3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(5):1167-1175. doi:10.7150/ijms.108397 This issue Cite
Research Paper
Comparative Efficacy of Silicone Sheets and Hyperbaric Oxygen Therapy in Post-Surgical Scar Prevention: A Prospective Observational Study
1. Graduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei, Taiwan.
2. Department of Orthopedics, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
3. Division of Plastic surgery, Department of Surgery, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
4. Division of Biostatistics and Medical Informatics, Department of Epidemiology, School of Public Health, National Defense Medical Center, Taipei, Taiwan.
5. Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan.
6. Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
7. Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
8. Department of Life Sciences, National Central University, Taoyuan, Taiwan.
Received 2024-12-7; Accepted 2025-2-3; Published 2025-2-11
Abstract
Background: Postoperative scarring can significantly impact physical function, aesthetic outcomes, and overall quality of life. Effective scar management is crucial to mitigate these effects. This study aimed to compare the efficacy of silicone sheets (SS) and hyperbaric oxygen therapy (HBOT) in minimizing postoperative scar formation and improving wound healing outcomes.
Methods: A total of 40 patients with clean, non-infected linear postoperative wounds were enrolled in a 12-week prospective observational study. Participants were randomly assigned to either the HBOT or SS group. The HBOT group underwent seven sessions of HBOT starting 4 weeks postoperatively, while the SS group applied silicone sheets to the wound from 4 to 12 weeks postoperatively. Scar outcomes were evaluated at 4, 8, and 12 weeks using the Patient and Observer Scar Assessment Scale (POSAS), which measures various parameters, including vascularity, pigmentation, thickness, relief, pliability, surface area and overall opinion.
Results: After applying exclusion criteria, 33 patients completed the study, with 18 in the HBOT group and 15 in the SS group. Both interventions significantly improved scar parameters such as vascularity, thickness, relief, pliability, and overall opinion over 12 weeks. SS was particularly effective in reducing pigmentation, while HBOT achieved greater reductions in scar surface area. By the study's conclusion, SS demonstrated superior outcomes in vascularity, pigmentation, scar thickness, relief, pliability, and overall appearance, whereas HBOT excelled in reducing surface area.
Conclusion: Both HBOT and SS are effective scar management options, each with unique benefits. Selecting the appropriate treatment based on patient-specific needs and wound characteristics is essential to achieve optimal outcomes and enhance patient satisfaction.
Keywords: hyperbaric oxygen therapy, silicone sheets, scar prevention, Patient and Observer Scar Assessment Scale (POSAS)
Introduction
Scarring is an inevitable outcome of surgical procedures and traumatic injuries, often resulting in physical discomfort and adversely impacting patients' quality of life [1, 2]. Scars are broadly categorized as immature or mature, with the latter further classified as “normal,” “atrophic,” or “hypertrophic.” Among these, hypertrophic scars and keloids pose significant challenges due to their propensity for recurrence and associated symptoms, such as pain, pruritus, and psychological distress [3]. These issues underscore the critical need for effective strategies to mitigate scar formation, given the substantial aesthetic and functional implications. The pathophysiology of scar formation is complex, involving multifactorial processes such as inflammation, collagen deposition, and extracellular matrix (ECM) remodeling [4]. The inflammatory phase, characterized by immune cell infiltration and increased vascular permeability, initiates the wound-healing cascade, followed by the proliferative phase, during which fibroblasts synthesize collagen and other ECM components [5]. Dysregulation at any stage of this process can lead to pathological scarring, emphasizing the importance of timely and effective therapeutic interventions.
Silicone-based treatments, including silicone gel sheets and gels, are among the most widely adopted methods for scar prevention in clinical practice [6]. These products create a protective barrier that maintains optimal hydration at the wound site, facilitating an environment conducive to healing and reducing the risk of aberrant scar formation [7]. Silicone therapy typically begins a few weeks postoperatively and is sustained for several months, demonstrating efficacy in preventing hypertrophic scars and keloids [8-11]. However, challenges such as patient adherence and discomfort, particularly in highly mobile regions like joints, may limit the practicality of silicone sheet application [8, 12]. Hyperbaric oxygen therapy (HBOT) has garnered attention as an alternative modality for enhancing wound healing and minimizing scar development [13-15]. HBOT involves administering 100% oxygen in a pressurized chamber, significantly enhancing tissue oxygenation and promoting angiogenesis [16, 17]. Beyond oxygenation, HBOT has been shown to modulate inflammatory pathways, improve collagen organization, and accelerate the healing process, potentially reducing scar thickness and enhancing scar quality [18]. Nonetheless, its widespread use is constrained by reliance on specialized infrastructure and the need for multiple sessions, posing logistical challenges for patients [19].
Given the respective benefits and limitations of both silicone therapy and HBOT, comparative analyses are essential to evaluate their relative efficacy in scar prevention. This study aimed to systematically compare the effectiveness of silicone sheets (SS) and HBOT in preventing postoperative scar formation. By providing evidence-based insights, this research seeks to guide clinicians in optimizing scar management strategies, aligning treatment approaches with patient needs, wound characteristics, and specific therapeutic goals.
