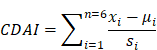3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(5):1184-1193. doi:10.7150/ijms.107332 This issue Cite
Research Paper
Association between composite dietary antioxidant index and rheumatoid arthritis: results from NHANES 2003-2018
1. Department of Joint Surgery, Shandong Provincial Hospital, Shandong University, Jinan, Shandong 250012, China.
2. Department of Joint Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong 250021, China.
3. Orthopaedic Research Laboratory, Medical Science and Technology Innovation Center, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong 250117, China.
4. Department of Stomatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong 250021, China.
5. School of Stomatology, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong 250117, China.
Received 2024-11-21; Accepted 2025-2-6; Published 2025-2-18
Abstract
Background: Increasing evidence has revealed oxidative stress as an essential risk factor in the development of rheumatoid arthritis (RA). Composite dietary antioxidant index (CDAI) is an important tool for assessing dietary antioxidant capacity. However, the association between CDAI and RA is still unclear.
Method: The data of 26501 participants from the NHANES database 2003-2018 cycle were collected to investigate the relationship between CDAI and RA risk. Logistic regression was used to examine the odds ratio (OR) of CDAI to RA risk. Restricted cubic spline (RCS) was utilized to test for potential nonlinear relationship. Stratified analysis and sensitivity analysis were also performed to strengthen the reliability of results.
Results: Multivariate logistic regression with full adjustment for covariates showed that the OR (95% confidence interval (CI)) for CDAI to RA was 0.974 (0.955, 0.993). A nonlinear negative correlation was identified by the RCS (p for nonlinearity=0.046). In both the subgroup and sensitivity analysis, this relationship was still present.
Conclusion: Our work suggests that higher dietary antioxidants intake is correlated with a lower RA incidence, thus providing some dietary recommendations for daily diets. Further clinical studies are acquired to better validate the current findings.
Keywords: CDAI, NHANES, cross-sectional study, rheumatoid arthritis, oxidative stress.
Introduction
Rheumatoid arthritis (RA) is a major health problem worldwide [1]. Globally, it is estimated that 17.6 million people are affected by RA, and prevalence of women is approximately 2.45 times higher than that of men [2]. Currently, treatments for RA are categorized as non-surgical and surgical. Non-surgical treatment is suitable for the early stage, but it is unable to cure the disease [3, 4]. When RA progresses to the late stage, appropriate surgical treatment is necessary, with total joint arthroplasty (TJA) being the last approach for severe patients [5, 6]. However, due to the durability of the prosthesis and associated complications, TJA is not an optimal choice for middle-aged or active individuals [7, 8]. Therefore, it is urgent to identify protective factors for RA to prevent the disease.
Oxidative stress (OS) is caused by excessive levels of reactive oxygen species (ROS) due to an imbalance between antioxidants and pro-oxidants [9-11]. Previous studies have emphasized the important role of OS in the pathogenesis of RA, and weaker antioxidant capacity is associated with a higher risk of RA [12-14]. Rheumatoid inflammation is accompanied by increased production of oxidants, which subsequently leads to oxidative damage to cartilage, tissue destruction and increased inflammation [15, 16]. Conversely, antioxidant intake has been reported to help ameliorate the development of RA [17, 18]. Composite dietary antioxidant index (CDAI) is a tool to evaluate an individual's dietary total antioxidants profile, which is based on the intake levels of multiple antioxidants (vitamins A, C, E, zinc, selenium and carotenoids) [19, 20]. Previous researches have demonstrated that high CDAI is correlated with low mortality rates [21, 22]. Moreover, CDAI exhibits a negative correlation with the development of several diseases, including hypertension [23], chronic kidney disease [24], diabetes [25], and osteoporosis [26]. In addition, intaking antioxidants can also alleviate RA. Zhou et al. reported that dietary supplementation of vitamin A may have a potential preventive effect in RA [27]. Regular vitamin E intake is effective for alleviating symptoms of RA [28]. However, the relationship between CDAI and RA is currently unknown.
Our study used data from the National Health and Nutrition Examination Survey (NHANES) 2003-2018. We aimed to discover the potential association between CDAI and RA, with the goal to provide an appropriate dietary suggestion to prevent the disease.
Method
Data source and population
The NHANES is a series of sample surveys of representative health data from the general population of the United States conducted by the National Center for Health Statistics (NCHS). It employs a sophisticated, multistage stratified probability sampling design to collect demographics, dietary, examination, laboratory and questionnaire data and has been published biennially since 1999 [29]. The NHANES protocol was approved by the NCHS Research Ethics Review Board, and all participants provided informed consent.
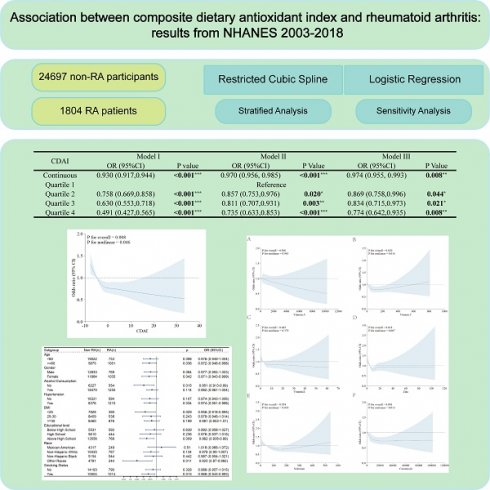
We used data from NHANES 2003-2018 cycle, which included detailed dietary and disease questionnaire information. In the 1999-2002 cycle, unfortunately, the carotenoid categories of the dietary questionnaire were not comprehensive compared to the 2003-2018 cycle and might cause analytic bias, so we discarded 1999-2002 cycle. Figure 1 illustrates the sample selection process. Totally, 80312 participants finished the survey during 2003-2018 cycle. We excluded individuals with missing dietary data (n=6337) or questionnaire information of RA (n=12606). In addition, participants with missing covariates were also excluded, including body measure index (BMI) (n=465), hypertension (n=4956), smoking status (n=21007), drinking status (n=5242), education level (n=35), marital status (n=14), and poverty-income ratio (PIR) (n=3149). Finally, 26501 subjects were included in our analysis.
