3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(3):745-753. doi:10.7150/ijms.104622 This issue Cite
Research Paper
Unveiling the role of risk factors and predictive models in acute type-a aortic dissection surgery: OI downregulation and its association with immune disorders
1. Department of Vascular Diseases Intensive Care Unit, People's Hospital of Zhengzhou University, Zhengzhou University Central China Fuwai Hospital, Zhengzhou 450000, China.
2. Department of Vascular Surgery, People's Hospital of Zhengzhou University, Zhengzhou University Central China Fuwai Hospital, Zhengzhou 450000, China.
3. Department of Children's Heart Center, People's Hospital of Zhengzhou University, Zhengzhou University Central China Fuwai Hospital, Zhengzhou 450000, China.
# These authors have contributed equally to this work.
Received 2024-10-3; Accepted 2025-1-3; Published 2025-1-13
Abstract
Background and Objective: Acute type A aortic dissection (ATAAD) represents a critical and life-threatening condition requiring urgent surgical intervention, which is often life-saving. However, postoperative acute lung injury (ALI) has emerged as a prominent complication that significantly impacts patient outcomes and prognosis. This study aims to systematically analyze the risk factors associated with the development of severe ALI following ATAAD surgery, providing insights to improve postoperative management strategies.
Methods: A retrospective analysis was conducted using a comprehensive database comprising 483 patients diagnosed with ATAAD. Patients were stratified into two groups based on the severity of postoperative ALI: severe ALI group (n = 182) and non-severe ALI group (n = 301). Clinical data were systematically collected and compared between the two cohorts. Binary logistic regression analysis was employed to identify independent predictors of severe ALI following ATAAD surgery. The diagnostic accuracy of these risk factors was assessed using receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) serving as the metric for prognostic performance.
Results: The severe ALI group exhibited a higher prevalence of preoperative oxygenation index (OI) ≤ 200 mmHg, smoking history, and coronary artery disease compared to the non-severe ALI group (P < 0.001, P = 0.032, and P = 0.039, respectively), while the prevalence of Marfan syndrome was lower (P = 0.033). Moreover, significant differences were observed in several clinical and intraoperative parameters, including body mass index (BMI), C-reactive protein (CRP), procalcitonin (PCT), D-dimer, white blood cell count (WBC), aortic cross-clamp time, moderate hypothermic circulatory arrest (MHCA) time, cardiopulmonary bypass (CPB) duration, and ICU length of stay (all P < 0.05). Multivariate logistic regression identified preoperative OI [P = 0.008, OR (95% CI): 0.002 (0.000-0.183)], BMI [P = 0.037, OR (95% CI): 1.569 (1.027-2.397)], CRP [P = 0.022, OR (95% CI): 1.292 (1.037-1.609)], D-dimer [P < 0.001, OR (95% CI): 3.841 (1.820-8.108)], MHCA time [P = 0.001, OR (95% CI): 3.306 (1.670-6.544)], and CPB duration [P = 0.017, OR (95% CI): 1.117 (1.020-1.223)] as independent predictors of severe ALI. ROC curve analysis revealed the diagnostic performance of preoperative OI, BMI, CRP, D-dimer, MHCA time, and CPB duration, with AUC values of 0.715, 0.844, 0.871, 0.955, 0.944, and 0.833, respectively (all P < 0.001).
Conclusion: Preoperative oxygenation index, BMI, CRP, D-dimer levels, MHCA time, and CPB duration are independent risk factors for the development of severe ALI following ATAAD surgery. These findings underscore the importance of preoperative risk assessment and perioperative optimization to mitigate the risk of severe ALI and improve patient outcomes.
Keywords: Acute type A aortic dissection, Acute lung injury, Risk factors, Clinical factors, Predictive efficacy
1. Introduction
Aortic dissection (AD) is characterized by a disruption in the innermost layer of the aorta, resulting in blood entering the space between the intima and media to form a false lumen. The pressure-driven blood flow within the false lumen can extend the dissection along the longitudinal axis in an anterograde or retrograde direction, potentially causing rupture of the aorta. This process often establishes communication between the true and false lumens through one or more breaches[1, 2]. Based on the extent of aortic involvement, AD is classified into two categories: Stanford type A and Stanford type B. Stanford type A, involving the ascending aorta, accounts for approximately 60-70% of all cases [3, 4].
Acute type A aortic dissection (ATAAD) is one of the most severe and life-threatening cardiovascular emergencies, necessitating prompt surgical intervention to prevent fatal complications such as aortic rupture or multi-organ ischemia. The surgical repair of ATAAD, while often life-saving, remains a formidable challenge due to its inherent complexity[5, 6], prolonged cardiopulmonary bypass (CPB) times, and the need for deep hypothermic circulatory arrest (DHCA) to create a bloodless surgical field[7, 8]. Despite significant advancements in surgical techniques and perioperative care, the postoperative outcomes for ATAAD remain suboptimal[9-11], with acute lung injury (ALI) emerging as a frequent and serious complication [12-14].
ALI is a critical form of acute respiratory failure characterized by widespread pulmonary inflammation, increased vascular permeability, and impaired gas exchange, often resulting in intractable hypoxemia [15, 16]. In the context of ATAAD surgery, ALI can be triggered by several factors, including the systemic inflammatory response associated with CPB, ischemia-reperfusion injury during DHCA, and hemodynamic fluctuations inherent to the procedure. These pathophysiological insults collectively compromise the integrity of the alveolar-capillary membrane, leading to pulmonary edema and respiratory dysfunction. Reports indicate that ALI occurs in 30-50% of ATAAD surgical cases and is associated with prolonged ICU stays, higher morbidity, and increased mortality rates.
