3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(5):1064-1071. doi:10.7150/ijms.106651 This issue Cite
Research Paper
Routine Clinic Surveillance on Arteriovenous Graft Patency in Hemodialysis Patients with Previous Access Complications
1. Division of Nephrology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan.
2. Department of Public Health and Environmental Medicine, School of Medicine, College of Medicine, Kaohsiung Medical University, Taiwan.
3. Nurse Practitioner, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan.
4. Department of Nursing, Chung-Jen Junior College of Nursing, Health Sciences and Management, Taiwan.
5. Division of Cardiology, Department of Internal Medicine, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan.
6. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
7. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
8. Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
Received 2024-11-9; Accepted 2025-1-28; Published 2025-2-3
Abstract
Background: Arteriovenous grafts (AVGs) are an alternative for hemodialysis (HD) access in patients with inadequate vasculature or advanced age. The effect of routine surveillance for AVG maintenance remains unclear. This study assesses the clinical and economic outcomes of routine surveillance at a collaborative clinic in patients with previous access complications.
Methods: We recruited HD patients from the initiation of the clinic in 2020, and divided them into two groups: those receiving routine surveillance and those without. Primary outcomes included AVG interventions (e.g., arteriovenous access [AVA] reconstruction, graft-anastomosis stenting, percutaneous transluminal angioplasty [PTA]). Other outcomes included AVG secondary patency and costs associated with the interventions.
Results: Twenty-two patients with routine surveillance and 65 without were recruited. There was no significant difference in AVA reconstruction rate between the surveillance and non-surveillance groups (0.46 vs. 0.5 per 100 patient-months, p = 0.99), however, rates of graft-anastomosis stenting (0.66 vs. 0.2 per 100 patient-months, p = 0.02) and PTA (30.19 vs. 14.17 per 100 patient-months, p < 0.01) were significantly higher in the surveillance group. No significant difference was observed in secondary patency (hazard ratio: 0.83, p = 0.79). The total costs of AVG interventions were more than double in the surveillance group (110672 New Taiwan Dollar [NTD] vs. 51874 NTD, p < 0.01).
Conclusions: Routine clinic surveillance in HD patients with AVGs and previous access complications resulted in significantly higher rates of graft-anastomosis stenting, PTA, and associated costs, without significant differences in AVA reconstruction rates or secondary patency. These results highlight the need for further assessment of the cost-effectiveness of routine AVG monitoring.
Keywords: arteriovenous graft, outcomes, routine surveillance
Introduction
Hemodialysis (HD) is the primary renal replacement therapy for patients with end-stage renal disease (1). This treatment necessitates the use of a vascular access (VA) such as arteriovenous fistulas (AVFs), arteriovenous grafts (AVGs), and both cuffed and non-cuffed catheters, to effectively remove metabolic waste and excess fluid from the patient's bloodstream. The maintenance of a well-functioning VA is crucial for the optimal management of patients undergoing HD. AVFs are preferred due to their lower thrombosis rates, longer patency, and superior patient outcomes compared to other modalities (2). However, for patients with poor vascular anatomy or advanced age, AVGs may be a suitable alternative (3), despite their significantly lower patency rates compared to AVFs (4-7).
The importance of clinical evaluation of arteriovenous access (AVA) during HD sessions is well-supported by current guidelines (3). The potential benefit of routine surveillance is the ability to preserve AVA patency by identifying dysfunction before full occlusion occurs. Routine surveillance includes monitoring intra-dialysis venous pressure and access flow, allowing for timely preemptive interventions such as percutaneous transluminal angioplasty (PTA), stenting, thrombectomy, bypass surgery, and ultimately access reconstruction. However, studies on AVA surveillance have yielded inconsistent results, with some indicating a beneficial impact on patency (8-10), whereas the outcomes of routine surveillance for AVGs remain ambiguous (11, 12). In addition, the economic impact of these surveillance measures on healthcare systems remains unclear (13-15).
The objective of this study was to assess the impact of routine surveillance at a multidisciplinary clinic in a tertiary teaching hospital on the clinical and economic outcomes of AVGs in HD patients with previous access complications.
Material and Methods
Statement of ethical approval
This study was approved by the Institutional Review Board of Ditmanson Medical Foundation Chia-Yi Christian Hospital (approval number: IRB2023067). The need for informed consent was waived due to the retrospective nature of the study. All methods were conducted in compliance with applicable guidelines and regulations (16).