CDAI measurement
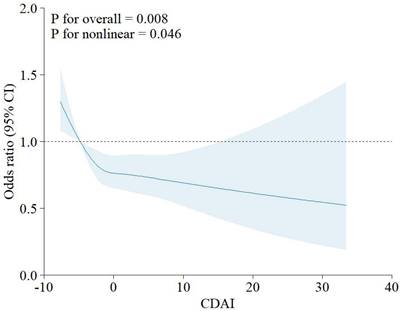
In NHANES, dietary intake was recorded for each participant by nonconsecutive 2-day 24-hour interviews. The first recording was performed at a mobile examination center, and the second was conducted online 3 to 10 days later. We extracted intake levels of 6 antioxidants (zinc, selenium, carotenoids, vitamin A, C, and E) based on these dietary recall data. CDAI is the sum of six standardized antioxidants intakes [19, 30, 31], as calculated below:
In this formula, xi represents the daily antioxidants intake; µi represents the mean of xi; si represents the standard deviation (SD) of µi.
Outcome ascertainment
RA data was collected from personal interview data. Participants were asked if doctor told they had arthritis. If they answered "yes", the participant was then asked the type of arthritis and then categorized into RA, or other types of arthritis.
Covariates
To evaluate the interference of potential confounding factors, this study included the following covariates: gender (Male, Female), age (<60, ≥60), race (Mexican American, Non-Hispanic White, Non-Hispanic Black, Other races) [32], educational level (Below High School, High School, Above High School) [33], marital status (married/living with partner, widowed/divorced/separated, never married) [34], PIR (<1.3, 1.3-3.5, ≥3.5) [30], total energy intake [35], hypertension status (Yes or No), BMI (<25.0, 25.0 to 30.0, ≥30.0) [36], alcohol consumption (Yes or No), and smoking status (Yes or No).
Gender, age, race, educational level, marital status and PIR information were obtained from demographics data. Total energy intake data was collected from dietary questionnaire. Hypertension was defined as self-reported hypertension or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Alcohol consumption was considered as “yes” if a participant had consumed alcohol, while BMI data was from body measurements profile. Smoking status was defined as “Yes”, if the participant had smoked.
Statistical analyses
Continuous variables were expressed as mean (SD) and compared using independent samples t-tests. Categorical variables were described using frequency (percentage) and compared using the chi-square test or Fisher's exact test, as appropriate. CDAI was regarded as continuous variable or categorical variable converted by quartiles. Logistic regression analysis was performed to explore the relationship between CDAI and RA, in which three models were employed. Model I was a crude model with no confounding variables adjusted. Model II was adjusted for gender, age, race, educational level, marital status and PIR. Model III was further adjusted for total energy intake, hypertension, alcohol consumption, smoke status and BMI based on Model II. Then, Restricted cubic spline (RCS) was employed to analyze the nonlinear correlation between CDAI and RA. The correlation between CDAI and RA was further examined stratified by gender (male/female), age (<60/≥60 years), alcohol consumption (yes/no), hypertension (yes/no), BMI (<25, 25-30, ≥30), PIR (<1.3, 1.3-3.5, ≥3.5) and educational level (below high school, high school, above high school). Finally, we adopted a sensitivity analysis to evaluate the robustness of our results. RCSs were applied to investigate the relationship between each antioxidant and RA, respectively.
All analysis were conducted using SPSS (version 25.0) and R (version 4.2.3). P<0.05 was considered statistically significant.
Results
Population characteristics
26501 individuals were finally enrolled in the study, including 1804 participants with RA. Table 1 demonstrates the population baseline characteristics. Compared with non-RA individuals, RA patients tended to be female, over 60 years old, diagnosed with hypertension, have a higher BMI, and own less total energy. Significant differences existed in the distribution of race, education level, marital status, and PIR between RA and non-RA individuals. In addition, participants suffering from RA had significantly less intakes of all the antioxidants (vitamin A, vitamin C, vitamin E, zinc, selenium, and carotenoids) and lower CDAI level than non-RA participants.
Flowchart of sample selection.
Population characteristics. Data was presented as mean (SD) for continuous variables or frequencies (percentages) for categorical variables.
