs.
Materials and methods: Hypertensive CKD patients (stages 3b-5) from Taiwan's National Health Insurance Research Database (2012-2016) were selected. Inverse probability treatment weighting (IPTW) was applied to balance baseline characteristics between patients treated with and without spironolactone. In this study, adherence to low-dose spironolactone (25 mg/day) was assessed using the medication possession ratio (MPR) over a continuous 3-month period within the first 12 months after initiation. Multivariate Cox regression models were used to compare clinical outcomes between two groups with MPR ≥80% and MPR <80%. The subgroup including poor adherence (MPR ≥40% and MPR <40%) was also evaluated.
Results: Of the 2,623 advanced CKD patients on ACEIs/ARBs and spironolactone, 55.5% (n=1,456) had an MPR ≥80% over a median follow-up of 3.9 years. Post-IPTW, both groups were balanced. Patients with MPR ≥80% showed a lower risk of major adverse cardiovascular events (MACEs; aHR = 0.71, 95% CI = 0.57-0.89), nonfatal myocardial infarction (aHR = 0.54, 95% CI = 0.39-0.75), and heart failure hospitalization (aHR = 0.84, 95% CI = 0.72-0.98). No significant risk was observed for acute renal failure (aHR = 0.87, 95% CI = 0.75-1.02), chronic renal failure (aHR = 0.84, 95% CI = 0.71-1.00), or hyperkalaemia (aHR = 0.86, 95% CI = 0.69-1.07) in the MPR ≥80% group. Patients with MPR ≥40% also showed a lower risk of MACEs (aHR =0.78, 95% CI = 0.62-0.99) and nonfatal MI (aHR = 0.66, CI = 0.47-0.93).
Conclusion: In Taiwan, higher adherence to low-dose spironolactone (25 mg/day) in ACEI/ARB-treated patients with CKD stages 3b-5 may reduce cardiovascular disease risk without increasing the risk of renal failure or hyperkalaemia.
Keywords: acute renal failure, acute kidney injury, chronic kidney disease, spironolactone, cardiovascular disease, dialysis, hyperkalaemia
Introduction
In patients with advanced chronic kidney disease (CKD), hypertension is common and usually remains poorly controlled. Spironolactone, a mineralocorticoid receptor antagonist, is recommended as an add-on therapy for hypertension and heart failure (HF) [1-3]. Spironolactone reduces cardiovascular events and mortality in HF patients and effectively lowers blood pressure (BP) in hypertensive patients [4-9]. Moreover, the addition of spironolactone has a beneficial effect on BP in patients with resistant hypertension [10-13]. A double-blind, placebo-controlled, crossover trial in the UK indicated that spironolactone is the most effective add-on medicine for ameliorating resistant hypertension despite the concurrent use of an angiotensin-converting enzyme inhibitor (ACEI), an angiotensin receptor blocker (ARB), a calcium-channel blocker (CCB), or a diuretic [14]. Thus, the hypertension guidelines from America, Britain and Europe recommend spironolactone as an add-on therapy when other drugs fail to control BP [15, 16]. Spironolactone is effective in patients with HF and CKD, but the risk of adverse events and worsening renal function may be greater in patients with advanced CKD. In addition, spironolactone is associated with an increased risk of adverse effects, including biochemical abnormalities (mainly hyperkalaemia), worsening renal function, and discontinuation of the drug because of anaphylactic reactions or gynaecomastia [17]. Therefore, the use of spironolactone in this high-risk population must be closely monitored to achieve clinical benefit.
Patients with hypertension or HF frequently have comorbid advanced CKD, which may alter the efficacy, tolerability, or safety of spironolactone. Spironolactone is a medication that is often used as a diuretic and for treating conditions such as HF, high BP, and certain types of edema. It works by blocking the action of aldosterone, a hormone that causes the kidneys to retain sodium and water. By blocking this hormone, spironolactone helps remove excess fluid from the body and lowers BP. One of the potential side effects of spironolactone is hyperkalaemia, as it reduces the amount of potassium that is excreted by the kidneys. Therefore, CKD patients taking spironolactone are usually advised to monitor their potassium intake to avoid hyperkalaemia [14-16]. To date, there is little evidence of the effectiveness and safety of spironolactone in patients with advanced CKD stages (3b-5), especially those with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2. In this study, data from real-world databases in Taiwan were analysed to explore the effectiveness of spironolactone on the risk of major adverse cardiovascular events (MACEs), hospitalization for HF (HHF), acute (ARF) and chronic (CRF) renal failure requiring dialysis, and hyperkalaemia among advanced CKD patients who were treated with ACEIs/ARBs.
Materials and methods
Study design and database
This was a retrospective cohort study using data from the National Health Insurance Research Data (NHIRD), a population-based claims database covering almost all healthcare services and drug prescriptions under the regulation of the NHI program in Taiwan. Since 1995, all residents of Taiwan have been required by law to enrol in the NHI, resulting in an over 99% coverage rate [18]. We also used the National Death Registry (NDR), a population-based registry for the cause of death, to obtain the death information of all residents. In addition, two datasets can be linked by unique encrypted identifiers under the regulation of the Health and Clinical Science Data Center, Ministry of Health and Welfare in Taiwan. The NHIRD is one of the highest-quality databases of its type in the world and has been widely used for longitudinal cohort studies, including several of our previous investigations [19-30].
Study cohort
First, patients were included if they were enrolled in the pre-end-stage renal disease (pre-ESRD) pay-for-performance (P4P) program between 2012 and 2016, a patient care and education program involving health management of high-risk groups to improve healthcare and delay the onset of ESRD and dialysis initiated in November 2006 [31, 32]. In this study, all CKD patients received their regular medications such as cardiovascular drugs and antidiabetic drugs. The eGFR was more commonly calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation in Taiwan. Patients were eligible for the program if they met the following criteria: CKD stage 3b, 4, or 5 with eGFRs of 30-44.9, 15-29.9, or <15 mL/min/1.73 m2, respectively; and proteinuria, defined as daily urinary protein levels of >1000 mg or a urine protein/creatinine ratio of >1000 mg/g. In addition, multidisciplinary medical teams, including nephrologists, health education nurses, and nutritionists, provided comprehensive medical assessments, laboratory examinations, and patient education every three months. Those enrolled in the program were also cared for according to applicable clinical guidelines at different stages of CKD [33]. The date of initial enrolment in the program was treated as the index date.
