3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(2):252-259. doi:10.7150/ijms.101733 This issue Cite
Research Paper
The association between grade of coronary heart disease and risk of developing keratopathy: a nationwide cohort study
1. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
2. Nobel Eye Institute, Taipei, Taiwan.
3. Department of Ophthalmology, Jen-Ai Hospital Dali Branch, Taichung, Taiwan.
4. Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan.
5. Department of Optometry, Yuanpei University of Medical Technology, Hsinchu, Taiwan
† The authors contribute equally and share the first authorship.
Received 2024-7-31; Accepted 2024-11-14; Published 2025-1-1
Abstract
Purpose: To evaluate the association between coronary heart disease (CHD) severity and the risk of developing keratopathy.
Method: A retrospective cohort study was conducted with data from the Taiwan National Health Insurance Research Database (NHIRD). A total of 593100, 593100 and 296500 patients were included in the control, mild CHD and severe CHD groups, respectively. The primary outcomes were the development of superficial keratopathy and infectious keratitis with antibiotic usage. Cox proportional hazard regression was used to calculate the adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the primary outcomes among the groups.
Results: A total of 30697, 32134, and 15977 superficial keratopathy episodes and 6021, 6010, and 2982 infectious keratitis episodes were recorded in the control, mild CHD, and severe CHD groups, respectively. The incidence of superficial keratopathy was significantly greater in the severe CHD group (P = 0.037), and both groups presented a greater risk of developing superficial keratopathy than did the control group (both P < 0.05). The cumulative incidence of superficial keratopathy was also significantly greater in the severe CHD group than in the mild CHD group (P < 0.001). In the subgroup analyses, the incidence of superficial keratopathy was significantly greater in severe CHD patients than in mild CHD patients older than 70 years, and the correlation between CHD severity and superficial keratopathy incidence was significantly greater in those older than 70 years of age (P = 0.002).
Conclusions: Severe CHD is related to a greater risk of developing superficial keratopathy, especially in those older than 70 years of age.
Keywords: coronary heart disease, superficial keratopathy, age, infectious keratitis, epidemiology
Introduction
Coronary heart disease (CHD) is a disease characterized by coronary arterial obstruction and cardiac ischemia [1]. People with mild CHD experience a relatively chronic course, and medications such as antiplatelet and antilipidemic drugs are often used [2, 3]. Nevertheless, severe CHD can result in fatal coronary arterial occlusion; thus, surgical management is usually advocated to prevent death [4]. Percutaneous coronary intervention (PCI) can be used to expand the occluded coronary artery in severe CHD patients, and PCI results in improved survival rate [5, 6].
In addition to the heart and coronary vasculatures, CHD may increase the incidence of certain diseases [7-9]. According to an earlier study, periodontitis is correlated with the formation and severity of CHD [10]. Furthermore, Behcet's disease is a systemic inflammatory defect that increases CHD risk and other cardiovascular impairments [11, 12]. On the other hand, CHD is significantly correlated with metabolic syndromes, including hypertension, hyperlipidemia and type 2 diabetes mellitus [13, 14]. With respect to the relationship between ophthalmic diseases and CHD, a higher risk of open angle glaucoma was found in CHD patients [15].
Corneal diseases, such as superficial keratopathy, present frequently in people and can result in irritation and ocular pain [16]. The severe form of corneal disease, infectious keratitis, can corrupt the corneal structure and contribute to subsequent eyeball rupture and loss of vision [17]. Among the causes of keratopathy, dry eye disease is a common etiology for the development of ocular surface damage and superficial keratopathy [18], and viral infection and immunological dysfunction can also lead to the development of superficial keratopathy [19, 20]. With respect to the etiology of infectious keratitis, microorganism invasion is the fundamental component of infectious keratitis, which includes bacterial, fungal, protozoan, and viral infections [21]. Contact lens wear, ocular trauma and ocular surgery could contribute to corneal insult and the development of infectious keratitis [17]. Nevertheless, few studies have evaluated the correlation between CHD status and the risk of developing keratopathy. In addition, the association between CHD severity and subsequent dry eye disease has been illustrated in previous research [22]. Thus, the severity of CHD may influence the severity of subsequent keratopathy, which warrants further investigation.