Methods
Patients and study design
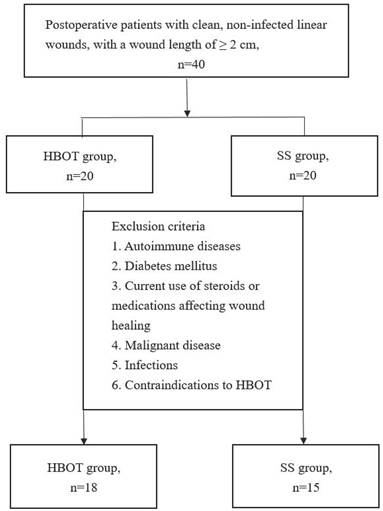
This 12-week prospective observational study included 40 postoperative patients. Eligibility criteria required participants to have clean, non-infected linear wounds (Class 1 wounds) [20, 21] with a minimum length of 2 cm. Patients with autoimmune diseases, diabetes mellitus, current use of steroids or other medications affecting wound healing, malignant disease, infections, or contraindications to HBOT were excluded [10]. Eligible patients were randomly assigned to one of two groups: the HBOT Group or the SS Group.
- HBOT Group: Participants underwent seven HBOT sessions starting 4 weeks postoperatively. Each session was conducted once daily, at a pressure of 2.5 atmospheres absolute (ATA), with a duration of 100 min.
- SS Group: Participants applied a silicone sheet (FoamLite™; ConvaTec, Singapore) to the wound starting 4 weeks after surgery. The silicone sheet was replaced every 24 h and used continuously until 12 weeks postoperatively.
Assessment
Clinical outcomes were evaluated using the Patient and Observer Scar Assessment Scale (POSAS) 2.0 Observer Scale (Figure S1) [22]. The Observer Scale includes seven parameters: vascularity, pigmentation, thickness, relief, pliability, surface area, and overall opinion. Trained healthcare professionals, including specialized nurses, assessed these parameters at the 4th, 8th, and 12th weeks postoperatively to objectively evaluate scar characteristics.
Statistical analysis
Descriptive statistics were used to summarize demographic and baseline characteristics. Continuous variables were expressed as mean ± standard deviation [12]. Between-group comparisons at the 4th, 8th, and 12th weeks were conducted using independent sample t-tests, while paired sample t-tests assessed within-group changes over time. The p-values for each parameter reflect two types of comparisons: within-group comparisons, where the changes in scores at 8 weeks and 12 weeks are compared to the baseline (4 weeks) within each group (HBOT or SS). Between-group comparisons, where the scores at 4 weeks, 8 weeks and 12 weeks are compared between the SS group and the HBOT group at each corresponding time point. Statistical significance was set at p < 0.05. Additionally, in order to minimize the impact of baseline differences, percentage improvements in each parameter from 4 weeks to 12 weeks were calculated. The percentage improvement is calculated by determining the change from baseline (4 weeks) to the final assessment (12 weeks) as a percentage of the baseline value. It can provide a clearer picture of the relative treatment effects between the two groups.
Results
Participant enrollment
A total of 40 patients were enrolled, 7 patients were excluded due to meeting the exclusion criteria, leaving 18 patients in the HBOT group and 15 patients in the SS group (Figure 1). The mean ages of the HBOT and SS groups were 42.39 and 42.87 years, respectively, with average body mass index of 25.59 kg/m2 and 23.94 kg/m2. Baseline characteristics were comparable between the two groups. Detailed demographic and baseline data are presented in Table 1.
Study enrollment and allocation flowchart for the randomized controlled trial. Of the 40 patients screened, 7 were excluded based on predefined criteria. The remaining 33 participants were randomized into two groups: the hyperbaric oxygen therapy (HBOT) group (n = 18) and the silicone sheets (SS) group (n = 15).
POSAS
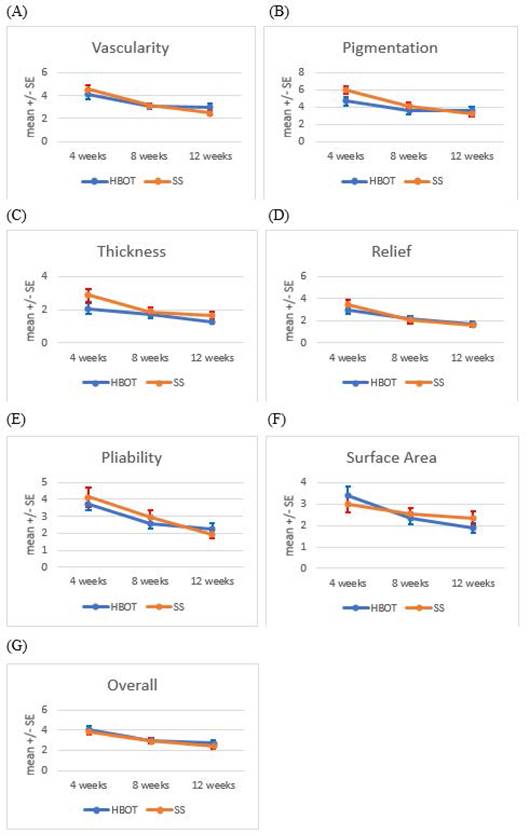
Changes in POSAS scores over time for both groups are summarized in Table 2. No significant differences in any parameters were observed between the two groups at any of the three time points. A detailed analysis of each variable, including within-group comparisons and percentage improvements as below are shown in Table 3 and Figure 2 (A-G).
