3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(3):641-650. doi:10.7150/ijms.98194 This issue Cite
Review
Differences of the Chest Images Between Coronavirus Disease 2019 (COVID-19) Patients and Influenza Patients: A Systematic Review and Meta-analysis
1. Department of Neurology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China, 130000, ORCID: 0000-0002-3583-0448.
2. Department of Radiology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China, 130000.
3. Department of Ultrasound, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China, 130000.
4. Department of Orthopaedics, The Second Hospital of Jilin University, Changchun, Jilin Province, China, 130000.
Received 2024-5-7; Accepted 2025-1-3; Published 2025-1-13
Abstract
Background: Coronavirus disease 2019 (COVID-19) and influenza are two infectious diseases that can pose a great threat to human health. We aimed to compare the differences in chest images between patients with COVID-19 and influenza to deepen the understanding of these two diseases.
Methods: We searched PubMed, Embase and Web of Science for articles published before December 25, 2023, and performed a meta-analysis using Stata 14.0 with a random-effects model. The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
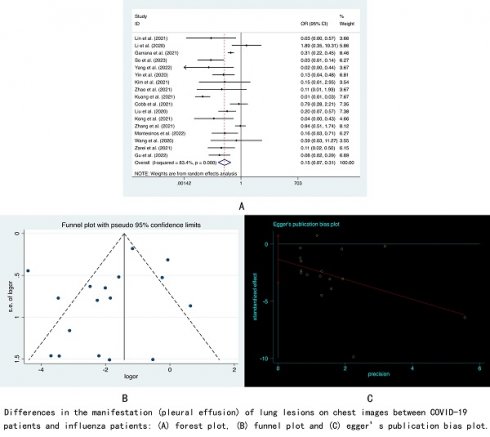
Results: Twenty-six articles with 2,159 COVID-19 patients and 1,568 influenza patients were included in the meta-analysis. By comparing chest computed tomography (CT) and chest X-ray, we found that COVID-19 patients had more peripheral lung lesions (OR=3.66, 95% CI: 1.84-7.31). Although COVID-19 patients had more bilateral lung involvement (OR=1.74, 95% CI: 0.90-3.38) and less unilateral lung involvement (OR=0.67, 95% CI: 0.44-1.02), these two results were not statistically significant. Patients with COVID-19 showed more ground-glass opacities (OR=2.83, 95% CI: 1.85-4.32), reverse halo signs (OR=3.47, 95% CI: 2.37-5.08), interlobular septal thickening (OR=2.16, 95% CI: 1.55-3.01), vascular enlargement (OR=5.00, 95% CI: 1.80-13.85) and crazy-paving patterns (OR=2.63, 95% CI: 1.57-4.41) on chest images than patients with influenza. We also found that compared with influenza patients, pleural effusion was rare in COVID-19 patients (OR=0.15, 95% CI: 0.07-0.31).
Conclusions: There are some differences in the manifestations and distributions of lesions between patients with COVID-19 and influenza on chest images, which is helpful to distinguish these two infectious diseases.
Keywords: Computed tomography, X-ray, COVID-19, influenza.
Introduction
In December 2019, a group of patients were unfortunately infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and most of them had been exposed to the Huanan Seafood Market in Wuhan, China [1]. Subsequently, the coronavirus disease 2019 (COVID-19) epidemic swept the world. On January 30, 2020, the WHO listed COVID-19 as a public health emergency of international concern and then listed it as a pandemic on March 11, 2020 [2]. Influenza virus is an RNA virus that causes influenza in humans and animals and belongs to the orthomyxoviridae family. The family has four genera; however, only genera A and B are clinically relevant to humans [3]. Prior to the current COVID-19 pandemic, there were multiple global outbreaks of acute respiratory disease caused by influenza viruses. As early as 1918, the H1N1 influenza virus rapidly erupted and spread in Europe, North America and Asia, infecting 500 million people and causing more than 50 million deaths [4]. The most recent influenza pandemic was in 2009, which spread to 214 countries between March 2009 and August 2010, resulting in 18,449 laboratory-confirmed deaths worldwide [5]. Although the influenza pandemic is over, H1N1 and other influenza virus strains have been with us as seasonal viruses, leading to annual seasonal influenza epidemics [6]. It is also estimated that COVID-19 will not go away and will eventually show the same seasonal peak similar to influenza [7]. COVID-19 and influenza have many similarities, especially in the early stage of the COVID-19 epidemic, and patients may be misdiagnosed with influenza [8].
CT and X-ray can be used to evaluate many chest disorders, including viral chest infections. Chest radiography has been shown to be helpful in determining the prognosis of patients in previous influenza outbreaks [9]. In the early stage of the COVID-19 pandemic, some countries used abnormal chest images as the sole diagnostic criteria for COVID-19 before antigen or antibody tests were widely available [10-12]. With the wide application of artificial intelligence with the topics of machine learning, artificial neural network and deep learning in medicine [13], X-ray images combined with artificial intelligence in the diagnosis of COVID-19 has received more attention [14]. Chest CT data can also be used in modeling with other clinical data to assess unfavorable outcomes in patients with COVID-19 [15]. We aimed to compare the differences in chest images between patients with COVID-19 and influenza to deepen the understanding of these two diseases and provide some guidance for clinicians to make differential diagnoses.