To date, limited research has systematically examined the risk factors contributing to the development of ALI in ATAAD patients. Preoperative oxygenation index (OI), body mass index (BMI), and biomarkers such as C-reactive protein (CRP) and D-dimer have been implicated as potential predictors in other forms of respiratory failure. However, their specific relevance in the context of ATAAD remains underexplored. Furthermore, intraoperative parameters, including CPB and DHCA durations, are known to exacerbate systemic inflammation and ischemia-reperfusion injury, potentially heightening the risk of ALI.
Understanding the interplay of these factors is essential for improving risk stratification and perioperative management. Early identification of high-risk patients could enable targeted interventions, such as optimizing preoperative lung function, mitigating inflammation, and refining intraoperative strategies, thereby reducing the incidence and severity of ALI. Moreover, a comprehensive evaluation of these risk factors could guide the development of predictive models to support clinical decision-making and improve outcomes.
This study aims to bridge these knowledge gaps by systematically analyzing clinical, biochemical, and intraoperative variables associated with ALI in ATAAD surgery. Using a robust retrospective dataset and multivariate statistical modeling, this research seeks to identify independent risk factors and assess their diagnostic value through receiver operating characteristic (ROC) curve analysis. These findings will not only contribute to the understanding of ALI pathogenesis but also offer practical insights for improving perioperative care and prognosis in ATAAD patients.
2. Methods
2.1 Inclusion criteria
The inclusion criteria for this study required patients to have a confirmed diagnosis of acute type A aortic dissection (ATAAD) using advanced imaging techniques such as computed tomographic angiography (CTA), magnetic resonance angiography (MRA), or transesophageal echocardiography (TEE), with hallmark imaging features including intimal tears and false lumen formation[17]. Furthermore, all cases adhered to the established diagnostic criteria for ATAAD and presented within 14 days of symptom onset, aligning with the recognized temporal definition of acute dissection. Eligible patients underwent surgical intervention for ATAAD, and to ensure comprehensive analysis, complete preoperative clinical records and postoperative monitoring data were required for inclusion. This meticulous selection process ensured a robust dataset for subsequent analyses [18].
2.2 Exclusion criteria
Exclusion criteria were established to maintain homogeneity within the study cohort. Patients with severe comorbid conditions, such as advanced pulmonary disease characterized by FEV₁/FVC < 0.5, were excluded, as were those with end-stage renal disease necessitating long-term dialysis, given the associated risks of metabolic derangements and fluid imbalance. Additionally, cases involving advanced hepatic failure (Child-Pugh grade C) were excluded due to significant impairments in coagulation, detoxification, and metabolic functions. Patients with preexisting advanced malignancies were also excluded, as their fragile physiological state, exacerbated by oncological therapies, could confound outcomes. Furthermore, those with unrelated severe congenital heart diseases, traumatic or iatrogenic aortic dissections, or significant intraoperative complications—such as uncontrollable hemorrhage or prolonged cardiac arrest—were excluded to ensure that the results specifically reflected the natural history and outcomes of ATAAD surgery [19, 20].
2.3 Grouping
To stratify patients based on postoperative complications, a retrospective review was conducted on 483 cases of acute lung injury (ALI) following surgical repair of ATAAD, performed at our institution between January 2020 and December 2023. Severe ALI was defined as an oxygenation index (OI) ≤ 100 mmHg within the first 72 hours postoperatively. The study population was divided into two groups: those who developed severe ALI (n = 182) and those who did not (n = 301). This stratification facilitated a focused investigation into risk factors associated with severe ALI, allowing for detailed comparisons between the two groups [21, 22].
2.4 Clinical information collection
Clinical data were comprehensively collected and included information on demographic characteristics, preoperative medical history, laboratory findings, and intraoperative parameters. Specific variables of interest included cardiopulmonary bypass (CPB) duration, moderate hypothermic circulatory arrest (MHCA) time, and aortic cross-clamp time, as well as biomarkers of inflammation and coagulation such as C-reactive protein (CRP), procalcitonin (PCT), D-dimer, and white blood cell (WBC) counts. This systematic data collection ensured a robust dataset for statistical analysis [23, 24].
2.5 Statistical methods
Statistical analyses were performed to identify meaningful associations and predictors of severe ALI. Normally distributed continuous variables were expressed as mean ± standard deviation and compared between groups using two-sample t-tests. To identify independent predictors of severe ALI, multivariate logistic regression analysis was employed, with results reported as odds ratios (ORs) and 95% confidence intervals (CIs). The diagnostic performance of these predictors was further evaluated using receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) providing a measure of predictive accuracy[25]. A significance level of p < 0.05 was applied throughout the analysis to ensure robust statistical validity. These comprehensive methods were designed to provide a detailed understanding of the factors contributing to severe ALI in ATAAD patients, with the ultimate goal of informing strategies to improve clinical outcomes [26, 27].
