3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(2):383-397. doi:10.7150/ijms.99958 This issue Cite
Research Paper
A Combination of Punica granatum Fruit Rind and Theobroma cacao Seed Extracts Enhances Sexual Function in Aging Males in a Randomized, Double-blind, Placebo-controlled Study
1. Department of General Medicine, Kashi Medicare, Varanasi-221001, Uttar Pradesh, India.
2. Department of General Medicine, Upendra Medicare, Varanasi-221001, Uttar Pradesh, India.
3. Department of Medicine, Sapthagiri Institute of Medical Sciences and Research Centre, Bengaluru-560090, Karnataka, India.
4. Department of General Medicine, Yalamanchi Hospitals and Research Centre, Vijayawada-520002, Andhra Pradesh, India.
Received 2024-6-22; Accepted 2024-12-4; Published 2025-1-1
Abstract
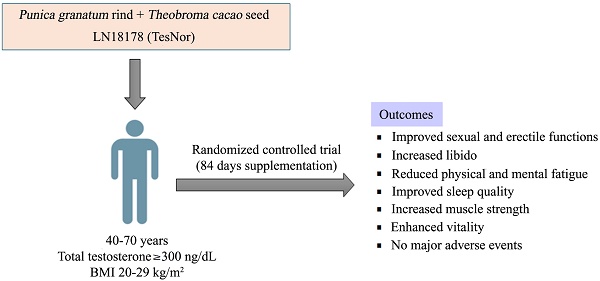
Introduction: LN18178 is a standardized, synergistic combination of Punica granatum fruit rind and Theobroma cacao seed extracts, which has been reported to increase serum testosterone levels in young and aging males.
Methods: The present 84-day randomized, double-blind, placebo-controlled study assessed the efficacy of LN18178 on the sexual function of aging male volunteers (age: 40-70 years; serum total testosterone: ≥ 300 ng/dL). The subjects with mild to moderate erectile dysfunction [5-item version of the International Index of Erectile Function (IIEF-5) scores 17-25] and low sexual desire (score < 3 on items 11 and 12 of IIEF) participated in this investigation. One hundred and twenty men were randomly allocated into either the LN18178 or placebo group (n=60); they took either 400 mg of LN18178 or a matched placebo capsule daily with breakfast.
Results: Post-trial, the LN18178-supplemented participants reported significant (P < 0.05) improvements in total and domain scores of the Derogatis Interview for Sexual Functioning-Self Reporting Male (DISF-SR-M) questionnaire, as well as substantial improvements in IIEF-5 (International Index of Erectile Function-5) and erection hardness scores (EHS). Comparative analysis also revealed significant improvements in the multi-dimensional fatigue inventory (MFI) and general health survey (GHS) scores. LN18178 supplementation substantially (P < 0.05) increased the six-minute walk distance and hand-grip strength compared to placebo. The participants' hemato-biochemical parameters, urinalysis, and vitals were within the normal range.
Conclusion: LN18178 enhances sexual function, libido and improves psychological well-being, as well as neuromotor function and general well-being in aging males. LN18178 supplementation is safe and well tolerated by the participants.
Keywords: Comprehensive safety, Derogatis Interview for Sexual Functioning-Male (DISF-SR-M), International Index of Erectile Function (IIEF), Testosterone, Punica granatum, Theobroma cacao
Introduction
Advancing age in men declines endocrine functions that result in a complex and multifaceted array of reduced physiological and biochemical events, including psychological functions [1]. Sexual desire and performance reduce with advancing age and cause dissatisfaction, impairing sexual health that negatively influences men's and their partner's quality of life and social functionality. According to the World Health Organization (WHO), “Good sexual and reproductive health is a state of complete physical, mental, and social well-being in all matters relating to the reproductive system.” [2]. In men, among various biological and psychological factors, endocrine function plays a major role in regulating sexual wellness, including sexual function and performance [3].
Generally, testosterone levels in males fall by 0.4 to 2% per year after age 30 [4]. Testosterone is the major androgen responsible for the growth and development of the male reproductive system, which helps maintain typical male sexual characteristics, including sexual function, desire, performance, vigor, healthy sperm profile, bone and muscle mass, metabolic homeostasis, and psychological wellness [5]. Reduced testosterone levels or hypogonadism results in sexual dysfunction that includes erectile dysfunction (ED), premature ejaculation (PE), and decreased interest or lack of desire are the most common sexual dysfunctions [6, 7]. Globally, 322 million men are predicted to have ED by 2025, an increase from 152 million in 1995 [8]. PE affects 30% to 50% of males [9]. ED and PE are intricately related to each other with a bidirectional relationship. ED has been reported as the major risk factor for about 36% to 50% of incidences of PE [10].
Numerous Indian medicinal herbs have been used in traditional medicine to treat sexual disorders and improve men's quality of life, sperm count and motility, and sexual performance [11, 12]. A proprietary blend of Punica granatum fruit rind and the seeds of Theobroma cacao, LN18178, synergistically increased steroidogenesis in mouse MA-10 Leydig cells and decreased aromatase enzyme activity in JEG-3 human choriocarcinoma cells. Furthermore, in a proof-of-concept preclinical study, LN18178 supplementation increased serum testosterone, luteinizing hormone levels, and semen quality (volume, sperm count, and motility) in young adult male Sprague Dawley rats (unpublished observation). Earlier, a clinical study in young male volunteers (age 21-35 yrs.) demonstrated that LN18178 supplementation increased testosterone (total and free) levels, and the volunteers enhanced their muscle mass and strength [13]. Next, in another independent human trial, LN18178 also significantly increased serum testosterone (total and free) levels and ameliorated the aging male symptoms (AMS) that suggested improvements in psychological, physical, and sexual behavior and activities in the participants (age: 36-55 yrs.) [14].
Pomegranates (Punica granatum L.) are associated with fertility, regeneration, and endurance of life [15]. Pomegranate is a rich source of active antioxidant phytochemical compounds like ellagic acid, gallic acid, quercetin, myricetin, and flavonoids such as anthocyanidins, cyanidins, luteolin, and pelargonidins, etc. [16]. Pomegranate juice has been reported to increase sperm count, motility, and viability in vivo [17]. Furthermore, pomegranate juice supplementation increased sperm count and mobility in the epididymis and reduced poor-quality sperm in male rats. These beneficial effects include increased intra-cavernosal blood flow, smooth muscle relaxation, and erectile activity against oxidative stress [18]. Earlier, a clinical study demonstrated improved erectile function in pomegranate juice-supplemented individuals compared to placebo [19].
Theobroma cacao or cocoa seeds are rich in phenolic antioxidant flavonoids like catechins, epicatechins, procyanidin B1 and -B2, quercetin, luteolin, vitexin, phenolic acids, etc. [20]. Epicatechins are primarily responsible for their beneficial impact on the vascular endothelium by upregulating nitric oxide (NO) production. T. cacao improves insulin sensitization. Additionally, T. cacao stimulates changes in redox-sensitive signaling pathways and the immune response. It also benefits nerve injury, skin protection from ultraviolet (UV) radiation, satiety, cognitive function, and mood elevation [21]. A diet containing T. cacao seeds significantly improved the semen quality in rabbit bucks [22].
The objective of the present study was to explore whether LN18178 supplementation improves sexual function in aging male volunteers. We conducted an 84-day randomized, double-blind, placebo-controlled clinical study that demonstrated enhanced sexual and erectile functions in LN18178-supplemented aging male participants with low sexual desire and mild to moderate levels of erection difficulty.
Materials and methods
A proprietary phytoceutical composition, LN18178 (TesNor®)
LN18178, a patented (PCT/IN2019/050361) synergistic combination of Punica granatum fruit rind and Theobroma cacao seed extracts (4:1 w/w) (batch # N22050248, mfg. on May 2022), was manufactured at a good manufacturing practice (cGMP)-certified facility of Laila Nutraceuticals, Vijayawada, India. Taxonomically authenticated voucher specimens of Punica granatum fruit rind (LNH6341) and Theobroma cacao seeds (LNH6924) are archived in the Taxonomy Department, Laila Nutraceuticals (Vijayawada, India). The extract blend was formulated to a free-flowing powder using 25% excipients (w/w) and standardized to a minimum of 3.5% punicalagins and 0.5% theobromine, affirmed using high-performance liquid chromatography (HPLC). Detailed descriptions of the raw materials, collection, extraction procedures, standardization, and phytochemical analysis of the plant were provided earlier [13].
Ethical conduct
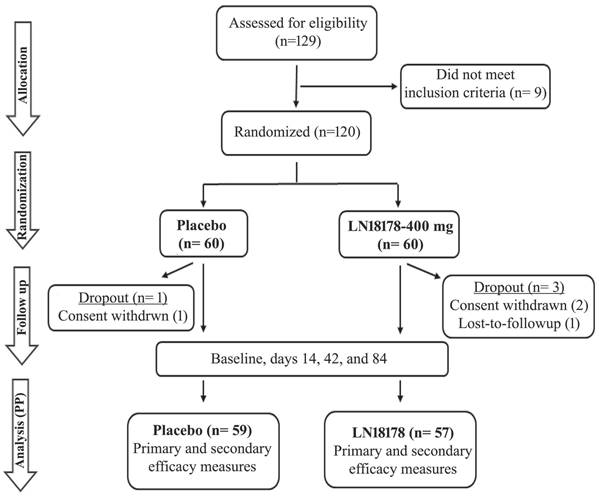
This double-blind, placebo-controlled clinical trial was registered with the Clinical Trial Registry of India (Registration No: CTRI/2023/03/050315; March 03, 2023) and approved by the institutional ethics committee (IEC) (ECR/1611/Inst/UP/2021) of Kashi Medicare and Upendra Medicare (Varanasi, Uttar Pradesh, India) on January 28, 2023. The study strictly followed the ethical principles of the Declaration of Helsinki, International Conference on Harmonization (ICH) - Good Clinical Practice (GCP) guidelines. The study flow is presented following the recommendations of Consolidated Standards of Reporting Trials (CONSORT) (Figure 1).