Materials and methods
Eligibility criteria
Articles that met the following requirements were included in this meta-analysis: 1) the chest image characteristics, including distributions and manifestations of lesions displayed by the patients, were recorded in the sample, 2) patients were divided into an experimental group and a control group, which were COVID-19 patients and influenza patients, respectively and 3) there was no restriction on the language of the article text.
The exclusion criteria: 1) nonhuman studies, 2) case reports, 3) reviews, comments or abstracts, 4) focused on children, 5) data duplication, and 6) the sample size of the experimental group or control group was less than 10.
Information sources
We searched PubMed, Embase and Web of Science for articles published before December 25, 2023. To collect as much data as possible, we did not restrict the language of the articles and searched for topics in both titles and abstracts.
Search strategy
The search strategy in PubMed was as follows: (((((Flu[Title/Abstract]) OR (influenza[Title/Abstract])) OR (Influenzas[Title/Abstract])) AND ((((COVID-19[Title/Abstract]) OR (2019-nCoV[Title/Abstract])) OR (Coronavirus Disease 2019[Title/Abstract])) OR (SARS-CoV-2[Title/Abstract]))) AND (((((((Chest Images[Title/Abstract]) OR (Chest Image[Title/Abstract])) OR (CT[Title/Abstract])) OR (Computed Tomography[Title/Abstract)) OR (X-ray[Title/Abstract])) OR (Radiology[Title/Abstract])) OR (Radiological[Title/Abstract])). The search strategies used for Embase and Web of Science databases are listed in File S1.
Study selection process
All the articles retrieved from the databases were imported into NoteExpress software, and duplicate articles were removed by matching titles. We then conducted a preliminary screening of articles by reading the titles or abstracts. For the articles that passed the initial screening, we conducted further screening by reading the full text and finally determined which articles could be used for this meta-analysis.
Data selection process and items
Data extraction was performed by three authors to ensure accuracy. The first two authors screened the data independently, and disagreements were adjudicated by the third author. The items recorded were mainly distributions and manifestations of lesions displayed by the patients on chest images at the time of admission.
Study risk of bias assessment
The Newcastle-Ottawa quality assessment scale was used to assess the quality and risk of bias of the included articles. Each article had a perfect score of nine, and a total score of seven or more meant that the article had a low risk of bias and high quality.
Reporting bias assessment
We used funnel plots and Egger's test to evaluate reporting bias assessment, and a p value<0.05 indicated that there was no reporting bias.
Statistical analysis
This meta-analysis was in alignment with the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines. Since only dichotomous variables were included in our results, odds ratios (ORs) were used for data analysis and evaluation, and the confidence interval (CI) was set at 95%. The I2 statistic was used to quantify heterogeneity, and subgroup analysis was used to explore the source of heterogeneity: I2≤50% indicated low heterogeneity, 50<I2≤75% indicated moderate heterogeneity, and I2>75% indicated high heterogeneity [16]. The statistical software was Stata 14.0, and we used a random-effects model to estimate the effect value. A p value of z test<0.05 was considered statistically significant.
Results
Study selection
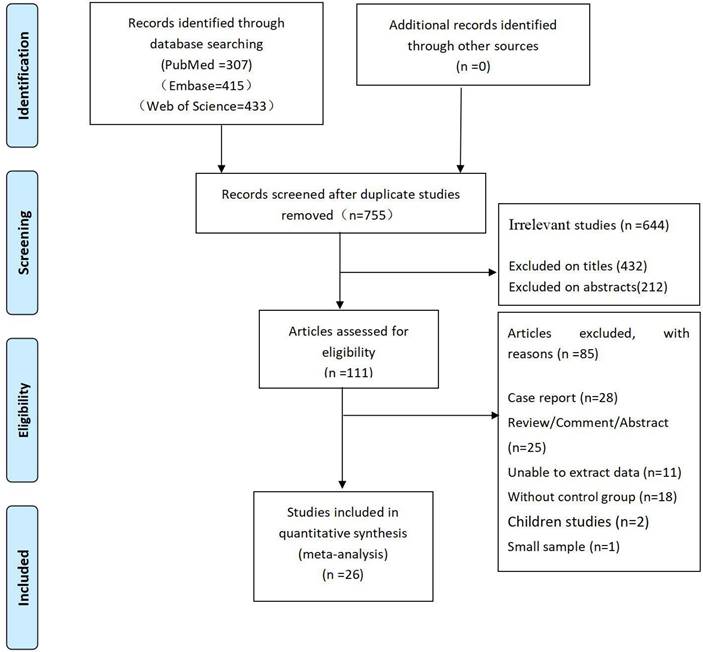
A total of 1,155 articles were retrieved: 307 from PubMed, 415 from Embase, and 433 from Web of Science. A total of 400 duplicate articles were removed through the duplicate identification function of NoteExpress software. Next, we removed 432 and 212 unrelated articles by reading the titles and abstracts, respectively. Among the remaining 111 articles, we further removed 85 articles by reading the full text. The detailed screening procedure is shown in Figure 1.
Risk of bias in studies
The Newcastle-Ottawa quality assessment scale is listed in Table S1. We found that all of the studies included were of high quality and had a low risk of bias.