Data source
To enhance the quality of dialysis VA care, a specialized outpatient clinic was established in our hospital on May 1, 2020, through the collaborative efforts of nephrology and cardiology teams (17). This clinic was designed to address the needs of HD patients with VA complications, such as maturation failure, challenging cannulation, elevated intra-dialysis venous pressure, reduced intra-dialysis blood flow, prolonged post-dialysis hemostasis, unexplained swelling of the limb on the side of the AVA, and other warning signs identified through guideline-directed physical examinations performed by dialysis staff. Nephrologists and dialysis staff actively encourage patients with previous VA complications undergoing interventions to attend routine surveillance at the clinic, which is predominantly led by cardiologists receiving specialized training in vascular access assessment. Following referral, the clinic provides comprehensive and objective access monitoring through physical examinations, supplemented by ultrasound when necessary. Salvage interventions are performed for patients whose VA demonstrates complications or imaging abnormalities, such as inadequate flow to achieve dialysis adequacy or stenosis exceeding 50% as detected by ultrasound. In addition, cardiologists at the clinic engage in periodic discussions with nephrologists and dialysis staff to address complex cases and optimize management plans.
We gathered demographic data including sex, age, HD vintage, and characteristics of AVGs. All AVGs in this study were constructed using expanded polytetrafluoroethylene grafts. We also collected information on comorbidities, history of parathyroidectomy, use of far-infrared radiation therapy, administration of antiplatelet and antihypotensive agents, and laboratory data at the time of recruitment. Furthermore, data on episodes and costs associated with AVG interventions, including AVA reconstruction, graft-anastomosis stenting, PTA, ultrasound for the AVG interventions and clinic visits were extracted from the hospital's electronic medical records system. The datasets generated or analyzed in this study are available from the corresponding author upon reasonable request.
Study design
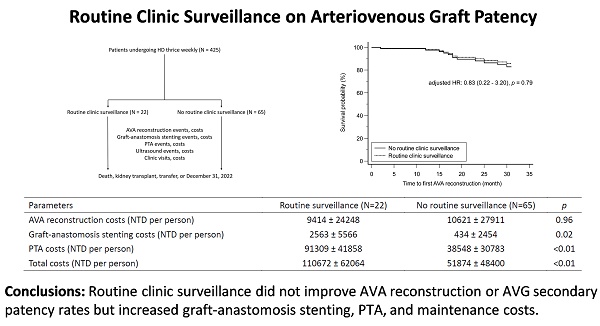
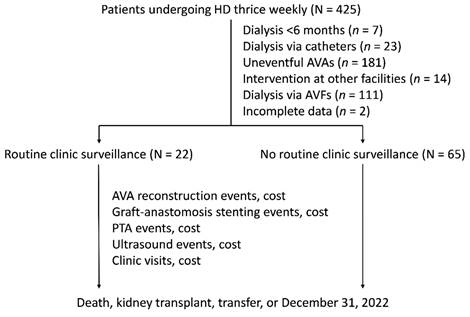
We recruited patients undergoing HD thrice weekly from the clinic's launch date. The exclusion criteria were patients undergoing dialysis for less than 6 months (n = 7), those using catheters or AVFs (n = 134) for dialysis, those who had not undergone salvage interventions for their AVAs (n = 181), those receiving interventions at other facilities (n = 14), and those with incomplete data (n = 2). The recruited patients were then categorized into two groups: those receiving routine clinic surveillance and those without. Routine clinic surveillance was defined as regular clinic visits scheduled at intervals ranging from 3 to 6 months, commencing from the establishment date of the clinic. Patients in the routine clinic surveillance group who experienced VA complications outside of the scheduled visit intervals could arrange additional appointments as needed. The follow-up period ended at the patient's death, kidney transplant, transfer to another medical facility, or December 31, 2022, whichever occurred first. Figure 1 depicts the recruitment, allocation, and follow-up process. In total, the study analyzed 87 patients, with 22 (25.3%) receiving routine clinic surveillance and 65 (74.7%) not receiving routine clinic surveillance.
Flow diagram of the recruitment, allocation, and follow-up. AVA: arteriovenous access; AVF: arteriovenous fistula; HD: hemodialysis; PTA: percutaneous transluminal angioplasty.
The primary outcomes of this study were the rates of different AVG interventions among the recruited patients throughout the follow-up period. AVA reconstruction was defined as the creation of a new VA after the previous one failed to support adequate dialysis treatment. Sensitivity analysis was performed to validate our findings. We also compared the secondary patency of AVGs between groups, defined as the duration from recruitment to AVG abandonment, and analyzed the costs associated with AVG interventions. Furthermore, we evaluated correlations between patient demographic and AVA reconstruction.
Statistical analysis
Statistical analyses were conducted using MedCalc Statistical Software (version 23.0.6, MedCalc Software Ltd., Ostend, Belgium). Categorical variables were expressed as frequencies or percentages, while continuous variables were presented as means and standard deviations. The chi-square test or Mann-Whitney test was used for comparisons between variables, as appropriate. A Cox proportional hazards regression model was used to assess the impact of routine clinic follow-up on time to AVA reconstruction. The model estimated hazard ratios after adjusting for potential confounders and significant variables between groups, to provide robust estimates of the association between routine follow-up and the risk of AVA reconstruction. Logistic regression analysis was used to determine the crude odds ratios between categorical variables and outcomes, while Spearman's rank correlation coefficients were calculated to assess correlations between continuous variables and outcomes. Statistical significance was set at a two-tailed p value of less than 0.05.
