3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(3):708-715. doi:10.7150/ijms.103916 This issue Cite
Research Paper
Spinal Metastasis Pain Surveillance: A Comprehensive Imaging-Based Tool Design for Evaluating Metastatic Burden and Guiding Therapeutic Strategies
1. Department of Breast Surgery, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, China.
2. Department of Breast and Thyroid Surgery, Peking University ShenZhen Hospital, China.
3. Tongxinling Community Health Service Center, Futian District, The 8 th affiliated hospital of Sun yet-sen University, China.
4. Department of Imaging, Zhengzhou University People's Hospital (Henan Provincial People's Hospital), Zhengzhou, Zhengzhou, China.
5. Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, 100053, China.
6. Department of Hepato-Biliary-Pancreatic Surgery, Henan Provincial People's Hospital (People's Hospital of Zhengzhou University), Zhengzhou, China.
# These authors contributed equally to this article.
Received 2024-9-19; Accepted 2025-1-3; Published 2025-1-13
Abstract
Background: The current research aims to elucidate the interplay between the anatomical distribution of spinal metastases, MRI features, and the intensity of bone pain in patients with breast cancer.
Methods: A retrospective analysis was used on a cohort of 45 breast cancer patients with verified spinal metastases, examining the relationship between metastatic locations, MRI-derived metrics, and bone pain scores. The Visual Analogue Scale (VAS) was conducted to measure the severity of bone pain.
Results: The results revealed a significant association between lumbar spine metastases and elevated pain scores, outpacing those observed in thoracic and cervical regions. Furthermore, a strong correlation was found between the multiplicity of metastatic sites and the ratio of high-intensity areas on MRI, both of which were predictive of increased pain severity.
Conclusions: The study's outcomes indicate that distinct MRI profiles, including the number and location of spinal metastases, can serve as prognostic indicators of bone pain intensity in breast cancer patients. Our data highlighted the need for personalized pain management strategies and targeted interventions tailored to specific imaging characteristics. Ultimately, this research underscores the dual role of MRI in both detecting spinal metastases and informing symptom management, with the potential to augment the overall well-being of breast cancer patients with spinal involvement.
Keywords: Spine metastases, Bone pain, MRI
Introduction
Breast cancer is a complex and multifaceted disease that can have far-reaching consequences for patients, including the development of bone metastases [1]. The skeletal system is a common site for metastatic spread, with approximately 70% of patients experiencing bone involvement at some point during their illness [2, 3]. The spine, in particular, is a frequent location for metastases [4-6]. However, migration can also happen in the cervical and lumbar spine, each with its own unique set of challenges and implications for patient care.
The presence of spinal metastases might make a profound influence on a patient, causing a range of symptoms including local pain, spinal instability, and neurological impairment. In some cases, spinal metastases can lead to neurologically compromising spinal lesions [7]. This can cause significant pain, neurological dysfunction, and even paralysis, all of which will make a devastating influence on a personal lifestyle and habits and overall well-being [8, 9].
Despite the availability of treatments such as radiological treatments and orthopedic procedures, the management of symptomatic spinal cord compression remains a significant challenge [10, 11]. The development of spinal metastases can require unscheduled treatments, which can disrupt the treatment schedule for the primary cancer and impede the patient's overall care. As a result, there is a growing recognition of the importance of bone management through a comprehensive strategy to mitigate bone-related complications and to improve patient outcomes [12].
A key aspect of bone management is the early diagnosis of symptomatic changes and the effective management of related pain or system therapy. However, there is still much to be learned about the factors that contribute to spinal metastasis and the relationship between the location of metastases, imaging characteristics, and the severity of associated bone pain [12, 13]. For example, research has shown that individuals with malignant spinal canal encroachment are more likely to experience symptoms in the thoracic spine, although a significant minority of cases occur in the cervical spine, potentially leading to greater morbidity and mortality [14].
Employment of cutting-edge diagnostic modalities like high-field nuclear magnetic resonance has revolutionized the diagnosis and characterization of spinal metastases [15]. MRI provides superior soft tissue contrast and detailed bone marrow imaging, making it an essential tool in the investigation of suspected cases of metastatic spinal cord compression. However, there is still a need for further research into the potential roles of specific MRI features in the assessment of bone pain caused by spinal cord compression.