The flow diagram of the article selection process.
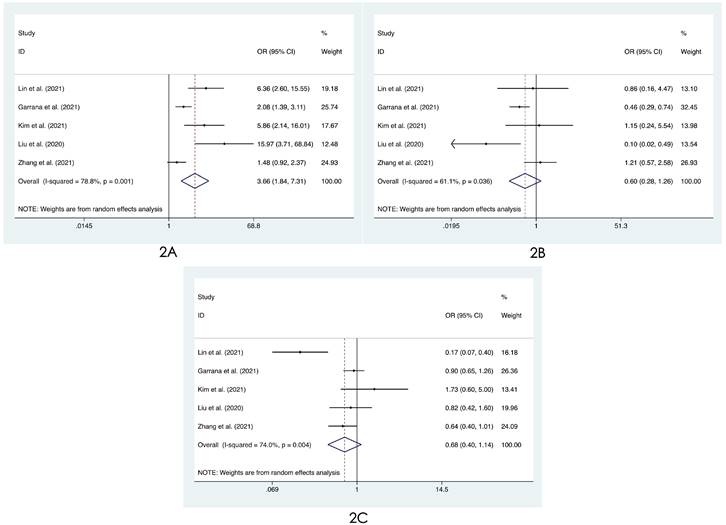
Forest plot of differences in the distribution of lung lesions on chest images between COVID-19 patients and influenza patients: (2A) only peripheral, (2B) only central, and (2C) both peripheral and central.
Characteristics and results of individual studies
A total of 2,159 patients with COVID-19 and 1,568 patients with influenza were included in this study. The data were collected from 26 articles, covering 12 countries or regions. Except for two articles that did not describe the type of study design, the remaining articles were retrospective studies. The data on influenza patients first came from 2009, with nine articles in which patients were infected with type A and 11 articles in which patients were infected with type A/B; six articles did not describe the influenza subtype; and no articles studied patients with influenza B separately. All data on patients with COVID-19 were obtained after the COVID-19 pandemic. Most of the chest images of the patients were obtained from CT, and a few were obtained from X-ray. Most articles described the number of radiologists who reviewed the images, with a minimum of one and a maximum of six. Detailed information is provided in Table 1.
Results of syntheses
Distributions of lesions
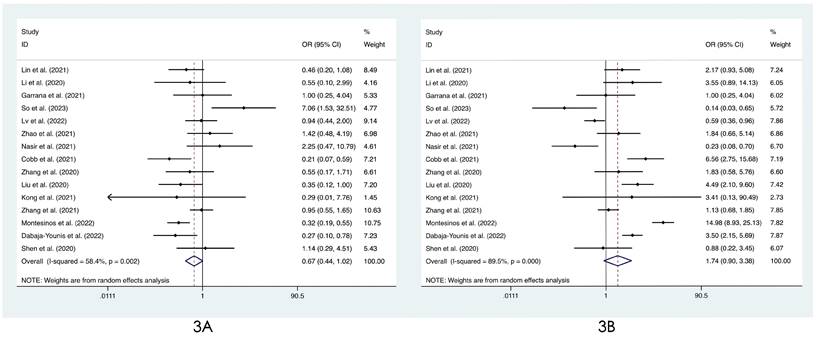
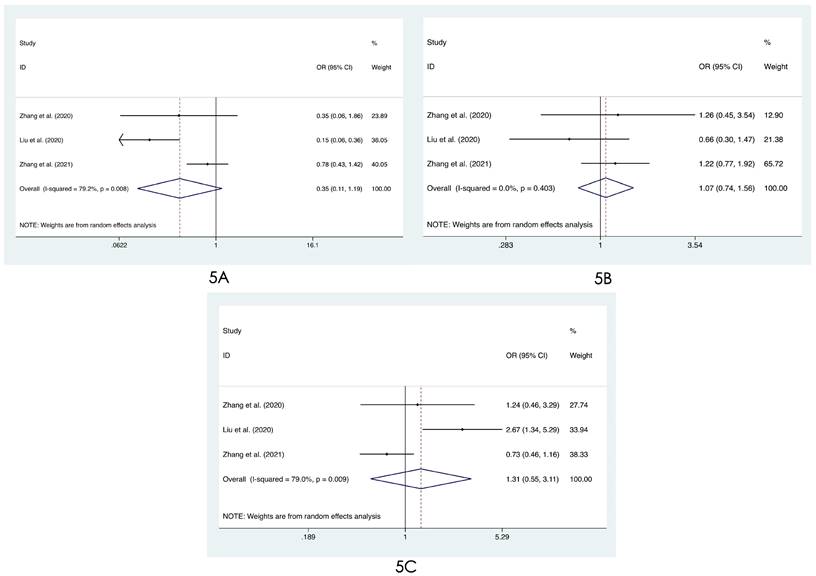
We compared the distribution of lung lesions on chest images between COVID-19 patients and influenza patients in four ways (Tables S2-S3). 1) We classified the location of lesions as only peripheral, only central, and both peripheral and central. We found that COVID-19 patients had more peripheral lesions (OR=3.66, 95% CI: 1.84-7.31:, I2=78.8%, p<0.001, Figure 2A), but there was no obvious difference in the distribution in the other two categories between patients with COVID-19 and influenza ((OR=0.60, 95% CI: 0.28-1.26, I2=61.1%, p=0.175, Figure 2B) and (OR=0.68, 95% CI: 0.40-1.14, I2=74.0%, p=0.145, Figure 2C)). 2) Compared with patients with influenza, COVID-19 patients had less unilateral lung involvement (OR=0.67, 95% CI: 0.44-1.02, I2=58.4%, p=0.059, Figure 3A) and more bilateral lung involvement (OR=1.74, 95% CI: 0.90-3.38:, I2=89.5%, p=0.100, Figure 3B). Although these two results were not statistically significant, both p values were near the critical value, and the results were likely to show significance when new articles were included in a future study. 3) We compared the involvement of each lobe between COVID-19 patients and influenza patients, and there were no significant differences in any of the five lobes (right upper lobe (OR=1.04, 95% CI: 0.43-2.52, I2=87.8%, p=0.927, Figure 4A), right middle lobe (OR=1.19, 95% CI: 0.44-3.24, I2=90.8%, p=0.729, Figure 4B), right lower lobe (OR=1.31, 95% CI: 0.46-3.73, I2=87.7%, p=0.609, Figure 4C), left upper lobe (OR=1.52, 95% CI: 0.55-4.20, I2=89.9%, p=0.417, Figure 4D), and left lower lobe (OR=1.11, 95% CI: 0.44-2.80, I2=85.2%, p=0.820, Figure 4E). 4) According to the number of involved lobes, we divided patients with COVID-19 and patients with influenza into three categories: 0-1 lobes involved (OR=0.35, 95% CI: 0.11-1.19, I2=79.2%, p=0.093, Figure 5A), 2-3 lobes involved (OR=1.07, 95% CI: 0.74-1.56, I2=0.0%, p=0.704, Figure 5B) and 4-5 lobes involved (OR=1.31, 95% CI: 0.55-3.11, I2=79.0%, p=0.541, Figure 5C), and no significant difference was found.