| Characteristics | Overall | Non-RA | RA | P Value | |
|---|---|---|---|---|---|
| N | 26501 | 24697 | 1804 | ||
| Gender (%) | Male | 13602 (51.3) | 12833 (52.0) | 769 (42.6) | <0.001*** |
| Female | 12899 (48.7) | 11864 (48.0) | 1035 (57.4) | ||
| Age (%) | <60 | 19775 (74.6) | 19022 (77.0) | 753 (41.7) | <0.001*** |
| ≥60 | 6726 (25.4) | 5675 (23.0) | 1051 (58.3) | ||
| Race (%) | Mexican American | 4566 (17.2) | 4317 (17.5) | 249 (13.8) | <0.001*** |
| Non-Hispanic White | 11202 (42.3) | 10435 (42.3) | 767 (42.5) | ||
| Non-Hispanic Black | 5698 (21.5) | 5154 (20.9) | 544 (30.2) | ||
| Other | 5035 (19.0) | 4791 (19.4) | 244 (13.5) | ||
| Educational level (%) | <High School | 6123 (23.1) | 5531 (22.4) | 592 (32.8) | <0.001*** |
| High School | 6054 (22.8) | 5610 (22.7) | 444 (24.6) | ||
| >High School | 14324 (54.1) | 13556 (54.9) | 768 (42.6) | ||
| Marital status (%) | Married/Living with partner | 16150 (60.9) | 15165 (61.4) | 985 (54.6) | <0.001*** |
| Widowed/Divorced/Separated | 4956 (18.7) | 4287 (17.4) | 669 (37.1) | ||
| Never married | 5395 (20.4) | 5245 (21.2) | 150 (8.3) | ||
| PIR (%) | <1.3 | 8085 (30.5) | 7364 (29.8) | 721 (40.0) | <0.001*** |
| 1.3-3.5 | 10023 (37.8) | 9341 (37.8) | 682 (37.8) | ||
| ≥3.5 | 8393 (31.7) | 7992 (32.4) | 401 (22.2) | ||
| Vitamin A(µg/day) (mean (SD)) | 612.37 (588.23) | 614.96 (589.31) | 576.97 (572.22) | 0.008** | |
| Vitamin C (mg/day) (mean (SD)) | 86.58 (82.79) | 87.20 (82.90) | 78.09 (80.86) | <0.001*** | |
| Vitamin E (mg/day) (mean (SD)) | 7.89 (5.41) | 7.96 (5.46) | 6.98 (4.58) | <0.001*** | |
| Zn (mg/day) (mean (SD)) | 11.47 (7.03) | 11.56 (7.08) | 10.28 (6.10) | <0.001*** | |
| Se (µg/day) (mean (SD)) | 113.64 (55.25) | 114.60 (55.31) | 100.51 (52.71) | <0.001*** | |
| Carotenoid (µg/day) (mean (SD)) | 9466.24 (10293.96) | 9568.64 (10377.93) | 8064.27 (8952.00) | <0.001*** | |
| CDAI (mean (SD)) | 0.13 (4.04) | 0.19 (4.06) | -0.76 (3.67) | <0.001*** | |
| Energy(kcal/day) (median [IQR]) | 2106.03 (883.65) | 2123.21 (886.42) | 1870.82 (809.15) | <0.001*** | |
| Hypertension (%) | No | 16915 (63.8) | 16321 (66.1) | 594 (32.9) | <0.001*** |
| Yes | 9586 (36.2) | 8376 (33.9) | 1210 (67.1) | ||
| Alcohol Consumption (%) | No | 6781 (25.6) | 6227 (25.2) | 554 (30.7) | <0.001*** |
| Yes | 19720 (74.4) | 18470 (74.8) | 1250 (69.3) | ||
| BMI (%) | <25 | 8218 (31.0) | 7828 (31.7) | 390 (21.6) | <0.001*** |
| 25-30 | 8947 (33.8) | 8409 (34.0) | 538 (29.8) | ||
| ≥30 | 9336 (35.2) | 8460 (34.3) | 876 (48.6) | ||
| Smoke (%) | No | 14983 (56.5) | 14193 (57.5) | 790 (43.8) | <0.001*** |
| Yes | 11518 (43.5) | 10504 (42.5) | 1014 (56.2) | ||
*p<0.05, **p<0.01, ***p<0.001.
Abbreviation: RA: rheumatoid arthritis; PIR: poverty-income ratio; SD: standard deviation; CDAI: composite dietary antioxidant index; BMI: body measure index.
Relationship between CDAI and RA
Logistic regression models were established to explore the relationship between CDAI and RA. As mentioned in methods, three models were used in this study. As shown in Table 2, Continuous CDAI level was negatively associated with RA risk. In Model I, the odds ratio (OR) and 95% confidence interval (CI) were 0.930 (0.917,0.944; p<0.001), suggesting for per CDAI unit increase, the risk of RA reduced by 7.0%. This association was also significant in both Model II (OR: 0.970 (0.956, 0.985), p<0.001), and Model III (OR: 0.974 (0.955, 0.993), p=0.008) (Table 2). In addition, after dividing the CDAI levels into quartiles, the ORs of Q2 (Model I: 0.758 (0.669,0.858) p<0.001; Model II: 0.857 (0.753,0.976), p=0.020; Model III: 0.869 (0.758,0.996), p=0.044), Q3 (Model I: 0.630 (0.553,0.718), p<0.001; Model II: 0.811 (0.707,0.931), p=0.003; Model III: 0.834 (0.715,0.973), p=0.021), and Q4 (Model I: 0.491 (0.427,0.565), p<0.001; Model II: 0.735 (0.633,0.853), p<0.001; Model III: 0.774 (0.642,0.935), p=0.008) were also significantly lower in all three models compared to the Q1 (Table 2).
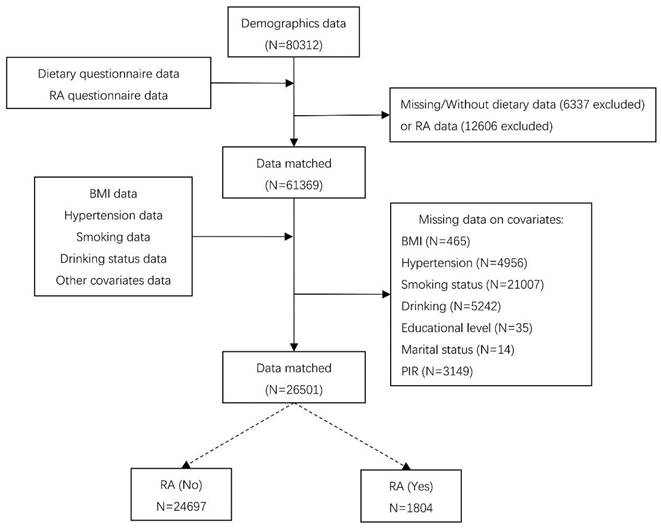
In addition, RCS showed the relationship between CDAI and RA is non-linear (p for nonlinearity<0.046, Figure 2).