Participant enrollment, consent, randomization, and blinding
Healthy and recreationally active aging men (age: 40-70 years; BMI: 20-29 kg/m2; serum total testosterone levels: ≥ 300 ng/dL) with mild erectile dysfunction (IIEF scores between 17 and 25) and low sexual desire, as assessed using self-reported International Index of Erectile Function (IIEF) questionnaire. The participants were in monogamous sexual relationships, and all of them complied with the inclusion-exclusion criteria of the study (Supplementary Table S1).
Each participant read the subject information sheet detailing the study procedures, aims, methodology, potential risks, and anticipated benefits. Then, all participants signed the IEC-approved informed consent form.
The enrolled participants (n=120) were equally allocated to either placebo or LN18178 groups using permuted block-randomization codes generated by the PROC-PLAN procedure in the Statistical Analysis System (SAS) program. The randomization details were controlled by an independent authorized designatory; the investigators, study monitors, and study participants were blinded to the randomization and treatments. Randomization codes were broken only after locking the data following the completion of the study.
A CONSORT diagram shows the flow of the study. The primary and secondary efficacy measures are described in the materials and methods. The safety evaluations, which included complete serum biochemistry, hematology, urine analysis, and vitals, were performed at the screening and the end of the study (day 84).
Sample size and Power calculation
The sample size was calculated using Stata Statistical Software (StataCorp LLC. 2019, College Station, TX). Sixty subjects per treatment group were assumed to provide 90% power to detect a treatment effect at the end of the study (day 84) Rmax(µm) change from baseline at a one-sided significance level of 0.05%. Based on an earlier study [23], assuming a pooled standard deviation of 1.31 to achieve the power of 90% and at 95% CI for detecting a difference in means between two groups of 0.96, the total minimum sample size was estimated to be 110 (55 in each group, two groups). This study recruited 120 participants (60 per group) with an assumption of a 10% dropout during the study.
Placebo or LN18178 Supplementation
The coded placebo or LN18178 capsules supplied to all recruited subjects contained either the investigational products (IP), LN18178, or matched placebo capsules of identical sizes, weights, and colors. Recruited subjects were advised to orally consume one placebo or LN18178 capsule (400 mg/day) with breakfast for 84 consecutive days. Each placebo capsule contained (w/w) brown dextrin (50%) and maltodextrin (50%).
Follow-up visits
Following screening and enrollment (visit 1), the enrolled subjects visited the site for baseline evaluations (visit 2). This study had three follow-up evaluations on days 14 (visit 3), 42 (visit 4), and 84 (visit 5) of treatment.
Compliance
The placebo and LN18178 capsules were stored at room temperature in a dry, cool, and dark place. The project coordinators exclusively distributed the placebo and treatment capsules to the recruited volunteers at baseline and on days 14 and 42 of the study. They maintained the data entry and were endorsed regularly by the principal investigator (PI). The PI regularly signed the accountability log. Study participants were advised to keep their routine regular diets and refrain from consuming any vitamins or beverages that were claimed to be ergogenic and enhance sexual function. All subjects regularly maintained individual daily diaries and recorded details of food and capsule intake, daily activities, and any or all adverse or untoward events. These daily diaries were routinely checked by the project coordinators and endorsed by the PI.
The project coordinators and PI periodically counseled the study participants to ascertain the maximum possible adherence to the study protocol. The participants returned all unused capsules at each follow-up visit, and their attendance at each visit was recorded to ensure the participants' IP-related and participant compliance with the study protocol.
The PI determined the physical health of all study participants by checking for signs of any adverse drug reaction. Safety was ascertained by medical checkups and laboratory evaluations at baseline and each follow-up visit.
Subject withdrawal criteria
The withdrawal of subjects from the study was considered if the subject had withdrawn consent or the investigator considered withdrawal in case of non-compliance with IP or protocol violation or loss to follow-up. The withdrawal was also considered in the subjects' interest due to tolerability issues, including serious adverse events. The reasons for withdrawal or dropout from the study were recorded in the case report form.
Concomitant medication
All subjects routinely maintained the intake records of all concomitant medications, including prescription, non-prescription, and over the counter (OTC) medications, and the study coordinators recorded these details on the case report forms (CRFs).
Efficacy measurements
Derogatis Interview for Sexual Functioning-Self Reported-Male (DISF-SR-M)
The scores of the DISF-SR-M questionnaire [24] were the primary efficacy outcome measure that evaluated the improvements in sexual functions of the participants on days 14, 42, and 84 of LN18178 supplementation compared to the baseline. The 25-question DISF-SR-M questionnaire is divided into five domains: sexual cognition/fantasy, sexual arousal, sexual behavior/experience, orgasm, and sexual drive/relationship. The sexual cognition/fantasy, sexual arousal, sexual behavior/experience domain questions, and two sexual drive/relationship-related questions are scored on a 9-point scale between 0 and 8. The orgasm-related questions and two questions from the sexual drive/relationship domain are scored between 0 (not at all) and 4 (extreme).
The International Index of Erectile Function (IIEF)
The IIEF questionnaire is a self-reported 15-item instrument to assess male sexual function. The IIEF consists of five domains: erectile function, orgasmic function, sexual desire, intercourse pleasure, and overall satisfaction. IIEF score evaluation is the gold standard for clinical efficacy assessment in erectile and sexual function studies [25, 26].
Erection hardness score (EHS)
EHS is a valid and reliable tool for scoring erection hardness [27]. This single-item self-reported score classifies the severity of erectile dysfunction (ED) into four grades:
(a) Grade 1 represents no enlargement and lack of hardness upon sexual stimulation.
(b) Grade 2 indicates that it is not hard enough to penetrate.
(c) Grade 3 indicates sufficient for penetration but not completely hard.
(d) Grade 4 indicates normal erection in hardness and rigidity.
The Multidimensional Fatigue Inventory (MFI)
The MFI is a 20-item self-reported instrument designed procedure to assess fatigue. It measures general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity [28]. The participants scored based on their perception of fatigue on a scale of 1 to 5, the higher the score, the higher the level of fatigue. MFI scores were evaluated at baseline and on days 42 and 84 of the study.
The Pittsburgh Sleep Quality Index (PSQI)
The participants were assessed for their sleep quality at baseline and on days 42 and 84 of treatment using a self-rated Pittsburgh Sleep Quality Index (PSQI) questionnaire. The PSQI reliably assesses sleep quality and disturbances in clinical practice and research setups. The PSQI measures sleep quality, latency, duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction [29]. Each item is scored between 0 and 3; the combined score of seven domains presents the global score. A global score greater than five indicates poor sleep quality.
General Health Survey (GHS)
The participants' feedback on their libido, muscular mass, muscle strength, energy, stamina, and sleep were captured on a 10-cm scale of a general health survey (GHS) questionnaire. A score of 1 indicates "not satisfied," and a 10 indicates "extremely satisfied.”
Hand-grip strength
The hand-grip strength of the dominant hand of the participants was measured using a digital dynamometer (INCO Instruments & Medical Devices Pvt. Limited, Ambala, India). The measurements were taken in the sitting position with the forearm extended on a table and bent at 90° with the elbow. The participants squeezed their grips as hard as possible without any jerking motion; the best measurement was recorded among three performances at 2-minute intervals.
Six-minute walk test (6MWT)
6MWT was performed to measure the submaximal level of functional activity of the participants following the American Thoracic Society (ATS) guidelines [30]. The participants walked on a 25-meter length of a well-ventilated and flat surface as quickly as possible for six minutes; the walked distance was recorded as a six-minute walk distance (6MWD). They were allowed for rest periods, but the time was included in the test duration. The participants were verbally encouraged during the test. They were allowed to withdraw from the test in case of any discomfort, including chest pain, extreme shortness of breath, or leg cramps.
The IIEF, EHS, 6MWT, GHS, and hand-grip strength evaluations were performed at baseline and on each follow-up visit of the study.
Safety assessments
Total blood chemistry was carried out during screening and at the end of the study, including an array of hematological, serum biochemical, and urinary analyses. Urine and blood chemistry analyses were conducted using the VITROS® 5600 integrated system (Dry Chemistry analyzer, Ortho Clinical Diagnostics, Linden, NJ, USA). Fasting blood glucose, serum creatinine, uric acid, creatine kinase (CK), blood urea nitrogen (BUN), serum bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), sodium, potassium, and serum albumin were the clinical chemistry parameters. The hematological parameters included hemoglobin, platelet count, total leukocyte count (TLC), red blood cells (RBC), erythrocyte sedimentation rate (ESR), and differential count. Color, specific gravity, pH, glucose, protein, and RBC were evaluated in urinalysis. Microscopic examinations were conducted using a light microscope (Olympus Opto Systems India Pvt. Ltd., New Delhi, India). At each visit, the participants' vital signs, blood pressure (systolic and diastolic), pulse rate, respiration rate, and oral temperature were recorded.
Statistical analysis
The data are presented as mean ± SD. The per-protocol (PP) analysis was performed using the data of the subjects who completed the study: placebo (n=59) and LN18178 (n=57). Intragroup comparisons were analyzed using paired t-test, and Wilcoxon signed rank test for normal and nonnormal data, respectively. Intergroup comparisons were performed using Analysis of Covariance (ANCOVA) and rank ANCOVA for normal and non-normal data respectively. ANCOVA was used to adjust the baseline differences between the groups. For non-normal data, rANCOVA was performed for covariate adjustments and to reject the type 1 error (false positives). For safety analysis, paired t-tests and independent t-tests were used for intragroup and intergroup analysis. All hypotheses were tested at a significance level of 0.05 and 95% confidence interval (CI). The effect size (Cohen's d) was calculated as the product of the mean difference between the groups to pooled standard deviation (pooled SD).