Characteristics of individual studies.
| Author | Publication year | Region | Study design | Influenza subtype | Chest images | Radiologists | Study period of COVID-19 | Study period of influenza |
|---|---|---|---|---|---|---|---|---|
| Fischer et al.[7] | 2022 | Switzerland | retrospective | NA | CT | 2 | 2020.3-2021.3 | 2017-2018 2019-2020 |
| Lin et al.[17] | 2021 | China | retrospective | A/B | CT | 2 | 2020.1.17-2020.2.13 | 2018.2.20-2020.2.9 |
| Li et al.[18] | 2020 | China | retrospective | A/B | CT | 2 | 2020.1.19-2020.2.24 | 2020.1.19-2020.2.24 |
| Garrana et al.[19] | 2021 | USA | retrospective | A/B | CT | 6 | 2020.3.3-2020.5.1 | 2011.1.1-2019.12.31 |
| So et al.[20] | 2023 | Hong Kong | retrospective | A/B | CT | 2 | 2020.1.24-2020.4.16 | 2018.2.20-2020.1.30 |
| Yang et al.[21] | 2022 | China | retrospective | NA | CT | 2 | 2020.1.1-2020.2.15 | 2015.1.1-2019.9.30 |
| Yin et al.[22] | 2020 | China | retrospective | A | CT | 2 | 2020.2.7-2020.2.14 | 2018.12-2019.2 |
| Lv et al.[23] | 2022 | China | retrospective | A | NA | NA | 2020.1.17-2020.3.10 | 2017.11.1-2018.3.31 |
| Kim et al.[24] | 2021 | Korea | retrospective | NA | CT | NA | 2020.2.25-2020.4.1 | 2016.1-2020.3 |
| Zhao et al.[25] | 2021 | China | retrospective | A/B | CT | 2 | 2020.1.21-2020.2.9 | 2020.1.21-2020.2.9 |
| Kuang et al.[26] | 2021 | China | retrospective | A | CT | 2 | 2020.1.21-2020.2.20 | 2017.1.1-2020.2.29 |
| Nasir et al.[27] | 2021 | Pakistan | retrospective | A | CT or X-ray | NA | 2020 | 2017-2019 |
| Cobb et al.[28] | 2021 | USA | retrospective | A/B | NA | NA | -2020.4.5 | 2019.1.1-2020.4.5 |
| Zhang et al.[29] | 2020 | China | NA | A | CT | 2 | NA | NA |
| Liu et al.[30] | 2020 | China | retrospective | A/B | CT | 2 | 2020.1-2020.2 | 2015.1-2020.2 |
| Yildirim et al.[31] | 2022 | Turkey | retrospective | NA | CT | NA | 2020.3.20-2020.8.1 | 2015.1.1-2020.2.1 |
| Kong et al.[32] | 2021 | China | retrospective | A | CT | NA | 2020.1.10-2020.3.1 | 2009.11.27-2009.12.312013.4.3-2013.4.30 |
| Zhang et al.[33] | 2021 | China | NA | A/B | CT | NA | 2020.1-2020.4 | 2018.10-2020.3 |
| Montesinos et al.[34] | 2022 | Spain | retrospective | A/B | X-ray | NA | 2020.3.1-2020.5.1 | 2017.1.1-2019.12.1 |
| Faury et al.[35] | 2021 | France | retrospective | NA | CT | NA | 2020.1.1-2020.3.25 | 2020.1.1-2020.3.25 |
| Wang et al.[36] | 2020 | China | retrospective | A/B | CT | 2 | 2020.1.16-2020.2.25 | 2019.1.1-2020.2.25 |
| Dabaja-Younis et al.[37] | 2022 | Israel | retrospective | NA | X-ray | NA | 2020.6.1-2020.8.31 | 2019.11.1-2020.8.31 |
| Zarei et al.[36] | 2021 | Iran | retrospective | A | CT | 3 | 2020.3.1-2020.7.20 | 2020.3.1-2020.7.20 |
| Marcoux et al.[39] | 2022 | Belgium | retrospective | A/B | CT | NA | -2020.3.13 | 2015.1.1-2020.4.20 |
| Shen et al.[40] | 2020 | China | retrospective | A | CT | 1 | 2020.1.22-2020.2.20 | 2018-2019 |
| Gu et al.[41] | 2022 | China | retrospective | A | CT | NA | 2020.1-2020.3 | 2014-2016 |
COVID-19: coronavirus disease 2019, NA: not applicable.