We selected patients who were diagnosed with hypertension and excluded those who 1) were aged less than 20 years or had missing sex information, 2) had a catastrophic illness (including cancer) other than dialysis during the overall study period, or 3) had a history of dialysis or liver cirrhosis before the index date. Finally, we limited inclusion to patients treated with ACEIs/ARBs within three months after the index date because we aimed to examine the effectiveness of spironolactone as an add-on therapy to ACEIs/ARBs, which are the preferred agents for treating advanced CKD patients with hypertension. We also used a three-month window to calculate medication compliance. Thus, patients who experienced outcomes within that window were also excluded.
Study variables
The identification of low-dose (25 mg per day) spironolactone use was based on prescription claims from the NHIRD. Patients were defined as spironolactone users if they received a pharmacy claim for spironolactone in combination with ACEIs/ARBs within three months of the start of the study. We used the medication possession ratio (MPR) to measure compliance with spironolactone treatment. In this study, the MPR represents the ratio of days covered by medication supply across all prescription fills within a specified time interval (continuous 3-month use during the 12 months following the index date). In this study, if a participant does not continue their prescription for more than 90 days during the follow-up period, they will be considered "censored" and will exit the study group, and we will no longer continue observing them. The MPR was further classified into the categories, with <40% as the lower bound and ≥80% as the upper bound. An MPR of 80% is a reasonable threshold for compliance, as it suggests very few days without the drug on hand and, consequently, fairly continuous medication use [34].
Outcome definition
The outcomes of interest were MACEs, HHF, requiring dialysis, and hyperkalaemia during the follow-up periods. The definition of MACEs was a composite of nonfatal ischaemic stroke (IS), nonfatal myocardial infarction (MI), and cardiovascular death. The occurrence of stroke, myocardial infarction, or HHF was indicated by a discharge record from the NHIRD. ARF requiring dialysis was defined as a payment code for dialysis. If a patient continuously had payment codes for at least three months, the patient was regarded as having CRF. Moreover, the occurrence of hyperkalaemia was defined as a diagnosis of hyperkalaemia after the index date. Finally, all the patients were followed up from the index date to the date of outcome, death or the study end date of December 31st, 2018.
Covariates
The covariates included sex, age, CKD stage, ACEI/ARB MPR, history of hospitalization, comorbidities, medication use, Charlson comorbidity index (CCI), and CHA2DS2-VASc score (congestive HF, hypertension, age ≥75 years, diabetes mellitus (DM), stroke/transient ischaemic attack, vascular disease, age 65 to 74 years, sex category [female]). Comorbidities associated with the risk of adverse cardiovascular outcomes were defined only if patients had at least two diagnostic claims within one year before the index date. For medication use, patients who had received the medication continuously for at least three months within a year were considered to have a specific prescription. We also used the CCI and CHA2DS2-VASc score to adjust for the severity of comorbidities.
Statistical analysis
Several factors might be associated with the effectiveness of spironolactone, resulting in a significant difference in baseline characteristics between the two groups. Thus, we used inverse probability treatment weighting (IPTW) to adjust the imbalance. IPTW, a method based on propensity scoring, was used to balance baseline variables without loss of samples. IPTW is also an appropriate method for estimating treatment effects on time-to-event outcomes [35]. The stabilized IPTW was weighted for each patient after the propensity score was generated. This method has been widely adopted in many observational studies. The standardized mean difference (SMD) is presented, and SMD >0.1 indicates nonnegligible differences between groups.
The incidence per 100 person-years of outcomes was calculated via Kaplan‒Meier estimation. A Cox proportional hazards regression model was used to evaluate the hazard ratio (HR) for risk outcomes associated with spironolactone (25 mg/day), after controlling for demographic and clinical factors [36]. Patients who died, were lost to follow-up, or were discharged before the event of interest occurred during the follow-up period were censored. Subgroup analysis was also performed by age group, DM status, and CHA2DS2-VASc score. None of the models violated the assumption of proportional hazards. All analyses were performed via SAS/STAT 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered to indicate statistical significance.
Results
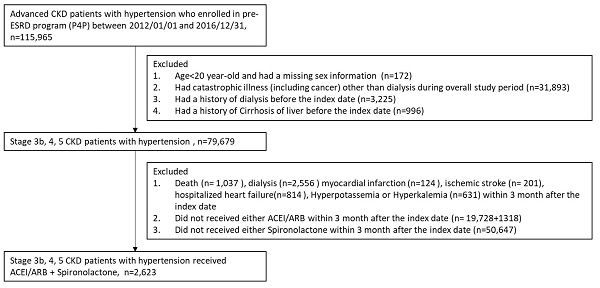
The patient selection process is shown in detail in Figure 1. Close to half of the 115,965 advanced CKD patients with hypertension who met the inclusion criteria were eligible for the analyses in this study, as shown in Figure 1. Ultimately, a total of 2,623 participants with CKD stages 3b, 4, and 5 and hypertension treated with ACEIs/ARBs plus spironolactone were enrolled and divided into 2 groups: patients with a spironolactone MPR <80% (n = 1,167) and those with a spironolactone MPR ≥80% (n = 1,456) (see Table 1 for the baseline characteristics of the study cohort). After IPTW, there were no differences in age, sex, CKD stage, MPR, CCI, CHA2DS2-VASc score, comorbidities, or medication use between the two groups, except during the follow-up period.
Table 2 presents the incidence (per 100 person-years) and adjusted HR (aHR) of cardiovascular and dialysis events for the MPR <80% and ≥80% groups. The incidences of MACEs were 4.84 and 3.43 per 100 person-years for patients with an MPR of <80% and ≥80%, respectively, with an aHR of 0.71 (95% confidence interval [CI] of 0.57-0.89). Differences in nonfatal MI (aHR = 0.54, CI = 0.39-0.75) and HHF (aHR = 0.91, CI = 0.72-0.98) were also detected between the two groups. The result of nonfatal IS showed no significance between the two groups (aHR = 0.83, CI = 0.59-1.16). Moreover, patients with an MPR ≥80% did not experience an increased risk of ARF requiring dialysis, CRF requiring dialysis, or hyperkalaemia.
Patient selection process.