Results
One hundred and twenty aging males (age: 52.95 ± 8.52 Y; BMI: 24.71 ± 1.32 kg/m2; Race: Asian) were enrolled in the study, and they were equally allocated to the placebo and LN18178 groups; each group consisted of 60 participants. None of them had a smoking or tobacco consumption history. The comparative analyses of the baseline demographic parameters (age, height, body weight, BMI, serum total testosterone) of the groups suggest that there was no statistical difference between the groups (Table 1). Overall, four participants dropped out from the study; one from the placebo group and two from the LN18178 group withdrew their consents after recruitment, and one subject in the LN18178 group did not report after the baseline visit. The per protocol (PP) analyses are presented using the placebo (n= 59) and LN18178 (n= 57) for the efficacy evaluation of supplementation.
DISF-SR-M scores
Table 2 summarizes gradual increases of the DISF-SR-M total and domain scores in the LN18178-supplemented volunteers from day 14 through the end of the study. Although the improvements in the placebo are significant (vs. baseline), the between-the-group comparison analysis reveals that LN18178 supplementation significantly (vs. placebo) increased the domain and total DISF-SR-M scores in the participants. Post-trial, the LN18178 and placebo groups showed 121.93% and 42.42% increases in DISF-SR scores, respectively, from baseline. The improvement in LN18178 (vs. placebo) is significant (P < 0.0001; Cohen'd: 0.88, 2.06, and 4.99 on days 14, 42, and 84, respectively) (Table 2). Interestingly, the comparisons (placebo vs. LN18178) between the improvements (from baseline) in scores of two specific questions on morning erection (Q 2.1) and frequency of sexual activity (Q 3.5) are significant (P < 0.001: Cohen'd: 2.77 and 3.03 respectively for Q 2.1 and 3.5, respectively) starting from day 14 till the end of the study (Table 2). In men, morning erection is negatively associated with physical and psychological stress [31], and the frequency of sexual activity determines the sexual relationship with a partner, general well-being, and health [32].
Baseline and demographic characteristics
| Mean ± SD | P value (vs. placebo) | 95% CI (vs. placebo) | ||
|---|---|---|---|---|
| Age (years) | ||||
| Placebo | 51.88 ± 8.28 | - | ||
| LN18178 | 54.02 ± 8.69 | 0.1710 | -0.93, 5.21 | |
| Race (Gender) | ||||
| Placebo | 60 Asian (male) | - | - | |
| LN18178 | 60 Asian (male) | - | - | |
| Height (cm) | ||||
| Placebo | 163.42 ± 4.06 | - | ||
| LN18178 | 163.72 ± 5.36 | 0.7304 | -1.42, 2.02 | |
| Weight (Kg) | ||||
| Placebo | 66.20 ± 4.61 | - | ||
| LN18178 | 66.02 ± 4.77 | 0.8353 | -1.52, 1.88 | |
| BMI (kg/m2) | ||||
| Placebo | 24.78 ± 1.38 | - | ||
| LN18178 | 24.63 ± 1.27 | 0.5210 | -0.33, 0.63 | |
| Total Testosterone (ng/dL) | ||||
| Placebo | 418.52 ± 40.76 | - | ||
| LN18178 | 433.40 ± 43.56 | 0.0557 | -0.37, 30.14 | |
| Fasting blood glucose (mg/dL) | ||||
| Placebo | 87.65 ± 6.16 | - | - | |
| LN18178 | 87.60 ± 7.08 | 0.9672 | -2.35, 2.45 | |
| Smoking history (past/present) | ||||
| Placebo | 0 | - | - | |
| LN18178 | 0 | - | - | |
Values present mean ± standard deviation (SD); placebo (n=60) and LN18178 (n=60). CI: Confidence interval. A P value < 0.05 (independent t-test) was considered significant.
IIEF scores
Table 3 demonstrates gradual and significant improvements in all domains (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction) and total IIEF scores in the LN18178-supplemented subjects. LN18178 supplementation increased the total IIEF scores by 21.25%, 42.30%, and 72.02%. In contrast, compared to baseline, the placebo group showed 6.79%, 18.80%, and 35.97% increases on days 14, 42, and 84, respectively. These changes are significant (P < 0.0001) in the within-the-group (vs. baseline) and between-the-group (vs. placebo) comparison analyses (Table 3). The Cohen'd values for improvement in IIEF total scores are 0.15, 2.52, and 3.98, respectively. The comparison analysis between the changes (from baseline) in the groups showed significant improvements in total and the IIEF domain scores in the LN18178-supplemented participants on the follow-up visits of the study (Table 3).
Assessment of DISF-SR-M scores
| Evaluation | Mean ± SD | P value (vs. baseline) | P value (vs. placebo) | 95% CI vs. Baseline | 95% CI vs. Placebo | P value, change from baseline (vs. placebo) | |
|---|---|---|---|---|---|---|---|
| Sexual cognition | |||||||
| Placebo | Baseline | 11.64 ± 2.77 | - | - | - | - | - |
| Day 14 | 12.69 ± 2.45 | < 0.0001 | - | 0.10, 2.00 | - | - | |
| Day 42 | 12.37 ± 2.37 | 0.0287 | - | -0.21, 1.67 | - | - | |
| Day 84 | 14.41 ± 3.92 | < 0.0001 | - | 1.53, 4.01 | - | - | |
| LN18178 | Baseline | 12.07 ± 2.08 | - | 0.4120 | - | -0.47, 1.33 | - |
| Day 14 | 15.04 ± 3.01 | < 0.0001 | < 0.0001 | 2.01, 3.93 | 1.34, 3.36 | < 0.0001 | |
| Day 42 | 17.88 ± 2.54 | < 0.0001 | < 0.0001 | 4.95, 6.67 | 4.61, 6.41 | < 0.0001 | |
| Day 84 | 26.21 ± 2.79 | < 0.0001 | < 0.0001 | 13.23, 15.05 | 10.54, 13.06 | < 0.0001 | |
| Sexual arousal | |||||||
| Placebo | Baseline | 10.98 ± 3.03 | - | - | - | - | - |
| Day 14 | 12.05 ± 2.35 | < 0.0001 | - | 0.08, 2.06 | - | - | |
| Day 42 | 15.08 ± 2.52 | < 0.0001 | - | 3.08, 5.12 | - | - | |
| Day 84 | 15.20 ± 2.5 | < 0.0001 | - | 3.21, 5.23 | - | - | |
| LN18178 | Baseline | 10.91 ± 3.01 | - | 0.8999 | - | -1.04, 1.18 | - |
| Day 14 | 14.58 ± 3.01 | < 0.0001 | < 0.0001 | 2.55, 4.79 | 1.54, 3.52 | < 0.0001 | |
| Day 42 | 18.39 ± 2.88 | < 0.0001 | < 0.0001 | 6.39, 8.57 | 2.32, 4.30 | < 0.0001 | |
| Day 84 | 26.54 ± 2.8 | < 0.0001 | < 0.0001 | 14.55, 16.71 | 10.36, 12.32 | < 0.0001 | |
| Sexual behaviour | |||||||
| Placebo | Baseline | 10.54 ± 2.36 | - | - | - | - | - |
| Day 14 | 12.02 ± 1.94 | < 0.0001 | - | 0.69, 2.27 | - | ||
| Day 42 | 15.17 ± 2.21 | < 0.0001 | - | 3.80, 5.46 | - | - | |
| Day 84 | 15.08 ± 1.99 | < 0.0001 | - | 3.74, 5.34 | - | - | |
| LN18178 | Baseline | 10.12 ± 2.88 | - | 0.3916 | - | -0.55, 1.39 | - |
| Day 14 | 13.53 ± 2.67 | < 0.0001 | < 0.0001 | 2.38, 4.44 | 0.65, 2.37 | < 0.0001 | |