3. Results
3.1 Comparisons of Clinical Characteristics Between Severe and Non-Severe ALI Groups
The comparison of clinical characteristics between patients with and without severe acute lung injury (ALI) revealed notable distinctions. No significant differences were observed in demographic variables such as age, gender, or in the prevalence of diabetes and hypertension between the severe ALI and non-severe ALI groups. However, the severe ALI group exhibited significantly higher proportions of patients with a preoperative oxygenation index (OI) ≤ 200 mmHg, smoking history, and coronary artery disease compared to their non-severe counterparts (P < 0.001, P = 0.032, and P = 0.039, respectively). In contrast, the prevalence of Marfan syndrome was notably lower in the severe ALI group (P = 0.033). Furthermore, elevated levels of body mass index (BMI), C-reactive protein (CRP), procalcitonin (PCT), D-dimer, and white blood cell count (WBC) were observed in the severe ALI group compared to the non-severe group (all P < 0.001, except PCT with P = 0.043). Additionally, key surgical parameters, including aortic cross-clamp duration, moderate hypothermic circulatory arrest (MHCA) time, cardiopulmonary bypass (CPB) duration, and length of ICU stay, were significantly longer in the severe ALI group (P < 0.01 for all comparisons) (Table 1).
3.2 Multivariate Logistic Regression Analysis of Risk Factors for Severe ALI
To identify predictors of severe ALI following ATAAD repair, a multivariate logistic regression model was constructed. Initially, univariate analyses were performed to evaluate the relationship between each variable and severe ALI. This preliminary analysis identified several potential risk factors, including preoperative OI, BMI, smoking history, coronary artery disease, Marfan syndrome, CRP, PCT, D-dimer, WBC count, aortic cross-clamp time, MHCA duration, and CPB time (Table 2). These candidate variables were subsequently analyzed using multivariate logistic regression to ascertain their independent contributions to severe ALI risk. The results demonstrated that preoperative oxygenation index was a significant independent predictor of severe ALI [P = 0.008, OR (95% CI): 0.002 (0.000-0.183)]. Similarly, BMI [P = 0.037, OR (95% CI): 1.569 (1.027-2.397)], CRP [P = 0.022, OR (95% CI): 1.292 (1.037-1.609)], D-dimer [P < 0.001, OR (95% CI): 3.841 (1.820-8.108)], MHCA time [P = 0.001, OR (95% CI): 3.306 (1.670-6.544)], and CPB duration [P = 0.017, OR (95% CI): 1.117 (1.020-1.223)] were identified as significant independent predictors (Table 3).
Differences in clinical data between the severe ALI and non-severe ALI groups
| Metric | Severe ALI group (n=182) | Non-severe ALI group (n=301) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 45.96 ± 4.63 | 46.17 ± 4.55 | 0.488 | 0.626 |
| Gender, n (%) | 0.256 | 0.613 | ||
| Male | 90 (49.45%) | 156 (51.83%) | ||
| Female | 92 (50.55%) | 145 (48.17%) | ||
| Preoperative OI, n (%) | 83.595 | <0.001 | ||
| ≤200 mmHg | 141 (77.5%) | 104 (33.9%) | ||
| >200 mmHg | 41 (22.5%) | 197 (66.1%) | ||
| BMI, kg/m2 | 26.43 ± 3.49 | 21.93 ± 2.59 | 16.186 | <0.001 |
| Smoking history, n (%) | 4.619 | 0.032 | ||
| Yes | 82 (45.1%) | 106 (35.2%) | ||
| No | 100 (54.9%) | 195 (64.8%) | ||
| Coronary heart disease, n (%) | 4.267 | 0.039 | ||
| Yes | 32 (17.6%) | 33 (11.0%) | ||
| No | 150 (82.4%) | 268 (89.0%) | ||
| Diabetes, n (%) | 0.325 | 0.596 | ||
| Yes | 50 (27.47%) | 90 (29.90%) | ||
| No | 132 (72.53%) | 211 (70.10%) | ||
| Hypertension, n (%) | 0.006 | 0.936 | ||
| Yes | 35 (19.2%) | 57 (18.9%) | ||
| No | 147 (80.8%) | 244 (81.1%) | ||
| Marfan syndrome, n (%) | 4.571 | 0.033 | ||
| Yes | 3 (1.6%) | 17 (5.6%) | ||
| No | 179 (98.4%) | 284 (94.4%) | ||
| C-Reactive protein (mg/L) | 37.64 ± 7.95 | 26.31 ± 6.12 | 17.574 | <0.001 |
| PCT (ng/L) | 10.36 ± 2.26 | 9.86 ± 2.82 | 2.030 | 0.043 |
| D-dimer (mg/L) | 12.47 ± 2.59 | 7.62 ±1.21 | 27.859 | <0.001 |
| WBC (109/L) | 12.98 ± 2.75 | 10.87 ± 2.63 | 8.398 | <0.001 |
