3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(3):551-557. doi:10.7150/ijms.105022 This issue Cite
Research Paper
The relationship between long noncoding RNA H19 genotypes and the clinical features of diabetic retinopathy
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Department of Ophthalmology, Show Chwan Memorial Hospital, Changhua, Taiwan.
3. Department of Ophthalmology, Cathay General Hospital, Taipei, Taiwan.
4. Departments of Ophthalmology, Sijhih Cathay General Hospital, New Taipei City, Taiwan.
5. School of Medicine, National Tsing Hua University, Hsinchu, Taiwan.
6. School of Medicine, College of Medicine, Fu Jen Catholic University, New Taipei, Taiwan.
7. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
8. Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan.
9. Department of Optometry, Chung Shan Medical University, Taichung, Taiwan.
Received 2024-10-11; Accepted 2024-12-14; Published 2025-1-1
Abstract
Diabetic retinopathy (DR) is a microvascular complication of diabetes characterized by an inflammatory response. The H19 gene plays a role in regulating inflammation and is associated with chronic systemic inflammation. This study aims to investigate the potential correlation between single-nucleotide polymorphisms (SNPs) in the H19 gene and the development of DR. Five loci of H19 SNPs—rs3024270 (C/G), rs2839698 (C/T), rs3741219 (A/G), rs2107425 (C/T), and rs217727 (C/T)—were genotyped using TaqMan allelic discrimination in 454 individuals without DR and 272 DR participants. The results indicate that the H19 SNP rs3741219 AG (p = 0.030) and AG+GG (p = 0.037) alleles are significantly associated with an increased risk of developing DR in individuals with diabetes onset before the age of 45. Additionally, diabetic individuals with the H19 SNP rs3741219 AG+GG genotype also showed significantly higher serum creatinine (p = 0.034), lower glomerular filtration rate (GFR) (p = 0.013), higher total cholesterol/HDL ratio (p = 0.031), and higher triglycerides (p = 0.012). In an age-based subgroup analysis, GFR was significantly lower in diabetic patients with an onset of diabetes before 45 years and with the H19 SNP rs3741219 AG+GG genotype (p = 0.012). In conclusion, the presence of the H19 SNP rs3741219 variant is associated with a higher risk of DR in individuals with early-onset diabetes, and the relationship between the rs3741219 variant and decreased GFR is particularly pronounced in this population.
Keywords: diabetic retinopathy, H19, single-nucleotide polymorphisms, glomerular filtration rate, age
Introduction
Diabetes mellitus is a widespread disease characterized by insulin deficiency, pancreatic β-cell dysfunction, and insulin resistance in the human body [1]. It can lead to several vascular complications, one of which is diabetic retinopathy (DR), a condition with high prevalence and an annual incidence exceeding 10 percent among diabetic patients [2-4]. In Asia, the risk of DR and DR-related visual impairment has increased in recent years, and advanced DR can result in significant visual disability [5, 6]. The most critical and foundational treatment for DR is the management of hyperglycemia, while intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) and panretinal photocoagulation may be necessary for severe cases of DR [7].
Certain parameters have been correlated with the development of DR in previous publications [8]. Hyperglycemia and elevated glycated hemoglobin (HbA1c) concentrations are well-established indicators for the development of DR in individuals with diabetes [5, 9]. Specifically, the incidence of DR increases by 15 percent for each 1 percent rise in HbA1c levels [8]. Additionally, dyslipidemia, including elevated serum LDL and triglyceride levels, has also been associated with the development of DR in earlier studies [10]. Inflammatory biomarkers, such as interleukin and C-reactive protein, are predictors for the onset of DR [11]. Regarding the genetic aspect, single nucleotide polymorphisms (SNPs) in the CDKN2B-AS1, MEG3, and GAS5 genes have been linked to a higher incidence of advanced DR [12-14].
The H19 gene is a long noncoding RNA that regulates cell proliferation and tumorigenesis in humans [15-18]. The presence of the H19 gene has been associated with an increased risk of colorectal and gastric cancers [16], while polymorphisms in the H19 gene have been shown to influence the incidence of bladder cancer [19].
However, the relationship between H19 gene polymorphisms and the risk of DR remains unclear. Given that the H19 gene is also involved in inflammation, a key pathophysiological factor in DR [5, 20], a potential correlation between them may exist.
Therefore, the objective of the present study is to evaluate the potential association between H19 gene SNPs and the clinical manifestations of DR in diabetic patients. A subgroup analysis will be conducted to examine patients with different ages separately.
Materials and Methods
Ethic declarations
All interventions in the present study adhered to the Declaration of Helsinki (1964) and its subsequent amendments. The study was approved by the Institutional Review Board of Chung Shan Medical University Hospital (project identification code: CS2-22190). Written informed consent was obtained from all participants in the study.
Participant selection
A total of 726 diabetic participants were recruited from Chung Shan Medical University Hospital in Taichung, Taiwan. Of these, 454 participants were assigned to the non-DR group, and 272 participants to the DR group. Medical records for all participants were reviewed, and the presence of DR was defined by the existence of any of the following ophthalmic conditions: dot or flame-shaped hemorrhages, microaneurysms, hard exudates, intraretinal microvascular abnormalities, cotton-wool spots, venous beading, or retinal neovascularization. Among the study population, 223 participants were aged 45 years or younger, and 503 participants were older than 45 years.