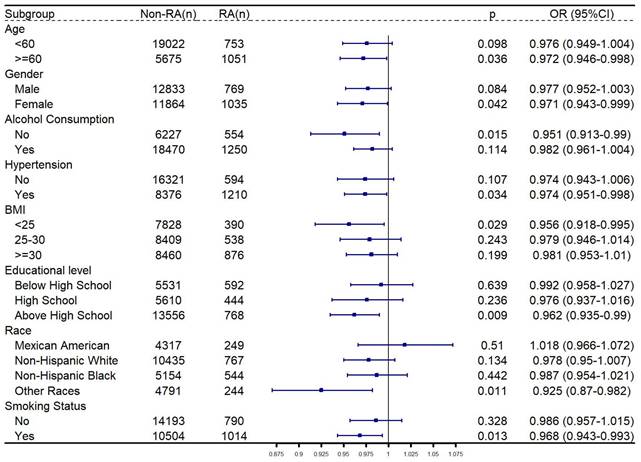
Subgroup analysis
The relationship between CDAI and RA was then explored in subgroup analysis. As shown in Figure 3, after adjusting the confounders, CDAI was significantly negatively related to RA among participants who were older than 60 years old, female, other races, had no history of alcohol consumption, no diagnosis of hypertension, and had a BMI < 25, highest level of education was high school, or had smoked.
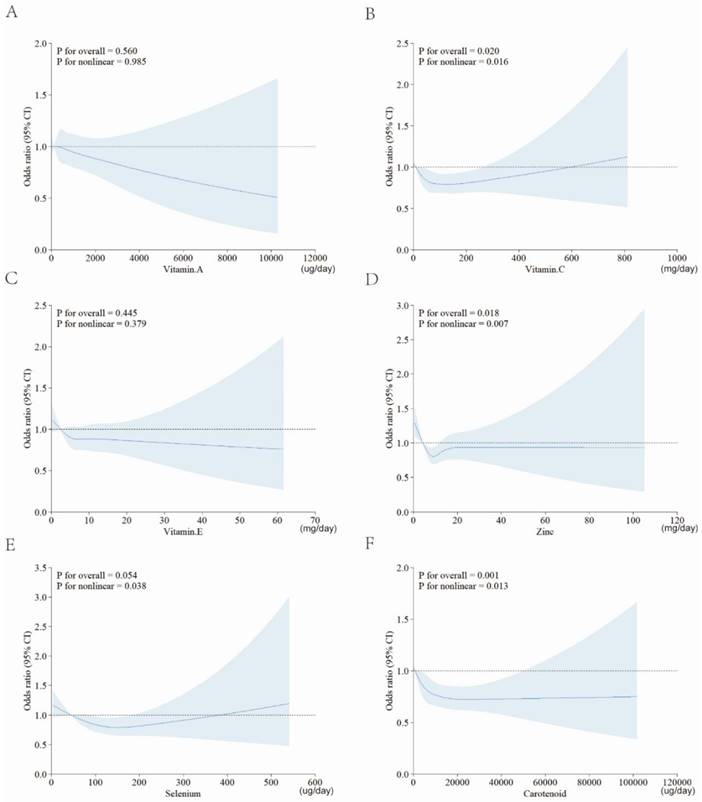
Sensitivity analysis
To validate the robustness of the results, we conducted sensitivity analysis. As shown in Figure 4, we focused on the role of single antioxidant dietary supplement, and also applied RCSs to explore their association with RA. Except for vitamin A and vitamin E, there were still statistically significant nonlinear relationship between each antioxidant intake and RA risk.
RCS for the relationship between CDAI and RA. Abbreviation: CDAI: composite dietary antioxidant index; CI: confidence interval.
Association between CDAI and RA. Model I: no covariates were adjusted. Model II: gender, age, race, educational level, marital status and PIR were adjusted. Model III: gender, age, race, educational level, marital status, PIR, BMI, total energy intake, hypertension, alcohol consumption, and smoking status were adjusted.
| CDAI | Model I | Model II | Model III | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P Value | OR (95%CI) | P Value | |
| Continuous | 0.930 (0.917,0.944) | <0.001*** | 0.970 (0.956, 0.985) | <0.001*** | 0.974 (0.955, 0.993) | 0.008** |
| Quartile 1 | Reference | |||||
| Quartile 2 | 0.758 (0.669,0.858) | <0.001*** | 0.857 (0.753,0.976) | 0.020* | 0.869 (0.758,0.996) | 0.044* |
| Quartile 3 | 0.630 (0.553,0.718) | <0.001*** | 0.811 (0.707,0.931) | 0.003** | 0.834 (0.715,0.973) | 0.021* |
| Quartile 4 | 0.491 (0.427,0.565) | <0.001*** | 0.735 (0.633,0.853) | <0.001*** | 0.774 (0.642,0.935) | 0.008** |
*p<0.05, **p<0.01, ***p<0.001.
Abbreviation: CDAI: composite dietary antioxidant index; OR: odds ratio; CI: confidence interval.
Subgroup analysis for relationship between CDAI and RA. Gender, age, race, educational level, marital status, PIR, total energy intake, hypertension, alcohol consumption, BMI and smoking status were adjusted, except the grouping criteria for each respective group (for example, “gender” was not adjusted in the gender-stratified group). Abbreviation: RA: rheumatoid arthritis; BMI: body measure index; PIR: poverty-income ratio; OR: odds ratio; CI: confidence interval.