| Aortic cross clamp duration (min) | 85.52 ± 7.60 | 83.18 ± 8.14 | 3.138 | 0.002 |
| MHCA time (min) | 33 (30, 35) | 23 (21, 26) | 16.371 | <0.001 |
| CPB duration (min) | 200.62 ± 14.76 | 181.15 ± 13.70 | 14.697 | <0.001 |
| Length of ICU (h) | 216.61 ± 24.33 | 96.76 ± 18.35 | 61.356 | <0.001 |
| Length of hospitalization (day) | 19 (17, 20) | 18 (15, 20) | 1.917 | 0.055 |
ALI: acute lung injury; OI: oxygenation index; BMI: Body mass index; PCT: Procalcitonin; WBC: White blood cell count; CPB: cardiopulmonary bypass; MHCA: moderate hypothermic circulatory arrest
Univariate logistic regression analysis
| Factor | Univariate logistic regression analysis | ||||
|---|---|---|---|---|---|
| B | S.E. | Wald | P | OR (95%CI) | |
| Preoperative OI | 1.874 | 0.215 | 76.058 | <0.001 | 6.514 (4.275-9.926) |
| BMI | 0.444 | 0.041 | 118.556 | <0.001 | 1.559 (1.439-1.689) |
| Smoking history | 0.411 | 0.192 | 4.598 | 0.032 | 0.663 (0.455-0.965) |
| Coronary heart disease | 0.550 | 0.268 | 4.198 | 0.040 | 0.577 (0.341-0.976) |
| Marfan syndrome | 1.273 | 0.633 | 4.039 | 0.044 | 3.572 (1.032-12.361) |
| C-Reactive protein | 0.233 | 0.021 | 118.158 | <0.001 | 1.262 (1.210-1.316) |
| PCT | 0.073 | 0.036 | 4.071 | 0.044 | 1.076 (1.002-1.155) |
| D-dimer | 1.401 | 0.138 | 103.798 | <0.001 | 4.060 (3.101-5.316) |
| WBC | 0.294 | 0.040 | 54.162 | <0.001 | 1.342 (1.241-1.452) |
| Aortic cross clamp duration | 0.037 | 0.012 | 9.495 | 0.002 | 1.038 (1.014-1.063) |
| MHCA time | 0.564 | 0.051 | 121.297 | <0.001 | 1.758 (1.590-1.943) |
| CPB duration | 0.098 | 0.009 | 106.584 | <0.001 | 1.103 (1.083-1.124) |
| Length of ICU | 1.020 | 1.189 | 0.736 | 0.391 | 2.774 (0.270-28.531) |
B: Beta coefficient; S.E.: Standard Error; OR: Odds Ratio; CI: Confidence Interval
Multivariate logistic regression analysis
| Factor | Multivariate logistic regression analysis | ||||
|---|---|---|---|---|---|
| B | S.E. | Wald | P | OR (95%CI) | |
| Preoperative OI | 6.484 | 2.443 | 7.043 | 0.008 | 0.002 (0.000-0.183) |
| BMI | 0.450 | 0.216 | 4.332 | 0.037 | 1.569 (1.027-2.397) |
| Smoking history | 0.432 | 1.176 | 0.135 | 0.713 | 0.649 (0.065-6.510) |
| Coronary heart disease | 0.596 | 2.809 | 0.045 | 0.832 | 0.551 (0.002-135.452) |
| Marfan syndrome | 1.579 | 5.445 | 0.084 | 0.772 | 4.852 (0.00-8887.211) |
| C-Reactive protein | 0.256 | 0.112 | 5.213 | 0.022 | 1.292 (1.037-1.609) |
| PCT | 0.009 | 0.245 | 0.001 | 0.971 | 1.009 (0.624-1.630) |
| D-dimer | 1.346 | 0.381 | 12.468 | <0.001 | 3.841 (1.820-8.108) |
| WBC | 0.188 | 0.261 | 0.521 | 0.471 | 1.207 (0.724-2.011) |
| Aortic cross clamp duration | 0.186 | 0.101 | 3.436 | 0.064 | 1.205 (0.989-1.467) |
| MHCA time | 1.196 | 0.348 | 11.783 | 0.001 | 3.306 (1.670-6.544) |
| CPB duration | 0.110 | 0.046 | 5.662 | 0.017 | 1.117 (1.020-1.223) |
B: Beta coefficient; S.E.: Standard Error; OR: Odds Ratio; CI: Confidence Interval
ROC analysis for severe ALI after ATAAD surgery
| Variable | AUC | P value | 95% CI |
|---|---|---|---|
| Preoperative OI | 0.715 | <0.001 | 0.667-0.762 |
| BMI | 0.844 | <0.001 | 0.806-0.882 |
| C-Reactive protein | 0.871 | <0.001 | 0.837-0.905 |
| D-dimer | 0.955 | <0.001 | 0.933-0.977 |
| MHCA time | 0.944 | <0.001 | 0.921-0.966 |
| CPB duration | 0.833 | <0.001 | 0.796-0.870 |
AUC: Area under the curve; CI: Confidence Interval
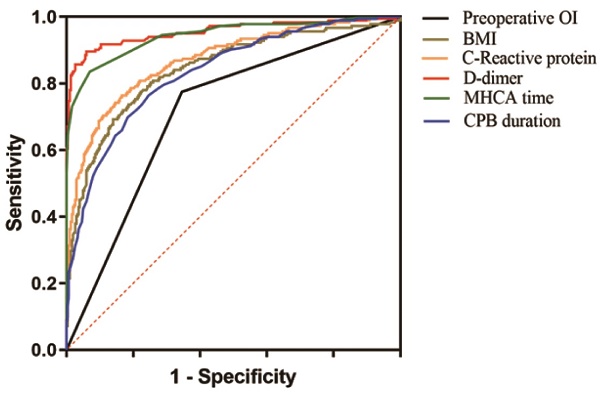
ROC analysis for severe ALI after ATAAD surgery.
3.3 Diagnostic Accuracy of Predictive Variables for Severe ALI
The diagnostic performance of the predictive variables for severe ALI was evaluated through receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC) values indicated that the predictive models based on preoperative oxygenation index, BMI, CRP, D-dimer, MHCA duration, and CPB time demonstrated excellent diagnostic capabilities, with AUC values of 0.715, 0.844, 0.871, 0.955, 0.944, and 0.833, respectively (all P < 0.001). These findings highlight the robustness of the identified predictors in assessing the likelihood of severe ALI following ATAAD surgery (Table 4 and Figure 1).