Medical profiles and samples obtainment
Medical information, including age at DR onset, sex, diabetes duration, HbA1c level, serum lipid concentrations, and kidney function (specifically serum creatinine and glomerular filtration rate, or GFR), was collected. For the H19 gene and genetic polymorphism analysis, blood samples were drawn from the vein of each participant and stored in tubes containing ethylenediaminetetraacetic acid (EDTA). The samples were then centrifuged and stored in a special freezer at -80°C. If a participant's genomic DNA or blood sample degraded before DNA analysis, that participant was excluded from the study.
DNA determination of H19 SNPs by Real-Time PCR
Five H19 SNPs, including rs3024270 (C/G), rs2839698 (C/T), rs3741219 (A/G), rs2107425 (C/T), and rs217727 (C/T), were selected based on the fact that the genotype frequencies of the minor alleles of these SNPs were greater than 5%, and because previous studies have linked these SNPs to the occurrence of specific diseases [21-25]. The DNA extraction procedures in this study followed the methods described in a previous publication [26]. Genomic DNA from leukocytes in venous blood samples was extracted using QIAamp DNA kits (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The isolated DNA was stored at -20°C. The polymorphisms of the H19 SNPs (rs3024270, assay ID: C_15833426_10; rs2839698, assay ID: C_2603701_10; rs3741219, assay ID: C_27492510_10; rs2107425, assay ID: C_16032886_10; rs217727, assay ID: C_2603707_10) were subsequently analyzed using the ABI StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA), and the results were further processed using SDS version 3.0 software (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
Statistical analyses in the present study were conducted using SAS version 9.4 (SAS Institute Inc., NC, USA). The independent t-test and Chi-square test were used to assess differences in baseline characteristics between the two groups. Subsequently, multiple logistic regression analysis was performed to estimate the adjusted odds ratios (AORs) with 95% confidence intervals (CIs) for the frequencies of five SNPs between the non-DR and DR groups. The multiple logistic regression model accounted for potential confounders, including age, duration of diabetes, HbA1c levels, serum creatinine, glomerular filtration rate (GFR), HDL cholesterol, and the total cholesterol/HDL ratio. A p-value of less than 0.05 was considered statistically significant.
Results
Initial features between the non-DR group and DR group
The baseline characteristics of the two groups are presented in Table 1. The mean age was significantly lower in the non-DR group (60.22 ± 11.22 years) compared to the DR group (62.59 ± 10.79 years; p = 0.005). However, there was no significant difference in the age of diabetes onset between the two groups (50.84 ± 10.78 years versus 50.59 ± 11.17 years; p = 0.767), and the sex distribution did not differ significantly between the groups (p = 0.550). Regarding laboratory data, the DR group exhibited significantly longer duration of diabetes, higher HbA1c levels, higher serum creatinine, lower glomerular filtration rate (GFR), lower HDL cholesterol, and a lower total cholesterol/HDL ratio compared to the non-DR group (all p < 0.05) (Table 1).
Clinical and laboratory characteristics of patients with diabetic retinopathy and no diabetic retinopathy.
| Variable | Non-DR group (N=454) | DR group (N=272) | p value |
|---|---|---|---|
| Age (years) | 60.22 ± 11.22 | 62.59 ± 10.79 | 0.005* |
| Age of onset (years) | 50.84 ± 10.78 | 50.59 ± 11.17 | 0.767 |
| Male gender [n (%)] | 240 (52.9%) | 150 (55.1%) | 0.550 |
| Duration of diabetes (years) | 9.38 ± 7.03 | 12.00 ± 7.98 | <0.001* |
| HbA1c [% (mmol/mol)] | 6.96 ± 0.99 | 7.58 ± 1.42 | <0.001* |
| Serum creatinine [mg/dL] | 0.89 ± 0.35 | 1.56 ± 1.85 | <0.001* |
| GFR [ml/min] | 78.44 ± 27.69 | 62.49 ± 33.95 | <0.001* |
| Total cholesterol [mmol/L] | 160.52 ± 43.04 | 165.38 ± 47.65 | 0.165 |
| HDL cholesterol [μmol/L] | 46.29 ± 12.65 | 43.65 ± 13.45 | 0.009* |
| Total cholesterol/HDL ratio | 3.70 ± 2.08 | 4.06 ± 1.51 | 0.016* |
| LDL cholesterol [μmol/L] | 86.60 ± 28.22 | 86.44 ± 32.78 | 0.948 |
| Triglycerides [μmol/L] | 140.19 ± 164.95 | 158.08 ± 118.39 | 0.127 |
DR: diabetic retinopathy, GFR: glomerular filtration rate, HbA1c: glycated hemoglobin, N: number
* denotes significant difference between groups
The genotype frequency of H19 SNP between the non-DR group and DR group
Regarding the distribution of H19 SNP genotypes between the non-DR and DR populations, none of the five H19 SNPs showed a significant association with the development of DR (all p > 0.05) (Table 2). In the age-based subgroup analysis, the H19 SNP rs3741219 AG genotype (AOR: 1.888, 95% CI: 1.065-3.348, p = 0.030) and the AG+GG genotype combination (AOR: 1.778, 95% CI: 1.034-3.057, p = 0.037) were significantly associated with an increased risk of DR development in individuals with diabetes onset before the age of 45 (Table 3). In contrast, no other H19 SNPs showed a significant association with DR development in this age subgroup (all p > 0.05). In the subgroup of individuals with diabetes onset after the age of 45, none of the H19 SNPs were significantly associated with an increased risk of DR (all p > 0.05) (Table 3).