Results
The baseline demographic and clinical characteristics of the recruited patients are presented in Table 1. No significant difference was observed in sex distribution between the two groups, and there were also no significant differences in age, HD vintage, AVG characteristics, comorbidities, history of parathyroidectomy, use of far-infrared radiation therapy, or administration of antiplatelet or antihypotensive agents. Regarding laboratory data, the patients undergoing routine clinic surveillance had a significantly higher hemoglobin level compared to those not receiving routine follow-up (10.8 g/dL vs. 10.1 g/dL, p < 0.01). Other laboratory parameters were comparable between the two groups.
The primary outcomes of the patients with and without routine clinic surveillance are detailed in Table 2. The rates of AVG interventions were expressed as occurrence per 100 patient-months, adjusted for HD vintage. No significant difference in the rate of AVA reconstruction was observed between the patients with and without routine clinic surveillance (0.46 / 100 patient-months vs. 0.5 / 100 patient-months, p = 0.99). Conversely, the rates of graft-anastomosis stenting (0.66 / 100 patient-months vs. 0.2 / 100 patient-months, p = 0.02) and PTA (30.19 / 100 patient-months vs. 14.17 / 100 patient-months, p < 0.01) were significantly higher in the patients with routine clinic surveillance. Sensitivity analysis, including patients matched for hemoglobin level, patients aged over 65 years, and those receiving antiplatelet agents, corroborated our primary findings.
Baseline demographic and clinical characteristics of patients with and without routine clinic surveillance
| Parameters | Routine surveillance (N=22) | No routine surveillance (N=65) | p |
|---|---|---|---|
| Sex (male / female) | 9 / 13 | 29 / 36 | 0.76 |
| Age (years) | 75 ± 14 | 72 ± 10 | 0.10 |
| HD vintage (months) | 108 ± 72 | 112 ± 109 | 0.51 |
| AVG at left / right side | 19 / 3 | 57 / 8 | 0.87 |
| AVG at forearm / arm | 14 / 8 | 44 / 21 | 0.73 |
| Hypertension | 19 | 59 | 0.56 |
| DM | 9 | 37 | 0.20 |
| Heart failure | 3 | 10 | 0.84 |
| Cardiovascular disease | 7 | 30 | 0.24 |
| Cerebrovascular disease | 8 | 12 | 0.09 |
| Peptic ulcer disease | 16 | 45 | 0.76 |
| Gout / hyperuricemia | 11 | 25 | 0.35 |
| Cancer | 4 | 18 | 0.38 |
| HBV carrier | 1 | 7 | 0.39 |
| HCV carrier | 4 | 15 | 0.63 |
| Parathyroidectomy | 6 | 7 | 0.06 |
| FIR therapy | 3 | 4 | 0.27 |
| Antiplatelet agents | 11 | 32 | 0.95 |
| Antihypotensive agents | 5 | 11 | 0.55 |
| Leukocyte (103/μL) | 7.02 ± 2.20 | 6.66 ± 2.14 | 0.54 |
| Hemoglobin (g/dL) | 10.8 ± 1.2 | 10.1 ± 0.9 | <0.01 |
| Platelet (103/μL) | 169 ± 47 | 195 ± 64 | 0.13 |
| Glucose (mg/dL) | 151 ± 52 | 151 ± 76 | 0.34 |
| HbA1c (%, DM patients) | 6.5 ± 1.4 | 6.9 ± 2.0 | 0.74 |
| Albumin (g/dL) | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.75 |
| ALK-P (U/L) | 274 ± 124 | 329 ± 180 | 0.16 |
| Blood urea nitrogen (mg/dL) | 69 ± 17 | 73 ± 20 | 0.42 |