4. Discussion
The underlying mechanisms of acute lung injury (ALI) following acute type A aortic dissection (ATAAD) surgery are highly complex, with cardiopulmonary bypass (CPB) playing a pivotal role in its pathogenesis [28, 29]. During CPB, a systemic inflammatory response is triggered, accompanied by complement system activation. This cascade produces anaphylatoxins that attract neutrophils to the lungs, where they release pro-inflammatory mediators such as reactive oxygen species, proteolytic enzymes, and cytokines [30, 31]. These substances compromise the integrity of the alveolar-capillary membrane, leading to increased permeability and subsequent lung injury [32, 33]. Additionally, ischemia-reperfusion injury (IRI) exacerbates this damage. The ischemic state of the lungs during CPB disrupts energy metabolism, and the subsequent reperfusion generates large amounts of oxygen free radicals, which attack cellular membranes and aggravate alveolar damage. Beyond the surgical process itself, postoperative factors also contribute to pulmonary damage [34, 35]. For instance, a sharp rise in cardiac afterload during surgery increases left ventricular end-diastolic pressure and worsens pulmonary congestion [36, 37]. Following aortic opening, hemodynamic fluctuations, hypotension, and reperfusion further aggravate the release of inflammatory mediators and microemboli, leading to pulmonary microvascular embolism and an inflammatory cascade [38].
Our findings identified preoperative oxygenation index (OI), body mass index (BMI), C-reactive protein (CRP), D-dimer levels, CPB duration, and moderate hypothermic circulatory arrest (MHCA) time as independent risk factors for ALI [39, 40]. The mechanisms underlying these associations are multifaceted. Higher BMI reflects increased adipose tissue, which secretes inflammatory cytokines and mediators, predisposing obese patients to heightened inflammatory responses post-surgery [41, 42]. This intensified inflammation damages vascular endothelial cells, increasing vascular permeability and promoting alveolar fluid leakage, which impairs gas exchange and elevates the risk of ALI [43, 44]. Furthermore, poor thoracic compliance in obese patients limits respiratory function, compounding the difficulty of postoperative recovery and elevating ALI susceptibility [45, 46].
Elevated CRP levels, an acute-phase reactant, signify a robust inflammatory response. In the context of ATAAD, CRP >33.4 mg/L indicates systemic inflammation induced by surgical trauma, the implantation of artificial grafts, and postoperative stress responses [47]. CRP activates the complement system, which recruits neutrophils to the lungs. The neutrophils release proteases and reactive oxygen species, damaging alveolar epithelial and pulmonary vascular endothelial cells [48]. This increases alveolar-capillary permeability, leading to pulmonary edema and ALI. Similarly, elevated D-dimer levels (>10,109 ng/mL) reflect hyperactivation of the coagulation and fibrinolytic systems [49, 50]. The dissection process itself exposes subendothelial collagen, triggering coagulation. Persistent hypercoagulable states promote microthrombus formation in pulmonary vasculature, causing localized ischemia and hypoxia [51-53]. Concurrently, fibrinolytic system activation produces degradation products that damage lung tissue and perpetuate inflammation, further increasing vascular permeability and exacerbating ALI [54-57].
Preoperative OI serves as a critical indicator of baseline pulmonary function, and poor preoperative lung function, potentially due to underlying chronic pulmonary diseases or cardiac insufficiency, increases the risk of postoperative ALI. Reduced lung reserve impairs the ability to tolerate surgical trauma, CPB, and other intraoperative stressors, making the lungs more vulnerable to alveolar collapse, pulmonary edema, and subsequent ALI. Additional perioperative factors, such as anesthetic drugs and CPB-induced hemodilution, may further compromise lung function [58-61].
Prolonged CPB time (>5.69 hours) significantly increases the risk of ALI due to prolonged exposure of blood to artificial surfaces, which activates leukocytes and platelets, triggering the release of inflammatory cytokines like interleukin-1 and interleukin-6. These inflammatory mediators damage pulmonary vascular endothelial cells, increasing vascular permeability [62]. Moreover, extended CPB time exacerbates ischemia-reperfusion injury, as ischemic lung tissue generates oxygen free radicals during reperfusion, which attack the alveolar and vascular endothelial cells, causing structural damage [63]. Similarly, extended MHCA time disrupts pulmonary vascular regulation, increasing vascular permeability and promoting the leakage of fluid into the alveoli and interstitium, thereby impairing gas exchange and heightening ALI risk [64]. In conclusion, the interplay between preoperative pulmonary function, systemic inflammation, coagulation abnormalities, and intraoperative factors such as CPB and MHCA significantly contributes to the development of ALI following ATAAD surgery. These findings underscore the importance of optimizing preoperative and perioperative strategies to mitigate risk factors and improve postoperative outcomes.
Despite the significant findings, this study has several limitations that must be acknowledged. First, the retrospective nature of the analysis may introduce inherent biases in data collection and interpretation. Although the inclusion and exclusion criteria were strictly defined, unmeasured confounding factors might still influence the results. Second, the study was conducted at a single institution, which may limit the generalizability of the findings to other populations and healthcare settings. Moreover, some variables, such as preoperative pulmonary function and inflammatory markers, were not dynamically monitored, which could have provided additional insights into the progression of acute lung injury (ALI). Lastly, while we identified independent risk factors for ALI, causality cannot be definitively established due to the observational design of the study.