Baseline characteristics of late-CKD patients treated with AECIs/ARBs + spironolactone before and after IPTW.
| Spironolactone, MPR | Before IPTW | After IPTW | ||||
|---|---|---|---|---|---|---|
| MPR < 80%n = 1,167, % | MPR ≥ 80%n = 1,456, % | SMD | MPR < 80%n = 1,167, % | MPR ≥ 80%n = 1,456, % | SMD | |
| Male | 54.4 | 56.2 | 0.036 | 54.3 | 54.2 | < 0.001 |
| Age (years), [mean, SD] | [69.0, 14.1] | [68.1, 14.1] | 0.068 | [68.6, 14.1] | [68.8, 13.9] | 0.015 |
| 20-44 | 5.2 | 6.6 | 0.058 | 5.8 | 5.8 | < 0.001 |
| 45-64 | 30.6 | 33 | 0.053 | 31.6 | 31.2 | 0.008 |
| 65-74 | 21.6 | 21.8 | 0.004 | 21.9 | 21.7 | 0.005 |
| ≥ 75 | 42.6 | 38.6 | 0.081 | 40.7 | 41.3 | 0.012 |
| Stage | ||||||
| 3b, 4 | 65.9 | 78.8 | 0.293 | 72.6 | 73.3 | 0.017 |
| 5 | 34.1 | 21.2 | 0.293 | 27.4 | 26.7 | 0.017 |
| AECIs/ARBs, MPR [mean, SD] | [0.8, 0.3] | [0.9, 0.2] | 0.517 | [0.9, 0.2] | [0.9, 0.2] | 0.012 |
| MPR ≥ 80% | 65.3 | 89.3 | 0.598 | 78.3 | 78.2 | 0.001 |
| MPR 40-80% | 21.3 | 6.4 | 0.441 | 13.3 | 13.3 | 0.002 |
| MPR < 40% | 13.5 | 4.3 | 0.325 | 8.4 | 8.4 | 0.001 |
| CCI [mean, SD] | [4.1, 1.9] | [4.0, 1.8] | 0.071 | [4.0, 1.9] | [4.0, 1.8] | 0.004 |
| CHA2DS2-VASc [mean, SD] | [3.7, 1.4] | [3.7, 1.5] | 0.054 | [3.7, 1.4] | [3.7, 1.5] | 0.005 |
| 0-2 | 20.3 | 26.2 | 0.139 | 20.8 | 24 | 0.078 |
| 3-5 | 68.6 | 62.5 | 0.129 | 67.5 | 63.8 | 0.078 |
| ≥ 6 | 11.1 | 11.3 | 0.009 | 11.8 | 12.2 | 0.014 |
| History of hospitalization | ||||||
| AMI | 1.1 | 1.6 | 0.040 | 1.3 | 1.5 | 0.017 |
| Heart failure | 9.9 | 11.5 | 0.049 | 10.8 | 10.6 | 0.005 |
| Stroke | 6.4 | 4.7 | 0.077 | 5.5 | 5.8 | 0.011 |
| Comorbidities | ||||||
| Diabetes mellitus | 64.3 | 64.1 | 0.004 | 65.2 | 65.1 | 0.003 |
| IHD | 28.5 | 33.4 | 0.106 | 31.4 | 31.5 | < 0.001 |
| Atrial fibrillation | 5.6 | 7.1 | 0.062 | 6.4 | 6.5 | 0.001 |
| Hyperlipidaemia | 48.1 | 47.9 | 0.004 | 47.8 | 47.9 | < 0.001 |
| PVD | 3.3 | 3 | 0.022 | 3.2 | 3 | 0.009 |
| COPD | 17.5 | 16 | 0.040 | 17.2 | 17.1 | 0.003 |
| CLD | 5.3 | 3.8 | 0.070 | 4.7 | 4.6 | 0.004 |
| Dementia | 4.3 | 4.3 | 0.001 | 4 | 4.3 | 0.015 |
| Medication use | ||||||
| Clopidogrel | 3.5 | 4.2 | 0.035 | 3.8 | 3.9 | 0.007 |
| Dipyridamole | 6 | 4 | 0.093 | 5 | 4.9 | 0.008 |
| Warfarin | 1.5 | 2.7 | 0.090 | 2.3 | 2.2 | 0.006 |
| ACEIs/ARBs | 42.2 | 40.6 | 0.032 | 41.3 | 41.2 | 0.003 |
| Loop diuretics | 29.2 | 28 | 0.028 | 29.3 | 29.2 | 0.003 |
| Beta-2 blockers | 23 | 20.9 | 0.049 | 21.8 | 21.9 | 0.003 |
| CCBs | 28.2 | 22.8 | 0.124 | 25.5 | 25.5 | < 0.001 |
| Antiplatelet drugs | 24.7 | 23.3 | 0.033 | 24.8 | 24.7 | 0.002 |
| Statins | 16.9 | 14.8 | 0.056 | 16.2 | 15.9 | 0.009 |
| NSAIDs | 26.5 | 21.2 | 0.124 | 24.1 | 24.2 | 0.002 |
| Metformin | 8.3 | 6.5 | 0.068 | 7.6 | 7.4 | 0.008 |
| Thiazolidinedione | 3.1 | 1.9 | 0.079 | 2.3 | 2.1 | 0.015 |
| Sulfonylureas | 12.8 | 11.9 | 0.027 | 12.6 | 12.5 | 0.003 |
| AGIs | 5.1 | 4.1 | 0.045 | 4.7 | 4.7 | 0.002 |
| DPP4is | 12.4 | 10.9 | 0.049 | 11.6 | 11.3 | 0.008 |
| Insulin | 11.2 | 10.3 | 0.030 | 11 | 10.9 | 0.002 |
| Follow-up period (years) [mean, SD] | [3.5, 1.9] | [3.9, 1.9] | 0.216 | [3.5, 1.9] | [3.9, 1.9] | 0.174 |
Abbreviations: ACEIs/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AGIs: alpha-glucosidase inhibitors; AMI: acute myocardial infarction; CCBs: calcium channel blockers; CCI: Charlson comorbidity index; CKD: chronic kidney disease; CLD: chronic liver disease; COPD: chronic obstructive pulmonary disease; DPP4is: dipeptidyl peptidase 4 inhibitors; IHD: ischaemic heart disease; IPTW: inverse probability of treatment weighting; NSAIDs: nonsteroidal anti-inflammatory drugs; PVD: peripheral vascular disease; SD: standard deviation; SMD: standardized mean difference.