This research seeks to bridge the existing information deficit by exploring the relationship between specific MRI features, such as the ratio of high-intensity areas in spinal metastases, and the intensity of bone pain. Additionally, the study will investigate the anatomical site of the metastasis within the spinal column and its potential impact on pain severity [16]. By elucidating the complex interplay between imaging characteristics of spinal metastasis and the clinical manifestation of bone pain, this research endeavors to inform the creation of enhanced analgesic protocols and improve patient care and quality of life [17].
Ultimately, the goal of this Investigation is to offer clinicians with a more nuanced understanding of the factors that contribute to spinal metastasis and the symptoms that patients experience. By informing clinicians about the potential severity of pain based on MRI findings, this study aims to aid in anticipatory pain management strategies and improve patient outcomes. Furthermore, the study seeks to provide insights into the pathophysiological mechanisms of pain generation in spinal metastasis, guiding future therapeutic interventions and contributing to the development of more effective treatments for metastatic breast cancer.
Materials and Methods
Patients
To ensure the integrity and confidentiality of patient information, a comprehensive data retrieval process was implemented, involving the collection of medical records from patients who had undergone surgical procedures for breast cancer at these two hospitals. As an added precaution, all patient records were thoroughly anonymized and deidentified prior to analysis, thereby safeguarding patient privacy and confidentiality. In light of the study's archival nature, the Human Subjects Committee dispensed with the necessity for formal patient assent, as the research was conducted using existing medical records and did not involve any direct patient interaction or intervention [18].
A rigorous patient selection process was undertaken to identify a cohort of patients who met the specified inclusion criteria. Specifically, the study focused on patients who had been diagnosed with breast cancer and had subsequently developed spine metastases, as confirmed by clinical diagnosis and histological examination [19]. To be eligible for inclusion, patients were required to have undergone MRI examinations at either Peking University Shenzhen Hospital or Henan Provincial People's Hospital between November 2014 and October 2023. A total of 45 patients met the inclusion criteria, which included: (1) a confirmed primary breast cancer diagnosis, as verified through histological examination; (2) a clinical diagnosis of spine metastases, as evidenced by relevant medical records and imaging data; and (3) the availability of comprehensive clinicopathologic features and image data for MRI examinations, which were meticulously recorded and stored in the patient database.
To guarantee the validity and trustworthiness of the research outcomes, a set of exclusion criteria was established to identify patients who did not meet the specified requirements. Patients were excluded from the study if they had been diagnosed with another type of malignancy, as this could potentially confound the results and introduce unnecessary variability [20]. Additionally, patients with unclear or uncertain diagnoses of spine metastases were excluded, as were those without access to electronic image files of their MRI examinations, which were essential for conducting a thorough analysis of the data [21]. By applying these strict inclusion and exclusion criteria, the study aimed to create a well-defined and homogeneous patient cohort, thereby enhancing the validity and generalizability of the research findings.
Image analysis protocol
A thorough examination of the images was conducted by two seasoned medical radiologists, who utilized an advanced Xeleris workstation (GE Healthcare) to meticulously review the scans. The radiologists carefully documented the locations and quantities of spine metastases, as reported in the medical records [22]. To quantify the extent of high-intensity areas within the spinal cord, the radiologists employed ImageJ software to calculate the proportion of high-intensity areas and the entire cord in the T2 scan images. This calculation enabled the determination of the Ratio of High Intensity Area (RHI), which was computed as the proportion of high-intensity area divided by the proportion of the whole cord [23]. This metric provided a standardized measure of the high-intensity area within the spinal cord, allowing for a more nuanced understanding of the metastatic lesions.
Pain assessment methodology
A comprehensive review of the medical records was conducted to determine the pain assessments for each patient. The Visual Analogue Scale (VAS) was used to evaluate the pain intensity associated with each lesion, with scores ranging from 0 (indicating no pain) to 10 (representing maximum pain) [24]. A pair of medical specialists, including a junior doctor and an experienced clinician, independently assessed the pain intensity and recorded their findings in the medical records. Notably, both physicians were blinded to the changes in the MRI examination, ensuring that their assessments were unbiased and based solely on the clinical presentation of the patient. The initial pain intensity was subsequently categorized into four distinct levels: none (0), mild (1-3), moderate (4-7), or severe (8-10) [25]. This categorization facilitated a more detailed analysis of the relationship between pain intensity and clinical features of the spine metastases.