| Day 42 | 17.54 ± 2.24 | < 0.0001 | < 0.0001 | 6.46, 8.38 | 1.55, 3.19 | < 0.0001 | |
| Day 84 | 26.12 ± 2.54 | < 0.0001 | < 0.0001 | 14.99, 17.01 | 10.20, 11.88 | < 0.0001 | |
| Orgasm | |||||||
| Placebo | Baseline | 11.32 ± 2.87 | - | - | - | - | |
| Day 14 | 12.64 ± 3.23 | < 0.0001 | 0.21, 2.43 | - | |||
| Day 42 | 13.75 ± 3.06 | < 0.0001 | - | 1.35, 3.51 | - | - | |
| Day 84 | 17.39 ± 2.29 | < 0.0001 | - | 5.12, 7.02 | - | - | |
| LN18178 | Baseline | 11.56 ± 3.05 | 0.6644 | - | -0.85, 1.33 | - | |
| Day 14 | 13.81 ± 2.46 | <0.0001 | <0.0001 | 1.22, 3.28 | -0.60, 1.52 | 0.0018 | |
| Day 42 | 15.16 ± 2.22 | <0.0001 | 0.0018 | 2.61, 4.59 | 0.42, 2.40 | 0.0195 | |
| Day 84 | 20.88 ± 2.01 | <0.0001 | <0.0001 | 8.36, 10.28 | <0.0001 | ||
| Sexual drive | |||||||
| Placebo | Baseline | 8.97 ± 1.61 | - | - | - | - | - |
| Day 14 | 10.34 ± 1.94 | <0.0001 | - | 0.72, 2.02 | - | - | |
| Day 42 | 11.05 ± 1.98 | <0.0001 | - | 1.42, 2.74 | - | - | |
| Day 84 | 14.05 ± 2.37 | <0.0001 | - | 4.34, 5.82 | - | - | |
| LN18178 | Baseline | 8.49 ± 1.91 | 0.1496 | - | -0.17, 1.13 | - | |
| Day 14 | 10.95 ± 1.85 | <0.0001 | 0.00411 | 1.76, 3.16 | -0.09, 1.31 | 0.0004 | |
| Day 42 | 14.56 ± 2.08 | <0.0001 | <0.0001 | 5.33, 6.81 | 2.76, 4.26 | <0.0001 | |
| Day 84 | 18.23 ± 1.6 | <0.0001 | <0.0001 | 9.09, 10.39 | 3.43, 4.93 | <0.0001 | |
| Total score | |||||||
| Placebo | Baseline | 53.46 ± 9.25 | - | - | - | - | - |
| Day 14 | 59.75 ± 8.18 | <0.0001 | - | 4.81, 10.53 | - | - | |
| Day 42 | 67.42 ± 7.47 | <0.0001 | - | 10.89, 17.03 | - | - | |
| Day 84 | 76.14 ± 7.22 | <0.0001 | - | 13.58, 19.20 | - | - | |
| LN18178 | Baseline | 53.16 ± 10.34 | 0.8694 | - | -3.31, 3.91 | - | |
| Day 14 | 67.89 ± 10.37 | <0.0001 | <0.0001 | 10.89, 18.57 | 4.71, 11.57 | <0.0001 | |
| Day 42 | 83.53 ± 8.16 | <0.0001 | <0.0001 | 26.91, 33.83 | 13.23, 18.99 | <0.0001 | |
| Day 84 | 117.98 ± 9.56 | <0.0001 | <0.0001 | 61.12, 68.52 | 38.73, 44.95 | <0.0001 | |
| Morning erection (Q 2.1) | |||||||
| Placebo | Baseline | 2.34 ± 0.92 | - | - | - | - | - |
| Day 14 | 2.53 ± 0.73 | 0.0245 | - | -0.11, 0.49 | - | - | |
| Day 42 | 3.02 ± 0.75 | < 0.0001 | - | 0.37, 0.99 | - | - | |
| Day 84 | 2.95 ± 0.86 | < 0.0001 | - | 0.29, 0.93 | - | - | |
| LN18178 | Baseline | 2.19 ± 0.95 | - | - | - | -0.19, 0.49 | - |
| Day 14 | 3.04 ± 0.84 | < 0.0001 | < 0.0001 | 0.52, 1.18 | 0.22, 0.80 | < 0.0001 | |
| Day 42 | 3.58 ± 1.02 | < 0.0001 | 0.0004 | 1.02, 1.76 | 0.23, 0.89 | 0.0009 | |
| Day 84 | 5.26 ± 0.81 | < 0.0001 | < 0.0001 | 2.74, 3.40 | 2.00, 2.62 | < 0.0001 | |
| Sexual activity (Q 3.5) | |||||||
| Placebo | Baseline | 2.22 ± 0.91 | - | - | - | - | - |
| Day 14 | 2.63 ± 0.72 | < 0.0001 | - | 0.11, 0.71 | - | - | |
| Day 42 | 3.22 ± 0.85 | < 0.0001 | - | 0.68, 1.32 | - | - | |
| Day 84 | 3.22 ± 0.67 | < 0.0001 | - | 0.71, 1.29 | - | - | |
| LN18178 | Baseline | 2.28 ± 0.96 | - | - | - | -0.28, 0.40 | - |
| Day 14 | 2.95 ± 0.88 | < 0.0001 | 0.0186 | 0.33, 1.01 | 0.03, 0.61 | 0.0738 | |
| Day 42 | 3.88 ± 0.87 | < 0.0001 | < 0.0001 | 1.26, 1.94 | 0.34, 0.98 | 0.0097 | |
| Day 84 | 5.37 ± 0.75 | < 0.0001 | < 0.0001 | 2.77, 3.41 | 1.89, 2.41 | < 0.0001 | |
Values present mean ± SD. placebo (n=59) and LN18178 (n=57). CI: Confidence interval; P < 0.05 was considered as statistically significant for 'within the group' and 'between the groups' comparison analysis using paired t test and ANCOVA, respectively, as described in materials and methods.
Assessment of International Index of Erectile Function scores (IIEF) & Erection Hardness Scores (EHS)
| Evaluation | Mean ± SD | P value (vs. baseline) | P value (vs. placebo) | 95% CI (vs. Baseline) | 95% CI (vs. Placebo) | P value, change from baseline (vs. placebo) | |
|---|---|---|---|---|---|---|---|
| IIEF- Erectile function | |||||||
| Placebo | Baseline | 17.80 ± 0.98 | - | - | - | - | - |
| Day 14 | 18.22 ± 1.47 | 0.0722 | - | -0.04, 0.88 | - | - | |
| Day 42 | 18.78 ± 1.47 | < 0.0001 | - | 0.52, 1.44 | - | - | |
| Day 84 | 20.44 ± 1.67 | < 0.0001 | - | 2.14, 3.14 | - | - | |
| LN18178 | Baseline | 17.81 ± 0.81 | 0.9341 | - | -0.32, 0.34 | - | |
| Day 14 | 20.07 ± 1.49 | < 0.0001 | < 0.0001 | 1.81, 2.71 | 1.31, 2.39 | < 0.0001 | |
| Day 42 | 22.28 ± 1.71 | < 0.0001 | < 0.0001 | 3.97, 4.97 | 2.91, 4.09 | < 0.0001 | |
| Day 84 | 25.54 ± 1.73 | < 0.0001 | < 0.0001 | 7.23, 8.23 | 4.47, 5.73 | < 0.0001 | |
| IIEF- Orgasmic function | |||||||
| Placebo | Baseline | 4.88 ± 1.02 | - | - | - | - | - |
| Day 14 | 5.1 ± 0.86 | 0.0459 | - | -0.12, 0.56 | - | - | |
| Day 42 | 5.85 ± 0.89 | < 0.0001 | - | 0.62, 1.32 | - | - | |
| Day 84 | 7.12 ± 0.91 | < 0.0001 | - | 1.89, 2.59 | - | - | |
| LN18178 | Baseline | 4.67 ± 0.83 | 0.1491 | - | -0.13, 0.55 | - | |
| Day 14 | 5.77 ± 1.09 | < 0.0001 | < 0.0001 | 0.74, 1.46 | 0.31, 1.03 | <0.0001 | |
| Day 42 | 6.79 ± 0.77 | < 0.0001 | < 0.0001 | 1.82, 2.42 | 0.63, 1.25 | <0.0001 | |
| Day 84 | 8.81 ± 0.72 | < 0.0001 | < 0.0001 | 3.85, 4.43 | 1.39, 1.99 | <0.0001 | |
| IIEF- Sexual desire | |||||||
| Placebo | Baseline | 3.71 ± 0.87 | - | - | - | - | - |
| Day 14 | 4.81 ± 1.14 | < 0.0001 | - | 0.73, 1.47 | - | ||
| Day 42 | 5.47 ± 0.92 | < 0.0001 | - | 1.43, 2.09 | - | - | |
| Day 84 | 6.64 ± 1 | < 0.0001 | - | 2.59, 3.27 | - | - | |
| LN18178 | Baseline | 3.86 ± 0.77 | 0.2635 | - | -0.15, 0.45 | - | |
| Day 14 | 5.65 ± 0.81 | < 0.0001 | < 0.0001 | 1.50, 2.08 | 0.48, 1.20 | 0.0014 | |
| Day 42 | 6.81 ± 0.67 | < 0.0001 | < 0.0001 | 2.68, 3.22 | 1.04, 1.64 | < 0.0001 | |
| Day 84 | 8.6 ± 0.88 | < 0.0001 | < 0.0001 | 4.43, 5.05 | 1.61, 2.30 | < 0.0001 | |
| IIEF- Intercourse satisfaction | |||||||
| Placebo | Baseline | 6.97 ± 1.33 | - | - | - | - | - |
| Day 14 | 7.32 ± 1.29 | 0.0149 | -0.13, 0.83 | - | |||
| Day 42 | 8.85 ± 1.26 | < 0.0001 | - | 1.41, 2.35 | - | - | |
| Day 84 | 10.64 ± 1.24 | < 0.0001 | - | 3.20, 4.14 | - | - | |
| LN18178 | Baseline | 6.6 ± 1.36 | 0.1237 | - | -0.12, 0.86 | - | |
| Day 14 | 8.25 ± 1.81 | < 0.0001 | < 0.0001 | 1.06, 2.24 | 0.35, 1.51 | < 0.0001 | |
| Day 42 | 10.33 ± 1.27 | < 0.0001 | < 0.0001 | 3.24, 4.22 | 1.01, 1.95 | < 0.0001 | |