RCS for the relationships between single antioxidant and RA. A, vitamin A; B, vitamin C; C, vitamin E; D, zinc; E, selenium; F, carotenoid. Abbreviation: CDAI: composite dietary antioxidant index; CI: confidence interval.
Discussion
In the present study, we examined relationship between CDAI and RA. After adjusting for possible confounding variables, we found that high CDAI level was correlated with lower risk of RA, suggesting that CDAI is a protective factor against RA. We then performed an RCS and found that this negative association was nonlinear. In addition, our subgroup analysis also demonstrated that in population who were older than 60 years old, female, other races, had no history of alcohol consumption, no diagnosis of hypertension, and had a BMI < 25, highest level of education was high school, or had smoked, CDAI was also significantly negatively associated with RA. In sensitivity analysis, dietary intake of vitamin C, zinc, selenium and carotenoid showed nonlinear correlations with RA. This is, to our knowledge, the first study to explore the relationship between CDAI (calculated using these six antioxidants) and RA using such a large sample.
OS occurs as a result of an imbalance between antioxidants and pro-oxidants and is rooted in excessive accumulation of ROS [9, 37]. OS causes cellular damage in many tissues, which leads to the progression of many diseases [38-40], including orthopedic disorders [41, 42]. Many studies have proved that OS can cause the bone homeostasis to tend to pathological states, and excessive ROS can lead to the apoptosis of osteocytes, osteoblasts and chondrocytes, and elevate osteoclasts formation and function [43-47]. And OS also leads to inflammation via activating overproduction of some pro-inflammatory factors in the joints [48, 49]. The cellular dysfunction and inflammatory environments will stimulate the development and progression of RA [48-50]. As an external factor, diet is a feasible way to regulate the redox state and facilitate the prevention of various diseases [51-53]. Some researches revealed the negative relationship between dietary antioxidants and RA, which is consistent with our study results. In active RA, the consumption of vitamin A, C, zinc and selenium is significantly and negatively correlated with some pro-inflammatory factors [54]. Additionally, dietary supplement of vitamin A or vitamin E supplement might be a potential way to alleviate RA [27, 28]. However, given the combination of food components, assessing the total dietary antioxidant intake may provide a more accurate and thorough understanding. Wang et al. calculated a dietary antioxidant index using intake levels of three vitamins and three metal ions and demonstrated a negative association with RA [55]. In fact, there has been extensive studies on the relationship between CDAI and health or disease, and there are minor differences in the choice of the six antioxidants used for the calculation [55-61]. In this study, we used intake levels of vitamins A, C, E, zinc, selenium, and carotenoids to calculate CDAI, in which carotenoids include α-carotene, β-carotene, cryptoxanthin, lycopene, lutein, and zeaxanthin. This type of calculation is also employed in a large number of studies and is used to explore correlations to multiple disorders [56, 57, 59, 61-63]. High CDAI is correlated with low levels of cytokines involved in OS and inflammation, including TNF-α and IL-1β [64, 65]. In young or middle-age adults and non-smokers, CDAI has the potential to reduce the risk of aging [66]. Many studies have reported negative associations between CDAI and various diseases, including hypertension, diabetes, chronic kidney disease, hyperuricemia, depression, and heart failure [23-25, 31, 35, 67]. Higher level of CDAI is also related to lower cardiovascular and mortality all-cause risk [22, 64, 68]. In addition, several studies also reported that CDAI is positively related to bone mineral density, and prevents bone loss [69-72].
Multivariate logistic regression is powerful for studying the role of dietary factors played in disease development [73, 74]. In our study, logistic regression showed that the OR of CDAI to RA was significantly lower than 1, suggesting CDAI as a potential protective factor. However, in the dose-effect curve of RCS, we find that this negative relationship is nonlinear. With rising CDAI, the risk of RA declined rapidly, then elevated mildly, followed by a slow decline. This indicates that keeping the CDAI at a proper level is profitable. In reality, it is not true that a higher intake of antioxidants is always better. Excessive supplementation can increase the risk of some disorders, including lung cancer and dyslipidemia [75, 76]. In addition, excessive intake of some antioxidants sometimes increases the risk of arthritis. It is reported that excessive selenium intake is related to higher risk of osteoarthritis (OA), and that it may be safe to keep the daily intake below 100ug [77]. In addition, people with a high zinc or selenium intake are also have an increased risk of developing OA, as indicated by Perri et al. [78]. The CDAI was calculated by taking the intake of the six antioxidants, so we think that due to compositional interference, the risk of RA was not always decreasing as the CDAI increased. However, those with higher CDAI still showed fewer subjects with RA compared to the lowest quartile. Therefore, we believe that a low level of CDAI intake is not recommended, while moderate level of CDAI is preferable. Then, in order to increase the efficiency of data utilization and reveal the underlying truth, we conducted subgroup analysis. Without any confounders adjusted, a negative relationship between CDAI and RA could be found in every subgroup. After adjusting for all covariates in the study, this significant negative relationship existed among women, older than 60 years, without a history of alcohol consumption, without a diagnosis of hypertension, BMI < 25, those with the highest level of education at high school or had not smoked.
Our study has some advantages and shortcomings. The primary advantage is the large sample size, which makes our study more stable and convincing. Secondly, we explored the nonlinear relationship between CDAI and RA, providing evidence for rational dietary intake. However, several limitations should also be mentioned. Firstly, because of the cross-sectional layout, the study cannot clarify the causality between CDAI and RA. Secondly, although we tried to adjust for potential confounders, residual confounders might still exist. Additionally, we were unable to access disease severity in RA patients, which limited the exploration of the correlation of the CDAI with RA severity. Finally, the evaluation indicators were collected mainly from subjective questionnaires, and the individual dietary recall was conducted through two separate recall interviews, which may have been biased.