Statistical analysis approach
To investigate the relationships between pain scores and clinical features of the spine metastases, the researchers employed Pearson's correlation coefficient [26]. This statistical method enabled the identification of correlations between the pain intensity and various clinical parameters, such as the distribution and quantity of tumor spread, along with the high-intensity area ratio [27, 28]. Additionally, one-way analysis of variance was used to examine the relationships among the metastasis location, numbers, RHI, and severity of pain. Data analysis was conducted utilizing a specialized statistical package, specifically version 19.0 of a prominent software platform developed by a leading analytics firm based in Chicago, which provided a robust and reliable platform for data analysis. The researchers also conducted other analyses, including descriptive statistics and data visualization, to provide a comprehensive understanding of the data and to identify potential trends and patterns [29].
Results
Clinical characteristics
A cohort of 45 patients with diagnosed breast cancer and radiologically confirmed spinal metastatic disease were included in this study, all of whom were female, with a median age of 51 years (Table 1). The median time from breast cancer diagnosis to the development of spinal metastases was 27 months. The most common histologic subtype was Luminal B (69%), followed by TNBC (13%), Luminal A (9%), and Her-2 positive (9%). Among these patients, 87% experienced bone pain, and 90% developed pain in a metachronous pattern following spinal metastasis diagnosis. Regarding the distribution of metastases, 58% of patients had lesions in multiple spinal regions, whereas the lumbar spine was the most frequently affected single region (16%). Systemic therapy included chemotherapy in 98% of patients, while only 13% underwent radiotherapy, either alone or in combination with chemotherapy.
Pain severity and metastatic factors
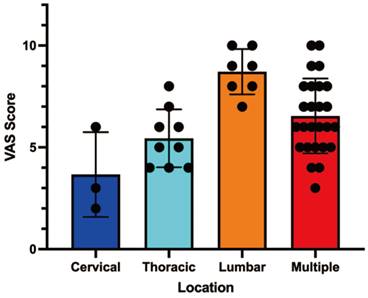
The mean initial Visual Analogue Scale (VAS) pain score was 5 (range: 0-10). TThe severity of pain significantly differed depending on the location of spinal metastases (Table 2). Patients with lumbar spine involvement reported the highest mean pain score (8, range: 5-10), while those with cervical spine metastases had the lowest mean score (4, range: 0-6; P = 0.0003). Patients with multiple metastases also experienced high pain scores, similar to those with isolated lumbar lesions.
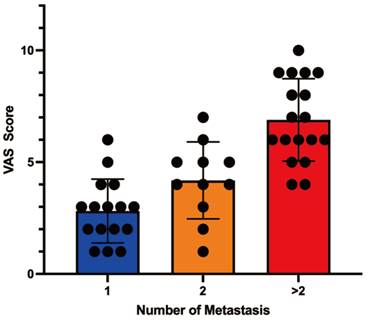
A strong correlation was obtained between the number of spinal metastases and the severity of pain (P < 0.0001). Patients with a single metastasis had a mean VAS score of 4 (range: 1-6), while those with two metastases reported a mean score of 5 (range: 1-7). Patients with more than two metastases exhibited a mean score of 8 (range: 4-10) (Table 2; Figure 2).
Patient (n= 45) and Metastatic Lesion (n= 45) clinical Characteristics
| Characteristic | Number of Patients(% or Range) |
|---|---|
| Gender | |
| Male | N=0(0%) |
| Female | N=45(100%) |
| Median age (years) | 51 |
| Median time of spine metastatic (months) | 27 |
| Histologic type | |
| Luminal A | 4(9%) |
| Luminal B | 31(69%) |
| Her-2 positive | 4(9%) |
| TNBC | 6(13%) |
| Bone metastases (n) | |
| One | 16(36%) |
| Multiple | 29(46%) |
| Location | |
| Cervical | 3(6%) |
| Thoracic | 9(20%) |
| Lumbar | 7(16%) |
| Multiple | 26(58%) |