Baseline characteristics of participants
| Variable | HBOT group (n=18) | SS Group (n=15) | p-value | |
|---|---|---|---|---|
| Age (years) | 42.39 ± 13.84 | 42.87 ± 13.23 | 0.92 | |
| Gender, n (%) | 0.77 | |||
| Female | 10 (55.6%) | 10 (66.7%) | ||
| Male | 8 (44.4%) | 5 (33.3%) | ||
| Height (m) | 1.63 ± 0.07 | 1.63 ± 0.08 | 0.993 | |
| Weight (kg) | 68.22 ± 16.49 | 63.93 ± 11.68 | 0.405 | |
| Body Mass Index (kg/m2) | 25.59 ± 5.37 | 23.94 ± 2.87 | 0.272 | |
| Surgical site, n (%) | 0.38 | |||
| Face | 2 (11.1%) | 3 (20.0%) | ||
| Limbs | 9 (50.0%) | 4 (26.7%) | ||
| Trunk | 7 (38.9%) | 8 (53.3%) | ||
| Wound length (cm) | 4.71 ± 3.03 | 3.33 ± 1.75 | 0.131 | |
i. Vascularity
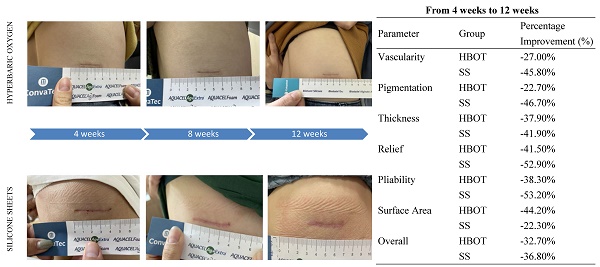
Both groups exhibited significant improvements in vascularity scores at 8 and 12 weeks compared to 4 weeks post-surgery (HBOT group: p < 0.01 at 8 weeks, p < 0.05 at 12 weeks; SS group: p < 0.01 at 8 weeks, p < 0.001 at 12 weeks). SS group demonstrating a more percentage improvement (45.8%) compared to the HBOT group (27.0%) in vascularity scores from 4 weeks to 12 weeks post-surgery.
ii. Pigmentation
The SS group showed a significant reduction in pigmentation scores at 8 and 12 weeks compared to 4 weeks post-surgery (p < 0.001 for both time points). The SS group had a greater percentage improvement in pigmentation scores (46.7%) compared to the HBOT group (22.7%) from 4 weeks to 12 weeks post-surgery.
iii. Thickness
Scar thickness decreased significantly by 12 weeks in the HBOT group compared to 4 weeks post-surgery (p < 0.05). In the SS group, significant reductions in thickness were observed at both 8 weeks (p < 0.05) and 12 weeks (p < 0.01). The percentage improvement in scar thickness was better in the SS group (41.9%) than in the HBOT group (37.9%) from 4 weeks to 12 weeks post-surgery.
iv. Relief
Relief scores improved significantly within both groups at 8 and 12 weeks compared to 4 weeks post-surgery (HBOT group: p < 0.01 at both time points; SS group: p < 0.01 at 8 weeks, p < 0.001 at 12 weeks). SS therapy resulted in a greater percentage improvement in relief scores (52.9%) compared to HBOT (41.5%) from 4 weeks to 12 weeks post-surgery.
v. Pliability
Pliability scores improved significantly at 8 and 12 weeks in both groups (HBOT group: p < 0.001 at both time points; SS group: p < 0.05 at 8 weeks, p < 0.001 at 12 weeks). The SS group had a more percentage improvement than HBOT in pliability scores (53.2% vs 38.3%) from 4 weeks to 12 weeks post-surgery.
vi. Surface area
The HBOT group demonstrated significant reductions in scar surface area at 8 weeks (p < 0.01) and 12 weeks (p < 0.001) compared to 4 weeks post-surgery. HBOT demonstrated a greater percentage improvement in surface area (44.2%) compared to SS (22.3%) from 4 weeks to 12 weeks post-surgery.
Trends in Patient and Observer Scar Assessment Scale (POSAS) scores over time. POSAS parameters, including vascularity, pigmentation, thickness, relief, pliability, surface area and overall observer opinion, were assessed at 4, 8, and 12 weeks postoperatively in the HBOT and SS groups, illustrating temporal changes in scar characteristics. The data are presented as mean ± standard errors.