In conclusion, our cross-sectional study based on eight cycles (2003-2018) of NHANES database revealed that CDAI was negatively associated with RA. This study provides new avenues for exploring dietary interventions to reduce RA risk. Future randomized controlled trials or cohort studies are necessary to confirm this finding.
Abbreviations
RA: rheumatoid arthritis; CDAI: Composite Dietary Antioxidant Index; OR: Odds Ratio; RCS: Restricted Cubic Spline; CI: Confidence Interval; TJA: Total Joint Arthroplasty; NHANES: National Health and Nutrition Examination Survey; NCHS: National Center for Health Statistics; BMI: Body Measure Index; PIR: Poverty-Income Ratio; SD: Standard Deviation; OS: Oxidative Stress; ROS: Reactive Oxygen Species; OA: Osteoarthritis.
Acknowledgements
We sincerely thank the participants and staff of NHANES for their valuable work and dedication.
Funding
This work was supported by the National Natural Science Foundation of China (no.82272485), Shandong Provincial Natural Science Foundation (no. ZR2021MH352) and Medical and Health Science and Technology Development Project of Shandong Province (no. 202308030310).
Data availability statement
All NHANES data for this study are publicly available and can be found here: https://wwwn.cdc.gov/nchs/nhanes (accessed on 15 November 2023).
Ethics approval and consent to participate
The research involving humans were approved by the Research Ethics Review Board of the National Center for Health Statistics. The studies were carried out in accordance with local legislative and institutional requirements. The participants provided informed written consent to participate in this study.
Institutional review board statement
The study depended on publicly available dataset from NHANES and therefore did not seek or obtain ethical approval and review.
Author contributions
Q.M. was responsible for data curation, formal analysis, writing—original draft, review and editing; S.D. and J.G. contributed to conceptualization, and writing—original draft; C.Q. and G.Z. contributed to writing—original draft; C.F. and S.S. were responsible for validation, visualization, supervision and writing—review and editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Venetsanopoulou AI, Alamanos Y, Voulgari PV, Drosos AA. Epidemiology of rheumatoid arthritis: genetic and environmental influences. Expert Rev Clin Immunol. 2022;18:923-31
2. Collaborators GBDRA. Global, regional, and national burden of rheumatoid arthritis, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5:e594-e610
3. Singh JA. Treatment Guidelines in Rheumatoid Arthritis. Rheum Dis Clin North Am. 2022;48:679-89
4. Lu Q, Wang H, Zhang X, Yuan T, Wang Y, Feng C. et al. Corydaline attenuates osteolysis in rheumatoid arthritis via mitigating reactive oxygen species production and suppressing calcineurin-Nfatc1 signaling. Int Immunopharmacol. 2024;142:113158
5. Goodman SM, Springer BD, Chen AF, Davis M, Fernandez DR, Figgie M. et al. 2022 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res (Hoboken). 2022;74:1399-408
6. Cao J, Wang W, Feng W, Xu H, Wang D, Zhou Z. Staged replacement of both hips and both knees in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2023;24:231
7. Murray DW, Parkinson RW. Usage of unicompartmental knee arthroplasty. Bone Joint J. 2018 100-B: 432-5
8. Walker-Santiago R, Tegethoff JD, Ralston WM, Keeney JA. Revision Total Knee Arthroplasty in Young Patients: Higher Early Reoperation and Rerevision. J Arthroplasty. 2021;36:653-6
9. Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234-46
10. Meng Q, Wang Y, Yuan T, Su Y, Li Z, Sun S. Osteoclast: The novel whistleblower in osteonecrosis of the femoral head. Gene Reports. 2023;33:101833
11. Li Z, Xu D, Li X, Deng Y, Li C. Redox Imbalance in Chronic Inflammatory Diseases. Biomed Res Int. 2022;2022:9813486
12. Balogh E, Veale DJ, McGarry T, Orr C, Szekanecz Z, Ng CT. et al. Oxidative stress impairs energy metabolism in primary cells and synovial tissue of patients with rheumatoid arthritis. Arthritis Res Ther. 2018;20:95
13. Ozturk HS, Cimen MY, Cimen OB, Kacmaz M, Durak I. Oxidant/antioxidant status of plasma samples from patients with rheumatoid arthritis. Rheumatol Int. 1999;19:35-7
14. Knekt P, Heliovaara M, Aho K, Alfthan G, Marniemi J, Aromaa A. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology. 2000;11:402-5
15. Bauerova K, Bezek A. Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys. 1999 18 Spec No: 15-20
16. Lopez-Armada MJ, Fernandez-Rodriguez JA, Blanco FJ. Mitochondrial Dysfunction and Oxidative Stress in Rheumatoid Arthritis. Antioxidants (Basel). 2022;11:1151
17. Hu H, Wang X, Ren Y, Zhang T, Sun L. Association Between Composite Dietary Antioxidant Index and the Risk of Endometriosis-Related Rheumatoid Arthritis in Women of Childbearing Age: A Cross-Sectional Study Based on the National Health and Nutrition Examination Survey Database. Int J Womens Health. 2024;16:717-26
18. Hagfors L, Leanderson P, Skoldstam L, Andersson J, Johansson G. Antioxidant intake, plasma antioxidants and oxidative stress in a randomized, controlled, parallel, Mediterranean dietary intervention study on patients with rheumatoid arthritis. Nutr J. 2003;2:5
19. Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR. et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160:68-76