| Pain occurrence | |
| With pain | 39(87%) |
| Without pain | 6(13%) |
| Time of bone metastasis and pain | |
| Metachronous | 35(90%) |
| Synchronous | 4(10%) |
| Systematic therapy | |
| Chemotherapy | 44(98%) |
| Radiotherapy | 6(13%) |
| Both | 6(13%) |
| None | 0 |
Correlations of Spine Metastasis to Pain Scores
| Factor | Mean | Range | P Value |
|---|---|---|---|
| Initial pain score (VSA) | 5 | 0-10 | - |
| Location | 0.0003 | ||
| Cervical | 4 | 0-6 | |
| Thoracic | 6 | 2-8 | |
| Lumbar | 8 | 5-10 | |
| Multiple | 8 | 5-10 | |
| Nunber of Metastasis | <0.0001 | ||
| 1 | 4 | 1-6 | |
| 2 | 5 | 1-7 | |
| over two | 8 | 4-10 |
Correlations of Spine Metastasis high-intensity zone rates to Pain Scores
| Factor | Median | Range | P Value |
|---|---|---|---|
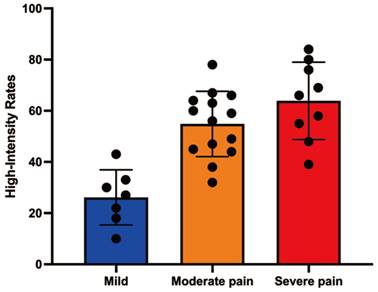
| High-intensity zone rates | 47 | 10-84 | |
| Pain serious | <0.0001 | ||
| Mild pain (1-3) | 27 | 10-43 | |
| Moderate pain (4-7) | 59 | 32-78 | |
| Severe pain (8-10) | 66 | 39-84 |
MRI findings and pain correlations
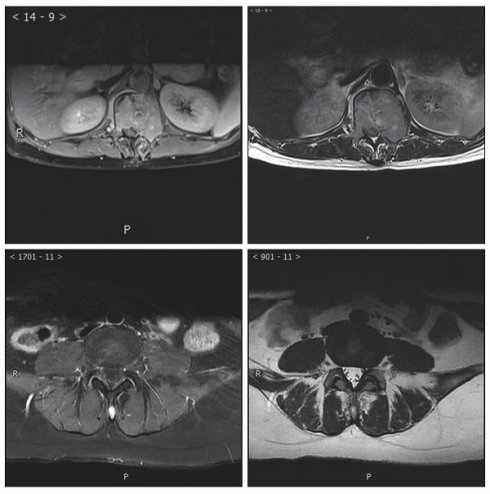
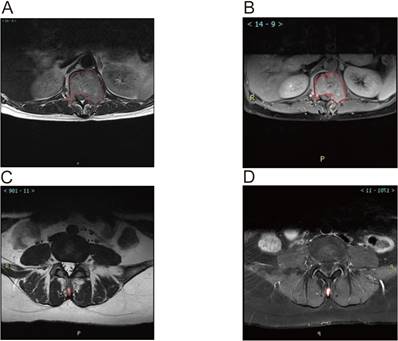
MRI analysis revealed significant associations between the ratio of high-intensity zones (RHI) and pain severity (Table 3). The median RHI was 47% (range: 10-84%). Patients with mild pain (VAS 1-3) exhibited a median RHI of 27% (range: 10-43%), whereas those with moderate pain (VAS 4-7) and severe pain (VAS 8-10) had significantly higher median RHIs of 59% (range: 32-78%) and 66% (range: 39-84%), respectively (P < 0.0001). Figure 3 illustrates representative MRI images showing the locations of metastatic lesions and associated high-intensity zones. Correlation analysis confirmed a significant linear relationship between RHI and pain scores (Figure 4).
Correlation analysis between Metastasis location and pain score.
Correlation analysis between Metastasis numbers and pain score.
Magnetic Resonance Imaging (MRI) Images showed Metastatic Lesion location and high-intensity zone in Patients With spine metastasis of breast cancer. A. Calulate the proportion of whole cord in the T2 scan image of the lumbar metastasis. B. Calulate the proportion of high indesity area in the T2 scan image of the lumbar metastasis. C. Calulate the proportion of whole cord in the T2 scan image of the Spinous process metastasis. D. Calulate the proportion of high indesity area in the T2 scan image of the Spinous process metastasis.
Correlation analysis between high-intensity zone rates and pain score.
Discussion
This study provides evidence that the combination of metastatic sites and MRI-derived features, particularly the ratio of high-intensity zones (RHI), is markedly correlated with the severity of bone pain in breast cancer patients with spinal metastases. These results hold significant practical relevance for elucidating the pathophysiology of bone pain and for optimizing patient management [30-33].
Our results showed that spinal metastases in the lumbar region are associated with significantly higher pain scores compared to those in the cervical or thoracic regions. This may be due to the biomechanical and structural differences in these regions, with the lumbar spine bearing greater mechanical loads, thereby amplifying pain when metastases are present [34, 35]. Parallel observations have been made in various forms of neoplastic disease emphasizing the impact of spinal biomechanics on metastatic pain severity [36-38].