Building on the findings of this study, several avenues for future research are evident. Prospective, multicenter studies with larger sample sizes are warranted to validate the identified risk factors and enhance the generalizability of the results. Furthermore, the inclusion of serial measurements of biomarkers, such as inflammatory cytokines and oxidative stress markers, could provide a more comprehensive understanding of the pathophysiological mechanisms underlying ALI. Investigating the role of preoperative interventions, such as optimized respiratory therapy or targeted anti-inflammatory treatments, may also yield strategies to mitigate ALI risk. Additionally, advanced imaging techniques and machine learning models could be employed to develop predictive algorithms, enabling early identification of high-risk patients and facilitating personalized perioperative management.
This study offers a novel contribution to the understanding of ALI in the context of acute type A aortic dissection (ATAAD) surgery. By systematically identifying preoperative oxygenation index, body mass index, C-reactive protein, D-dimer levels, CPB duration, and MHCA time as independent predictors of ALI, this research provides actionable insights that could inform perioperative decision-making. The use of receiver operating characteristic (ROC) curve analysis further underscores the predictive value of these factors, offering a practical framework for risk stratification. Importantly, the study highlights the interplay between inflammatory responses, coagulation abnormalities, and mechanical factors in ALI pathogenesis, advancing the current understanding of this multifaceted complication. These findings not only contribute to the academic literature but also hold significant clinical implications for improving patient outcomes in ATAAD surgery.
This study identified preoperative oxygenation index (OI), body mass index (BMI), C-reactive protein (CRP), D-dimer levels, moderate hypothermic circulatory arrest (MHCA) time, and cardiopulmonary bypass (CPB) duration as independent risk factors for severe acute lung injury (ALI) following ATAAD surgery. Specifically, a preoperative OI ≤ 200 mmHg, elevated BMI, increased CRP levels, and high D-dimer concentrations were associated with a significantly elevated risk of developing severe ALI. Conversely, shorter MHCA and CPB durations were found to mitigate the likelihood of severe ALI. These findings highlight the potential diagnostic and therapeutic value of these risk factors in predicting and managing severe ALI in patients undergoing ATAAD surgery, offering valuable insights for improving patient outcomes through targeted perioperative strategies.
Acknowledgements
Funding
This study was supported by the Medical Science and Technology Research Project of Henan Province, China (Grant No. LHGJ20220110).
Ethics statement
The research protocol was reviewed and approved by the institutional ethics committee, ensuring adherence to ethical guidelines. All participants provided written informed consent prior to their inclusion in the study. The procedures conducted in this research comply fully with the principles outlined in the Declaration of Helsinki.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Biancari F, Juvonen T, Fiore A, Perrotti A, Hervé A, Touma J. et al. Current Outcome after Surgery for Type A Aortic Dissection. Ann Surg. 2023;278:e885-e92
2. Zhou J, Chen S, Liu J, Du J, Li J. Knockdown of hnRNPAB reduces the stem cell properties and enhances the chemosensitivity of human colorectal cancer stem cells. Oncol Rep. 2023;49:129
3. Sherk WM, Khaja MS, Williams DM. Anatomy, Pathology, and Classification of Aortic Dissection. Tech Vasc Interv Radiol. 2021;24:100746
4. Zhao B, Li M, Su Y, Shan S, Qian W, Zhu D. et al. Role of transcription factor FOXM1 in diabetes and its complications (Review). Int J Mol Med. 2023;52:101
5. Rylski B, Schilling O, Czerny M. Acute aortic dissection: evidence, uncertainties, and future therapies. Eur Heart J. 2023;44:813-21
6. Zhang Z, Ni P, Tang M, Song Y, Liu C, Zhao B. Dapagliflozin alleviates renal podocyte pyroptosis via regulation of the HO-1/NLRP3 axis. Mol Med Rep. 2023;28:200
7. Wang TKM, Wei D, Evans T, Ramanathan T, Haydock D. Surgery for Type A Aortic Dissection: 14-Year Contemporary Cohort Study. Heart Lung Circ. 2020;29:1210-6
8. Zhang Y, Zhang Y, Liu S, Li B, Song Z, Han Q. et al. Acupuncture for cancer pain: a scoping review of systematic reviews and meta-analyses. Front Oncol. 2023;13:1169458
9. Guan XL, Li L, Li HY, Gong M, Zhang HJ, Wang XL. Risk factor prediction of severe postoperative acute kidney injury at stage 3 in patients with acute type A aortic dissection using thromboelastography. Front Cardiovasc Med. 2023;10:1109620
10. Zhang L, Jiang B, Zhu N, Tao M, Jun Y, Chen X. et al. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCalpha/ERK1/2 and PI3K/Akt pathway. Med Oncol. 2019;37:5
11. Xiong T, Xia L, Song Q. Circular RNA SPI1 expression before and after induction therapy and its correlation with clinical features, treatment response, and survival of acute myeloid leukemia patients. J Clin Lab Anal. 2023;37:e24835