Variability of POSAS observer scale across groups and time
| Parameter | Group | Time (weeks) | Mean ± SD |
|---|---|---|---|
| Vascularity | HBOT | 4 | 4.11 ± 1.64 |
| 8 | 3.06 ± 1.06** | ||
| 12 | 3.00 ± 1.37* | ||
| SS | 4 | 4.53 ± 1.51 | |
| 8 | 3.13 ± 0.83** | ||
| 12 | 2.47 ± 0.92*** | ||
| Pigmentation | HBOT | 4 | 4.67 ± 2.09 |
| 8 | 3.61 ± 1.85 | ||
| 12 | 3.61 ± 1.82 | ||
| SS | 4 | 6.00 ± 1.65 | |
| 8 | 4.13 ± 1.51*** | ||
| 12 | 3.20 ± 1.15*** | ||
| Thickness | HBOT | 4 | 2.06 ± 1.31 |
| 8 | 1.72 ± 0.90 | ||
| 12 | 1.28 ± 0.58* | ||
| SS | 4 | 2.87 ± 1.51 | |
| 8 | 1.87 ± 0.99* | ||
| 12 | 1.67 ± 0.90** | ||
| Relief | HBOT | 4 | 2.94 ± 1.31 |
| 8 | 2.17 ± 0.99** | ||
| 12 | 1.72 ± 1.13** | ||
| SS | 4 | 3.40 ± 1.72 | |
| 8 | 2.07 ± 1.10** | ||
| 12 | 1.60 ± 0.74*** | ||
| Pliability | HBOT | 4 | 3.72 ± 1.64 |
| 8 | 2.56 ± 1.20*** | ||
| 12 | 2.28 ± 1.27*** | ||
| SS | 4 | 4.13 ± 2.13 | |
| 8 | 2.93 ± 1.58* | ||
| 12 | 1.93 ± 0.80*** | ||
| Surface Area | HBOT | 4 | 3.39 ± 1.69 |
| 8 | 2.33 ± 1.19** | ||
| 12 | 1.89 ± 0.96*** | ||
| SS | 4 | 3.00 ± 1.51 | |
| 8 | 2.53 ± 1.13 | ||
| 12 | 2.33 ± 1.23 | ||
| Overall | HBOT | 4 | 4.06 ± 1.31 |
| 8 | 2.94 ± 0.80*** | ||
| 12 | 2.72 ± 1.49** | ||
| SS | 4 | 3.80 ± 1.08 | |
| 8 | 2.93 ± 1.10** | ||
| 12 | 2.40 ± 0.91** |
*p < 0.05, **p < 0.01, ***p < 0.001 compared to 4 months (within-group comparisons)
#p < 0.05, ##p < 0.01, ###p < 0.001, compared to the HBOT group (between-group comparisons0)
vii. Overall opinion
Both groups exhibited significant improvements in overall opinion scores at 8 and 12 weeks compared to 4 weeks post-surgery (HBO group: p < 0.001 at 8 weeks, p < 0.01 at 12 weeks; SS group: p < 0.01 at both time points). The SS group showed a slightly higher percentage improvement in overall opinion scores (36.8%) compared to the HBOT group (32.7%) from 4 weeks to 12 weeks post-surgery.
Percentage improvements of parameters (from 4 weeks to 12 weeks)
| Parameter | Group | Percentage Improvement (%) |
|---|---|---|
| Vascularity | HBOT | -27.00% |
| SS | -45.80% | |
| Pigmentation | HBOT | -22.70% |
| SS | -46.70% | |
| Thickness | HBOT | -37.90% |
| SS | -41.90% | |
| Relief | HBOT | -41.50% |
| SS | -52.90% | |
| Pliability | HBOT | -38.30% |
| SS | -53.20% | |
| Surface Area | HBOT | -44.20% |
| SS | -22.30% | |
| Overall | HBOT | -32.70% |
| SS | -36.80% |
Discussion
Mechanisms of action
Understanding the mechanisms underlying HBOT and SS is essential for optimizing their clinical applications. HBOT enhances oxygen delivery to hypoxic tissues, stimulating angiogenesis, facilitating ECM remodeling, and promoting collagen maturation - key processes in wound repair and scar modulation [23-25]. By improving tissue oxygenation, HBOT alleviates local ischemia, a contributing factor to delayed healing and excessive scarring [26]. Elevated oxygen levels also regulate growth factors and cytokines, enhancing cellular proliferation and tissue regeneration [24].
Silicone therapy primarily functions through occlusion, hydration, cellular signaling modulation, and potential thermoregulation [27, 28]. These actions optimize the wound healing environment, reduce scar formation, and improve aesthetic outcomes. By forming a semi-permeable barrier, SS reduces transepidermal water loss (TEWL) and maintains stratum corneum hydration, which is essential for effective epithelialization and scar modulation [29-31]. This hydration state attenuates pro-inflammatory cytokine activity [32, 33] and suppresses fibroblast-mediated excessive collagen synthesis, mitigating the risk of hypertrophic scar and keloid formation [34].