| Day 84 | 12.74 ± 1.06 | < 0.0001 | < 0.0001 | 5.69, 6.59 | 1.68, 2.53 | < 0.0001 | |
| IIEF- Overall satisfaction | |||||||
| Placebo | Baseline | 4.63 ± 0.98 | - | - | - | - | - |
| Day 14 | 5.1 ± 0.84 | < 0.0001 | - | 0.14, 0.80 | - | - | |
| Day 42 | 6.17 ± 0.85 | < 0.0001 | - | 1.21, 1.87 | - | - | |
| Day 84 | 6.8 ± 0.89 | < 0.0001 | - | 1.83, 2.51 | - | - | |
| LN18178 | Baseline | 4.49 ± 1.05 | 0.3834 | - | -0.23, 0.51 | - | |
| Day 14 | 5.63 ± 1.14 | < 0.0001 | 0.0004 | 0.73, 1.55 | 0.16, 0.90 | 0.0026 | |
| Day 42 | 7.04 ± 0.73 | < 0.0001 | < 0.0001 | 2.21, 2.89 | 0.58, 1.16 | < 0.0001 | |
| Day 84 | 8.68 ± 0.74 | < 0.0001 | < 0.0001 | 3.85, 4.53 | 1.58, 2.18 | < 0.0001 | |
| IIEF- Total score | |||||||
| Placebo | Baseline | 37.98 ± 3.07 | - | - | - | - | |
| Day 14 | 40.56 ± 3.59 | < 0.0001 | - | 1.36, 3.80 | - | ||
| Day 42 | 45.12 ± 3.13 | < 0.0001 | - | 6.01, 8.27 | - | ||
| Day 84 | 51.64 ± 3.31 | < 0.0001 | - | 12.50, 14.82 | - | ||
| LN18178 | Baseline | 37.42 ± 2.66 | 0.2511 | -0.5, 1.62 | - | ||
| Day 14 | 45.37 ± 4.74 | < 0.0001 | < 0.0001 | 6.52, 9.38 | 3.27, 6.35 | < 0.0001 | |
| Day 42 | 53.25 ± 3.33 | < 0.0001 | < 0.0001 | 14.71, 16.95 | 6.94, 9.32 | < 0.0001 | |
| Day 84 | 64.37 ± 3.09 | < 0.0001 | < 0.0001 | 25.88, 28.02 | 11.55, 13.91 | < 0.0001 | |
| EHS scores | |||||||
| Placebo | Baseline | 1.97 ± 0.61 | - | - | - | ||
| Day 14 | 1.98 ± 0.68 | 0.9813 | - | -0.23, 0.25 | - | ||
| Day 42 | 2.08 ± 0.6 | 0.2453 | -0.11, 0.33 | ||||
| Day 84 | 2.12 ± 0.74 | 0.2233 | - | -0.10, 0.40 | - | ||
| LN18178 | Baseline | 2.02 ± 0.67 | 0.6632 | -0.19, 0.29 | - | ||
| Day 14 | 2.47 ± 0.57 | < 0.0001 | < 0.0001 | 0.22, 0.68 | 0.26, 0.72 | 0.0079 | |
| Day 42 | 3.12 ± 0.71 | < 0.0001 | < 0.0001 | 0.84, 1.36 | 0.80, 1.28 | < 0.0001 | |
| Day 84 | 3.65 ± 0.48 | < 0.0001 | <0.0001 | 1.41, 1.85 | 1.30, 1.76 | < 0.0001 | |
Values present mean ± SD. placebo (n=59) and LN18178 (n=57). CI: Confidence interval; P < 0.05 was considered as statistically significant for 'within the group' and 'between the groups' comparison analysis using paired t test and ANCOVA, respectively, as described in materials and methods.
EHS scores
Similarly, on days 14, 42, and 84 of LN18178 supplementation, the erection hardness (EHS) scores were significantly (P < 0.0001) increased by 22.28%, 54.46%, and 80.69% from baseline, whereas the placebo group showed only 0.51%, 5.58%, and 7.61% increases, respectively. The changes in the placebo group are not significant (vs. baseline). Between-the-group comparison analysis on the net scores and the changes from baseline reveal that the improvements in the LN18178 group on the follow-up visits are significant (P < 0.0001, vs. placebo) (Table 3). The Cohen's d values for improvement in EHS scores are 0.78, 1.59, and 2.51, respectively,
MFI scores
Data analysis of multi-dimensional fatigue inventory (MFI) scores revealed that post-trial LN18178 significantly decreased the total MFI and selected domain scores (general fatigue, physical fatigue, and mental fatigue) as compared to baseline (Table 4). No significant change in total MFI scores was recorded in the placebo group following 84 days of supplementation. Relative to placebo, the MFI total scores were significantly decreased on days 42 (Cohen's d -0.26) and 84 (Cohen's d -0.62). However, post-trial, the MFI total score and its improvement (change from baseline) in the LN18178-supplemented group was not statistically significant (vs. placebo) (Table 4).
PSQI scores
Similarly, the PSQI global score in the LN18178-supplemented group was significantly (P < 0.05) reduced on days 42 and at the end of the study as compared to baseline and placebo (Table 5). Post-trial, the domain scores, such as subjective sleep quality, sleep disturbances, and daytime dysfunction in the LN18178-supplemented group, were significantly improved (p<0.05) when compared directly to baseline and placebo (Table 5). Also, post-trial, analysis showed significant improvements (change from baseline) in the global score, and these domain scores in the LN18178 group (vs. placebo) were substantial. Compared to placebo, the Cohen's 'd' values for improving sleep quality (Global PSQI scores) were 0.65 and 1.08, respectively (Table 5).
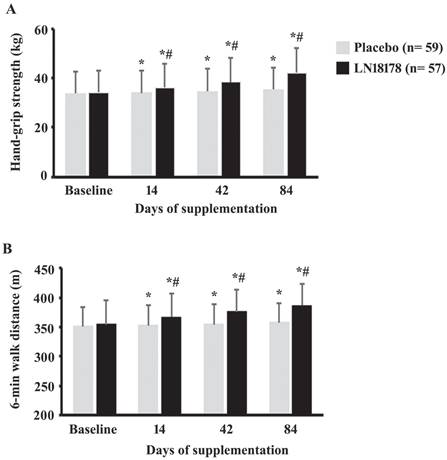
Hand-grip strength
On days 14, 42, and 84 of the investigation, the hand-grip strength in the LN18178 group were increased by 5.98% (P < 0.0001), 12.72% (P < 0.0001), and 23.83% (P < 0.0001), in comparison, the increases in the placebo group were 0.77% (P = 0.0029), 2.70% (P < 0.0001), and 5.01% (P < 0.0001), respectively, as compared to baseline (Figure 2A). These improvements in the LN18178-supplemented volunteers are significant (P < 0.0001) when compared with the placebo on days 14, 42, and 84 of the study (Figure 2A).
Six-minute walk test
LN18178-supplemented participants significantly (P < 0.0001) improved (P < 0.0001) the absolute walked distance in the six-minute walk test (SMWT) (Figure 2B). From baseline, the increases in the ADW by the LN18178 subjects were 3.56%, 5.93%, and 9.06% on days 14, 42, and 84 of supplementation. These improvements were also significant (P < 0.0001) compared to the placebo. In placebo, on days 14, 42 and 84, the improvements were 0.44% (P = 0.0003), 0.99% (P < 0.0001, and 1.97% (P < 0.0001), respectively, from baseline (Figure 2B).
GHS scores
Comparative analysis of general healthy survey (GHS) scores revealed that the GHS total and the domain scores were significantly (P < 0.0001) increased in the LN18178 group on days 14, 42, and 84 of supplementation as compared to the baseline and placebo (Supplementary Table S2).
Adverse events and concomitant medication
During the intervention, in the placebo, two subjects (one each) reported bloating and abdominal pain), and in the LN18178 group, three subjects (one each) reported an incidence of vomiting, nausea, or headache. However, these events were minor and transient (Supplementary Table S3). No participant reported any concomitant medication usage during the study.