| Creatinine (mg/dL) | 9.9 ± 3.1 | 9.7 ± 2.4 | 0.98 |
| Potassium (mmol/L) | 4.3 ± 0.7 | 4.7 ± 0.7 | 0.05 |
| Phosphorus (mg/dL) | 4.8 ± 1.6 | 5.0 ± 1.6 | 0.63 |
| Total calcium (mg/dL) | 9.2 ± 0.7 | 9.0 ± 0.8 | 0.08 |
| Sodium (mmol/L) | 139 ± 4 | 138 ± 4 | 0.43 |
| Kt/V | 1.7 ± 0.3 | 1.6 ± 0.2 | 0.20 |
| Urea reduction ratio (%) | 76 ± 4 | 74 ± 5 | 0.15 |
| Uric Acid (mg/dL) | 7.2 ± 1.9 | 7.1 ± 2.1 | 0.86 |
| Cholesterol (mg/dL) | 165 ± 31 | 158 ± 38 | 0.29 |
| Triglyceride (mg/dL) | 177 ± 124 | 158 ± 109 | 0.58 |
| HDL-C (mg/dL) | 43 ± 13 | 45 ± 16 | 0.69 |
| LDL-C (mg/dL) | 94 ± 28 | 88 ± 32 | 0.27 |
| Serum iron (μg/dL) | 73 ± 22 | 68 ± 31 | 0.23 |
| TIBC (μg/dL) | 226 ± 36 | 231 ± 55 | 0.93 |
| Transferrin saturation (%) | 33 ± 10 | 30 ± 13 | 0.22 |
| Ferritin (ng/mL) | 558 ± 273 | 504 ± 356 | 0.26 |
| PTH-I (pg/mL) | 327 ± 266 | 319 ± 368 | 0.44 |
| hs-CRP (mg/dL) | 0.86 ± 1.09 | 1.74 ± 2.80 | 0.30 |
Abbreviations: ALK-P: alkaline phosphatase; AVG: arteriovenous graft; DM: diabetes mellitus; FIR: far-infrared radiation; HbA1c: glycated hemoglobin; HBV: hepatitis B virus; HCV: hepatitis C virus; HD: hemodialysis; HDL-C: high density lipoprotein cholesterol; hs-CRP: high sensitivity C-reactive protein; LDL-C: low density lipoprotein cholesterol; PTH-I: parathyroid hormone intact; TIBC: total iron-binding capacity.
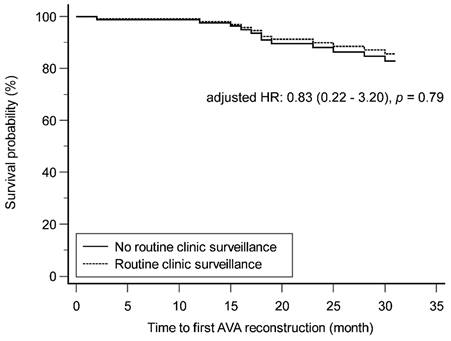
The secondary patency of AVGs between the patients with and without routine clinic surveillance is depicted in Figure 2, using the Cox proportional hazards regression model. After adjusting for hemoglobin level, the survival probability did not differ significantly between the two groups (adjusted hazard ratio: 0.83, p = 0.79).
AVG interventions rates and associated sensitivity analyses in patients with and without routine clinic surveillance
| Parameters | Routine surveillance | No routine surveillance | p |
|---|---|---|---|
| N = 22 | N = 65 | ||
| AVA reconstruction rate (per 100 patient-months) | 0.46 ± 1.19 | 0.50 ± 1.30 | 0.99 |
| Graft-anastomosis stenting rate (per 100 patient-months) | 0.66 ± 1.45 | 0.20 ± 1.30 | 0.02 |
| PTA rate (per 100 patient-months) | 30.19 ± 13.68 | 14.17 ± 10.43 | <0.01 |
| Sensitivity analyses | |||
| Matched with hemoglobin | N = 22 | N = 22 | |
| AVA reconstruction rate | 0.46 ± 1.12 | 0.73 ± 1.70 | 0.68 |
| Graft-anastomosis stenting rate | 0.66 ± 1.45 | 0.00 ± 0.00 | 0.04 |
| PTA rate | 30.19 ± 13.68 | 13.81 ± 8.25 | <0.01 |
| Aged over 65 years | N = 19 | N = 52 | |
| AVA reconstruction rate | 0.36 ± 1.10 | 0.62 ± 1.43 | 0.53 |
| Graft-anastomosis stenting rate | 0.76 ± 1.53 | 0.19 ± 1.39 | <0.01 |
| PTA rate | 31.56 ± 14.26 | 14.62 ± 11.14 | <0.01 |
| Receiving antiplatelet agents | N = 11 | N = 32 | |
| AVA reconstruction rate | 0.00 ± 0.00 | 0.30 ± 0.96 | 0.30 |
| Graft-anastomosis stenting rate | 0.29 ± 0.97 | 0.00 ± 0.00 | 0.09 |
| PTA rate | 28.39 ± 9.48 | 14.78 ± 12.75 | <0.01 |
Abbreviations: AVA: arteriovenous access; AVG: arteriovenous graft; PTA: percutaneous transluminal angioplasty.