Comparative efficacy of HBOT and SS
Both HBOT and SS demonstrated efficacy in mitigating post-surgical scar formation, albeit with distinct mechanistic advantages. Across POSAS parameters, both modalities yielded comparable improvements in vascularity, thickness, relief, pliability, and overall opinion.
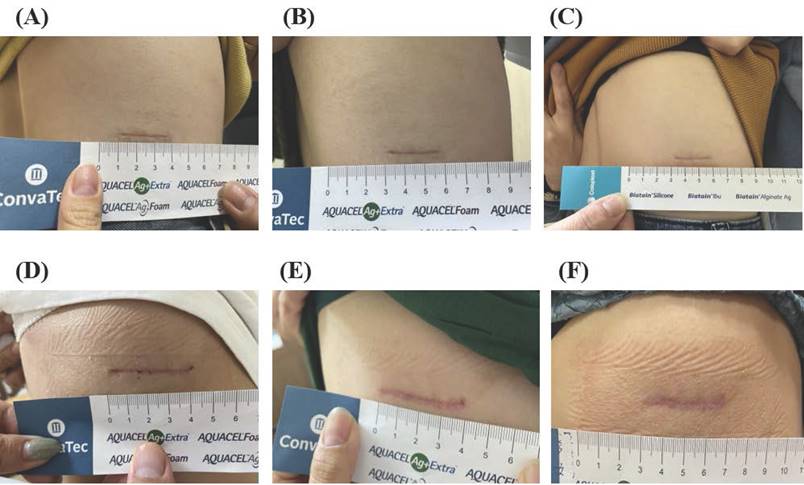
Comparative pre- and post-treatment outcomes in the HBOT and SS groups. (A-C) Representative scar images from the HBOT group at (A) 4 weeks, (B) 8 weeks, and (C) 12 weeks postoperatively. (D-F) Representative scar images from the SS group at (D) 4 weeks, (E) 8 weeks, and (F)12 weeks postoperatively.
However, differences emerged in specific scar characteristics. The SS group showed significant improvements in pigmentation, a key concern for patients prioritizing aesthetic outcomes. SS's ability to modulate skin hydration and reduce TEWL likely contributes to minimizing pigmentary changes [35]. Silicone gel sheeting forms a semi-permeable barrier, maintaining optimal moisture levels, enhancing hydration, and attenuating inflammatory responses [27, 28], which prevent hyperpigmentation and promote cosmetically acceptable outcomes.
In contrast, the HBOT group exhibited superior improvements in scar surface area, highlighting its role in enhancing tissue oxygenation and accelerating wound healing [23-25]. HBOT's multifactorial mechanisms include stimulation of angiogenesis, enhanced fibroblast activity, and improved collagen organization, contributing to reduced scar surface area and improved overall scar quality [36]. Increased oxygenation during HBOT facilitates collagen synthesis and deposition [37, 38], promoting improved collagen organization and density. These effects result in thinner, more pliable scars [39].
Implications for clinical practice
Our findings validate the efficacy of HBOT and SS as distinct yet effective interventions for scar prevention. Clinicians should tailor treatment plans based on patient-specific factors, including surgical type, wound characteristics, and individual healing responses. HBOT may be preferable for patients with a history of hypertrophic scars or keloids, particularly when wound healing is impaired by hypoxia [16, 17]. In contrast, SS may be ideal for patients seeking noninvasive options or for scars in less mobile areas [27]. We posit that engaging patients in discussions about the advantages and limitations of each modality will foster adherence, a key determinant of therapeutic success.
Timing of treatment initiation
Optimal timing of therapy initiation is critical for maximizing outcomes. While this study initiated treatments at 4 weeks postoperatively, earlier intervention might yield greater benefits, especially for patients at high risk of hypertrophic scarring [40]. Evidence suggests that initiating HBOT during the inflammatory phase enhances oxygen delivery to healing tissues [41], potentially mitigating excessive collagen deposition and scarring [40, 42, 43].
Similarly, SS is most effective when applied to fully healed wounds [6], but early initiation within the first few weeks of healing can stabilize the process and minimize pathological scarring [44]. Future studies should explore the benefits of initiating both therapies earlier in the postoperative period to determine the optimal timing for scar prevention.
Considerations for patient adherence
Patient adherence significantly influences the success of scar management strategies. Both HBOT and SS require patient commitment, which various factors, including the perceived efficacy, comfort, and practicality of the treatment, can influence. For instance, HBOT requires multiple sessions in a hyperbaric chamber, posing logistical challenges [45], such as accessibility issues and frequent appointments [46]. Clinicians should assess the feasibility of this approach and address potential barriers to compliance.