20. Zhang J, Lu X, Wu R, Ni H, Xu L, Wu W. et al. Associations between composite dietary antioxidant index and estimated 10-year atherosclerotic cardiovascular disease risk among U.S. adults. Front Nutr. 2023;10:1214875
21. Xu Q, Qian X, Sun F, Liu H, Dou Z, Zhang J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. 2023;322:84-90
22. Wang L, Yi Z. Association of the Composite dietary antioxidant index with all-cause and cardiovascular mortality: A prospective cohort study. Front Cardiovasc Med. 2022;9:993930
23. Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. 2023;45:2233712
24. Wang M, Huang ZH, Zhu YH, He P, Fan QL. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011-2018. Food Funct. 2023;14:9279-86
25. Chen X, Lu H, Chen Y, Sang H, Tang Y, Zhao Y. Composite dietary antioxidant index was negatively associated with the prevalence of diabetes independent of cardiovascular diseases. Diabetol Metab Syndr. 2023;15:183
26. Chen Y, Tang W, Li H, Lv J, Chang L, Chen S. Composite dietary antioxidant index negatively correlates with osteoporosis among middle-aged and older US populations. Am J Transl Res. 2023;15:1300-8
27. Zhou X, Mi J, Liu Z. Causal association of diet-derived circulating antioxidants with the risk of rheumatoid arthritis: A Mendelian randomization study. Semin Arthritis Rheum. 2022;56:152079
28. Kou H, Qing Z, Guo H, Zhang R, Ma J. Effect of vitamin E supplementation in rheumatoid arthritis: a systematic review and meta-analysis. Eur J Clin Nutr. 2023;77:166-72
29. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr. 2016;7:121-34
30. Wu D, Wang H, Wang W, Qing C, Zhang W, Gao X. et al. Association between composite dietary antioxidant index and handgrip strength in American adults: Data from National Health and Nutrition Examination Survey (NHANES, 2011-2014). Front Nutr. 2023;10:1147869
31. Lin Z, Chen H, Lan Q, Chen Y, Liao W, Guo X. Composite Dietary Antioxidant Index Is Negatively Associated with Hyperuricemia in US Adults: An Analysis of NHANES 2007-2018. Int J Endocrinol. 2023;2023:6680229
32. Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999-2018. Arthritis Res Ther. 2023;25:34
33. Meng Q, Wang Y, Yuan T, Su Y, Ge J, Dong S. et al. Association between combined exposure to dioxins and arthritis among US adults: a cross-sectional study. Environ Sci Pollut Res Int. 2024;31:5415-28
34. Mahemuti N, Jing X, Zhang N, Liu C, Li C, Cui Z. et al. Association between Systemic Immunity-Inflammation Index and Hyperlipidemia: A Population-Based Study from the NHANES (2015-2020). Nutrients. 2023;15:1177
35. Zhao L, Sun Y, Cao R, Wu X, Huang T, Peng W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. 2022;10:988727
36. Xia F, Li Q, Luo X, Wu J. Identification for heavy metals exposure on osteoarthritis among aging people and Machine learning for prediction: A study based on NHANES 2011-2020. Front Public Health. 2022;10:906774
37. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180-3
38. van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019;21:425-35
39. Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer's disease. Ageing Res Rev. 2022;77:101619
40. Kuo CL, Ponneri Babuharisankar A, Lin YC, Lien HW, Lo YK, Chou HY. et al. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: foe or friend? J Biomed Sci. 2022;29:74
41. Zhu C, Shen S, Zhang S, Huang M, Zhang L, Chen X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front Endocrinol (Lausanne). 2022;13:898634
42. Kimball JS, Johnson JP, Carlson DA. Oxidative Stress and Osteoporosis. J Bone Joint Surg Am. 2021;103:1451-61
43. Geng Q, Gao H, Yang R, Guo K, Miao D. Pyrroloquinoline Quinone Prevents Estrogen Deficiency-Induced Osteoporosis by Inhibiting Oxidative Stress and Osteocyte Senescence. Int J Biol Sci. 2019;15:58-68
44. Domazetovic V, Falsetti I, Ciuffi S, Iantomasi T, Marcucci G, Vincenzini MT. et al. Effect of Oxidative Stress-Induced Apoptosis on Active FGF23 Levels in MLO-Y4 Cells: The Protective Role of 17-beta-Estradiol. Int J Mol Sci. 2022;23:2103
45. Deng S, Dai G, Chen S, Nie Z, Zhou J, Fang H. et al. Dexamethasone induces osteoblast apoptosis through ROS-PI3K/AKT/GSK3beta signaling pathway. Biomed Pharmacother. 2019;110:602-8
46. Wang Y, Yuan T, Wang H, Meng Q, Li H, Feng C. et al. Inhibition of Protein Disulfide Isomerase Attenuates Osteoclast Differentiation and Function via the Readjustment of Cellular Redox State in Postmenopausal Osteoporosis. Inflammation. 2024;47:626-48
47. Yuan T, Wang H, Wang Y, Dong S, Ge J, Li Z. et al. Inhibition of insulin degrading enzyme suppresses osteoclast hyperactivity via enhancing Nrf2-dependent antioxidant response in glucocorticoid-induced osteonecrosis of the femoral head. Mol Med. 2024;30:111
48. Phull AR, Nasir B, Haq IU, Kim SJ. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121-36
49. Jing W, Liu C, Su C, Liu L, Chen P, Li X. et al. Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs. Front Immunol. 2023;14:1107670
50. Komatsu N, Takayanagi H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat Rev Rheumatol. 2022;18:415-29
51. Scoditti E, Massaro M, Garbarino S, Toraldo DM. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients. 2019;11:1357
52. Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L. et al. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int J Environ Res Public Health. 2019;16:3011