The costs of AVG interventions expressed in New Taiwan Dollars (NTD) per patient during the follow-up period in both groups are detailed in Table 3. The cost of AVA reconstruction was lower in the patients with routine clinic surveillance compared to those without, although the difference was not significant (9414 NTD per patient vs. 10620 NTD per patient, p = 0.96). However, the costs of graft-anastomosis stenting (2563 NTD per patient vs. 434 NTD per patient, p = 0.02) and PTA (91309 NTDs per patient vs. 38548 NTD per patient, p < 0.01) were significantly higher in the patients with routine clinic surveillance. Overall, the total costs of AVG interventions, including the costs of ultrasound and clinic visits (shown in Table 4), were significantly higher in the routine surveillance group compared to the group without routine surveillance (110672 NTD per patient vs. 51874 NTD per patient, p < 0.01).
The correlations between patient demographic information and AVA reconstruction are presented in Supplementary Table 1 and 2. Analysis revealed no significant correlations between categorical variables (Supplementary Table 1) or continuous variables (Supplementary Table 2) and AVA reconstruction in the patients with routine clinic surveillance.
Secondary patency of AVG in patients with and without routine clinic surveillance. AVA: arteriovenous access; AVG: arteriovenous graft; HR: hazard ratio.
AVG interventions costs in patients with and without routine clinic surveillance
| Parameters | Routine surveillance (N=22) | No routine surveillance (N=65) | p |
|---|---|---|---|
| AVA reconstruction costs (NTD per person) | 9414 ± 24248 | 10621 ± 27911 | 0.96 |
| Graft-anastomosis stenting costs (NTD per person) | 2563 ± 5566 | 434 ± 2454 | 0.02 |
| PTA costs (NTD per person) | 91309 ± 41858 | 38548 ± 30783 | <0.01 |
| Total costs (NTD per person) | 110672 ± 62064 | 51874 ± 48400 | <0.01 |
Abbreviations: AVA: arteriovenous access; AVG: arteriovenous graft; NTD: New Taiwan Dollar; PTA: percutaneous transluminal angioplasty.
Ultrasound and clinic visits in patients with and without routine clinic surveillance
| Parameters | Routine surveillance (N=22) | No routine surveillance (N=65) | p |
|---|---|---|---|
| Ultrasound rate (per 100 patient-months) | 15.07 ± 12.64 | 3.36 ± 4.80 | <0.01 |
| Clinic rate (per 100 patient-months) | 50.10 ± 21.63 | 21.20 ± 17.19 | <0.01 |
| Ultrasound costs (NTD per patient) | 3382 ± 2910 | 714 ± 960 | <0.01 |
| Clinic costs (NTD per patient) | 4004 ± 1718 | 1558 ± 1349 | <0.01 |
Abbreviations: NTD: New Taiwan Dollar
Discussion
This study investigated the outcomes of routine clinic surveillance on AVG patency in HD patients with previous access complications. The results showed no significant differences in AVA reconstruction or secondary patency rates, but significant increases in graft-anastomosis stenting and PTA rates in the patients undergoing routine clinic surveillance compared to those without. Furthermore, the patients with routine clinic surveillance incurred significantly higher costs to maintain AVG patency. These findings underscore the need for more detailed evaluations of the cost-effectiveness of routine AVG monitoring in this patient cohort.
AVA reconstruction is typically required when an access fails to maintain adequate function despite salvage interventions (3), and routine surveillance may help reduce its occurrence. In this study, we observed no significant difference in AVA reconstruction rate between the patients undergoing routine clinic surveillance and those without, a finding validated by sensitivity analysis involving matched hemoglobin level, as well as subgroups of older patients and those on antiplatelet therapy. McCarley et al. evaluated the clinical and financial outcomes of different VA surveillance methods in a cohort of 132 HD patients (18). Their results showed variable costs in the patients with AVGs, reflecting differences in the rates of access reconstruction or revision across surveillance methods, compared to a control group without surveillance (18). The discrepancies between our findings and those of McCarley et al. may be attributed to differences in the definition of AVA reconstruction.