Forest plot of differences in the distribution of lung lesions on chest images between COVID-19 patients and influenza patients: (3A) unilateral lung and (3B) bilateral lung.
Forest plot of differences in the distribution of lung lesions on chest images between COVID-19 patients and influenza patients: (4A) right upper lobe, (4B) right middle lobe, (4C) right lower lobe, (4D) left upper lobe, and (4E) left lower lobe.

Manifestations of lesions (Tables S4-S6, Figures S1-S18)
Patients with COVID-19 showed more ground-glass opacities (GGO) (OR=2.83, 95% CI: 1.85-4.32, I2=69.7%, p<0.001) and crazy-paving patterns (OR=2.63, 95% CI: 1.57-4.41, I2=68.5%, p<0.001) on chest images than patients with influenza. However, there was no significant difference in the proportion of consolidation between the two groups (OR=0.78, 95% CI: 0.55-1.10, I2=66.9%, p=0.156). We divided the patients' pulmonary nodules into two categories, namely, nodules with non-tree-in-bud (OR=0.71, 95% CI: 0.34-1.49, I2=76.3%, p=0.369) and nodules with tree-in-bud (OR=0.42, 95% CI: 0.13-1.37, I2=88.5%, p=0.152). The results showed that patients with COVID-19 and patients with influenza had approximately the same probability of having these two types of nodules. In terms of effusion, we found that pleural effusion was rare in COVID-19 patients (OR=0.15, 95% CI: 0.07-0.31, I2=83.4%, p<0.001), while no significant difference was shown between the two types of patients regarding pericardial effusion (OR=0.25, 95% CI: 0.57-1.26, I2=0.0%, p=0.164). We also found that reverse halo signs (OR=3.47, 95% CI:2.37-5.08, I2=0.0%, p<0.001), interlobular septal thickening (OR=2.16, 95% CI: 1.55-3.01, I2=0.0%, p<0.001) and vascular enlargement (OR=5.00, 95% CI:1.80-13.85, I2=72.7%, p<0.001) were more common on chest images of COVID-19 patients, while compared with influenza patients, there was no significant difference in the characteristics of halo signs (OR=1.14, 95% CI: 0.80-1.63, I2=0.0%, p=0.479), linear opacities (OR=2.08, 95% CI:0.75-5.77, I2=92.7%, p=0.161), cavitation (OR=0.71, 95% CI: 0.22-2.30, I2=48.4%, p=0.573), lymphadenopathy (OR=0.79, 95% CI: 0.55-1.14, I2=5.0%, p=0.211), air bronchogram (OR=1.39, 95% CI:0.85-2.24, I2=74.8%, p=0.186), bronchiectasis (OR=0.33, 95% CI: 0.02-5.67, I2=87.7%, p=0.445), bronchial wall thickening (OR=1.21, 95% CI: 0.64-2.29, I2=66.8%, p=0.568) and pleural thickening (OR=1.27, 95% CI: 0.54-3.00, I2=61.0%, p=0.588).
Reporting biases
Funnel plots and Egger's test were used for reporting bias analysis, and most of the results were not found to have reporting bias (Figures S19-S80).
Heterogeneity
In our study, a few results showed high heterogeneity, and we tried to perform a subgroup analysis by using regions or influenza subtypes as the basis for classification. However, unfortunately, we did not find the exact source of heterogeneity.
Forest plot of differences in the distribution of lung lesions on chest images between COVID-19 patients and influenza patients: (5A) 0-1 lobes involved, (5B) 2-3 lobes involved, and (5C) 4-5 lobes involved.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to compare the differences in chest images between patients with COVID-19 and those with influenza based on case‒control studies. Pormohammad et al. [42] and Altmayer et al. [43] conducted similar studies, but their data were all from nonrandomized controlled trials, observational studies, and case series, which meant a lack of control groups. The data retrieved in both articles were up to April 2020, and related case‒control studies were lacking because the COVID-19 epidemic had just broken out at that time. Pormohammad et al. did not focus on chest images; they made only a simple comparison of patients' abnormal chest radiology and mainly studied the clinical characteristics and laboratory findings of the patients. Altmayer et al. compared adenovirus, rhinovirus, parainfluenza virus, respiratory syncytial virus and influenza virus as "other viruses" with SARS-CoV-2 and did not specifically study differences between patients with COVID-19 and those with influenza.