Assessment of Multidimensional Fatigue Inventory scores (MFI)
| Evaluation | Mean ± SD | P value (vs. baseline) | P value (vs. placebo) | 95% CI (vs. Baseline) | 95% CI (vs. Placebo) | P value, change from baseline (vs. placebo) | |
|---|---|---|---|---|---|---|---|
| General Fatigue | |||||||
| Placebo | Baseline | 10.54 ± 2.12 | - | - | - | - | |
| Day 42 | 10.68 ± 1.61 | 0.4063 | - | -0.55, 0.83 | - | - | |
| Day 84 | 10.58 ± 1.53 | 0.9458 | - | -0.63, 0.71 | - | - | |
| LN18178 | Baseline | 11.23 ± 2.41 | 0.1058 | -0.14, 1.52 | - | ||
| Day 42 | 11.02 ± 1.47 | 0.3739 | 0.5976 | -0.53, 0.95 | -0.22, 0.92 | 0.4170 | |
| Day 84 | 9.58 ± 2.44 | 0.0020 | 0.054 | 0.75, 2.55 | 0.25, 1.75 | 0.0336 | |
| Physical fatigue | |||||||
| Placebo | Baseline | 9.03 ± 1.36 | - | - | - | - | |
| Day 42 | 8.63 ± 1.27 | 0.0050 | - | -0.08, 0.88 | - | - | |
| Day 84 | 8.22 ± 1.39 | 0.0001 | - | 0.31, 1.31 | - | - | |
| LN18178 | Baseline | 8.86 ± 1.59 | 0.5267 | -0.37, 0.71 | - | ||
| Day 42 | 8.4 ± 1.12 | 0.0254 | 0.6051 | -0.05, 0.97 | -0.21, 0.67 | 0.8792 | |
| Day 84 | 6.47 ± 1.4 | <0.000 | <0.0001 | 1.83, 2.95 | 1.24, 2.26 | <0.0001 | |
| Reduced activity | |||||||
| Placebo | Baseline | 8.36 ± 1.44 | - | - | - | - | |
| Day 42 | 8.25 ± 1.21 | 0.6517 | - | -0.38, 0.60 | - | - | |
| Day 84 | 8.85 ± 1.49 | 0.1022 | - | -0.04, 1.02 | - | - | |
| LN18178 | Baseline | 8.53 ± 1.38 | 0.5157 | -0.35, 0.69 | - | ||
| Day 42 | 8.58 ± 1.13 | 0.8266 | 0.1663 | -0.42, 0.52 | -0.10, 0.76 | 0.5777 | |
| Day 84 | 7.98 ± 2.56 | 0.1061 | 0.1022 | -0.21, 1.31 | 0.10, 1.64 | 0.0954 | |
| Reduced motivation | |||||||
| Placebo | Baseline | 11.36 ± 1.84 | - | - | - | ||
| Day 42 | 11.25 ± 1.43 | 0.7078 | - | -0.49, 0.71 | - | - | |
| Day 84 | 11.86 ± 2.07 | 0.0773 | - | -0.21, 1.21 | - | - | |
| LN18178 | Baseline | 11.7 ± 2.1 | 0.3460 | -0.39, 1.07 | - | ||
| Day 42 | 11.11 ± 1.29 | 0.0410 | 0.3568 | -0.06, 1.24 | -0.36, 0.64 | 0.0783 | |
| Day 84 | 11.16 ± 3.95 | 0.3367 | 0.4424 | -0.63, 1.71 | -0.45, 1.85 | 0.3295 | |
| Mental fatigue | |||||||
| Placebo | Baseline | 11.36 ± 1.7 | - | - | - | - | |
| Day 42 | 11.32 ± 1.31 | 0.8442 | - | -0.51, 0.59 | - | - | |
| Day 84 | 10.56 ± 1.57 | 0.0039 | - | 0.20, 1.40 | - | - | |
| LN18178 | Baseline | 11.65 ± 2.07 | 0.4049 | -0.41, 0.99 | - | ||
| Day 42 | 10.53 ± 1.38 | < 0.0001 | < 0.0001 | 0.47, 1.77 | 0.30, 1.28 | 0.0005 | |
| Day 84 | 8.67 ± 1.52 | < 0.0001 | < 0.0001 | 2.31, 3.65 | 1.32, 2.46 | < 0.0001 | |
| Total score | |||||||
| Placebo | Baseline | 56.51 ± 6.17 | - | - | - | - | |
| Day 42 | 56.1 ± 3.74 | 0.4831 | - | -1.45, 2.27 | - | - | |
| Day 84 | 55.61 ± 5.12 | 0.4962 | - | -1.17, 2.97 | - | - | |
| LN18178 | Baseline | 58.07 ± 8.07 | 0.2430 | -1.08, 4.20 | - | ||
| Day 42 | 55.11 ± 3.94 | 0.0091 | 0.0100 | 0.60, 5.32 | -0.42, 2.40 | 0.0877 | |
| Day 84 | 50.09 ± 12.58 | 0.0009 | 0.3767 | 4.06, 11.90 | 2.01, 9.03 | 0.1866 | |
Values present mean ± SD. placebo (n=59) and LN18178 (n=57). CI: Confidence interval; P < 0.05 was considered as statistically significant for 'within the group' and 'between the groups' comparison analysis using paired t-test and ANCOVA, respectively, as described in materials and methods.
Assessment of PSQI scores
| Evaluation | Mean ± SD | P value (vs. baseline) | P value (vs. placebo) | 95% CI vs. Baseline | 95% CI vs. Placebo | P value, change from baseline (vs. placebo) | |
|---|---|---|---|---|---|---|---|
| Subjective sleep quality | |||||||
| Placebo | Baseline | 1.66 ± 0.86 | - | - | - | - | - |
| Day 42 | 1.85 ± 0.94 | 0.1805 | - | -0.14, 0.52 | - | - | |
| Day 84 | 1.51 ± 0.97 | 0.4089 | - | -0.18, 0.48 | - | - | |
| LN18178 | Baseline | 1.42 ± 0.80 | 0.1237 | - | -0.07, 0.55 | - | |
| Day 42 | 1.25 ± 0.79 | 0.2050 | 0.0015 | -0.13, 0.47 | 0.28, 0.92 | 0.0693 | |
| Day 84 | 0.72 ± 0.67 | < 0.0001 | < 0.0001 | 0.43, 0.97 | 0.48, 1.10 | 0.0160 | |
| Sleep latency | |||||||
| Placebo | Baseline | 2.22 ± 0.59 | - | - | - | - | - |
| Day 42 | 1.97 ± 0.56 | 0.0115 | - | 0.04, 0.46 | - | - | |
| Day 84 | 1.71 ± 0.53 | < 0.000 | - | 0.31, 0.71 | - | - | |
| LN18178 | Baseline | 2.18 ± 0.47 | 0.6510 | - | -0.16, 0.24 | - | |
| Day 42 | 1.96 ± 0.42 | 0.0075 | 0.8964 | 0.05, 0.39 | -0.17, 0.19 | 0.5119 | |
| Day 84 | 1.53 ± 0.50 | < 0.0001 | 0.0763 | 0.47, 0.83 | -0.01, 0.37 | 0.2870 | |
| Sleep duration | |||||||
| Placebo | Baseline | 0.83 ± 0.85 | - | - | - | - | |
| Day 42 | 0.56 ± 0.68 | 0.0389 | - | -0.01, 0.55 | - | - | |
| Day 84 | 0.47 ± 0.63 | 0.0026 | - | 0.09, 0.63 | - | - | |
| LN18178 | Baseline | 0.47 ± 0.68 | 0.0147 | - | 0.08, 0.64 | - | |
| Day 42 | 0.46 ± 0.71 | 0.8821 | 0.5223 | -0.25, 0.27 | -0.16, 0.36 | 0.1141 | |
| Day 84 | 0.35 ± 0.64 | 0.3930 | 0.2960 | -0.13, 0.37 | -0.11, 0.35 | 0.1663 | |
| Habitual sleep efficiency | |||||||
| Placebo | Baseline | 0.15 ± 0.36 | - | - | - | - | - |
| Day 42 | 0.10 ± 0.30 | 0.5488 | - | -0.07, 0.17 | - | - | |
| Day 84 | 0.07 ± 0.25 | 0.2266 | - | -0.03, 0.19 | - | - | |
| LN18178 | Baseline | 0.12 ± 0.38 | 0.6676 | - | -0.11, 0.17 | - | |
| Day 42 | 0.09 ± 0.29 | 0.7266 | 0.9276 | -0.10, 0.16 | -0.10, 0.12 | 0.8268 | |
| Day 84 | 0.19 ± 0.48 | 0.4978 | 0.1131 | -0.09, 0.23 | -0.02, 0.26 | 0.1092 | |
| Sleep disturbances | |||||||
| Placebo | Baseline | 2.03 ± 0.26 | - | - | - | - | - |
| Day 42 | 1.62 ± 0.49 | < 0.0001 | - | 0.27, 0.55 | - | - | |
| Day 84 | 1.63 ± 0.49 | < 0.0001 | - | 0.26, 0.54 | - | - | |
| LN18178 | Baseline | 2.00 ± 0.27 | 0.4904 | - | -0.07, 0.13 | - | |
| Day 42 | 1.77 ± 0.42 | 0.001 | 0.0849 | 0.10, 0.36 | -0.02, 0.32 | 0.0683 | |
| Day 84 | 1.28 ± 0.45 | <0.0001 | 0.0002 | 0.58, 0.86 | 0.18, 0.52 | 0.0019 | |
| Use of sleep medication | |||||||
| Placebo | Baseline | 0.00 ± 0.00 | - | - | - | - | - |
| Day 42 | 0.00 ± 0.00 | - | - | 0, 0 | - | - | |
| Day 84 | 0.00 ± 0.00 | - | - | 0, 0 | - | - | |
| LN18178 | Baseline | 0.00 ± 0.00 | - | - | - | - | - |
| Day 42 | 0.00 ± 0.00 | - | - | 0, 0 | - | - | |
| Day 84 | 0.00 ± 0.00 | - | - | 0, 0 | - | - | |
| Daytime dysfunction | |||||||
| Placebo | Baseline | 1.46 ± 0.68 | - | - | - | - | - |
| Day 42 | 1.68 ± 0.68 | 0.0039 | - | -0.03, 0.47 | - | - | |
| Day 84 | 1.51 ±0.73 | 0.6592 | - | -0.21, 0.31 | - | - | |
| LN18178 | Baseline | 1.70 ± 0.65 | 0.0509 | - | -0.004, 0.48 | - | |
| Day 42 | 1.12 ± 0.66 | < 0.0001 | < 0.0001 | 0.34, 0.82 | 0.31, 0.81 | < 0.0001 | |
| Day 84 | 1.05 ± 0.79 | < 0.0001 | 0.0070 | 0.38, 0.92 | 0.18, 0.74 | 0.0003 | |
| Global PSQI score | |||||||
| Placebo | Baseline | 8.36 ± 1.81 | - | - | - | - | - |
| Day 42 | 7.78 ± 1.89 | 0.0096 | - | -0.09, 1.25 | - | - | |
| Day 84 | 6.90 ± 1.60 | < 0.0001 | - | 0.84, 2.08 | - | - | |
| LN18178 | Baseline | 7.89 ± 1.71 | 0.1609 | - | -0.18, 1.12 | - | |
| Day 42 | 6.65 ± 1.60 | < 0.0001 | 0.0009 | 0.63, 1.85 | 0.48, 1.78 | 0.0346 | |
| Day 84 | 5.12 ± 1.69 | < 0.0001 | < 0.0001 | 2.14, 3.40 | 1.17, 2.39 | 0.0006 | |
Values present mean ± SD. placebo (n=59) and LN18178 (n=57). CI: Confidence interval; P < 0.05 was considered as statistically significant for 'within the group' and 'between the groups' comparison analysis using paired t-test and ANCOVA, respectively, as described in materials and methods.