53. Yammine A, Namsi A, Vervandier-Fasseur D, Mackrill JJ, Lizard G, Latruffe N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules. 2021;26:3483
54. Arablou T, Aryaeian N, Djalali M, Shahram F, Rasouli L. Association between dietary intake of some antioxidant micronutrients with some inflammatory and antioxidant markers in active Rheumatoid Arthritis patients. Int J Vitam Nutr Res. 2019;89:238-45
55. Ma G, Zhang S, Luo Y, Zhang C, Xu W, Wang L. The association between composite dietary antioxidant index and rheumatoid arthritis: evidence from NHANES 2001-2020. BMC Rheumatol. 2024;8:74
56. Min X, Kong X, Wang W. L-Shaped Associations Between Composite Dietary Antioxidant Index and Hearing Loss: A Cross-Sectional Study From the National Health and Nutrition Examination Survey. Biol Res Nurs. 2025;27:28-36
57. Jiang Y, Shen Y. Composite dietary antioxidant index is inversely and nonlinearly associated with cardiovascular disease, atherosclerotic cardiovascular disease, and cardiovascular mortality in people with dyslipidemia: evidence from NHANES 2001-2018. Front Nutr. 2024;11:1478825
58. He H, Chen X, Ding Y, Chen X, He X. Composite dietary antioxidant index associated with delayed biological aging: a population-based study. Aging (Albany NY). 2024;16:15-27
59. Wang T, Liu H, Wei X. Association between the Composite Dietary Antioxidant Index and Stroke: A cross-sectional Study. Biol Trace Elem Res. 2024;202:4335-44
60. Mao J, Hu H, Zhao Y, Zhou M, Yang X. Association Between Composite Dietary Antioxidant Index and Cognitive Function Among Aging Americans from NHANES 2011-2014. J Alzheimers Dis. 2024;98:1377-89
61. Cheng W, Wang Y, Ding N, Xie R. Association between composite dietary antioxidant index and Epstein-Barr virus infection in children aged 6-19 years in the United States: from the national health and nutrition examination survey 2007-2010. Front Nutr. 2024;11:1496410
62. Nie K, Deng T, Bai Y, Zhang Y, Chen Z, Peng X. et al. Association between composite dietary antioxidant index and hyperlipidemia in adults based on the NHANES. Sci Rep. 2025;15:2382
63. Zhang HQ, Shi J, Yue T, Weng JH, Wang XL, Wang H. et al. Association between composite dietary antioxidant index and stroke among individuals with diabetes. World J Diabetes. 2024;15:1742-52
64. Tan Z, Meng Y, Li L, Wu Y, Liu C, Dong W. et al. Association of Dietary Fiber, Composite Dietary Antioxidant Index and Risk of Death in Tumor Survivors: National Health and Nutrition Examination Survey 2001-2018. Nutrients. 2023;15:2968
65. Luu HN, Wen W, Li H, Dai Q, Yang G, Cai Q. et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signal. 2015;22:951-9
66. Wang H, Chen Y. Relationship between Composite Dietary Antioxidant Index and Aging. Healthcare (Basel). 2023;11:2722
67. Ma Y, Liu J, Sun J, Cui Y, Wu P, Wei F. et al. Composite dietary antioxidant index and the risk of heart failure: A cross-sectional study from NHANES. Clin Cardiol. 2023;46:1538-1543
68. Yang C, Yang Q, Peng X, Li X, Rao G. Associations of composite dietary antioxidant index with cardiovascular disease mortality among patients with type 2 diabetes. Diabetol Metab Syndr. 2023;15:131
69. Han H, Chen S, Wang X, Jin J, Li X, Li Z. Association of the composite dietary antioxidant index with bone mineral density in the United States general population: data from NHANES 2005-2010. J Bone Miner Metab. 2023;41:631-41
70. Liu J, Tang Y, Peng B, Tian C, Geng B. Bone mineral density is associated with composite dietary antioxidant index among US adults: results from NHANES. Osteoporos Int. 2023;34:2101-2110
71. Zhou Q, Chen X, Chen Q, Hao L. Independent and combined associations of dietary antioxidant intake with bone mineral density and risk of osteoporosis among elderly population in United States. J Orthop Sci. 2023;29:1064-1072
72. Zhang R, Ni Z, Wei M, Cui Y, Zhou H, Di D. et al. Composite dietary antioxidant intake and osteoporosis likelihood in premenopausal and postmenopausal women: a population-based study in the United States. Menopause. 2023;30:529-38
73. Li W, Song J, Chen Z. The association between dietary vitamin C intake and periodontitis: result from the NHANES (2009-2014). BMC Oral Health. 2022;22:390
74. Tonstad S, Nathan E, Oda K, Fraser G. Vegan diets and hypothyroidism. Nutrients. 2013;5:4642-52
75. Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M. et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560-70
76. Yehya A, Baer JT, Smiley W, Dollar A, Sperling L. Hypervitaminosis A altering the lipid profile in a hypercholesterolemic patient. J Clin Lipidol. 2009;3:205-7
77. Deng X, Tan Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: NHANES 2003-2016. Front Public Health. 2022;10:1047605
78. Yang WM, Lv JF, Wang YY, Xu YM, Lin J, Liu J. et al. The Daily Intake Levels of Copper, Selenium, and Zinc Are Associated with Osteoarthritis but Not with Rheumatoid Arthritis in a Cross-sectional Study. Biol Trace Elem Res. 2023;201:5662-70
Author contact
![]() Corresponding authors: Shui Sun, M.D., Ph.D., Professor, Tel.: +86(0)13808933938; Email: sunshuiedu.cn; Chuanyun Fu, M.D., Ph.D., Associate Professor, Tel.: +86(0)15168889803; Email: fuchuanyunedu.cn.
Corresponding authors: Shui Sun, M.D., Ph.D., Professor, Tel.: +86(0)13808933938; Email: sunshuiedu.cn; Chuanyun Fu, M.D., Ph.D., Associate Professor, Tel.: +86(0)15168889803; Email: fuchuanyunedu.cn.

 Global reach, higher impact
Global reach, higher impact