The strong correlation between the number of metastatic sites and pain severity further supports the role of tumor burden in exacerbating symptoms [39, 40]. Individuals with disseminated disease exhibited markedly elevated discomfort levels relative to those with solitary foci. This aligns with the hypothesis that cumulative skeletal involvement leads to increased mechanical instability, nerve compression, and heightened inflammatory responses [41, 42].
The MRI findings in this study highlight the role of RHI as a potential biomarker for bone pain severity. Higher RHI values may reflect increased tumor activity, bone marrow edema, or inflammatory processes within the metastases, all of which contribute to heightened pain perception. Previous research has suggested that advanced imaging features such as fluorine-18 fluorodeoxyglucose PET can serve as prognostic indicators for pain severity and disease progression in cancer patients16. These findings underscore the utility of MRI not only in diagnosing spinal metastases but also in guiding targeted pain management strategies [43, 44].
The integration of imaging data into routine clinical workflows could significantly improve patient outcomes. Identifying patients at high risk of severe pain based on metastatic location, RHI values, and lesion number enables the early initiation of aggressive pain management strategies. For instance, targeted radiotherapy or systemic treatments like bisphosphonates and denosumab may be more effectively applied when guided by these predictive imaging markers [45, 46]. Additionally, the ability to stratify patients by pain risk could aid in personalized care planning, potentially improving their overall quality of life [47].
Although this research offers meaningful contributions, certain methodological constraints warrant consideration. The look-back methodology and modest cohort size may constrain the broader applicability of our results [48]. Additionally, factors such as individual pain tolerance, psychological status, and concurrent treatments were not accounted for, which may have influenced pain scores [49]. Further forward-looking investigations involving expanded populations are required to corroborate these results and further elucidate the mechanisms linking MRI features to pain severity. Exploring the role of advanced imaging modalities, such as functional MRI or positron emission tomography (PET), could provide additional insights into the complex relationship between metastatic burden and pain.
This study provides compelling evidence linking the location of spinal metastases, the extent of high-intensity areas observed on MRI, and the intensity of bone pain experienced by patients with breast cancer [50]. Our findings underscore the complex interplay between tumor characteristics and patient-reported symptoms, offering valuable insights for clinical practice.
The observation that lumbar spine metastases are associated with significantly greater pain compared to cervical or thoracic involvement is noteworthy. This disparity likely stems from the unique biomechanical demands placed on the lumbar region, which bears a greater share of the body's weight and is subjected to more movement. The presence of a tumor in this area could exacerbate pain through increased mechanical stress, nerve compression, and local inflammation. This highlights the importance of considering the specific anatomical location of metastases when assessing and managing pain.
Furthermore, the study establishes a robust correlation between the number of metastatic sites and pain severity[51]. Patients with multiple spinal lesions reported markedly higher pain scores compared to those with solitary metastases. This finding reinforces the notion that the overall tumor burden contributes substantially to the intensity of symptoms[52]. The cumulative effect of multiple lesions may result in greater mechanical instability, more widespread nerve irritation, and a more pronounced inflammatory response, collectively leading to increased pain[53].
The significant correlation between the ratio of high-intensity areas (RHI) on MRI and pain severity adds another layer of complexity to our understanding of the relationship between imaging features and clinical manifestations[54-60]. A higher RHI may reflect increased tumor activity, bone marrow edema, or the presence of inflammatory mediators within the metastatic lesion. These factors could all contribute to the heightened pain sensitivity observed in patients with elevated RHI values. These observations underscore the potential of MRI as a tool not only for diagnosing metastases, but also for predicting the severity of associated pain.
The clinical implications of these findings are substantial. By identifying patients at higher risk of experiencing severe pain based on the location, number of metastases, and RHI values, clinicians can proactively implement more aggressive pain management strategies. This may include targeted radiation therapy, systemic treatments such as bisphosphonates or denosumab, or other novel approaches. Furthermore, factors such as individual pain tolerance, psychological status, and concurrent treatments were not accounted for, which may have influenced pain scores. In conclusion, this study demonstrates a significant association between spinal metastatic sites, MRI-derived RHI values, and the severity of bone pain in breast cancer patients. These findings highlight the critical role of MRI not only as a diagnostic tool but also as a predictor of symptomatic outcomes. Incorporating these imaging parameters into clinical practice could enhance symptom management and improve the quality of life for patients with metastatic breast cancer.