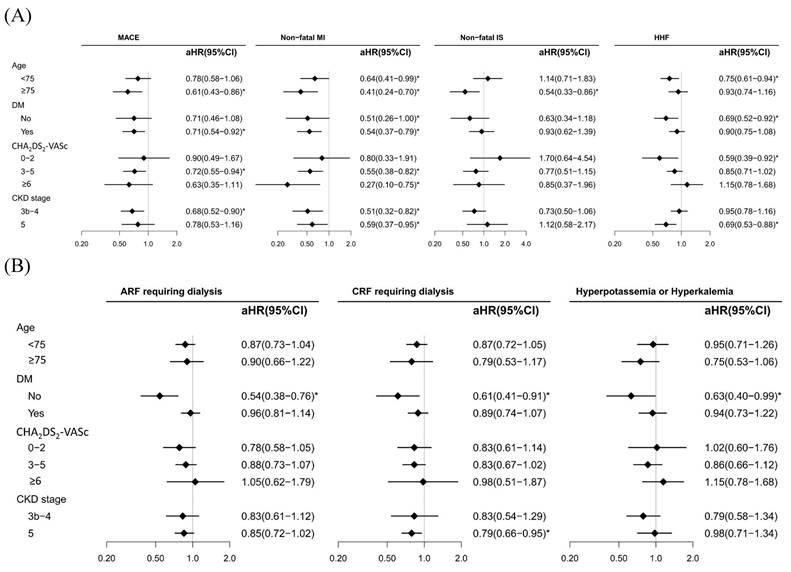
Figure 2A shows the subgroup analyses (age, DM, CHA2DS2-VASc score, and CKD stages 3b-4 and 5) for the benefits and risks associated with spironolactone use, with MPRs <80% (reference) and ≥80% for cardiovascular and dialysis events. For elderly individuals (≥75 years old), our data revealed a lower risk of MACEs, nonfatal MI, and nonfatal IS; however, a lower risk of HHF was observed in patients aged <75 years. DM status was associated with a decreased risk of MACEs and nonfatal MI, whereas non DM status was associated with a reduced risk of HHF. With respect to HHF severity, we utilized the CHA2DS2-VASc; a mild score (0-2) was associated with a decreased risk of HHF, and an advanced score (3-5 and ≥6) was associated with an increased risk of nonfatal MI. Finally, compared with CKD stage 5, CKD stages 3b-4 were associated with a lower risk of MACEs and nonfatal MI. The study results also demonstrated that there was no significant risk of ARF, CRF, and hyperkalaemia in patients who maintained a MPRs of 80% or higher (Figure 2B). Specifically, the adjusted hazard ratio (aHR) for ARF requiring dialysis was 0.87 with a 95% confidence interval (CI) ranging from 0.75 to 1.02. Similarly, the risk of CRF requiring dialysis was also not significantly elevated, with an aHR of 0.84 and a 95% CI of 0.71 to 1.00. Furthermore, the incidence of hyperkalaemia was not significantly higher in the high adherence group, with an aHR of 0.86 and a 95% CI of 0.69 to 1.07.
Table 3 presents the incidence (per 100 person-years) and adjusted HR (aHR) of cardiovascular and dialysis events for the MPR <40% and ≥40% groups. The incidences of MACEs were 4.77 and 3.72 per 100 person-years for patients with an MPR of <40% and ≥40%, respectively, with an aHR of 0.78 (95% confidence interval [CI] of 0.62-0.99). Differences in nonfatal MI (aHR = 0.66, CI = 0.47-0.93) and HHF (aHR = 0.91, CI = 0.77-1.07) were also detected between the two groups.
Discussion
In this study, we are the first to explore the associations between low-dose spironolactone use and the risk of cardiovascular events and dialysis in advanced CKD patients treated with ACEIs/ARBs on the basis of real-world data in Taiwan. After adopting IPTW, controlling for covariates, and performing subgroup and sensitivity analyses, our main findings were as follows. (1) Low-dose spironolactone with an MPR ≥80% was associated with a decreased risk of cardiovascular events (MACEs, nonfatal MI, and HHF) and no additional risk of dialysis events (ARF requiring dialysis, CRF requiring dialysis, and hyperkalaemia). (2) Subgroup analyses revealed a decreased risk of MACEs and nonfatal MI in elderly individuals (≥ 75 years old) and DM patients and a decreased risk of HHF in younger individuals (< 75 years old), non-DM patients, and patients with a mild CHA2DS2-VASc score.
Subgroup analysis for adjusted hazard ratios (aHRs) of cardiovascular (A) and dialysis events (B) between spironolactone MPR groups (<80% (ref.) and ≥80%) in patients with CKD stages 3b-5 treated with ACEIs/ARBs.
The incidence (per 100 PY) and adjusted HR of cardiovascular and dialysis events between spironolactone MPR groups (< 80% and ≥ 80%) in patients with CKD stages 3b-5 treated with ACEIs/ARBs.
| Outcomes | No. of events | PY | Incidence (95% CI) | Adjusted* HR (95% CI) | P value |
|---|---|---|---|---|---|
| MACEs | 0.003 | ||||
| MPR < 80% | 184 | 3,806 | 4.84 (4.19-5.59) | 1.00 (Ref.) | |
| MPR ≥ 80% | 183 | 5,329 | 3.43 (2.95-3.95) | 0.71 (0.57-0.89) | |
| Nonfatal MI | < 0.001 | ||||
| MPR < 80% | 94 | 3,942 | 2.39 (1.95-2.92) | 1.00 (Ref.) | |
| MPR ≥ 80% | 71 | 5,489 | 1.29 (1.03-1.63) | 0.54 (0.39-0.75) | |
| Nonfatal IS | 0.264 | ||||
| MPR < 80% | 74 | 3,937 | 1.88 (1.50-2.36) | 1.00 (Ref.) | |
| MPR ≥ 80% | 84 | 5,456 | 1.54 (1.23-1.89) | 0.83 (0.59-1.16) | |
| HHF | 0.025 | ||||
| MPR < 80% | 353 | 3,369 | 10.47 (9.41-11.60) | 1.00 (Ref.) | |
| MPR ≥ 80% | 412 | 4,809 | 8.57 (7.78-9.44) | 0.84 (0.72-0.98) | |
| ARF requiring dialysis | 0.077 | ||||
| MPR < 80% | 369 | 3,279 | 11.25 (10.14-12.43) | 1.00 (Ref.) | |
| MPR ≥ 80% | 452 | 4,690 | 9.64 (8.79-10.57) | 0.87 (0.75-1.02) | |
| CRF requiring dialysis | 0.051 | ||||
| MPR < 80% | 295 | 3,334 | 8.86 (7.90-9.92) | 1.00 (Ref.) | |
| MPR ≥ 80% | 354 | 4,793 | 7.38 (6.64-8.18) | 0.84 (0.71-1.00) | |
| Hyperkalaemia | 0.181 | ||||
| MPR < 80% | 178 | 3,735 | 4.76 (4.09-5.49) | 1.00 (Ref.) | |
| MPR ≥ 80% | 207 | 5,148 | 4.02 (3.49-4.59) | 0.86 (0.69-1.07) |
Abbreviations: ARF: acute renal failure; CRF: chronic renal failure; CI: confidence interval; HHF: hospitalized heart failure; HR: hazard ratio; IS: ischaemic stroke; MACEs: major adverse cardiac events; MI: myocardial infarction; PY: person-year; Ref.: reference group.