Odds ratio and 95% confidence interval of diabetic retinopathy associated with H19 genotypic frequencies.
| Variable | Non-DR group (N=454) | DR group (N=272) | AOR (95% CI) | p value |
|---|---|---|---|---|
| rs3024270 | ||||
| CC | 125 (27.5%) | 79 (29.0%) | 1.000 | |
| CG | 219 (48.2%) | 138 (50.7%) | 0.858 (0.577-1.275) | 0.448 |
| GG | 110 (24.3%) | 55 (20.3%) | 0.795 (0.493-1.280) | 0.345 |
| CG+GG | 329 (72.5%) | 193 (71.0%) | 0.915 (0.760-1.102) | 0.349 |
| rs2839698 | ||||
| CC | 212 (46.7%) | 127 (46.7%) | 1.000 | |
| CT | 196 (43.2%) | 117 (43.0%) | 0.829 (0.578-1.189) | 0.308 |
| TT | 46 (10.1%) | 28 (10.3%) | 1.084 (0.611-1.924) | 0.783 |
| CT+TT | 242 (53.3%) | 145 (53.3%) | 0.935 (0.788-1.108) | 0.438 |
| rs3741219 | ||||
| AA | 206 (45.4%) | 122 (44.9%) | 1.000 | |
| AG | 200 (44.1%) | 121 (44.5%) | 0.819 (0.571-1.176) | 0.279 |
| GG | 48 (10.5%) | 29 (10.6%) | 1.047 (0.592-1.851) | 0.875 |
| AG+GG | 248 (54.6%) | 150 (55.1%) | 0.927 (0.781-1.100) | 0.386 |
| rs2107425 | ||||
| CC | 170 (37.4%) | 102 (37.5%) | 1.000 | |
| CT | 213 (46.9%) | 128 (47.1%) | 0.860 (0.592-1.249) | 0.428 |
| TT | 71 (15.6%) | 42 (15.4%) | 1.084 (0.653-1.799) | 0.754 |
| CT+TT | 284 (62.6%) | 170 (62.5%) | 0.955 (0.802-1.138) | 0.605 |
| rs217727 | ||||
| CC | 192 (42.3%) | 119 (43.8%) | 1.000 | |
| CT | 206 (45.4%) | 122 (44.9%) | 0.909 (0.632-1.307) | 0.605 |
| TT | 56 (12.3%) | 31 (11.4%) | 1.052 (0.609-1.814) | 0.857 |
| CT+TT | 262 (57.7%) | 153 (56.3%) | 0.968 (0.816-1.150) | 0.713 |
AOR: adjusted odds ratio, CI: confidence intervals, N: number
The adjusted odds ratio (AOR) with their 95% confidence intervals were estimated by multiple logistic regression models after controlling for age, the duration of diabetes, HbA1c, serum creatinine levels, glomerular filtration rate, HDL cholesterol levels and total cholesterol/HDL ratio.
Correlation between the clinical features and the H19 SNP rs3741219 genotypes
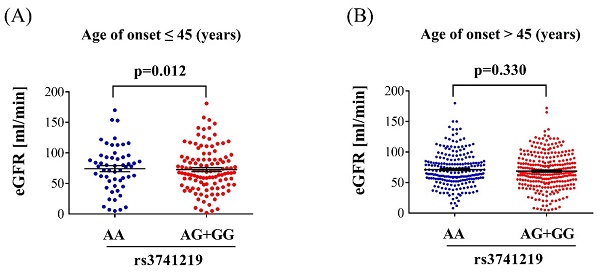
Regarding the clinical characteristics of individuals with diabetes and different H19 SNP rs3741219 genotypes, those with the H19 SNP rs3741219 AG+GG genotype was associated with significantly higher serum creatinine levels (p = 0.034), lower glomerular filtration rate (GFR) (p = 0.013), higher total cholesterol/HDL ratio (p = 0.031), and elevated triglyceride levels (p = 0.012) (Table 4). No significant associations were observed between the H19 SNP rs3741219 genotypes and other clinical characteristics (all p > 0.05) (Table 4). In the subgroup of individuals with diabetes onset before the age of 45, GFR was significantly lower in those with the H19 SNP rs3741219 AG+GG genotype compared to those with the AA genotype (72.66 ± 35.19 versus 84.59 ± 34.84, p = 0.012) (Figure 1A). In contrast, no significant association between GFR and H19 SNP rs3741219 genotypes was observed in individuals with diabetes onset after the age of 45 (68.85 ± 29.07 versus 71.37 ± 27.89, p = 0.330) (Figure 1B).