These two viruses primarily affect the respiratory system; SARS-CoV-2 can easily reach the periphery of the lung and, as does SARS, bind to angiotensin-converting enzyme 2 receptors in alveoli, bronchioles, and terminal bronchioles, which explains why the lesions associated with COVID-19 are mainly in the periphery of the lung. In contrast, the α2,6-linked sialic acid-bearing receptors, to which influenza viruses preferentially bind, are abundant in the human upper and lower respiratory tract, particularly in the tracheobronchial epithelium and type I alveolar cells. Thus, the pulmonary lesions of influenza are not distributed mainly in the peripheral lung but in the central or whole lung [25]. GGO are hazy areas with increased lung density that do not obscure bronchial and vascular markings. The pathological features of GGO can usually be attributed to the partial displacement of air from partial filling of air spaces, thickening of interstitial tissues from fluid or cells, partial alveolar collapse, or increased capillary blood volume [44]. Interlobular septal thickening is a common sign on chest CT and is visible in interstitial fluid, cellular infiltration, or fibrosis. This sign can be found in a variety of viral pneumonias [17]. Our study showed that compared with influenza patients, COVID-19 patients showed more GGO and interlobular septal thickening on chest images. Therefore, it is not surprising that our other finding, that is, the proportion of crazy-paving patterns in COVID-19 patients, is higher. The crazy-paving pattern is defined as interlobular septal thickening with superimposed GGO, which is one of the worsening lesions of GGOs [22]. Pleural effusion means that the pleural space is filled with fluid, which may be transudative-normal pleural fluid or exudative fluid from infection [45]. Pleural effusion may indicate bacterial superinfection, which is a serious complication of COVID-19 [46]. Chen et al. noted that in COVID-19 patients, pleural effusion showed an even higher odds ratio for severe course and mortality than pulmonary consolidation (3.31 versus 2.46) [47]. Our results showed that patients with influenza were more prone to pleural effusion than patients with COVID-19, which may be due to the tendency of influenza virus to affect large and small airways and lung parenchyma, leading to excessive mucus production [48]. Vascular enlargement is a common imaging finding in patients with COVID-19. In the study of Ghayda et al., the probability of vascular enlargement in the chest of COVID-19 patients was even higher than that of GGO (84.8% versus 60.1%) [49]. The reverse halo sign is defined as a focal, rounded area of ground-glass attenuation surrounded by a more or less complete consolidation ring [50]. Although the reverse halo sign is not as common as vascular enlargement in patients with COVID-19, we found that it still showed a significant difference compared with influenza patients and can be used as an imaging index to distinguish the two virus infections.
The health status after undergoing COVID-19 should also be paid enough attention. Infection with SARS-CoV-2 may have a long-term consequences on patients. Kozlik et al. pointed out that chronic kidney disease, diabetes mellitus, sex and vaccination could affect the quality of life after COVID-19 disease [51]. The chest images of patients with COVID-19 and influenza may change with disease progression [7]. Because most of the included articles were retrospective studies, we could not determine the stage of disease progression in all patients at the time of admission for imaging examination, but the patients in the experimental group and the control group were roughly at the same stage of disease at the time of screening in each included article. Therefore, the results of our study can reflect the differences in chest images of the two virus infections, which is of great significance for the diagnosis of other coronavirus diseases that may appear in the future. Accurate diagnosis of new infectious diseases during the first time can help local governments take corresponding measures to prevent the spread of the virus more quickly and control the epidemic in local areas, which can prevent the formation of a worldwide pandemic, such as COVID-19.
Limitations
This meta-analysis has the following limitations. 1) In some of the included studies, it was not clear whether the study used blinding correctly, that is, whether the radiologist was aware of the results of the RT‒PCR test or the laboratory results, which might lead to subjective interpretation of the obtained chest image results. 2) The data came from different medical institutions, and the scanning parameters and image quality of their equipment were different, which might affect the interpretation of certain imaging details.
Conclusions
There are some differences in the manifestations and distributions of lesions between patients with COVID-19 and influenza on chest images, which is helpful to distinguish these two infectious diseases.
Abbreviations
CI: confidence interval; COVID-19: coronavirus disease 2019; CT: computed tomography; GGO: ground-glass opacities; OR: odds ratio; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RT‒PCR: reverse-transcriptase polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Supplementary Material
Supplementary figures and tables.