The incidence (per 100 PY) and adjusted HR of cardiovascular and dialysis events between spironolactone MPR groups (< 40% and ≥ 40%) in patients with CKD stages 3b-5 treated with ACEIs/ARBs.
| Outcomes | No. of events | PY | Incidence (95% CI) | Adjusted* HR (95% CI) | P value |
|---|---|---|---|---|---|
| MACEs | 0.040 | ||||
| MPR < 40% | 122 | 2,564 | 4.77 (3.99-5.68) | 1.00 (Ref.) | |
| MPR ≥ 40% | 245 | 6,570 | 3.72 (3.28-4.21) | 0.78 (0.62-0.99) | |
| Nonfatal MI | 0.017 | ||||
| MPR < 40% | 61 | 2,658 | 2.31 (1.79-2.95) | 1.00 (Ref.) | |
| MPR ≥ 40% | 104 | 6,773 | 1.53 (1.25-1.84) | 0.66 (0.47-0.93) | |
| Nonfatal IS | 0.114 | ||||
| MPR < 40% | 54 | 2,636 | 2.05 (1.57-2.67) | 1.00 (Ref.) | |
| MPR ≥ 40% | 104 | 6,757 | 1.54 (1.26-1.85) | 0.76 (0.53-1.07) | |
| HHF | 0.243 | ||||
| MPR < 40% | 231 | 2,272 | 10.18 (8.94-11.57) | 1.00 (Ref.) | |
| MPR ≥ 40% | 533 | 5,905 | 9.03 (8.29-9.83) | 0.91 (0.77-1.07) | |
| ARF requiring dialysis | 0.023 | ||||
| MPR < 40% | 261 | 2,196 | 11.88 (10.49-13.37) | 1.00 (Ref.) | |
| MPR ≥ 40% | 560 | 5,773 | 9.70 (8.93-10.54) | 0.83 (0.71-0.97) | |
| CRF requiring dialysis | 0.013 | ||||
| MPR < 40% | 210 | 2,223 | 9.44 (8.21-10.77) | 1.00 (Ref.) | |
| MPR ≥ 40% | 439 | 5,904 | 7.44 (6.77-8.17) | 0.80 (0.67-0.95) | |
| Hyperkalaemia | 0.658 | ||||
| MPR < 40% | 107 | 2,526 | 4.22 (3.47-5.08) | 1.00 (Ref.) | |
| MPR ≥ 40% | 278 | 6,357 | 4.38 (3.89-4.92) | 1.06 (0.83-1.34) |
Abbreviations: ARF: acute renal failure; CRF: chronic renal failure; CI: confidence interval; HHF: hospitalized heart failure; HR: hazard ratio; IS: ischaemic stroke; MACEs: major adverse cardiac events; MI: myocardial infarction; PY: person-year; Ref.: reference group.
(3) Even when spironolactone with an MPR ≥40% was used, there was still a benefit in terms of MACEs and nonfatal MI. Low-dose spironolactone with an MPR ≥40% also had a protective effect in reducing the risk of MACEs and nonfatal MI. Similar to the MPR ≥80% group, patients with an MPR ≥40% did not experience an increased risk of ARF requiring dialysis, CRF requiring dialysis, or hyperkalaemia.
The use of spironolactone for heart disease treatment in patients with advanced CKD continues to be a challenge in clinical practice. According to a meta-analysis of randomized controlled trials (RCTs; N = 829), compared with the control group, ESRD patients with or without HF who were treated with spironolactone or eplerenone had reduced risks of cardiovascular (RR: 0.34; 95% CI: 0.15 to 0.75) and all-cause (RR: 0.40; 95% CI: 0.23 to 0.69) death [37]. Furthermore, a subgroup analysis from the TOPCAT trial, an international, double-blind RCT of spironolactone use in patients older than 50 years of age with symptomatic HFpEF (left ventricular ejection fraction ≥45%),17 revealed that patients (N=1767) on spironolactone had a decreased risk (HR 0.82, 95% CI: 0.69-0.98) of cardiovascular events (cardiovascular death, HHF, and aborted cardiac arrest); however, the TOPCAT trial did not include patients with more severe kidney disease (eGFR <30 mL/min/1.73 m2 or serum creatinine ≥2.5 mg/dL).
Our results regarding the effects of low-dose spironolactone use with an MPR ≥80% on lowering the risk of cardiovascular events (MACEs, nonfatal MI, and HHF) were similar to some other clinical studies in the Asian and Europe populations [38-40]. For example, in a single-centre, observational, retrospective, registry-based clinical study of 200 patients with acute MI and CKD (eGFR <60 mL/min/1.73 m2) who were treated with spironolactone, Qu et al. reported a reduced risk of both all-cause mortality (HR: 0.389; 95% CI: 0.276-0.548; p < 0.001) and readmission (HR: 0.664; 95% CI: 0.522-0.846; p = 0.004) after 30 months of follow-up. Additionally, compared with high-dose spironolactone use (more than 40 mg; HR: 0.429; 95% CI: 0.199-0.925; p = 0.007), low-dose spironolactone use (no more than 40 mg) was associated with a lower risk of all-cause mortality (HR: 0.309; 95% CI: 0.228-0.418; p < 0.001) [38]. In addition, the results from the MiREnDa trial (an RCT) and experiments on mice subjected to subtotal nephrectomy and cholecalciferol treatment supported the benefit of spironolactone treatment in ameliorating CKD-associated vascular calcification [39]. Moreover, in an RCT that included haemodialysis patients, spironolactone treatment at a dose of 12.5 to 25 mg for 6 months was associated with the regression of left ventricular hypertrophy [40]. Recent evidence has demonstrated that potassium binders can reduce the incidence of hyperkalaemia. The related guidelines address key areas, including monitoring, dietary restrictions, the use of potassium binders, and the concurrent prescription of renin‒angiotensin‒aldosterone system (RAAS) inhibitors. However, further research is needed to determine whether reduced-potassium diets or potassium binder therapy may improve patient-centred outcomes, especially in patients with advanced stages of CKD [41]. Although our data revealed the effectiveness and safety of spironolactone treatment in patients with advanced CKD, further prospective RCTs are needed to clarify the effect of spironolactone on cardiovascular events in this population.