Odds ratio and 95% confidence interval of H19 genotypic frequencies and diabetic retinopathy with different onset age.
| Variable | Age of onset ≤ 45 (years) (N=223) | Age of onset >45 (years) (N=503) | ||||
|---|---|---|---|---|---|---|
| Non-DR group (N=132) | DR group (N=91) | p value | Non-DR group (N=322) | DR group (N=181) | p value | |
| rs3024270 | ||||||
| CC | 43 (32.6%) | 28 (30.8%) | 82 (25.5%) | 51 (28.2%) | ||
| CG | 60 (45.4%) | 46 (50.5%) | 0.601 | 159 (49.4%) | 92 (50.8%) | 0.744 |
| GG | 29 (22.0%) | 17 (18.7%) | 0.788 | 81 (25.1%) | 38 (21.0%) | 0.288 |
| CG+GG | 89 (67.4%) | 63 (69.2%) | 0.776 | 240 (74.5%) | 130 (71.8%) | 0.508 |
| rs2839698 | ||||||
| CC | 71 (53.8%) | 41 (45.1%) | 141 (43.8%) | 86 (47.5%) | ||
| CT | 47 (35.6%) | 40 (44.0%) | 0.183 | 149 (46.3%) | 77 (42.5%) | 0.398 |
| TT | 14 (10.6%) | 10 (11.0%) | 0.643 | 32 (9.9%) | 18 (10.0%) | 0.803 |
| CT+TT | 61 (46.2%) | 50 (54.9%) | 0.200 | 181 (56.2%) | 95 (52.5%) | 0.420 |
| rs3741219 | ||||||
| AA | 71 (53.8%) | 36 (39.6%) | 135 (41.9%) | 86 (47.5%) | ||
| AG | 47 (35.6%) | 45 (49.4%) | 0.030*,a | 153 (47.5%) | 76 (42.0%) | 0.206 |
| GG | 14 (10.6%) | 10 (11.0%) | 0.458 | 34 (10.6%) | 19 (10.5%) | 0.680 |
| AG+GG | 61 (46.2%) | 55 (60.4%) | 0.037*,b | 187 (58.1%) | 95 (52.5%) | 0.226 |
| rs2107425 | ||||||
| CC | 49 (37.1%) | 29 (31.9%) | 121 (37.6%) | 73 (40.3%) | ||
| CT | 53 (40.2%) | 44 (48.4%) | 0.276 | 160 (49.7%) | 84 (46.4%) | 0.488 |
| TT | 30 (22.7%) | 18 (19.7%) | 0.971 | 41 (12.7%) | 24 (13.3%) | 0.919 |
| CT+TT | 83 (62.9%) | 62 (68.1%) | 0.419 | 201 (62.4%) | 108 (59.7%) | 0.543 |
| rs217727 | ||||||
| CC | 54 (40.9%) | 36 (49.6%) | 138 (42.9%) | 83 (45.9%) | ||
| CT | 55 (41.7%) | 41 (45.1%) | 0.708 | 151 (46.9%) | 81 (44.8%) | 0.559 |
| TT | 23 (17.4%) | 14 (15.4%) | 0.821 | 33 (10.2%) | 17 (9.4%) | 0.638 |
| CT+TT | 78 (59.1%) | 55 (60.4%) | 0.840 | 184 (57.1%) | 98 (54.1%) | 0.515 |
DR: diabetic retinopathy, N: number
* denotes significant difference between groups
aOR (95% CI): 1.888 (1.065-3.348)
bOR (95% CI): 1.778 (1.034-3.057)
Clinical characteristics of diabetes patients according to H19 rs3741219 genotypes.
| Variable# | H19 rs3741219 | ||
|---|---|---|---|
| AA (n=328) | AG+GG (n=398) | p value | |
| Duration of diabetes (years) | 10.11 ± 7.56 | 10.57 ± 7.45 | 0.411 |
| HbA1c [% (mmol/mol)] | 7.19 ± 1.26 | 7.19 ± 1.15 | 0.990 |
| Serum creatinine [mg/dL] | 1.03 ± 0.86 | 1.22 ± 1.41 | 0.034* |
| GFR [ml/min] | 75.74 ± 30.94 | 69.97 ± 31.01 | 0.013* |
| Total cholesterol [mmol/L] | 160.06 ± 37.86 | 164.10 ± 49.78 | 0.231 |
| HDL cholesterol [μmol/L] | 46.37 ± 13.33 | 44.49 ± 12.66 | 0.055 |
| Total cholesterol/HDL ratio | 3.66 ± 1.19 | 3.97 ± 2.33 | 0.031* |
| LDL cholesterol [μmol/L] | 86.53 ± 28.41 | 86.55 ± 31.16 | 0.995 |
| Triglycerides [μmol/L] | 131.11 ± 92.84 | 159.53 ± 183.58 | 0.012* |
# Quantitative data are represented as means ± standard deviation.
DR: diabetic retinopathy, GFR: glomerular filtration rate, HbA1c: glycated hemoglobin, N: number
* denotes significant difference between group
Discussion
In the present study, a significant association was identified between the H19 SNP rs3741219 variant and the risk of DR development in patients with diabetes onset before the age of 45. Furthermore, the H19 SNP rs3741219 variant was associated with an increased risk of dyslipidemia, elevated serum creatinine levels, and decreased GFR. Notably, decreased GFR was more commonly observed in individuals with diabetes onset before the age of 45 who possessed the H19 SNP rs3741219 variant genotypes.