Conclusion
In summary, our study highlights the significant association between spinal metastatic sites, MRI-derived RHI values, and bone pain severity in breast cancer patients. These findings emphasize the critical role of MRI not only as a diagnostic tool but also as a predictor of symptomatic outcomes. Incorporating these imaging parameters into clinical practice could enhance symptom management and improve the quality of life for patients with metastatic breast cancer.
Acknowledgements
Funding
The present study was supported by the Medical Research Grant funded by Health Commission of Guangdong Province, People's Republic of China (No. B2024021) and by National Natural Science Foundation of China (No. 82103130), Shenzhen Science and Technology Program (No. JCYJ20210324110402006).
Ethics statement
The investigative plan underwent rigorous scrutiny and received endorsement from the Ethics Committee of two prominent medical institutions in China: Peking University Shenzhen Hospital and Henan Provincial People's Hospital.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH. et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271-7
2. Manders K, van de Poll-Franse LV, Creemers GJ, Vreugdenhil G, van der Sangen MJ, Nieuwenhuijzen GA. et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6:179
3. Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br J Cancer. 1998;77:336-40
4. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-9s
5. Major PP, Cook RJ, Lipton A, Smith MR, Terpos E, Coleman RE. Natural history of malignant bone disease in breast cancer and the use of cumulative mean functions to measure skeletal morbidity. BMC Cancer. 2009;9:272
6. Briasoulis E, Karavasilis V, Kostadima L, Ignatiadis M, Fountzilas G, Pavlidis N. Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer. 2004;101:1524-8
7. Klimo P Jr, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9:188-96
8. Kakutani K, Sakai Y, Maeno K, Takada T, Yurube T, Kurakawa T. et al. Prospective Cohort Study of Performance Status and Activities of Daily Living After Surgery for Spinal Metastasis. Clin Spine Surg. 2017;30:E1026-e32
9. Miyazaki S, Kakutani K, Sakai Y, Ejima Y, Maeno K, Takada T. et al. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: prospective cohort study. Int Orthop. 2017;41:1265-71
10. Chhabra P, de Graaf M, Parra GI, Chan MC, Green K, Martella V. et al. Updated classification of norovirus genogroups and genotypes. J Gen Virol. 2019;100:1393-406
11. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2002:Cd004721
12. Kakutani K, Kanda Y, Yurube T, Takeoka Y, Miyazaki K, Ohnishi H. et al. The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers (Basel). 2023;15:1251
13. Lawrie I. Back Pain in Malignant Disease - Metastatic Spinal Cord Compression? Rev Pain. 2010;4:14-7
14. Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess. 2013;17:1-274
15. Yearley AG, McNulty JJ, Chalif EJ, Chalif JI, Lee SJ, Klinger NV. et al. Spinal Metastases from Colorectal Cancer at Mass General Brigham: A Twenty-Year Case Series With Literature Review. World Neurosurg. 2023;176:e246-e53
16. Zhao F, Ding G, Huang W, Li M, Fu Z, Yang G. et al. FDG-PET Predicts Pain Response and Local Control in Palliative Radiotherapy With or Without Systemic Treatment in Patients With Bone Metastasis From Non-small-cell Lung Cancer. Clin Lung Cancer. 2015;16:e111-9
17. Yao XC, Shi XJ, Xu ZY, Tan J, Wei YZ, Qi L. et al. Preliminary establishment of a spinal stability scoring system for multiple myeloma. World J Clin Cases. 2021;9:9023-37
18. Cao Y, Lan W, Wen L, Li X, Pan L, Wang X. et al. An effectiveness study of a wearable device (Clouclip) intervention in unhealthy visual behaviors among school-age children: A pilot study. Medicine (Baltimore). 2020;99:e17992
19. Chen L, Tian Q, Shi Z, Qiu Y, Lu Q, Liu C. Melatonin Alleviates Cardiac Function in Sepsis-Caused Myocarditis via Maintenance of Mitochondrial Function. Front Nutr. 2021;8:754235