The effects of spironolactone treatment on the risk of hyperkalaemia, ARF or acute kidney injury (AKI, acute deterioration of renal function) and the need for emergency dialysis in patients with advanced CKD remain unclear. In the TOPCAT Americas study (n = 1,767) on safety outcomes, including hyperkalaemia, worsening kidney function, and permanent drug discontinuation, the risk of safety outcomes was greater for patients with severe kidney dysfunction (eGFR <45 ml/min/1.73 m2: HR, 1.99; 95% CI, 1.62-2.45; p < 0.001) than for patients with relatively preserved eGFRs (≥60 ml/min/1.73 m2) during the 4-year follow-up [17]. This finding suggested an increased risk for intolerance to spironolactone with decreasing renal function. These data focus on the importance of close laboratory monitoring in patients with HFpEF and an eGFR less than 45 mL/min/1.73 m2 who are treated with spironolactone. Furthermore, meta-analyses revealed that the addition of spironolactone to an ACEI or an ARB was associated with an increased risk of side effects, mainly a two- to threefold greater risk of hyperkalaemia [42-44].
Feniman-De-Stefano et al. reported that spironolactone treatment at a dose of 12.5 to 25 mg for 6 months was not associated with the risk of hyperkalaemia in haemodialysis patients [40]. The safety of spironolactone in a randomized, placebo-controlled study of dialysis-dependent ESRD patients showed a dose-dependent increase related to the risk of hyperkalaemia, particularly when spironolactone was prescribed at a dosage of 50 mg/d. In this small study, spironolactone was found to be safe for well-monitored maintenance haemodialysis patients, though it did not significantly impact cardiovascular outcomes [45]. Rajagopalan et al. recently reported that spironolactone could reduce the progression of atherosclerosis in diabetic patients with advanced stages of CKD. These RCT results from the United States are also compatible with our study findings [46]. Our study results revealed low-dose spironolactone treatment had no additional risk of adverse events regarding dialysis or hyperkalaemia. We propose that these results may be influenced by potassium-restricted diets and drug dosages. In Taiwan, the pre-ESRD program has been effectively implemented due to the high prevalence of ESRD. A low-potassium diet is often recommended for patients in the pre-ESRD program to help manage potassium levels and reduce the risk of complications. Various clinical factors, such as sample size, follow-up duration, racial differences, low-potassium diets, drug dosages, and patient compliance, may impact the clinical outcomes. Long-term benefit-risk profile of low-dose spironolactone treatment in the patients with advanced CKD and ESRD needs further investigation. Finerenone is a new non-steroidal mineralocorticoid receptor antagonist used primarily in the treatment of CKD associated with type 2 DM. It works by blocking the effects of aldosterone and can further decrease kidney damage, fibrosis, and cardiovascular complications. From FIDELIO-DKD and FIGARO-DKD Trial, finerenone has been shown to reduce albuminuria, slow the progression of kidney disease, lower the risk of hyperkalemia and cardiovascular events such as heart failure and stroke in patients with diabetic kidney disease [47-50].
Thiazide and thiazide-like diuretics are widely prescribed in clinical practice. It was believed that these agents would lose effectiveness in patients with the GFR below 30 mL/min/1.73 m² previously [51]. Over time, this notion became widely accepted, and this threshold has long been cited in medical textbooks and guidelines to contraindicate thiazides in patients with advanced stages of CKD. The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for hypertension management advise against using thiazides in patients with GFR <30 mL/min/1.73 m² [52]. Similarly, the 2018 ESC/ESH Hypertension Guidelines state that thiazides and thiazide-like agents are less effective in patients with an eGFR <45 mL/min/1.73 m² and are ineffective at eGFR <30 mL/min/1.73 m² [53]. Therefore, thiazides are used less frequently among these patients. To avoid the selection bias, baseline medications did not include this drug in our study. However, recent data supporting cardiovascular risk reduction have renewed interest in the use of thiazides in the patients with advanced stages of CKD. These findings should spur new prospective randomized trials and spark discussions, particularly about upcoming hypertension guidelines in CKD patients [54, 55].
Strengths and limitations
This study is the first to use the MPR to assess the effects of low-dose spironolactone on cardiovascular events, emergency dialysis, and hyperkalaemia in Taiwanese patients with advanced stages of CKD. In our nationwide population-based cohort study, we observed that a low dose of 25 mg spironolactone per day combined ACEIs/ARBs use was safe in patients with CKD stages 3b-5. Our study results revealed that low-dose spironolactone was not associated with ARF or CKD requiring dialysis in these patients. However, there are several limitations in this investigation. First, in observational studies, confounding by indication has often been an intractable threat to validity because patients with poor prognoses are more likely to be treated aggressively. Second, we observed that the patients who were treated with spironolactone had more comorbidities and greater medication use (shown in Table 1); thus, confounding by indication was found. We then adopted IPTW, multivariable regression models, and patient subgroups to improve the validity of the findings in this study; however, no adjustment methods could fully resolve the bias. Therefore, the results of the present study should be interpreted with caution. Moreover, we used data from reimbursement claims; consequently, we could not obtain data on BP, body mass index, or lifestyle. Magnesium levels rise as kidney function declines. Hypermagnesemia is a predictor of cardiovascular events and all-cause mortality in individuals with reduced renal function [56]. Associated laboratory data could be investigated in the future study. Finally, the information was derived from individuals of Asian descent, and therefore, generalizability of the findings may be limited to other different races or ethnicities.