12. Wang Q, Feng W, Kuang J, Wu J, Yang J, Li C. et al. Prediction model for postoperative severe acute lung injury in patients undergoing acute type A aortic dissection surgery. J Card Surg. 2022;37:1602-10
13. Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W. et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752-69
14. Yang Y, Wu Q, Pan W, Wen L, Luo Z, Wu H. et al. Characteristics of the Ocular Surface in Myopic Child Candidates of Orthokeratology Lens Wear. Ophthalmol Ther. 2023;12:3067-79
15. Xu S, Wu Z, Liu Y, Zhu J, Gong M, Sun L. et al. Influence of Preoperative Serum Albumin on Acute Kidney Injury after Aortic Surgery for Acute Type A Aortic Dissection: A Retrospective Cohort Study. J Clin Med. 2023;12:1581
16. Wen L, Cao Y, Cheng Q, Li X, Pan L, Li L. et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol. 2020;104:1542-7
17. Malaisrie SC, Szeto WY, Halas M, Girardi LN, Coselli JS, Sundt TM 3rd. et al. 2021 The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162:735-58.e2
18. Wang W, Zhai T, Luo P, Miao X, Wang J, Chen Y. Beneficial effects of silibinin on serum lipids, bile acids, and gut microbiota in methionine-choline-deficient diet-induced mice. Front Nutr. 2023;10:1257158
19. Su H, Geng H, Cai L, Xu M, Xing W, Long W. et al. Immune-check blocking combination multiple cytokines shown curative potential in mice tumor model. Cancer Med. 2023;12:13573-85
20. Shao Y, Zhao T, Zhang W, He J, Lu F, Cai Y. et al. Presence of the apolipoprotein E-epsilon4 allele is associated with an increased risk of sepsis progression. Sci Rep. 2020;10:15735
21. Peng Y, Yan H, Mei W, Zhang P, Zeng C. Combining Radiotherapy with Immunotherapy in Cervical Cancer: Where Do We Stand and Where Are We Going? Curr Treat Options Oncol. 2023;24:1378-91
22. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128
23. Lu Y, Lin Z, Wen L, Gao W, Pan L, Li X. et al. The Adaptation and Acceptance of Defocus Incorporated Multiple Segment Lens for Chinese Children. Am J Ophthalmol. 2020;211:207-16
24. Liu Y, Shen D, Wang HY, Qi MY, Zeng QY. Development and validation to predict visual acuity and keratometry two years after corneal crosslinking with progressive keratoconus by machine learning. Front Med (Lausanne). 2023;10:1146529
25. Li J, Liang X, Wang X, Yang P, Jian X, Fu L. et al. A missense GDF5 variant causes brachydactyly type A1 and multiple-synostoses syndrome 2. JOR Spine. 2024;7:e1302
26. Liu J, Lu Y, Huang D, Yang J, Fan C, Chen C. et al. The Efficacy of Defocus Incorporated Multiple Segments Lenses in Slowing Myopia Progression: Results from Diverse Clinical Circumstances. Ophthalmology. 2023;130:542-50
27. Lin L, Wu Q, Lu F, Lei J, Zhou Y, Liu Y. et al. Nrf2 signaling pathway: current status and potential therapeutic targetable role in human cancers. Front Oncol. 2023;13:1184079
28. Fan L, Meng K, Meng F, Wu Y, Lin L. Metabolomic characterization benefits the identification of acute lung injury in patients with type A acute aortic dissection. Front Mol Biosci. 2023;10:1222133
29. Li H, Shi W, Shen T, Hui S, Hou M, Wei Z. et al. Network pharmacology-based strategy for predicting therapy targets of Ecliptae Herba on breast cancer. Medicine (Baltimore). 2023;102:e35384
30. Huang Z, Yu P, Tang J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020;13:5395-405
31. Gao WL, Li XH, Dun XP, Jing XK, Yang K, Li YK. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr Med Sci. 2020;40:434-43
32. Zhou Y, Li X, Chen H, Zhong X, Ren H. Efficacy and safety of sivelestat sodium for the treatment of inflammatory response in acute Stanford type A aortic dissection: a retrospective cohort study. J Thorac Dis. 2022;14:3975-82
33. Jiang L, Chen T, Xiong L, Xu JH, Gong AY, Dai B. et al. Knockdown of m6A methyltransferase METTL3 in gastric cancer cells results in suppression of cell proliferation. Oncol Lett. 2020;20:2191-8
34. Gao P, Rao ZW, Li M, Sun XY, Gao QY, Shang TZ. et al. Tetrandrine Represses Inflammation and Attenuates Osteoarthritis by Selective Inhibition of COX-2. Curr Med Sci. 2023;43:505-13
35. Dong H, Li H, Wang L, Yuan Y, Zhang D, Zhou L. et al. Clinical analysis of 175 cases of vaginal intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2023;287:232-6
36. Jiao R, Liu M, Lu X, Zhu J, Sun L, Liu N. A nomogram for reduced cardiac function in postoperative acute type A aortic dissection patients with acute kidney injury undergoing continuous renal replacement therapy. Front Cardiovasc Med. 2022;9:874715
37. Ding L, Lu S, Zhou Y, Lyu D, Ouyang C, Ma Z. et al. The 3' Untranslated Region Protects the Heart from Angiotensin II-Induced Cardiac Dysfunction via AGGF1 Expression. Mol Ther. 2020;28:1119-32
38. Cuny H, Bozon K, Kirk RB, Sheng DZ, Broer S, Dunwoodie SL. Maternal heterozygosity of Slc6a19 causes metabolic perturbation and congenital NAD deficiency disorder in mice. Dis Model Mech. 2023;16:dmm049647