20. Chen L, Zhan CZ, Wang T, You H, Yao R. Curcumin Inhibits the Proliferation, Migration, Invasion, and Apoptosis of Diffuse Large B-Cell Lymphoma Cell Line by Regulating MiR-21/VHL Axis. Yonsei Med J. 2020;61:20-9
21. Chen Z, Jin M, He H, Dong J, Li J, Nie J. et al. Mesenchymal stem cells and macrophages and their interactions in tendon-bone healing. J Orthop Translat. 2023;39:63-73
22. Cuny H, Bozon K, Kirk RB, Sheng DZ, Broer S, Dunwoodie SL. Maternal heterozygosity of Slc6a19 causes metabolic perturbation and congenital NAD deficiency disorder in mice. Dis Model Mech. 2023;16:dmm049647
23. Dang X, Fan C, Cui F, He Y, Sun G, Ruan J. et al. Interactions between ultrasonographic cervical length and placenta accreta spectrum on severe postpartum hemorrhage in women with placenta previa. Int J Gynaecol Obstet. 2023;161:1069-74
24. Ding L, Lu S, Zhou Y, Lyu D, Ouyang C, Ma Z. et al. The 3' Untranslated Region Protects the Heart from Angiotensin II-Induced Cardiac Dysfunction via AGGF1 Expression. Mol Ther. 2020;28:1119-32
25. Dong H, Li H, Wang L, Yuan Y, Zhang D, Zhou L. et al. Clinical analysis of 175 cases of vaginal intraepithelial neoplasia. Eur J Obstet Gynecol Reprod Biol. 2023;287:232-6
26. Gao P, Rao ZW, Li M, Sun XY, Gao QY, Shang TZ. et al. Tetrandrine Represses Inflammation and Attenuates Osteoarthritis by Selective Inhibition of COX-2. Curr Med Sci. 2023;43:505-13
27. Huang Z, Yu P, Tang J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. Onco Targets Ther. 2020;13:5395-405
28. Li H, Shi W, Shen T, Hui S, Hou M, Wei Z. et al. Network pharmacology-based strategy for predicting therapy targets of Ecliptae Herba on breast cancer. Medicine (Baltimore). 2023;102:e35384
29. Gao WL, Li XH, Dun XP, Jing XK, Yang K, Li YK. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr Med Sci. 2020;40:434-43
30. Li J, Liang X, Wang X, Yang P, Jian X, Fu L. et al. A missense GDF5 variant causes brachydactyly type A1 and multiple-synostoses syndrome 2. JOR Spine. 2024;7:e1302
31. Liu J, Lu Y, Huang D, Yang J, Fan C, Chen C. et al. The Efficacy of Defocus Incorporated Multiple Segments Lenses in Slowing Myopia Progression: Results from Diverse Clinical Circumstances. Ophthalmology. 2023;130:542-50
32. Liu Y, Shen D, Wang HY, Qi MY, Zeng QY. Development and validation to predict visual acuity and keratometry two years after corneal crosslinking with progressive keratoconus by machine learning. Front Med (Lausanne). 2023;10:1146529
33. Lu Y, Lin Z, Wen L, Gao W, Pan L, Li X. et al. The Adaptation and Acceptance of Defocus Incorporated Multiple Segment Lens for Chinese Children. Am J Ophthalmol. 2020;211:207-16
34. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128
35. Peng Y, Yan H, Mei W, Zhang P, Zeng C. Combining Radiotherapy with Immunotherapy in Cervical Cancer: Where Do We Stand and Where Are We Going? Curr Treat Options Oncol. 2023;24:1378-91
36. Shao Y, Zhao T, Zhang W, He J, Lu F, Cai Y. et al. Presence of the apolipoprotein E-epsilon4 allele is associated with an increased risk of sepsis progression. Sci Rep. 2020;10:15735
37. Wen L, Cao Y, Cheng Q, Li X, Pan L, Li L. et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol. 2020;104:1542-7
38. Yu W, Qin X, Zhang Y, Qiu P, Wang L, Zha W. et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752-69
39. Xiong T, Xia L, Song Q. Circular RNA SPI1 expression before and after induction therapy and its correlation with clinical features, treatment response, and survival of acute myeloid leukemia patients. J Clin Lab Anal. 2023;37:e24835
40. Yang Y, Wu Q, Pan W, Wen L, Luo Z, Wu H. et al. Characteristics of the Ocular Surface in Myopic Child Candidates of Orthokeratology Lens Wear. Ophthalmol Ther. 2023;12:3067-79
41. Su H, Geng H, Cai L, Xu M, Xing W, Long W. et al. Immune-check blocking combination multiple cytokines shown curative potential in mice tumor model. Cancer Med. 2023;12:13573-85