The glomerular filtration rate in different H19 SNP rs3741219 genotypes. (A) The glomerular filtration rate in the population with diabetes onset before 45 years. (B) The glomerular filtration rate in the population with diabetes onset after 45 years.
The development of DR is influenced by several biochemical and genetic factors, as demonstrated in earlier studies [11, 27, 28]. The hyperglycemic status is the basic component for DR development and the HbA1c can predicted the development of DR [5, 9, 29]. Additionally, inflammatory cytokines, including members of the interleukin family, VEGF, and tumor necrosis factor-alpha (TNF-α), are significantly elevated in individuals with diabetes and DR [11]. Regarding genetic factors, several loci have been linked to an increased risk of DR. Specifically, genetic variants at the PPARG and KCNJ11 loci are associated with a higher incidence of DR development [6]. Furthermore, MEG3 methylation has been shown to accelerate endothelial-mesenchymal transition, a critical process in DR formation [30]. Under hyperglycemic conditions, MEG3 expression in the human retina is reduced, suggesting that elevated MEG3 levels may have potential therapeutic implications for DR management [31]. The ALR2 gene, which regulates the polyol pathway, has also been implicated in DR pathogenesis [29]. With regard to genetic polymorphisms and DR, several SNPs have been identified as risk factors for DR. The SELP gene variants rs6128, rs6133, and rs3917779 have been associated with a significantly increased risk of DR development [27]. In addition, SNPs in the MMP gene family, particularly the MMP-2 SNP rs243864 variant, are significantly more prevalent in the DR population [32, 33]. The HOTAIR SNP rs12427129 has also been shown to contribute to a higher risk of proliferative DR in Asian populations [34]. On the other hand, the H19 gene has been implicated in the development of several disorders, including endometriosis and various malignancies [35-37]. The H19 gene is also associated with chronic inflammatory diseases [20], and lower expression of H19 may mitigate hyperglycemia-induced inflammation [38]. In terms of genetic polymorphisms, the H19 SNP rs2839698 variant has been significantly associated with an increased risk of hepatocellular carcinoma [39], and the H19 SNP rs217727 has been linked to a higher risk of breast cancer development [40]. Given that the H19 gene is involved in the pathophysiology of DR and that its genetic polymorphisms can influence the development of various diseases [20, 41], we hypothesize that H19 SNPs may also play a role in DR development in specific populations. This hypothesis is partially supported by the findings of the present study.
In relation to the association between H19 gene SNPs and the presence of DR in the diabetic population, the overall group analysis did not reveal a significant correlation. However, the H19 SNP rs3741219 variant was associated with an increased risk of DR development in patients with diabetes onset before the age of 45. Previous studies have shown a higher prevalence of DR in individuals with MTHFR gene variants [27], and the RAGE gene SNP rs2070600 has also been significantly correlated with DR development [29]. Nevertheless, the association between H19 gene SNPs and DR has not been fully elucidated. To the best of our knowledge, the findings of the present study provide preliminary evidence supporting a significant correlation between the H19 SNP rs3741219 variant and DR in younger diabetic patients (i.e., those under 45 years old). Furthermore, all participants were regularly followed up in the ophthalmology department by a single ophthalmologist, ensuring consistency in the diagnostic criteria for DR. Additionally, we controlled for potential confounding factors, such as age, sex, HbA1c levels, and other laboratory parameters, in the multiple logistic regression model. As a result, the H19 SNP rs3741219 variant may serve as an independent predictor for the development of DR in individuals with diabetes onset before the age of 45. Previous research has indicated that younger diabetic patients may experience more rapid progression of DR [42]. Moreover, diabetes patients under the age of 40 have been shown to have a fivefold increased risk of developing DR compared to those over the age of 60, after adjusting for potential confounders [43]. On the other hand, the H19 SNP rs3741219 variant has been linked to an increased risk of diseases such as breast cancer [40]. Therefore, since the H19 gene influences DR-related factors, including inflammatory and vascular components [38, 44], specific H19 gene polymorphisms may have a distinct impact on the incidence of DR in younger diabetic patients, who typically present with more severe baseline diabetes status compared to their older counterparts.