The bar diagrams present mean ± SD of the (A) hand-grip strength and (B) six-minute walk distance (m) in the placebo (n=59) and LN18178 (n=57) groups at baseline and on 14, 42 and 84 days of the study. * and # indicate significance (P < 0.05) in 'within the group' (vs. baseline) and 'between the group' (vs. placebo) comparison analyses using paired t-test and ANCOVA, respectively.
Safety assessments
At the screening visit the participants' total serum biochemistry, hematology, urine analysis, and vital parameters were within the normal ranges. At the end of the study, these safety parameters remained within the normal ranges (supplementary Table S4).
Discussion
The major outcome of the present study indicates that LN18178 supplementation is safe, and it increased overall sexual function (DISF-SR-M total score) and associated behavioral functions, including sexual cognition, arousal, sexual behavior, orgasm, and desire (Table 2). The daily consumption of LN18178 over 84 consecutive days is safe and well-tolerable by the participants. None of the study participants reported any major adverse events; their complete blood biochemistry, including liver, cardiovascular, and kidney functions, lipid profiles, hematology, and urinalysis parameters, were within the normal ranges. These safety observations were consistent with the earlier clinical studies [13, 14]. Importantly, LN18178 has shown comprehensive safety, as affirmed by a ninety-day sub-chronic oral toxicity study in rats and in vitro and in vivo genetic toxicology studies [33]. LN18178 is a food-derived ingredient; P granatum fruit rind powder and extracts are used in the dairy industry [34], and T. cacao beans are widely used in confectionaries [35].
Earlier clinical investigations conferred that LN18178, a synergistic phytoceutical composition, significantly increased serum total and free testosterone levels in young and aging male volunteers. LN18178 supplementation also enhanced the participants' muscle mass and strength [13. 14]. Cell-based in vitro studies demonstrated that this phytoceutical composition increased testosterone production via enhancing steroidogenesis in MA-10 mouse Leydig cells by upregulating Steroid acute regulatory protein (StAR) and cytochrome P450 family 17 subfamily A member 1 (CYP17A1) and reducing the conversion of testosterone to estradiol by inhibiting aromatase activity (unpublished data).
Testosterone is the integral androgenic and anabolic endocrine factor for men that regulates the development of male sexual traits and maintains and enhances sexual function and body composition [36]. In adult males, sexual function and performance are well-coordinated and regulated by physiological and psychological or emotional factors. Although sexual function is a multi-factorial process, testosterone plays a major and central role in modulating the emotional and neuro-physiological control of sexual arousal, erection and penetration, ejaculation, satisfaction, and overall performance [37]. Low testosterone levels are strongly associated with reduced sexual function, including impaired erectile function, libido, and desire in men. Testosterone replacement therapy (TRT) in hypogonadal men has been shown as a therapeutic strategy for the clinical management [37, 38]. However, due to serious side effects, including a rise in blood pressure, liver toxicity, increased risk of heart attack, and stroke, the US FDA recommendation limits the TRT or androgenic-anabolic steroids (AAS) therapy for selected medical conditions rather than age-related androgen deficiency [39]. In this context, a gradual rise in public attention to natural, plant-based diets and therapies for improving male sexual function, including increased hormone levels, erectile function, and libido, is worth mentioning [40, 41].
LN18178 supplementation has improved multi-dimensional fatigue inventory (MFI) and Pittsburgh Sleep Quality Index (PSQI) scores, indicative of reduced fatigue/stress and improved sleep quality. These improvements indicate the psychological benefits of LN18178 in the participants. Poor psychological status and low testosterone significantly contribute to impaired erectile function pathologies [42]. Interestingly, the increased frequency of morning erection and reduced physical and mental fatigue domain scores of the MFI questionnaire suggest reduced physical and psychological stress in the LN18178-supplemented participants. Morning erections in men are rapid eye movement (REM)-sleep-related normal physiological phenomena that are coordinated primarily by testosterone levels and have been negatively associated with physical and mental stress [43]. LN18178 improved the overall erectile function of the participants. Sustainable penile erection is a muscular response in coordination with the central and peripheral neural control involving endocrine factors like testosterone [44, 45]. Testosterone plays a pivotal role in regulating male sexual performances via central and autonomic responses [37] and indirectly increases penile blood flow and improves erection by reducing alpha-adrenergic activity in the vascular smooth muscles of the corpus cavernosum [44, 45].
Other important observations from this study are that LN18178 substantially increased the six-minute walk distance and hand-grip strength (HGS), suggesting an enhanced aerobic capacity and endurance [46] and increased muscle strength [47] in the participants. Improvements in the 6MWT indicate increased cardiopulmonary function with improved aerobic capacity and endurance [46, 48]. Improvements in the isometric muscular strength (as HGS measurement) in the LN18178-supplemented participants corroborate earlier observations [13, 14]. HGS determines aging-related muscle loss and measures neuromotor function and overall physical fitness in an aging population [49]. Testosterone is an anabolic hormone; a lower testosterone level is associated with muscle weakness in aging men [47]. Testosterone increases mitochondrial function in muscles via enhanced mitochondrial gene expression, thus helping improve energy metabolism in the muscles and preventing the gradual loss of skeletal muscle mass in aging. Elevated levels of mitochondrial function and prevention from muscle loss help improve muscle strength, endurance, and recovery [50]. Testosterone also plays a pivotal role in balancing multi-dimensional psychological networks of mood, behavior, self-perception, and perceived quality of life in men across the age range [51. Overall, the present and earlier observations [13, 14] on increased muscle strength and endurance affirm an anabolic effect of LN18178; also, the combined observations on increased HGS and GHS scores and global PSQI scores suggest a possible role of LN18178 in increasing vitality, vigor, and well-being in the participants.
The present study has a few limitations. This study did not measure the semen parameters, such as semen volume, sperm count, motility, etc. A future investigation on young male volunteers would be interesting. The present study did not test the efficacy of LN18178 supplementation on participants' body composition. Testosterone regulates metabolic function, age-related muscle and bone loss, and fat accumulation [52]. However, based on the present and earlier observations on the increased testosterone levels in the participants, we anticipate that LN18178 would improve body composition, thus warranting a more extended duration investigation.
Conclusion
LN18178 (TesNor®) is a safe and well-tolerated phytoceutical composition containing a combination of Punica granatum fruit rind and Theobroma cacao seed extracts. The present randomized, double-blind, placebo-controlled study data affirm that LN18178 consumption increases sexual function, erectile function, and libido and improves psychological well-being, muscle strength, neuromotor function, and general well-being in aging males. This botanical supplementation holds a potential promise to be an effective strategy in clinical practice to improve male sexual function and physical and psychological health in aging adults. Further research is warranted to evaluate the efficacy of LN18178 in male fertility.
Abbreviations
AAS: Anabolic-androgenic steroids; 3β-HSD: 3β-Hydroxysteroid Dehydrogenase; ANCOVA: Analysis of Covariance; BMI: Body Mass Index; CI: Confidence Interval; CTRI: Clinical Trial Registry of India; CYP17A1: Cytochrome P450 family 17 subfamily A member 1; DISF-SR-M: Derogatis Interview for Sexual Functioning-Male; ED: Erectile Dysfunction; EHS: Erection Hardness Score; GCP: Good Clinical Practice; GHS: General Health Survey; HGS: hand-grip strength; ICH: International Conference on Harmonization; IEC: Independent Ethics Committee; IIEF: International Index of Erectile Function; MFI: Multi-dimensional Fatigue Inventory; PP: Per-Protocol; PSQI: Pittsburgh Sleep Quality Index; SD: Standard Deviation; SMWT: Six-Minute Walk Test; StAR: Steroid Acute Regulatory protein; TRT: Testosterone replacement therapy; US FDA: United States Food and Drug Administration.
Supplementary Material
Supplementary tables.
Acknowledgements
The authors thank Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India, for providing the financial support to conduct the research. The authors also thank the participants and the coordinators of the study.
Funding
The present study was funded (LNTB1817818) by Laila Nutraceuticals, Vijayawada, Andhra Pradesh, India.