Several salvage interventions are available to restore the patency of AVGs, with graft-anastomosis stenting and PTA being the primary approaches in our hospital. Graft-anastomosis stenting is indicated for cases requiring frequent PTA at the stenotic graft-anastomosis site. In our study, both graft-anastomosis stenting and PTA rates were significantly higher in the routine clinic surveillance group, a finding that was further supported by sensitivity analysis. Moist et al. conducted a randomized controlled trial (RCT) involving 112 HD patients to assess the effect of monthly AVG flow monitoring on thrombosis and access loss, and found that the intervention rates in the treatment group were 1.65 times higher than those in the control group (19). Similarly, a prospective RCT by Robbin et al. assessed the impact of regular ultrasound surveillance on stenosis in 126 HD patients with AVGs, and found that the frequency of preemptive PTA was 64% higher in the ultrasound surveillance group compared to the control group (20). In addition, Hoeben et al. assessed the impact of routine surveillance on intervention rates in a cohort of 86 patients, and observed that the frequency of interventions was significantly higher in the group receiving regular surveillance compared to those without (21). Moreover, in a cohort of 363 patients, Plantinga et al. observed that those undergoing more frequent monitoring were 1.4 times more likely to require an intervention compared to those with less frequent monitoring (22). Furthermore, national data from the Netherlands reported by Tordoir et al. indicated that multidisciplinary discussions of AVA problems increased the rate of preemptive endovascular interventions (23). In a cohort of 60 patients, Mauro et al. assessed AVG secondary patency between patients in a surveillance program and those receiving clinical assessment (24). In the surveillance group, 15 AVG malfunctions were detected and treated with graft-anastomosis stenting and PTA, while no malfunctions were observed in the historical control group (24). Despite these findings, some studies have reported minimal differences in intervention rates. The Hemodialysis Access Surveillance Evaluation Study, a multicenter RCT by Salman et al. compared monthly ultrasound AVA flow surveillance with standard care in 436 HD patients, and found no statistically significant difference in the total number of procedures between the groups (25). Similarly, Schuman et al. compared AVA outcomes between ultrasound-based flow measurements and clinical criteria, and reported a modest 1.17-fold increase in intervention rate in the ultrasound group only (26). These discrepancies may be due to differences in study populations and design.
Maintaining AVA functionality is a crucial issue in HD-related research. In our study, secondary patency of AVGs was defined as the period from the initiation of the clinic to the date of AVG abandonment. Cox proportional hazards regression was used to compare secondary patency between the patients with and without routine clinic surveillance. After adjusting for baseline demographic and clinical variables, no significant difference in AVG secondary patency was identified between the two groups. Ram et al. conducted an RCT involving 101 patients, and applied criteria including clinical symptoms, AVG flow measurements, and ultrasound findings to guide referrals for PTA, and they observed no significant difference in 2-year AVG survival rate between the groups (27). Similarly, Dember et al. performed an RCT of 64 patients to compare the prophylactic repair of AVG stenosis with repair at the time of thrombosis (28). Over the 3.5-year study period, no significant differences were observed in AVG abandonment rates or time to abandonment between the intervention and observation groups (28). These findings are consistent with other RCTs (19, 20). In addition, Lumsden et al. compared a surveillance program involving prophylactic PTA for stenoses greater than 50% with a non-interventional approach in 65 patients, and found no significant differences in patency rates at 6 and 12 months between the groups (29). These outcomes align with similar findings from other cohort studies (22, 24). In contrast, Mauro et al. compared AVG secondary patency between patients in a surveillance program and those undergoing clinical assessment, and found that the 5-year patency rate was significantly higher in the surveillance group (24). However, it is important to acknowledge that the comparison groups were analyzed in different temporal and geographic contexts. Overall, our findings align with the existing literature, including subgroup analysis in systematic reviews and meta-analyses by Tonelli et al. (30) and Casey et al. (8) which found no significant difference in AVG abandonment when comparing AVA flow surveillance with standard care.
The economic burden of HD places a significant strain on healthcare systems (31), and the costs associated with AVA interventions further exacerbate this challenge (32). The costs related to graft-anastomosis stenting and PTA were significantly higher in the routine surveillance group compared to those without surveillance in this study. Consequently, the total costs of AVG-related interventions were more than twice as high in the routine surveillance group compared to the patients without routine surveillance. In the RCT by Ram et al. mentioned above, subgroup analysis revealed that costs related to monthly AVG flow monitoring, quarterly stenosis evaluations, and total PTAs were higher in the surveillance group (13). These findings align with the cost outcomes observed in our study. In contrast, McCarley et al. reported a 49% reduction in the total costs of managing thrombosis-related events in AVGs with ultrasound-assisted flow monitoring compared to no monitoring, and a 54% reduction compared to venous pressure monitoring (18). Given that AVG interventions were primarily managed on an outpatient basis and the demographic and clinical characteristics were comparable between the surveillance and non-surveillance groups, our study specifically examined the costs associated with outpatient interventions, excluding expenses associated with dialysis catheters and hospitalizations. Further investigations are warranted to evaluate the clinical benefits and cost-effectiveness of routine surveillance, including an analysis of potential long-term benefits, such as reduced hospitalizations and complication rates.
The Kidney Disease Outcomes Quality Initiative guidelines recommend regular physical examinations of AVGs by experienced practitioners to identify clinical signs of flow dysfunction. However, routine surveillance methods, including AVG flow measurement, pressure monitoring, or imaging for stenosis beyond standard clinical monitoring, are not advised for improving AVG patency (3). We have established a multidisciplinary clinic with bidirectional feedback, involving nephrologists, dialysis staff, and trained cardiologists, in conjunction with guideline-directed assessments in the dialysis unit, to optimize AVA patency in our HD patients. This collaborative approach offers a fresh perspective on AVG follow-up. However, our surveillance strategy did not significantly improve AVG secondary patency and was associated with higher intervention rates and increased costs of AVG-related care.