Regarding the association between clinical characteristics in the diabetic population and polymorphisms in the H19 gene, we found that reduced renal function and dyslipidemia were significantly correlated with the H19 SNP rs3741219 variant. Consistent with earlier studies, the H19 gene has been shown to influence the development of renal fibrosis in diabetic mice [45]. Additionally, the H19 gene is associated with an increased incidence of renal tubular epithelial cell injury [46] , and a higher risk of diabetic kidney disease has been observed in patients with elevated H19 gene expression [47]. LncRNA H19 expression has also been linked to a decreased GFR in patients with chronic kidney disease [48]. Given that the H19 gene contributes to worsening renal conditions, it is plausible that polymorphisms in the H19 gene could exacerbate renal dysfunction, leading to elevated serum creatinine levels and decreased GFR. In terms of lipid metabolism, the presence of the H19 SNP rs3741219 variant was associated with elevated serum lipids. Previous studies have shown that the H19 gene regulates lipid droplet metabolism in hepatic cells [49]. Furthermore, the H19 gene expression is involved in the regulation of atherosclerosis, a condition for which dyslipidemia is a known predictor [50]. Thus, the H19 SNP rs3741219 variant may contribute to higher serum lipid levels.
In the subgroup analysis stratified by age, reduced GFR was predominantly observed in the diabetic population diagnosed before the age of 45. This phenomenon has been rarely reported in the literature. As discussed earlier, younger individuals with diabetes tend to experience more rapid progression of DR [42], and the deterioration of renal function may be more pronounced in this group under the influence of the H19 SNP rs3741219 genotype. These findings align with previous research, demonstrating that the H19 SNP rs3741219 variant is associated with poorer health outcomes in the early-onset diabetes population. However, the precise mechanisms by which the H19 SNP rs3741219 variant contributes to these conditions require further investigation.
From an epidemiological perspective, diabetes is a widespread disease, affecting approximately four million people in China [51]. Among its complications, DR is the most common microvascular complication and affects a significant proportion of the diabetic population [6, 52]. In its advanced or proliferative form, DR can lead to intraocular hemorrhage, retinal detachment, and glaucoma, potentially resulting in permanent visual loss [5]. According to previous studies, advanced DR accounts for approximately 2.6% of cases of irreversible blindness worldwide [6]. Furthermore, diabetic kidney disease (DKD) also affects a large proportion of individuals with diabetes [53], and end-stage renal disease, which requires hemodialysis, is not uncommon in this population [54]. Therefore, the management of diabetic kidney disease is of paramount importance. Given the substantial economic burden associated with both DR and diabetic kidney disease, identifying genetic markers for these conditions is essential for better prevention and management strategies.
There are several limitations to the present study. First, the case-control design employed in this study precludes the evaluation of the relationship between H19 gene polymorphisms and the progression of DR, which would be more appropriately assessed in a cohort design. Additionally, several baseline laboratory parameters in the DR group were significantly worse compared to those in the non-DR group. While genetic polymorphisms are not influenced by these parameters and we controlled for them in the multiple logistic regression model, the substantial differences in baseline characteristics may still introduce potential bias. Moreover, inflammatory biomarkers, such as interleukins and tumor necrosis factor, were not measured in the present study, which could have influenced the results of our statistical analysis. Finally, all participants enrolled in this study were Han Taiwanese, which may limit the generalizability of the findings to other populations, thus reducing the external validity of the study.
In conclusion, the presence of the H19 SNP rs3741219 variant is associated with a higher risk of DR development in individuals with diabetes onset before the age of 45. Furthermore, the H19 SNP rs3741219 variant is also linked to reduced renal function, particularly in those with early-onset diabetes. Therefore, ophthalmic and nephrology referrals may be recommended for individuals with the H19 SNP rs3741219 variant and diabetes onset before 45 years. Future large-scale prospective clinical trials are necessary to further investigate the relationship between H19 gene SNPs and the therapeutic outcomes of DR.
Acknowledgements
We would like to thank the Human Biobank of Chung Shan Medical University Hospital for providing the biological specimens and related clinical data for our research.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239-51
2. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124-36
3. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N. et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-91
4. Sabanayagam C, Banu R, Chee ML, Lee R, Wang YX, Tan G. et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140-9
5. Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12:1322-5
6. Chong YH, Fan Q, Tham YC, Gan A, Tan SP, Tan G. et al. Type 2 Diabetes Genetic Variants and Risk of Diabetic Retinopathy. Ophthalmology. 2017;124:336-42
7. Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci. 2018;19:1816
8. Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8:010803
9. Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Medicine (Baltimore). 2020;99:e19236
10. Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in Diabetic Retinopathy. Rev Diabet Stud. 2015;12:159-95
11. Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović Antunica A. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochem Med (Zagreb). 2020;30:030502
12. Lee CM, Yang YS, Kornelius E, Huang CN, Hsu MY, Lee CY. et al. Association of Long Non-Coding RNA Growth Arrest-Specific 5 Genetic Variants with Diabetic Retinopathy. Genes (Basel). 2022;13:584
13. Chien HW, Wang K, Chao SC, Lee CY, Lin HY, Yang SF. The Genetic Variants of Long Noncoding RNA MEG3 and Its Association to the Clinical Features of Diabetic Retinopathy. Curr Eye Res. 2024;49:980-7
14. Yao YP, Chien HW, Wang K, Yang YS, Su SC, Chang LC. et al. Genetic association of diabetic retinopathy with long noncoding RNA CDKN2B-AS1 gene polymorphism. Eur J Ophthalmol. 2024: 11206721241266704.