Conclusion
This cohort study revealed that low-dose spironolactone treatment is associated with a reduced risk of cardiovascular events (MACEs, nonfatal MI, and HHF) and no change in the risk of adverse events (emergent dialysis and hyperkalaemia) in patients with stages 3b-5 CKD treated with ACEIs/ARBs. Moreover, we believe that prospective randomized trials further investigating these effects are warranted.
Acknowledgements
Funding
This study was supported by grants TYAFGH-D-113032, TYAFGH-E-113052, TYAFGH-D-114032, and TYAFGH-E-114051 from the Taoyuan Armed Forces General Hospital, Taiwan.
Author contributions
The authors declare that they have no conflicts of interest. All the authors contributed to the conception and design of the study. Material preparation was performed by LNC, PJH, CCC, WTN, CJC, LLT, YHK, CLC, and TCF; data collection was performed by LNC, PJH, and CCC; analysis was performed by PJH and FCL; and validation was performed by WTN, CJC, LLT, YHK and PJH. LNC, PJH, and CCC wrote the original drafts. PJH, CCC, CLC, YHK, and TCF reviewed and edited the manuscript. All authors commented on all versions of the manuscript and have read and approved the published version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Institutional review board statement
This study was approved by the Taipei Medical University Joint Institutional Review Board (TMU-JIRB No. N202106040).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR. et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90
2. Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, Ferreira JP. et al. Hypertension and heart failure with preserved ejection fraction: position paper by the European Society of Hypertension. J Hypertens. 2021;39(8):1522-45
3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628-51
4. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. 1999;341(10):709-17
5. Tsujimoto T, Kajio H. Spironolactone use and improved outcomes in patients with heart failure with preserved ejection fraction with resistant hypertension. J Am Heart Assoc. 2020;9(23):e018827
6. Wolf RL, Mendlowitz M, Roboz J, Styan GP, Kornfeld P, Weigl A. Treatment of hypertension with spironolactone. Double-blind study. JAMA. 1966;198(11):1143-9
7. Johnston LC, Grieble HG. Treatment of arterial hypertensive disease with diuretics. V. Spironolactone, an aldosterone antagonist. Arch Intern Med. 1967;119(3):225-31
8. Brown JJ, Davies DL, Ferriss JB, Fraser R, Haywood E, Lever AF. et al. Comparison of surgery and prolonged spironolactone therapy in patients with hypertension, aldosterone excess, and low plasma renin. Br Med J. 1972;2(5816):729-34
9. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892-96
10. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16(11 Pt 1):925-30
11. Sharabi Y, Adler E, Shamis A, Nussinovitch N, Markovitz A, Grossman E. Efficacy of add-on aldosterone receptor blocker in uncontrolled hypertension. Am J Hypertens. 2006;19(7):750-5
12. Chapman N, Dobson J, Wilson S, Dahlöf B, Sever PS, Wedel H. et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49(4):839-45
13. De Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55(1):147-52
14. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G. et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059-68
15. Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF. et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18(3):139-85
16. Grassi G, Calhoun DA, Mancia G, Carey RM. Resistant hypertension management: comparison of the 2017 American and 2018 European high blood pressure guidelines. Curr Hypertens Rep. 2019;21(9):67
17. Beldhuis IE, Myhre PL, Claggett B, Damman K, Fang JC, Lewis EF. et al. Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Fail. 2019;7(1):25-32
18. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH. et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol. 2019;11:349-58
19. Hwang DK, Chou YJ, Pu CY, Chou P. Epidemiology of uveitis among the Chinese population in Taiwan: a population-based study. Ophthalmology. 2012;119(11):2371-6
20. Wu KL, Shih CP, Chan JS, Chung CH, Lin HC, Tsao CH. et al. Investigation of the relationship between sensorineural hearing loss and associated comorbidities in patients with chronic kidney disease: A nationwide, population-based cohort study. PLoS One. 2020;15(9):e0238913
21. Hu CC, Ho JD, Lou HY, Keller JJ, Lin HC. A one-year follow-up study on the incidence and risk of endophthalmitis after pyogenic liver abscess. Ophthalmology. 2012;119(11):2358-63
22. Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. 2012;27(4):1585-90
23. Hung TH, Chou CL, Fang TC. Impact of renal dysfunction in cirrhotic patients with bacterial infections other than spontaneous bacterial peritonitis. Hepatol Res. 2014;44(8):863-70
24. Chou CL, Hsieh TC, Wang CH, Hung TH, Lai YH, Chen YY. et al. Long-term outcomes of dialysis patients after coronary revascularization: a population-based cohort study in Taiwan. Arch Med Res. 2014;45(2):188-94
25. Wang YC, Hsieh TC, Chou CL, Wu JL, Fang TC. Risks of adverse events following coprescription of statins and calcium channel blockers: a nationwide population-based study. Medicine (Baltimore). 2016;95(2):e2487
26. Hsieh TC, Chou CL, Chen JS, Kuo CH, Wang YC, Lai YH. et al. Risk of mortality and of atherosclerotic events among patients who underwent hemodialysis and subsequently developed retinal vascular occlusion: a Taiwanese retrospective cohort study. JAMA Ophthalmol. 2016;134(2):196-203
27. Kuo CH, Hsieh TC, Wang CH, Chou CL, Lai YH, Chen YY. et al. Increased risks of mortality and atherosclerotic complications in incident hemodialysis patients subsequently with bone fractures: a nationwide case-matched cohort study. PLoS One. 2015;10(4):e0121705
28. Shao YJ, Chen WT, Yu SM, Tsou LL, Hsu YH, Wu MS. et al. Investigation of cardiorenal outcomes and incidence of genitourinary tract infection after combined SGLT2 inhibitor and ACEI/ARB use in patients with chronic kidney disease stages 3-5: A real-world retrospective cohort study in Taiwan. Int J Med Sci. 2024;21(11):2109-18
29. Chou CL, Juan SH, Li CH, Chen HH, Kao CC, Chen LY. et al. Association between DPP-4 inhibitors and events of colorectal and liver cancers in patients with diabetes receiving second-line agents: a nested case-control study. Front Oncol. 2022;12:840142