42. Wang W, Zhai T, Luo P, Miao X, Wang J, Chen Y. Beneficial effects of silibinin on serum lipids, bile acids, and gut microbiota in methionine-choline-deficient diet-induced mice. Front Nutr. 2023;10:1257158
43. Zhang L, Jiang B, Zhu N, Tao M, Jun Y, Chen X. et al. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCalpha/ERK1/2 and PI3K/Akt pathway. Med Oncol. 2019;37:5
44. Zhang Y, Zhang Y, Liu S, Li B, Song Z, Han Q. et al. Acupuncture for cancer pain: a scoping review of systematic reviews and meta-analyses. Front Oncol. 2023;13:1169458
45. Zhao B, Li M, Su Y, Shan S, Qian W, Zhu D. et al. Role of transcription factor FOXM1 in diabetes and its complications (Review). Int J Mol Med. 2023;52:101
46. Zhou J, Chen S, Liu J, Du J, Li J. Knockdown of hnRNPAB reduces the stem cell properties and enhances the chemosensitivity of human colorectal cancer stem cells. Oncol Rep. 2023;49:129
47. Zhang Z, Ni P, Tang M, Song Y, Liu C, Zhao B. Dapagliflozin alleviates renal podocyte pyroptosis via regulation of the HO-1/NLRP3 axis. Mol Med Rep. 2023;28:200
48. McVeigh LG, Linzey JR, Strong MJ, Duquette E, Evans JR, Szerlip NJ. et al. Stereotactic body radiotherapy for treatment of spinal metastasis: A systematic review of the literature. Neurooncol Adv. 2024;6:iii28-iii47
49. Van den Brande R, Thijs D, Bilsky M, Peeters M, Billiet C, Van de Kelft E. Treatment of ambulatory patients with metastatic epidural spinal cord compression: a systematic review and meta-analysis. J Neurosurg Spine. 2024;40:175-84
50. Kurisunkal V, Gulia A, Gupta S. Principles of Management of Spine Metastasis. Indian J Orthop. 2020;54:181-93
51. Lin L, Wu Q, Lu F, Lei J, Zhou Y, Liu Y. et al. Nrf2 signaling pathway: current status and potential therapeutic targetable role in human cancers. Front Oncol. 2023;13:1184079
52. Zhu ZY, Liu YD, Gong Y, Jin W, Topchiy E, Turdi S. et al. Mitochondrial aldehyde dehydrogenase (ALDH2) rescues cardiac contractile dysfunction in an APP/PS1 murine model of Alzheimer's disease via inhibition of ACSL4-dependent ferroptosis. Acta Pharmacol Sin. 2022;43:39-49
53. Liang T, Zhang Y, Wu S, Chen Q, Wang L. The Role of NLRP3 Inflammasome in Alzheimer's Disease and Potential Therapeutic Targets. Front Pharmacol. 2022;13:845185
54. Feng MG, Liu CF, Chen L, Feng WB, Liu M, Hai H. et al. MiR-21 attenuates apoptosis-triggered by amyloid-β via modulating PDCD4/ PI3K/AKT/GSK-3β pathway in SH-SY5Y cells. Biomed Pharmacother. 2018;101:1003-7
55. Zhang Y, Zhai M, Chen Z, Han X, Yu F, Li Z. et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017;24:1045-55
56. Li H, Wang X, Liu Y, Pan D, Wang Y, Yang N. et al. Hepatoprotection and hepatotoxicity of Heshouwu, a Chinese medicinal herb: Context of the paradoxical effect. Food Chem Toxicol. 2017;108:407-18
57. Chen Q, Mo R, Wu N, Zou X, Shi C, Gong J. et al. Berberine Ameliorates Diabetes-Associated Cognitive Decline through Modulation of Aberrant Inflammation Response and Insulin Signaling Pathway in DM Rats. Front Pharmacol. 2017;8:334
58. Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z. et al. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065-76
59. Ma P, Wu Y, Zeng Q, Gan Y, Chen J, Ye X. et al. Oxidative damage induced by chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem Toxicol. 2013;58:177-83
60. Ma P, Luo Q, Chen J, Gan Y, Du J, Ding S. et al. Intraperitoneal injection of magnetic Fe₃O₄-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int J Nanomedicine. 2012;7:4809-18
Author contact
![]() Corresponding authors: Jinghua Liu, email: 707841972com; Tao Qin, email: taoqin173com; Zeng Fang, email: fangzsysu.edu.cn.
Corresponding authors: Jinghua Liu, email: 707841972com; Tao Qin, email: taoqin173com; Zeng Fang, email: fangzsysu.edu.cn.

 Global reach, higher impact
Global reach, higher impact