15. Ghafouri-Fard S, Esmaeili M, Taheri M. H19 lncRNA: Roles in tumorigenesis. Biomed Pharmacother. 2020;123:109774
16. Yang J, Qi M, Fei X, Wang X, Wang K. LncRNA H19: A novel oncogene in multiple cancers. Int J Biol Sci. 2021;17:3188-208
17. Gandhi P, Wang Y, Li G, Wang S. The role of long noncoding RNAs in ocular angiogenesis and vascular oculopathy. Cell Biosci. 2024;14:39
18. Su SC, Reiter RJ, Hsiao HY, Chung WH, Yang SF. Functional Interaction between Melatonin Signaling and Noncoding RNAs. Trends Endocrinol Metab. 2018;29:435-45
19. Verhaegh GW, Verkleij L, Vermeulen SH, den Heijer M, Witjes JA, Kiemeney LA. Polymorphisms in the H19 gene and the risk of bladder cancer. Eur Urol. 2008;54:1118-26
20. Wang B, Suen CW, Ma H, Wang Y, Kong L, Qin D. et al. The Roles of H19 in Regulating Inflammation and Aging. Front Immunol. 2020;11:579687
21. Wang YC, Tsao SM, Li YT, Lee CY, Tsao TC, Hsieh MJ. et al. The Relationship between Long Noncoding RNA H19 Polymorphism and the Epidermal Growth Factor Receptor Phenotypes on the Clinicopathological Characteristics of Lung Adenocarcinoma. Int J Environ Res Public Health. 2021;18:2862
22. Hu JC, Lin CY, Wang SS, Chiu KY, Li JR, Chen CS. et al. Impact of H19 Polymorphisms on Prostate Cancer Clinicopathologic Characteristics. Diagnostics (Basel). 2020;10:656
23. Wu ER, Chou YE, Liu YF, Hsueh KC, Lee HL, Yang SF. et al. Association of lncRNA H19 Gene Polymorphisms with the Occurrence of Hepatocellular Carcinoma. Genes (Basel). 2019;10:506
24. Yang PJ, Hsieh MJ, Hung TW, Wang SS, Chen SC, Lee MC. et al. Effects of Long Noncoding RNA H19 Polymorphisms on Urothelial Cell Carcinoma Development. Int J Environ Res Public Health. 2019;16:1322
25. Huang MC, Chou YH, Shen HP, Ng SC, Lee YC, Sun YH. et al. The clinicopathological characteristic associations of long non-coding RNA gene H19 polymorphisms with uterine cervical cancer. J Cancer. 2019;10:6191-8
26. Ting KH, Yang PJ, Su SC, Tsai PY, Yang SF. Effect of SDF-1 and CXCR4 gene variants on the development of diabetic kidney disease. Int J Med Sci. 2024;21:2851-61
27. Sienkiewicz-Szłapka E, Fiedorowicz E, Król-Grzymała A, Kordulewska N, Rozmus D, Cieślińska A. et al. The Role of Genetic Polymorphisms in Diabetic Retinopathy: Narrative Review. Int J Mol Sci. 2023;24:15865
28. Saleh AA, El-Hefnawy SM, Kasemy ZA, Alhagaa AA, Nooh MZ, Arafat ES. Mi-RNA-93 and Mi-RNA-152 in the Diagnosis of Type 2 Diabetes and Diabetic Retinopathy. Br J Biomed Sci. 2022;79:10192
29. Mishra B, Swaroop A, Kandpal RP. Genetic components in diabetic retinopathy. Indian J Ophthalmol. 2016;64:55-61
30. He Y, Dan Y, Gao X, Huang L, Lv H, Chen J. DNMT1-mediated lncRNA MEG3 methylation accelerates endothelial-mesenchymal transition in diabetic retinopathy through the PI3K/Akt/mTOR signaling pathway. Am J Physiol Endocrinol Metab. 2021;320:E598-e608
31. Qiu GZ, Tian W, Fu HT, Li CP, Liu B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochem Biophys Res Commun. 2016;471:135-41
32. Sarray S, Lamine LB, Dallel M, Jairajpuri D, Turki A, Sellami N. et al. Association of MMP-2 genes variants with diabetic retinopathy in Tunisian population with type 2 diabetes. J Diabetes Complications. 2022;36:108182
33. Singh K, Goyal P, Singh M, Deshmukh S, Upadhyay D, Kant S. et al. Association of functional SNP-1562C>T in MMP9 promoter with proliferative diabetic retinopathy in north Indian type 2 diabetes mellitus patients. J Diabetes Complications. 2017;31:1648-51
34. Chuang CC, Wang K, Yang YS, Kornelius E, Tang CH, Lee CY. et al. Association of Long Noncoding RNA HOTAIR Polymorphism and the Clinical Manifestations of Diabetic Retinopathy. Int J Environ Res Public Health. 2022;19:14592
35. Liu P, Huang X, Wu H, Yin G, Shen L. LncRNA-H19 gene plays a significant role in regulating glioma cell function. Mol Genet Genomic Med. 2021;9:e1480
36. Li W, Hua RX, Wang M, Zhang D, Zhu J, Zhang S. et al. H19 gene polymorphisms and Wilms tumor risk in Chinese children: a four-center case-control study. Mol Genet Genomic Med. 2021;9:e1584