Data availability statement
The supporting data are available on reasonable request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Elterman DS, Bhattacharyya SK, Mafilios M. et al. The Quality of Life and Economic Burden of Erectile Dysfunction. Res Rep Urol. 2021;13:79-86 doi:10.2147/RRU.S283097
2. World Health Organization. (2006). Defining sexual health: report of a technical consultation on sexual health, 28-31 January. 2002 Geneva. World Health Organization
3. Bhasin S, Enzlin P, Coviello A. et al. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597-611 doi:10.1016/S0140-6736(07)60280-3
4. McBride JA, Carson CC 3rd, Coward RM. Testosterone deficiency in the aging male. Ther Adv Urol. 2016;8(1):47-60 doi:10.1177/1756287215612961
5. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25-45 doi:10.1530/JOE-12-0455
6. Irfan M, Hussain NHN, Noor NM. et al. Epidemiology of Male Sexual Dysfunction in Asian and European Regions: A Systematic Review. Am J Mens Health. 2020;14(4):1557988320937200 doi:10.1177/1557988320937200
7. Lewis RW, Fugl-Meyer KS, Corona G. et al. Definitions/Epidemiology/Risk Factors for Sexual Dysfunction. J Sex Med. 2010;7(4):1598-1607 doi:10.1111/j.1743-6109.2010.01778.x
8. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50-56 doi:10.1046/j.1464-410x.1999.00142.x
9. Gao J, Zhang X, Su P. et al. Prevalence and impact of premature ejaculation in outpatients complaining of ejaculating prematurely: using the instruments of intravaginal ejaculatory latency time and patient-reported outcome measures. Int J Impot Res. 2014;26(3):94-99
10. Tsai WK, Chiang PK, Lu CC. et al. The comorbidity between premature ejaculation and erectile dysfunction—a cross-sectional internet survey. Sex Med. 2019;7(4):451-8 doi:10.1016/j.esxm.2019.06.014
11. Chauhan NS, Sharma V, Dixit VK. et al. A review on plants used for improvement of sexual performance and virility. Biomed Res Int. 2014;2014:868062 doi:10.1155/2014/868062
12. Sin VJ, Anand GS, Koh HL. Botanical Medicine and Natural Products Used for Erectile Dysfunction. Sex Med Rev. 2021;9(4):568-592 doi: 10.1016/j.sxmr.2020.10.005
13. Sreeramaneni PGA, Yalamanchi A, Konda MR. et al. A Proprietary Herbal Blend Containing Extracts of Punica granatum Fruit Rind and Theobroma cocoa Seeds Increases Serum Testosterone Level in Healthy Young Males: A Randomized, Double-Blind Placebo-Controlled Study. J Diet Suppl. 2023;20(3):411-427 doi: 10.1080/19390211.2022.2035037
14. Pandit SL, Yaligar D, Halemane M, Bhat A. A proprietary blend of standardized Punica granatum fruit rind and Theobroma cocoa seed extracts mitigates aging males' symptoms: A randomized, double-blind, placebo-controlled study. Int J Med Sci. 2022;19(8):1290-1299 doi: 10.7150/ijms.73645
15. Langley P. Why a pomegranate? Br Med J. 2000;321(7269):1153-1154 doi: 10.1136/bmj.321.7269.1153
16. Sreekumar S, Sithul H, Muraleedharan P. et al. Pomegranate fruit as a rich source of biologically active compounds. Biomed Res Int. 2014;2014:686921 doi:10.1155/2014/686921
17. Minisy FM, Shawki HH, El Omri A. et al. Pomegranate Seeds Extract Possesses a Protective Effect against Tramadol-Induced Testicular Toxicity in Experimental Rats. Biomed Res Int. 2020;2020:2732958 doi: 10.1155/2020/2732958
18. Zhang Q, Radisavljevic ZM, Siroky MB. et al. Dietary antioxidants improve arteriogenic erectile dysfunction. Int J Androl. 2011;34(3):225-235 doi: 10.1111/j.1365-2605.2010.01083.x
19. Forest CP, Padma-Nathan H, Liker HR. Efficacy and safety of pomegranate juice on improvement of erectile dysfunction in male patients with mild to moderate erectile dysfunction: a randomized, placebo-controlled, double-blind, crossover study. Int J Impot Res. 2007;19(6):564-567 doi: 10.1038/sj.ijir.3901570
20. Rusconi M, Conti A. Theobroma cacao L, the Food of the Gods: a scientific approach beyond myths and claims. Pharmacol Res. 2010;61(1):5-13 doi: 10.1016/j.phrs.2009.08.008
21. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011Nov15;15(10):2779-811 doi: 10.1089/ars.2010.3697
22. Ali LC, Ikeh NE, Amaefule BC. et al. Effects of discarded cocoa (Theobroma cacao) seed meal-based diets on semen traits, testicular morphometry and histomorphology of rabbit bucks. Adv Anim Vet Sci. 2022;10(6):1245-1254 doi:10.17582/journal.aavs/2022/10.6.1245.1254
23. Ambiye VR, Langade D, Dongre S. et al. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evid Based Complement Alternat Med. 2013;2013:571420 doi: 10.1155/2013/571420
24. Derogatis LR. The Derogatis Interview for Sexual Functioning (DISF/DISF-SR): an introductory report. J Sex Marital Ther. 1997;23(4):291-304 doi:10.1080/00926239708403933
25. Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226-244 doi:10.1038/sj.ijir.3900857
26. Montorsi F, Adaikan G, Becher E. et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7(11):3572-3588 doi:10.1111/j.1743-6109.2010.02062.x
27. Cappelleri JC, Stecher VJ. An assessment of patient-reported outcomes for men with erectile dysfunction: Pfizer's perspective. Int J Impot Res. 2008;20(4):343-357 doi:10.1038/ijir.2008.8
28. Smets EM, Garssen B, Bonke B. et al. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315-325 doi:10.1016/0022-3999(94)00125-o
29. Buysse DJ, Reynolds CF 3rd, Monk TH. et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213 doi:10.1016/0165-1781(89)90047-4
30. ATS Statement. Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117 doi:10.1164/ajrccm.166.1.at1102
31. Gahyun Y. Why Do Healthy Men Experience Morning Erections? Open Psychol J. 2017;10:49-54 doi:10.2174/1874350101710010049
32. Ueda P, Mercer CH, Ghaznavi C. et al. Trends in Frequency of Sexual Activity and Number of Sexual Partners Among Adults Aged 18 to 44 Years in the US, 2000-2018. JAMA Netw Open. 2020;3(6):e203833 doi:10.1001/jamanetworkopen.2020.3833
33. Madireddy RK, Alluri KV, Somepalli V. et al. Toxicological Assessments of a Proprietary Blend of Punica granatum Fruit Rind and Theobroma cacao Seed Extracts: Acute, Subchronic, and Genetic Toxicity Studies. J Toxicol. 2022. 2022 3903943. Article ID 903943, doi:10.1155/2022/3903943
34. Mahajan D, Bhat ZF, Kumar S. Pomegranate (Punica granatum) rind extract as a novel preservative in cheese. Food Biosci. 2015;12:47-53 doi: 10.1016/j.fbio.2015.07.005
35. Rusconi M, Conti A. Theobroma cacao L. the Food of the Gods: a scientific approach beyond myths and claims. Pharmacol Res. 2010;61(1):5-13 doi:10.1016/j.phrs.2009.08.008
36. Rommerts FFG. Testosterone: An overview of biosynthesis, transport, metabolism and nongenomic actions. In: Nieschlag E, Behre HM (eds) Testosterone. Springer, Berlin, Heidelberg. 1998 doi:10.1007/978-3-642-72185-4_1
37. Corona G, Maggi M. The role of testosterone in male sexual function. Rev Endocr Metab Disord. 2022;23(6):1159-1172 doi:10.1007/s11154-022-09748-3
38. Nguyen V, Leonard A, Hsieh TC. Testosterone and Sexual Desire: A Review of the Evidence. Androg Clin Res Ther. 2022;3(1):85-90 doi:10.1089/andro.2021.0034
39. Bond P, Smit DL, de Ronde W. Anabolic-androgenic steroids: How do they work and what are the risks? Front Endocrinol. 2022;13:1059473 doi:10.3389/fendo.2022.1059473
40. Eleazu C, Obianuju N, Eleazu K. et al. The role of dietary polyphenols in the management of erectile dysfunction-Mechanisms of action. Biomed Pharmacother. 2017;88:644-652 doi:10.1016/j.biopha.2017.01.125
41. Masuku NP, Unuofin JO, Lebelo SL. Promising role of medicinal plants in the regulation and management of male erectile dysfunction. Biomed Pharmacother. 2020;130:110555 doi:10.1016/j.biopha.2020.110555
42. Kshirsagar A, Seftel A, Ross L. et al. Predicting hypogonadism in men based upon age, presence of erectile dysfunction, and depression. Int J Impot Res. 2006;18(1):47-51 doi:10.1038/sj.ijir.3901369
43. Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379-95 v. doi:10.1016/j.ucl.2005.08.007
44. Schardein JN, Hotaling JM. The Impact of Testosterone on Erectile Function. Androg Clin Res Ther. 2022;3(1):113-124 doi:10.1089/andro.2021.0033
45. Park NC, Kim SW, Hwang SY. et al. Efficacy and safety of an herbal formula (KBMSI-2) in the treatment of erectile dysfunction: A preliminary clinical study. Investig Clin Urol. 2019;60(4):275-284 doi:10.4111/icu.2019.60.4.275
46. Holland AE, Spruit MA, Troosters T. et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446 doi:10.1183/09031936.00150314
47. Chiu HT, Shih MT, Chen WL. Examining the association between grip strength and testosterone. Aging Male. 2020;23(5):915-922 doi:10.1080/13685538.2019.1632282
48. Cazzoletti L, Zanolin ME, Dorelli G. et al. Six-minute walk distance in healthy subjects: reference standards from a general population sample. Respir Res. 2022;23(1):83 doi:10.1186/s12931-022-02003-y
49. Vaishya R, Misra A, Vaish A. et al. Hand grip strength as a proposed new vital sign of health: a narrative review of evidences. J Health Popul Nutr. 2024;43(1):7 doi:10.1186/s41043-024-00500-y
50. Pronsato L, Milanesi L, Vasconsuelo A. Modulation of mitochondrial gene expression by testosterone in skeletal muscle. Cell Signal. 2024;2(1):80-85 doi:10.46439/signaling.2.034
51. Zitzmann M. Testosterone, mood, behaviour and quality of life. Andrology. 2020;8:1598-1605 doi:10.1111/andr.12867
52. Dandona P, Dhindsa S, Ghanim H, Saad F. Mechanisms underlying the metabolic actions of testosterone in humans: A narrative review. Diabetes Obes Metab. 2021;23(1):18-28 doi: 10.1111/dom.14206
Author contact
![]() Corresponding author: Dr. Amulya Yalamanchi, Department of General Medicine, Yalamanchi Hospitals and Research Centre, Vijayawada-520002, Andhra Pradesh, India; Email: dramulyacom; ORCID: 0000-0001-6729-5331.
Corresponding author: Dr. Amulya Yalamanchi, Department of General Medicine, Yalamanchi Hospitals and Research Centre, Vijayawada-520002, Andhra Pradesh, India; Email: dramulyacom; ORCID: 0000-0001-6729-5331.

 Global reach, higher impact
Global reach, higher impact