In contrast, SS offers a more convenient, home-based option with minimal disruption to daily activities. However, adherence may be compromised in areas near joints, where the application is challenging [8], or in highly visible areas (e.g., face) due to aesthetic concerns [27]. Educating patients on the importance of consistent use and providing practical guidance can enhance adherence and treatment outcomes.
Socioeconomic factors
Socioeconomic factors also influence access to scar management therapies. HBOT availability is often limited by geographic location, healthcare infrastructure, and insurance coverage, necessitating travel and time off work [47]. SS, being more accessible and cost-effective, is a viable option for a broader patient population.
Addressing these disparities through advocacy for HBOT accessibility and financial support will ensure equitable access to effective scar management.
Strengths, limitations and future directions
This study contributes to the growing body of evidence on scar management, offering a direct comparison of two widely used interventions. The randomized controlled design strengthens the validity of the findings, reducing bias and ensuring a fair comparison. The use of the POSAS, a validated and comprehensive tool, adds reliability to the scar assessments by considering both objective and subjective perspectives. Furthermore, the inclusion of multiple time points allows for a dynamic evaluation of scar progression over time, providing valuable insights into the temporal effects of each treatment. Despite its strengths, this study has certain limitations that warrant consideration. First, the treatment initiation timeline of 4 weeks post-surgery may have precluded the potential benefits of earlier intervention, especially for HBOT, which could be more effective during the inflammatory phase of wound healing. Future research should explore the outcomes of initiating therapy immediately after wound closure to optimize results. Second, the limited sample size, while sufficient for preliminary findings, restricts the generalizability of the results. Larger studies are needed to validate these findings across diverse populations, particularly among individuals with varying skin types and predispositions to scarring. Additionally, this study focused solely on linear, clean, non-infected wounds, which may not represent the outcomes for more complex or irregular scars, such as hypertrophic or infected wounds. Third, compliance with treatment protocols, particularly in the SS group, could introduce variability in the results. The efficacy of silicone sheets depends heavily on patient adherence, which can be influenced by factors such as discomfort or inconvenience. Evaluating strategies to improve adherence could enhance future outcomes. Fourth, this study did not include a no-treatment control group, limiting the ability to assess the natural course of scar healing. A control group would provide a baseline for evaluating the relative benefits of each intervention. Furthermore, baseline data for scar characteristics at Week 0 were not available, which may be considered a limitation of the study design. This decision was made because the intervention was initiated at Week 4 postoperatively, based on the optimal timing for treatment initiation. Additionally, immediate objective measurements of postoperative wounds (such as vascularity, pigmentation, and pliability) present several challenges. For instance, during the early postoperative period, wounds typically undergo an inflammatory phase characterized by redness, swelling, heat, and pain. These inflammatory responses can alter the wound's appearance, leading to inaccurate measurements. As such, it is generally recommended to perform these measurements during later stages of wound healing to ensure more reliable data. Nevertheless, future studies should consider including baseline measurements at earlier time points, as this data would provide a more comprehensive understanding of the progression of scar characteristics and the relative impact of each treatment modality.
Finally, exploring the combined effects of HBOT and SS remains an intriguing prospect. These treatments address complementary aspects of scar prevention—oxygenation and hydration—which may synergistically reduce hypertrophic scarring and enhance skin pliability. Future studies should investigate this combined approach to uncover potentially superior clinical outcomes.
Conclusion
HBOT and SS both demonstrate significant efficacy in post-surgical scar prevention, offering distinct advantages through unique mechanisms of action. HBOT excels in improving tissue oxygenation and reducing scar surface area, while SS effectively addresses pigmentation and hydration, making each modality suited to different patient needs. Leveraging these complementary mechanisms allows clinicians to individualize scar management strategies, optimizing both aesthetic and functional outcomes. Future studies should refine treatment protocols, evaluate the synergistic potential of combining HBOT and SS, and investigate the effects of earlier treatment initiation on scar prevention.
Abbreviations
SS: silicone sheets; HBOT: hyperbaric oxygen therapy; ECM: extracellular matrix; POSAS: Patient and Observer Scar Assessment Scale.
Supplementary Material
Supplementary figure.
Acknowledgements
We thank the research teams from Taoyuan Armed Forces General Hospital and the National Taiwan University of Science and Technology for their valuable suggestions, guidance, and support throughout this study.
Funding
This work was supported by Taoyuan Armed Forces General Hospital (TYAFGH-D-114032 and TYAFGH-E-114051). The funder had no role in the study's design, execution, analysis, or the decision to submit the manuscript for publication.
Author contributions
P.C.C., P.J.H., and H.C.T. designed the study. T.C.L., and C.Y.C. assisted in providing clinical patient enrollment. P.C.C. performed the experiments and wrote the paper. P.J.H., and C.M.C. analyzed and visualized the data. P.C.C., P.J.H., and H.C.T. assisted in the overall manuscript revision and guidance. All authors read and approved the final manuscript.