37. Xin W, Wang Y, Hua K, Liu S. The role of long noncoding RNA H19 in gynecological pathologies: Insights into gene regulation and immune modulation (Review). Int J Mol Med. 2023;52:73
38. Cheng XW, Chen ZF, Wan YF, Zhou Q, Wang H, Zhu HQ. Long Non-coding RNA H19 Suppression Protects the Endothelium Against Hyperglycemic-Induced Inflammation via Inhibiting Expression of miR-29b Target Gene Vascular Endothelial Growth Factor a Through Activation of the Protein Kinase B/Endothelial Nitric Oxide Synthase Pathway. Front Cell Dev Biol. 2019;7:263
39. Yang ML, Huang Z, Wang Q, Chen HH, Ma SN, Wu R. et al. The association of polymorphisms in lncRNA-H19 with hepatocellular cancer risk and prognosis. Biosci Rep. 2018;38:BSR20171652
40. Li L, Huang Q, Yan F, Wei W, Li Z, Liu L. et al. Association between long non-coding RNA H19 polymorphisms and breast cancer risk: a meta-analysis. Women Health. 2022;62:565-75
41. Song Y, Xing H, Zhou L, Zhang N, Yang M. LncRNA H19 modulated by miR-146b-3p/miR-1539-mediated allelic regulation in transarterial chemoembolization of hepatocellular carcinoma. Arch Toxicol. 2021;95:3063-70
42. Lake AJ, Hateley-Browne JL, Rees G, Speight J. Effect of a tailored leaflet to promote diabetic retinopathy screening among young adults with type 2 diabetes: a randomised controlled trial. BMC Ophthalmol. 2020;20:80
43. Chen M, Wang Y, Feng P, Liang Y, Liu Q, Yang M. et al. Association between Age at Type 2 Diabetes Onset and Diabetic Retinopathy: A Double-Center Retrospective Study. J Diabetes Res. 2023;2023:5919468
44. Sun Y, Zhong L, He X, Wang S, Lai Y, Wu W. et al. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J Mol Cell Cardiol. 2019;131:66-81
45. Shi S, Song L, Yu H, Feng S, He J, Liu Y. et al. Knockdown of LncRNA-H19 Ameliorates Kidney Fibrosis in Diabetic Mice by Suppressing miR-29a-Mediated EndMT. Front Pharmacol. 2020;11:586895
46. Liu H, Ye T, Yang X, Liu J, Jiang K, Lu H. et al. H19 promote calcium oxalate nephrocalcinosis-induced renal tubular epithelial cell injury via a ceRNA pathway. EBioMedicine. 2019;50:366-78
47. Wu Q, Huang F. LncRNA H19: a novel player in the regulation of diabetic kidney disease. Front Endocrinol (Lausanne). 2023;14:1238981
48. Okuyan HM, Dogan S, Terzi MY, Begen MA, Turgut FH. Association of serum lncRNA H19 expression with inflammatory and oxidative stress markers and routine biochemical parameters in chronic kidney disease. Clin Exp Nephrol. 2021;25:522-30
49. Wang Z, Yang X, Kai J, Wang F, Wang Z, Shao J. et al. HIF-1α-upregulated lncRNA-H19 regulates lipid droplet metabolism through the AMPKα pathway in hepatic stellate cells. Life Sci. 2020;255:117818
50. Shi X, Wei YT, Li H, Jiang T, Zheng XL, Yin K. et al. Long non-coding RNA H19 in atherosclerosis: what role? Mol Med. 2020;26:72
51. Deng W, Zhao L, Chen C, Ren Z, Jing Y, Qiu J. et al. National burden and risk factors of diabetes mellitus in China from 1990 to 2021: Results from the Global Burden of Disease study 2021. J Diabetes. 2024;16:e70012
52. Pablo L, Garay-Aramburu G, García Layana A, Fernandez A, Vázquez I, Acebes X. et al. Assessing the economic burden of vision loss and irreversible legal blindness in Spain (2021-2030): a societal perspective. Health Econ Rev. 2024;14:70
53. Gupta S, Dominguez M, Golestaneh L. Diabetic Kidney Disease: An Update. Med Clin North Am. 2023;107:689-705
54. Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S. et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018
Author contact
![]() Corresponding author: Hung-Yu Lin, MD., PhD., Department of Ophthalmology, Show Chwan Memorial Hospital, Changhua, Taiwan. E-mail: anthonyhungyulincom.
Corresponding author: Hung-Yu Lin, MD., PhD., Department of Ophthalmology, Show Chwan Memorial Hospital, Changhua, Taiwan. E-mail: anthonyhungyulincom.

 Global reach, higher impact
Global reach, higher impact