This study also has several limitations. First, being a retrospective analysis from a single tertiary teaching hospital, the findings may not be widely generalizable. Future research involving larger sample sizes or multicenter data is recommended to validate these results and improve their applicability. Additionally, the follow-up period for the recruited patients was limited to a maximum of 1.5 years, and longer follow-up durations may be necessary to fully assess long-term outcomes. Second, the collaborative clinic adopted an individualized approach rather than a standardized protocol for the assessment and management of AVG. Furthermore, the multidisciplinary team lacked the inclusion of vascular surgeons, who play a critical role in AVA reconstruction. Third, critical variables such as AVG flow, intra-dialysis venous pressure, and outcomes such as dialysis catheter use and associated hospitalization data were not recorded. Fourth, the reasons for AVA reconstruction are not limited to AVG occlusion (28), however they were not specified in our analysis. Lastly, despite multivariate analysis was performed to account for known variables, the influence of unmeasured confounding factors, such as smoking status, on the outcomes cannot be completely excluded.
Conclusions
We observed no significant differences in AVA reconstruction or secondary patency rate between HD patients with AVGs receiving routine collaborative clinic surveillance and those without. However, routine clinic surveillance was associated with a marked increase in graft-anastomosis stenting and PTA, resulting in significantly higher costs for maintaining AVG patency. These findings highlight the need for further assessment of the cost-effectiveness of routine AVG surveillance in this patient population.
Supplementary Material
Supplementary tables.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Thurlow JS, Joshi M, Yan G, Norris KC, Agodoa LY, Yuan CM. et al. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. American journal of nephrology. 2021;52(2):98-107
2. Murakami M, Fujii N, Kanda E, Kikuchi K, Wada A, Hamano T. et al. Association of Four Types of Vascular Access Including Arterial Superficialization with Mortality in Maintenance Hemodialysis Patients: A Nationwide Cohort Study in Japan. American journal of nephrology. 2023;54(3-4):83-94
3. Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K. et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2020;75(4 Suppl 2):S1-s164
4. Jadlowiec CC, Lavallee M, Mannion EM, Brown MG. An Outcomes Comparison of Native Arteriovenous Fistulae, Polytetrafluorethylene Grafts, and Cryopreserved Vein Allografts. Ann Vasc Surg. 2015;29(8):1642-7
5. Park HS, Kim WJ, Kim YK, Kim HW, Choi BS, Park CW. et al. Comparison of Outcomes with Arteriovenous Fistula and Arteriovenous Graft for Vascular Access in Hemodialysis: A Prospective Cohort Study. American journal of nephrology. 2016;43(2):120-8
6. Choi J, Ban TH, Choi BS, Baik JH, Kim BS, Kim YO. et al. Comparison of vascular access patency and patient survival between native arteriovenous fistula and synthetic arteriovenous graft according to age group. Hemodialysis international International Symposium on Home Hemodialysis. 2020;24(3):309-16
7. Roetker NS, Guo H, Ramey DR, McMullan CJ, Atkins GB, Wetmore JB. Hemodialysis Access Type and Access Patency Loss: An Observational Cohort Study. Kidney Med. 2023;5(1):100567
8. Casey ET, Murad MH, Rizvi AZ, Sidawy AN, McGrath MM, Elamin MB. et al. Surveillance of arteriovenous hemodialysis access: a systematic review and meta-analysis. Journal of vascular surgery. 2008;48(5 Suppl):48s-54s
9. Ibrahim A, Ali H, Raza H, Mohamed M. Hemodialysis Access Surveillance: A Review of the Literature. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2022;33(Supplement):S66-s76
10. Evans LM, Raj R. A scoping review of outcomes with routine surveillance of arterio-venous fistulas. J Vasc Access. 2024;5:1409-1415
11. Whittier WL. We Perform Surveillance for Arteriovenous Graft Stenosis. Seminars in dialysis. 2016;29(4):287-8
12. Kingsmore DB, Thomson P, Stevenson K. Screening and surveillance of venous stenosis in AVG: Is it time to rethink our assumptions? J Vasc Access. 2023;24(5):873-8
13. Dossabhoy NR, Ram SJ, Nassar R, Work J, Eason JM, Paulson WD. Stenosis surveillance of hemodialysis grafts by duplex ultrasound reduces hospitalizations and cost of care. Seminars in dialysis. 2005;18(6):550-7