Ethics committee approval and patient consent
This study adhered to the principles outlined in the Declaration of Helsinki. The research protocol was reviewed and approved by the Institutional Review Board of the Tri-Service General Hospital, National Defense Medical Center (TSGHIRB no. B202105201). Written informed consent was obtained from all participants. Patient confidentiality and data security were maintained throughout the study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Leszczynski R, da Silva CA, Pinto A, Kuczynski U, da Silva EM. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2022;9:CD011642
2. Mustoe TA. International Scar Classification in 2019. In: Téot, L, Mustoe, T. A, Middelkoop, E, Gauglitz, G.G. (eds) Textbook on Scar Management. Springer, Cham. 2020:79-84
3. Rabello FB, Souza CD, Farina Júnior JA. Update on hypertrophic scar treatment. Clinics (Sao Paulo). 2014;69:565-73
4. Lee HJ, Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018;19:711
5. Xue M, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle). 2015;4:119-36
6. Hsu KC, Luan CW, Tsai YW. Review of Silicone Gel Sheeting and Silicone Gel for the Prevention of Hypertrophic Scars and Keloids. Wounds. 2017;29:154-8
7. Tziotzios C, Profyris C, Sterling J. Cutaneous scarring: Pathophysiology, molecular mechanisms, and scar reduction therapeutics Part II. Strategies to reduce scar formation after dermatologic procedures. J Am Acad Dermatol. 2012;66:13-24 quiz 25-6
8. de Oliveira GV, Gold MH. Silicone sheets and new gels to treat hypertrophic scars and keloids: A short review. Dermatol Ther. 2020;33:e13705
9. Khansa I, Harrison B, Janis JE. Evidence-Based Scar Management: How to Improve Results with Technique and Technology. Plast Reconstr Surg. 2016;138:165S-178S
10. Pangkanon W, Yenbutra P, Kamanamool N, Tannirandorn A, Udompataikul M. A comparison of the efficacy of silicone gel containing onion extract and aloe vera to silicone gel sheets to prevent postoperative hypertrophic scars and keloids. J Cosmet Dermatol. 2021;20:1146-53
11. Tran B, Wu JJ, Ratner D, Han G. Topical Scar Treatment Products for Wounds: A Systematic Review. Dermatol Surg. 2020;46:1564-71
12. Bleasdale B, Finnegan S, Murray K, Kelly S, Percival SL. The Use of Silicone Adhesives for Scar Reduction. Adv Wound Care (New Rochelle). 2015;4:422-30
13. Kirby JP. Hyperbaric Oxygen Therapy as an Elective Treatment. Mo Med. 2019;116:184-7
14. Hao Y, Dong X, Zhang M, Liu H, Zhu L, Wang Y. Effects of hyperbaric oxygen therapy on the expression levels of the inflammatory factors interleukin-12p40, macrophage inflammatory protein-1β, platelet-derived growth factor-BB, and interleukin-1 receptor antagonist in keloids. Medicine (Baltimore). 2020;99:e19857
15. Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2015;2015:CD004123
16. Lam G, Fontaine R, Ross FL, Chiu ES. Hyperbaric Oxygen Therapy: Exploring the Clinical Evidence. Adv Skin Wound Care. 2017;30:181-90
17. Lindenmann J, Kamolz L, Graier W, Smolle J, Smolle-Juettner F-M. Hyperbaric Oxygen Therapy and Tissue Regeneration: A Literature Survey. Biomedicines. 2022;10:3145
18. Song KX, Liu S, Zhang MZ, Liang WZ, Liu H, Dong XH. et al. Hyperbaric oxygen therapy improves the effect of keloid surgery and radiotherapy by reducing the recurrence rate. J Zhejiang Univ Sci B. 2018;19:853-62
19. Bassetto F, Bosco G, Brambullo T, Kohlscheen E, Tocco Tussardi I, Vindigni V. et al. Hyperbaric oxygen therapy in Plastic Surgery practice: case series and literature overview. G Chir. 2019;40:257-75
20. Herman TF, Popowicz P, Bordoni B. Wound Classification. [Updated 2023 Aug 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2024
21. Xu H, Huo Y, Zhou Q, Wang LA, Cai P, Doss B. et al. Geometry-mediated bridging drives nonadhesive stripe wound healing. Proc Natl Acad Sci U S A. 2023;120:e2221040120
22. van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg. 2005;116:514-22
23. Yamamoto N, Takada R, Maeda T, Yoshii T, Okawa A, Yagishita K. Microcirculation and tissue oxygenation in the head and limbs during hyperbaric oxygen treatment. Diving Hyperb Med. 2021;51:338-44
24. De Wolde SD, Hulskes RH, Weenink RP, Hollmann MW, Van Hulst RA. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules. 2021;11:1210