Availability of data and materials
All data relevant to the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhu N, Zhang D, Wang W. et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733
2. WHO. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
3. Venkatram S, Alapati A, Dileep A, Diaz-Fuentes G. Change in patterns of hospitalization for influenza during COVID-19 surges. Influenza Other Respir Viruses. 2022;16(1):72-78
4. Chowell G, Sullivan P, Rothenberg R. Introduction to symposium: a century after the 1918 influenza pandemic. Ann Epidemiol. 2018;28:265-266
5. ANZIC Influenze Investigators. Critical care services and the H1N1 (2009) influenza epidemic in Australia and New Zealand in 2010: the impact of the second winter epidemic. Crit Care. 2011;15(3):R143
6. Aquino SL, Dunagan DP, Chiles C, Haponik EF. Herpes simplex virus 1 pneumonia: patterns on CT scans and conventional chest radiographs. J Comput Assist Tomogr. 1998;22(5):795-800
7. Fischer T, Baz YE, Scanferla G. et al. Comparison of temporal evolution of computed tomography imaging features in COVID-19 and influenza infections in a multicenter cohort study. Eur J Radiol Open. 2022;9:100431
8. Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260(1):18-39
9. Dai J, Zhou X, Dong D. et al. Human infection with a novel avian-origin influenza A (H7N9) virus: serial chest radiographic and CT findings. Chin Med J (Engl). 2014;127(12):2206-2211
10. Rubin GD, Ryerson CJ, Haramati LB. et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020;158(1):106-116
11. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020;30(9):4930-4942
12. Duarte ML, Santos LRD, Contenças ACS, Iared W, Peccin MS, Atallah AN. Reverse-transcriptase polymerase chain reaction versus chest computed tomography for detecting early symptoms of COVID-19. A diagnostic accuracy systematic review and meta-analysis. Sao Paulo Med J. 2020;138(5):422-432
13. Kufel J, Bargieł-Łączek K, Kocot S. et al. What Is Machine Learning, Artificial Neural Networks and Deep Learning?-Examples of Practical Applications in Medicine. Diagnostics (Basel). 2023;13(15):2582
14. Kufel J, Bargieł K, Koźlik M. et al. Application of artificial intelligence in diagnosing COVID-19 disease symptoms on chest X-rays: A systematic review. Int J Med Sci. 2022;19(12):1743-1752
15. Parczewski M, Kufel J, Aksak-Wąs B. et al. Artificial neural network based prediction of the lung tissue involvement as an independent in-hospital mortality and mechanical ventilation risk factor in COVID-19. J Med Virol. 2023;95(5):e28787
16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560
17. Lin L, Fu G, Chen S. et al. CT Manifestations of Coronavirus Disease (COVID-19) Pneumonia and Influenza Virus Pneumonia: A Comparative Study. AJR Am J Roentgenol. 2021;216(1):71-79
18. Li Y, Long S, Zhao Y, Ge Z, Wu W, Xia J. High-Resolution Computed Tomography in the Differential Diagnosis between Imported COVID-19 and Seasonal Influenza Pneumonia. Iran Red Crescent Med J. 2020;22(9):e109
19. Garrana SH, Som A, Ndakwah GS. et al. Comparison of Chest CT Findings of COVID-19, Influenza, and Organizing Pneumonia: A Multireader Study. AJR Am J Roentgenol. 2021;217(5):1093-1102
20. So TY, Yu SCH, Wong WT, Wong JKT, Lee H, Wang YX. Chest computed tomography analysis of lung sparing morphology: differentiation of COVID-19 pneumonia from influenza pneumonia and bacterial pneumonia using the arched bridge and vacuole signs. Hong Kong Med J. 2023;29(1):39-48
21. Yang Z, Lin D, Chen X. et al. Distinguishing COVID-19 From Influenza Pneumonia in the Early Stage Through CT Imaging and Clinical Features. Front Microbiol. 2022;13:847836
22. Yin Z, Kang Z, Yang D, Ding S, Ding ZH, Xiao E. A Comparison of Clinical and Chest CT Findings in Patients With Influenza A (H1N1) Virus Infection and Coronavirus Disease (COVID-19). AJR Am J Roentgenol. 2020;215(5):1065-1071
23. Lv Y, Yu G, Zhang X. et al. Comparative analysis of elderly hospitalized patients with coronavirus disease 2019 or influenza A H1N1 virus infections. International Journal of Infectious Diseases. 2022;125:278-284
24. Kim SH, Wi YM, Lim S, Han KT, Bae IG. Differences in Clinical Characteristics and Chest Images between Coronavirus Disease 2019 and Influenza-Associated Pneumonia. Diagnostics (Basel). 2021;11(2):261
25. Zhao S, Huang Z, Zeng H. et al. Combining initial chest CT with clinical variables in differentiating coronavirus disease 2019 (COVID-19) pneumonia from influenza pneumonia. Sci Rep. 2021;11(1):6422
26. Kuang PD, Wang C, Zheng HP. et al. Comparison of the clinical and CT features between COVID-19 and H1N1 influenza pneumonia patients in Zhejiang, China. Eur Rev Med Pharmacol Sci. 2021;25(2):1135-1145
27. Nasir N, Khanum I, Habib K. et al. Comparison of clinical characteristics and outcomes between COVID-19 pneumonia and H1N1 influenza. Adv Respir Med. 2021;89(3):254-261