39. Jo Y, Anzai T, Sugano Y, Naito K, Ueno K, Kohno T. et al. Early use of beta-blockers attenuates systemic inflammatory response and lung oxygenation impairment after distal type acute aortic dissection. Heart Vessels. 2008;23:334-40
40. Dang X, Fan C, Cui F, He Y, Sun G, Ruan J. et al. Interactions between ultrasonographic cervical length and placenta accreta spectrum on severe postpartum hemorrhage in women with placenta previa. Int J Gynaecol Obstet. 2023;161:1069-74
41. Jin M, Cheng Y, Yang Y, Pan X, Lu J, Cheng W. Protection of xenon against postoperative oxygen impairment in adults undergoing Stanford Type-A acute aortic dissection surgery: Study protocol for a prospective, randomized controlled clinical trial. Medicine (Baltimore). 2017;96:e7857
42. Chen Z, Jin M, He H, Dong J, Li J, Nie J. et al. Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Translat. 2023;39:63-73
43. Chen L, Tian Q, Shi Z, Qiu Y, Lu Q, Liu C. Melatonin Alleviates Cardiac Function in Sepsis-Caused Myocarditis via Maintenance of Mitochondrial Function. Front Nutr. 2021;8:754235
44. Cao Y, Lan W, Wen L, Li X, Pan L, Wang X. et al. An effectiveness study of a wearable device (Clouclip) intervention in unhealthy visual behaviors among school-age children: A pilot study. Medicine (Baltimore). 2020;99:e17992
45. Xia L, Liu Y, Yang Z, Ge Y, Wang L, Du Y. et al. Obesity and acute type A aortic dissection: unraveling surgical outcomes through the lens of the upper hemisternotomy approach. Front Cardiovasc Med. 2024;11:1301895
46. Chen L, Zhan CZ, Wang T, You H, Yao R. Curcumin Inhibits the Proliferation, Migration, Invasion, and Apoptosis of Diffuse Large B-Cell Lymphoma Cell Line by Regulating MiR-21/VHL Axis. Yonsei Med J. 2020;61:20-9
47. Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-96
48. Zhao G, Wang L, Sandeep B. A review regarding the article 'Impact of previous cardiac operations in patients undergoing surgery for type A acute aortic dissection. Long-term follow up. '. Curr Probl Cardiol. 2024;49:102242
49. Xu CE, Zou CW, Zhang MY, Guo L. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2013;27:479-84
50. Chen Q, Mo R, Wu N, Zou X, Shi C, Gong J. et al. Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front Pharmacol. 2017;8:334
51. Ma P, Wu Y, Zeng Q, Gan Y, Chen J, Ye X. et al. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem Toxicol. 2013;58:177-83
52. Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z. et al. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065-76
53. Zhu ZY, Liu YD, Gong Y, Jin W, Topchiy E, Turdi S. et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer's disease via inhibition of ACSL4-dependent ferroptosis. Acta Pharmacol Sin. 2022;43:39-49
54. Zhao X, Bie M. Preoperative acute lung injury and oxygenation impairment occurred in the patients with acute aortic dissection. BMC Cardiovasc Disord. 2022;22:129
55. Feng MG, Liu CF, Chen L, Feng WB, Liu M, Hai H. et al. MiR-21 attenuates apoptosis-triggered by amyloid-β via modulating PDCD4/ PI3K/AKT/GSK-3β pathway in SH-SY5Y cells. Biomed Pharmacother. 2018;101:1003-7
56. Ma P, Luo Q, Chen J, Gan Y, Du J, Ding S. et al. Intraperitoneal injection of magnetic Fe₃O₄-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int J Nanomedicine. 2012;7:4809-18
57. Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li Z. et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017;24:1045-55
58. Guo S, Meng XW, Yang XS, Liu XF, Ou-Yang CH, Liu C. Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. Acta Pharmacol Sin. 2018;39:195-204
59. Li H, Wang X, Liu Y, Pan D, Wang Y, Yang N. et al. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem Toxicol. 2017;108:407-18
60. Liang T, Zhang Y, Wu S, Chen Q, Wang L. The Role of NLRP3 Inflammasome in Alzheimer's Disease and Potential Therapeutic Targets. Front Pharmacol. 2022;13:845185
61. Ding J, Tang Q, Luo B, Zhang L, Lin L, Han L. et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur J Pharmacol. 2019;859:172549
62. Pan X, Lu J, Cheng W, Yang Y, Zhu J, Jin M. Independent factors related to preoperative acute lung injury in 130 adults undergoing Stanford type-A acute aortic dissection surgery: a single-center cross-sectional clinical study. J Thorac Dis. 2018;10:4413-23
63. Gao Z, Pei X, He C, Wang Y, Lu J, Jin M. et al. Oxygenation impairment in patients with acute aortic dissection is associated with disorders of coagulation and fibrinolysis: a prospective observational study. J Thorac Dis. 2019;11:1190-201
64. Fu R, Cheng Z. Letter to the Editor: Prediction model for postoperative severe acute lung injury in patients undergoing acute type A aortic dissection surgery. J Card Surg. 2022;37:2486
Author contact
![]() Corresponding author: Taibing Fan, Department of Children's Heart Center, People's Hospital of Zhengzhou University, Zhengzhou University Central China Fuwai Hospital, Zhengzhou, China. E-mail: fantaibingvicucom.
Corresponding author: Taibing Fan, Department of Children's Heart Center, People's Hospital of Zhengzhou University, Zhengzhou University Central China Fuwai Hospital, Zhengzhou, China. E-mail: fantaibingvicucom.

 Global reach, higher impact
Global reach, higher impact