25. Hatibie MJ, Islam AA, Hatta M, Moenadjat Y, Susilo RH, Rendy L. Hyperbaric Oxygen Therapy for Second-Degree Burn Healing: An Experimental Study in Rabbits. Adv Skin Wound Care. 2019;32:1-4
26. Chang C, White C, Katz A, Hanna MK. Management of ischemic tissues and skin flaps in Re-Operative and complex hypospadias repair using vasodilators and hyperbaric oxygen. J Pediatr Urol. 2020;16:672.e1-672.e8
27. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32:82-92
28. Sangha MS, Deroide F, Meys R. Wound healing, scarring and management. Clin Exp Dermatol. 2024;49:325-36
29. Dyson E, Sikkink S, Nocita D, Twigg P, Westgate G, Swift T. Evaluating the Irritant Factors of Silicone and Hydrocolloid Skin Contact Adhesives Using Trans-Epidermal Water Loss, Protein Stripping, Erythema, and Ease of Removal. ACS Appl Bio Mater. 2024;7:284-96
30. De Decker I, Hoeksema H, Vanlerberghe E, Beeckman A, Verbelen J, De Coninck P. et al. Occlusion and hydration of scars: moisturizers versus silicone gels. Burns. 2023;49:365-79
31. Sato J, Yanai M, Hirao T, Denda M. Water content and thickness of the stratum corneum contribute to skin surface morphology. Arch Dermatol Res. 2000;292:412-7
32. Yang B, Lv C, Ye L, Wang Z, Kim Y, Luo W. et al. Stratum corneum hydration inversely correlates with certain serum cytokine levels in the elderly, possibly contributing to inflammaging. Immun Ageing. 2023;20:7
33. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585-601
34. Deng X, Zhao F, Zhao D, Zhang Q, Zhu Y, Chen Q. et al. Oxymatrine promotes hypertrophic scar repair through reduced human scar fibroblast viability, collagen and induced apoptosis via autophagy inhibition. Int Wound J. 2022;19:1221-31
35. De Paepe K, Sieg A, Le Meur M, Rogiers V. Silicones as nonocclusive topical agents. Skin Pharmacol Physiol. 2014;27:164-71
36. Fu Q, Duan R, Sun Y, Li Q. Hyperbaric oxygen therapy for healthy aging: From mechanisms to therapeutics. Redox Biol. 2022;53:102352
37. Ueng SW-N, Lee MS-S, Tai C-L, Hsu K-Y, Lin S-S, Chan Y-S. et al. Hyperbaric oxygen therapy improves medial collateral ligament healing in a rabbit model. Formos J Musculoskelet Disord. 2011;2:7-11
38. Eggleton P, Bishop A, Smerdon G. Safety and efficacy of hyperbaric oxygen therapy in chronic wound management: current evidence. Chronic Wound Care Manag Res. 2015;2:81-93
39. Mostaço-Guidolin L, Rosin NL, Hackett TL. Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications. Int J Mol Sci. 2017;18:1772
40. Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P. et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020;11:603187
41. Chang DH, Hsieh CY, Chang CW, Wang HH, Chang HT. The use of hyperbaric oxygen therapy in the treatment of hand crush injuries. Wound Repair Regen. 2024;32:146-54
42. Spielman AF, Griffin MF, Parker J, Cotterell AC, Wan DC, Longaker MT. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv Wound Care (New Rochelle). 2023;12:57-67
43. Almadani YH, Vorstenbosch J, Davison PG, Murphy AM. Wound Healing: A Comprehensive Review. Semin Plast Surg. 2021;35:141-4
44. Akaishi S, Akimoto M, Hyakusoku H, Ogawa R. The tensile reduction effects of silicone gel sheeting. Plast Reconstr Surg. 2010;126:109e-111e
45. MacInnes L, Baines C, Bishop A, Ford K. Patient knowledge and experience of hyperbaric oxygen treatment. Diving Hyperb Med. 2021;51:72-7
46. Hess CL, Howard MA, Attinger CE. A review of mechanical adjuncts in wound healing: hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann Plast Surg. 2003;51:210-8
47. Huang C, Zhong Y, Yue C, He B, Li Y, Li J. The effect of hyperbaric oxygen therapy on the clinical outcomes of necrotizing soft tissue infections: a systematic review and meta-analysis. World J Emerg Surg. 2023;18:23
Author contact
![]() Corresponding authors: Po-Jen Hsiao, MD, PhD, No. 168, Zhongxing Rd., Longtan Dist., Taoyuan City, 325, Taiwan; E-mail: doc10510gov.tw or a2005a660820com.tw. Hsieh-Chih Tsai, PhD, No. 43, Sec. 4, Keelung Rd., Da'an Dist., Taipei City 106335, Taiwan; E-mail: h.c.tsaintust.edu.tw.
Corresponding authors: Po-Jen Hsiao, MD, PhD, No. 168, Zhongxing Rd., Longtan Dist., Taoyuan City, 325, Taiwan; E-mail: doc10510gov.tw or a2005a660820com.tw. Hsieh-Chih Tsai, PhD, No. 43, Sec. 4, Keelung Rd., Da'an Dist., Taipei City 106335, Taiwan; E-mail: h.c.tsaintust.edu.tw.

 Global reach, higher impact
Global reach, higher impact