28. Cobb NL, Sathe NA, Duan KI. et al. Comparison of Clinical Features and Outcomes in Critically Ill Patients Hospitalized with COVID-19 versus Influenza. Ann Am Thorac Soc. 2021;18(4):632-640
29. Zhang M, Xu J, Wang Z, Li X, Yu Y, Huang G. Initial Chest CT Features Between COVID-19 and Influenza A Virus-Induced Pneumonia: A Comparative Study. Chinese Journal of Medical Imaging. 2020;28(12):891-895
30. Liu M, Zeng W, Wen Y, Zheng Y, Lv F, Xiao K. COVID-19 pneumonia: CT findings of 122 patients and differentiation from influenza pneumonia. Eur Radiol. 2020;30(10):5463-5469
31. Yildirim M, Halacli B, Pektezel MY. et al. Comparison of critically ill COVID-19 and influenza patients with acute respiratory failure. Acute Crit Care. 2022;37(2):168-176
32. Kong J, Hao Y, Wan S. et al. Comparative study of hematological and radiological feature of severe/critically ill patients with COVID-19, influenza A H7N9, and H1N1 pneumonia. J Clin Lab Anal. 2021;35(12):e24100
33. Zhang J, Ding D, Huang X. et al. Differentiation of COVID-19 from seasonal influenza: A multicenter comparative study. J Med Virol. 2021;93(3):1512-1519
34. Montesinos IL, Arrieta-Aldea I, Dicastillo A. et al. Comparison of Hospitalized Coronavirus Disease 2019 and Influenza Patients Requiring Supplemental Oxygen in a Cohort Study: Clinical Impact and Resource Consumption. Clin Infect Dis. 2022;75(12):2225-2238
35. Faury H, Courboules C, Payen M. et al. Medical features of COVID-19 and influenza infection: A comparative study in Paris, France. J Infect. 2021;82(2):e36-39
36. Wang H, Wei R, Rao G, Zhu J, Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID-19) from influenza pneumonia. Eur Radiol. 2020;30(9):4910-4917
37. Dabaja-Younis H, Fuchs E, Shorbaji N. et al. SARS-CoV-2 and seasonal influenza: similarities and disparities. Arch Virol. 2022;167(12):2761-2765
38. Zarei F, Jalli R, Iranpour P. et al. Differentiation of Chest CT Findings Between Influenza Pneumonia and COVID-19: Interobserver Agreement Between Radiologists. Acad Radiol. 2021;28(10):1331-1338
39. Marcoux D, Etienne I, Van Muylem A, Bogossian EG, Yin N, Taccone FS. A Retrospective, Monocentric Study Comparing Co and Secondary Infections in Critically Ill COVID-19 and Influenza Patients. Antibiotics (Basel). 2022;11(6):704
40. Shen C, Tan M, Song X. et al. Comparative Analysis of Early-Stage Clinical Features Between COVID-19 and Influenza A H1N1 Virus Pneumonia. Front Public Health. 2020;8:206
41. Gu B, Yao L, Zhu X, Tang P, Chen C. Comparison of hospitalized patients with severe pneumonia caused by COVID-19 and influenza A (H7N9 and H1N1): A retrospective study from a designated hospital. Open Med (Wars). 2022;17(1):1965-1972
42. Pormohammad A, Ghorbani S, Khatami A. et al. Comparison of influenza type A and B with COVID-19: A global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Reviews in Medical Virology. 2021;31(3):e2179
43. Altmayer S, Zanon M, Pacini GS. et al. Comparison of the computed tomography findings in COVID-19 and other viral pneumonia in immunocompetent adults: a systematic review and meta-analysis. European Radiology. 2020;30(12):6485-6496
44. Li P, Su DJ, Zhang JF, Xia XD, Sui H, Zhao DH. Pneumonia in novel swine-origin influenza A (H1N1) virus infection: high-resolution CT findings. Eur J Radiol. 2011;80(2):e146-152
45. Hoffman M. “What is a pleural effusion?”. https://www.webmd.com/lung/pleural-effusion-symptoms-causes-treatments
46. Meyer HJ, Wienke A, Surov A. Extrapulmonary CT Findings Predict In-Hospital Mortality in COVID-19. A Systematic Review and Meta-Analysis. Acad Radiol. 2022;29(1):17-30
47. Chen Q, Xu L, Zhu W, Ge J. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin Cardiol. 2020;43(10):1054
48. Deng J, Zheng Y, Li C, Ma Z, Wang H, Rubin BK. Plastic bronchitis in three children associated with 2009 influenza A (H1N1) virus infection. Chest. 2010;138(6):1486-1488
49. Ghayda RA, Lee KH, Kim JS. et al. Chest CT abnormalities in COVID-19: a systematic review. Int J Med Sci. 2021;18(15):3395-3402
50. Secrest S, Sakamoto K. Halo and reverse halo signs in canine pulmonary computed tomography. Vet Radiol Ultrasound. 2014;55(3):272-277
51. Koźlik M, Kaźmierski M, Kaźmierski W. et al. Quality of Life 6 Months after COVID-19 Hospitalisation:A Single-Centre Polish Registry. J Clin Med. 2023;12(16):5327
Author contact
![]() Corresponding author: Zhuan Zhong, Department of Orthopaedics, The Second Hospital of Jilin University, Changchun, Jilin Province, China. Postcode: 130000. Email: zhongzhuanedu.cn.
Corresponding author: Zhuan Zhong, Department of Orthopaedics, The Second Hospital of Jilin University, Changchun, Jilin Province, China. Postcode: 130000. Email: zhongzhuanedu.cn.

 Global reach, higher impact
Global reach, higher impact