30. Wang YC, Juan SH, Li CH, Chou CL, Chen LY, Chien LN, Fang TC. et al. Valacyclovir-associated neurotoxicity among patients on hemodialysis and peritoneal dialysis: a nationwide population-based study. Front Med (Lausanne). 2022;9:997379
31. Lin MY, Cheng LJ, Chiu YW, Hsieh HM, Wu PH, Lin YT. et al. Effect of national pre-ESRD care program on expenditures and mortality in incident dialysis patients: a population-based study. PLoS One. 2018;13(6):e0198387
32. Hsieh HM, Lin MY, Chiu YW, Wu PH, Cheng LJ, Jian FS. et al. Economic evaluation of a pre-ESRD pay-for-performance programme in advanced chronic kidney disease patients. Nephrol Dial Transplant. 2017;32(7):1184-94
33. Goldstein M, Yassa T, Dacouris N, McFarlane P. Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis. 2004;44(4):706-14
34. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449-57
35. Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C. et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14-20
36. Moolgavkar SH, Chang ET, Watson HN, Lau EC. An Assessment of the Cox Proportional Hazards Regression Model for Epidemiologic Studies. Risk Anal. 2018;38(4):777-94
37. Quach K, Lvtvyn L, Baigent C, Bueti J, Garg AX, Hawley C. et al. The safety and efficacy of mineralocorticoid receptor antagonists in patients who require dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68(4):591-8
38. Qu X, Yao H, Chen C, Kong S, Sun L, Du L. et al. Spironolactone improves the all-cause mortality and re-hospitalization rates in acute myocardial infarction with chronic kidney disease patients. Front Pharmacol. 2021;12:632978
39. Hammer F, Buehling SS, Masyout J, Malzahn U, Hauser T, Auer T. et al. Protective effects of spironolactone on vascular calcification in chronic kidney disease. Biochem Biophys Res Commun. 2021;582:28-34
40. Feniman-De-Stefano GM, Zanati-Basan SG, De Stefano LM, Xavier PS, Castro AD, Caramori JC. et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Ther Adv Cardiovasc Dis. 2015;9(4):158-67
41. Fishbane S, Charytan DM, Chertow GM, Ford M, Kovesdy CP, Pergola PE. et al. Consensus-based recommendations for the management of hyperkalemia in the hemodialysis setting. J Ren Nutr. 2022;32(4):e1-14
42. Navaneethan SD, Nigwekar SU, Sehgal AR, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009(3):CD007004
43. Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014(4):CD007004
44. Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC. et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020;10(10):CD007004
45. Charytan DM, Himmelfarb J, Ikizler TA, Raj DS, Hsu JY, Landis JR. et al. Safety and cardiovascular efficacy of spironolactone in dialysis-dependent ESRD (SPin-D): a randomized, placebo-controlled, multiple dosage trial. Kidney Int. 2019;95(4):973-82
46. Rajagopalan S, Dobre M, Dazard JE, Vergara-Martel A, Connelly K, Farkouh ME. et al. Mineralocorticoid Receptor Antagonism Prevents Aortic Plaque Progression and Reduces Left Ventricular Mass and Fibrosis in Patients With Type 2 Diabetes and Chronic Kidney Disease: The MAGMA Trial. Circulation. 2024;150(9):663-76
47. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al; FIDELIO-DKD Investigators. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383(23):2219-29
48. Al-Kindi S, Motairek I, Janus S, Deo S, Rahman M, Neeland IJ. et al. Time-Varying Cardiovascular Effects of Finerenone in Diabetic Kidney Disease: Insights From FIDELIO-DKD and FIGARO-DKD Trials. J Am Coll Cardiol. 2022;80(19):1855-6
49. Flack JM, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P. et al. FIDELIO-DKD and FIGARO-DKD Investigators. Finerenone in Black Patients With Type 2 Diabetes and CKD: A Post hoc Analysis of the Pooled FIDELIO-DKD and FIGARO-DKD Trials. Kidney Med. 2023;5(12):100730
50. Eissing T, Goulooze SC, van den Berg P, van Noort M, Ruppert M, Snelder N. et al. Pharmacokinetics and pharmacodynamics of finerenone in patients with chronic kidney disease and type 2 diabetes: Insights based on FIGARO-DKD and FIDELIO-DKD. Diabetes Obes Metab. 2024;26(3):924-36
51. Reubi FC, Cottier PT. Effects of reduced glomerular filtration rate on responsiveness to chlorothiazide and mercurial diuretics. Circulation. 1961;23:200-10
52. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269-324
53. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-104
54. Dussol B, Moussi-Frances J, Morange S, Somma-Delpero C, Mundler O, Berland Y. A pilot study comparing furosemide and hydrochlorothiazide in patients with hypertension and stage 4 or 5 chronic kidney disease. J Clin Hypertens (Greenwich). 2012;14(1):32-7
55. Teles F, Peçanha de Miranda Coelho JA, Albino RM, Verçosa Pacheco FC, Rodrigues de Oliveira E, Silveira MAD. et al. Effectiveness of thiazide and thiazide-like diuretics in advanced chronic kidney disease: a systematic review and meta-analysis. Ren Fail. 2023;45(1):2163903
56. Galán Carrillo I, Vega A, Goicoechea M, Shabaka A, Gatius S, Abad S. et al. Impact of Serum Magnesium Levels on Kidney and Cardiovascular Prognosis and Mortality in CKD Patients. J Ren Nutr. 2021;31(5):494-502
Author contact
![]() Corresponding authors: Te-Chao Fang, MD, PhD, Division of Nephrology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan, No. 252, Wuxing Street, Taipei City 110, Taiwan; Tel.: +886-2-27372181 Ext:3577; E-mail: fangtechaocom. Chu-Lin Chou, MD, PhD, Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, 250 Wuxing Street, Xinyi District, Taipei 11031, Taiwan; Tel.: 886-3-422-5180 ext. 606; Fax: 886-3-422-8925; E-mail: chulin.chouedu.tw.
Corresponding authors: Te-Chao Fang, MD, PhD, Division of Nephrology, Department of Internal Medicine, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan, No. 252, Wuxing Street, Taipei City 110, Taiwan; Tel.: +886-2-27372181 Ext:3577; E-mail: fangtechaocom. Chu-Lin Chou, MD, PhD, Division of Nephrology, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, 250 Wuxing Street, Xinyi District, Taipei 11031, Taiwan; Tel.: 886-3-422-5180 ext. 606; Fax: 886-3-422-8925; E-mail: chulin.chouedu.tw.

 Global reach, higher impact
Global reach, higher impact