14. Tessitore N, Bedogna V, Poli A, Mantovani W, Lipari G, Baggio E. et al. Adding access blood flow surveillance to clinical monitoring reduces thrombosis rates and costs, and improves fistula patency in the short term: a controlled cohort study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(11):3578-84
15. Bittl JA, Cohen DJ, Seek MM, Feldman RL. Economic analysis of angiography and preemptive angioplasty to prevent hemodialysis-access thrombosis. Catheter Cardiovasc Interv. 2010;75(1):14-21
16. World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-4
17. Whittier WL. Surveillance of hemodialysis vascular access. Seminars in interventional radiology. 2009;26(2):130-8
18. McCarley P, Wingard RL, Shyr Y, Pettus W, Hakim RM, Ikizler TA. Vascular access blood flow monitoring reduces access morbidity and costs. Kidney international. 2001;60(3):1164-72
19. Moist LM, Churchill DN, House AA, Millward SF, Elliott JE, Kribs SW. et al. Regular monitoring of access flow compared with monitoring of venous pressure fails to improve graft survival. Journal of the American Society of Nephrology: JASN. 2003;14(10):2645-53
20. Robbin ML, Oser RF, Lee JY, Heudebert GR, Mennemeyer ST, Allon M. Randomized comparison of ultrasound surveillance and clinical monitoring on arteriovenous graft outcomes. Kidney international. 2006;69(4):730-5
21. Hoeben H, Abu-Alfa AK, Reilly RF, Aruny JE, Bouman K, Perazella MA. Vascular access surveillance: evaluation of combining dynamic venous pressure and vascular access blood flow measurements. American journal of nephrology. 2003;23(6):403-8
22. Plantinga LC, Jaar BG, Astor B, Fink NE, Eustace JA, Klag MJ. et al. Association of clinic vascular access monitoring practices with clinical outcomes in hemodialysis patients. Nephron Clinical practice. 2006;104(4):c151-9
23. Tordoir JH, van Loon MM, ter Meer M, van Laanen J, Bode AS, Weijmer MC. et al. Hemodialysis vascular access management in the Netherlands. J Vasc Access. 2015;16(Suppl 9):S11-5
24. Mauro R, Pini R, Faggioli G, Donati G, Facchini MG, D'Amico R. et al. Impact of duplex ultrasound surveillance program on patency of prosthetic arteriovenous graft for hemodialysis: a single-center experience. Ann Vasc Surg. 2015;29(6):1211-7
25. Salman L, Rizvi A, Contreras G, Manning C, Feustel PJ, Machado I. et al. A Multicenter Randomized Clinical Trial of Hemodialysis Access Blood Flow Surveillance Compared to Standard of Care: The Hemodialysis Access Surveillance Evaluation (HASE) Study. Kidney Int Rep. 2020;5(11):1937-44
26. Schuman E, Ronfeld A, Barclay C, Heinl P. Comparison of clinical assessment with ultrasound flow for hemodialysis access surveillance. Arch Surg. 2007;142(12):1129-33
27. Ram SJ, Work J, Caldito GC, Eason JM, Pervez A, Paulson WD. A randomized controlled trial of blood flow and stenosis surveillance of hemodialysis grafts. Kidney international. 2003;64(1):272-80
28. Dember LM, Holmberg EF, Kaufman JS. Randomized controlled trial of prophylactic repair of hemodialysis arteriovenous graft stenosis. Kidney international. 2004;66(1):390-8
29. Lumsden AB, MacDonald MJ, Kikeri D, Cotsonis GA, Harker LA, Martin LG. Cost efficacy of duplex surveillance and prophylactic angioplasty of arteriovenous ePTFE grafts. Ann Vasc Surg. 1998;12(2):138-42
30. Tonelli M, James M, Wiebe N, Jindal K, Hemmelgarn B. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2008;51(4):630-40
31. Pockros BM, Finch DJ, Weiner DE. Dialysis and Total Health Care Costs in the United States and Worldwide: The Financial Impact of a Single-Payer Dominant System in the US. Journal of the American Society of Nephrology: JASN. 2021;32(9):2137-9
32. Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J, Allon M. The Clinical and Economic Effect of Vascular Access Selection in Patients Initiating Hemodialysis with a Catheter. Journal of the American Society of Nephrology: JASN. 2017;28(12):3679-87
Author contact
![]() Corresponding author: Szu-Chia Chen, MD, PhD, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, No. 482, Shanming Rd., Siaogang Dist., Kaohsiung City, Taiwan; Tel.: 886 7 8036783 #3441; E‑mail: scarchenonecom.tw.
Corresponding author: Szu-Chia Chen, MD, PhD, Department of Internal Medicine, Kaohsiung Municipal Siaogang Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan, No. 482, Shanming Rd., Siaogang Dist., Kaohsiung City, Taiwan; Tel.: 886 7 8036783 #3441; E‑mail: scarchenonecom.tw.

 Global reach, higher impact
Global reach, higher impact