Consequently, the objective of the current study was to survey the potential correlation between CHD severity and the risk of developing keratopathy of differing severities. The general health status of each participant was also included in the analyses. With respect to the terms and criteria for classification in the current study, superficial keratopathy referred to patients who received superficial keratopathy-related ICD-9 and ICD-10 diagnostic codes; infectious keratitis/keratopathy referred to patients who received infectious keratitis-related ICD-9 and ICD-10 diagnostic codes and antibiotic treatment; mild CHD referred to patients who received CHD-related ICD-9 and ICD-10 diagnostic codes and medical treatments; and severe CHD referred to patients who received CHD-related ICD-9 and ICD-10 diagnostic codes and PCI management.
Materials and Methods
Ethics declaration
The current study adhered to the Declaration of Helsinki and later amendments. The current study was also approved by both the National Health Insurance Administration of Taiwan and the Institutional Review Board of Chung Shan Medical University (Project code: CS1-20108, date of approval: 06/16/2020). The requirement of obtaining written informed consent was waived by the above two bureaus.
Data source
The Taiwan NHIRD saves the medical records of 23 million Taiwanese individuals from January 1, 2000, to December 31, 2020. The available records in the NHIRD include the International Classification of Diseases Ninth Revision (ICD-9) diagnostic code, the International Classification of Diseases Tenth Revision (ICD-10) diagnostic code, sex, age, socioeconomic status, place of residence, laboratory codes, image codes, medical department codes, procedure or surgical codes and international ATC codes for all medicines in the Taiwan National Health Insurance System.
Participant selection
A retrospective cohort study was performed, and patients were defined as having CHD occurrence if these conditions were met: (1) CHD was diagnosed according to ICD-9/ICD-10 codes from 20142019; (2) complete blood cell count, cholesterol, white blood cell differentiation count, high-density lipoprotein, triglyceride, low-density lipoprotein, cardiac angiography and electrocardiogram data were available before CHD diagnosis; (3) age ranged from 20 to 100 years; and (4) participants were regularly followed for more than three months. The index date of the current study was set at 6 months after CHD diagnosis. Additionally, the following exclusion criteria were utilized: (1) demographic data were unavailable, (2) CHD patients died before the index date, and (3) outcomes developed before the index date. To survey the correlation between CHD severity and the risk of developing keratopathy, CHD patients were divided into mild CHD patients who received other medical treatments and severe CHD patients who underwent PCI. One severe CHD patient with PCI was matched to two mild CHD patients with other medical treatments via the propensity score-matching (PSM) method, which was used to consider the distribution of age and sex between the severe CHD patients who underwent PCI and the mild CHD patients who received other medical treatments. Additionally, the mild CHD population mentioned above was matched to the non-CHD population at a 1:1 ratio. After the whole process, 593100, 593100 and 296500 patients were enrolled in the control, mild CHD and severe CHD groups, respectively.
Main outcomes
The main outcomes in this study were superficial keratopathy and infectious keratitis. Patients with superficial keratopathy met the following criteria: (1) received superficial keratopathy-related ICD-9 and ICD-10 diagnostic codes, (2) underwent slit-lamp biomicroscopy before superficial keratopathy was diagnosed according to the procedure code, and (3) superficial keratopathy was diagnosed by an ophthalmologist. In addition, infectious keratitis was defined as (1) the receipt of infectious keratitis-related ICD-9 and ICD-10 diagnostic codes, (2) the arrangement of slit-lamp biomicroscope tests before the infectious keratitis diagnosis according to the procedure code, (3) the receipt of topical antibiotic eyedrop or ointment prescriptions after the infectious keratitis diagnosis, and (4) the infectious keratitis diagnosis was performed by an ophthalmologist. Only superficial keratopathy and infectious keratitis events that emerged after the index date were considered as the primary outcomes in the current study.
Confounding factors
Multiple demographic factors, systemic diseases and medications were included in the statistical model of the current study to adjust for the influences of possible confounders of keratopathy development. These covariates include age, sex, hypertension, diabetes mellitus, hyperlipidemia, ischemic stroke, rheumatoid arthritis, systemic lupus erythematosus, Sjogren syndrome, corticosteroids, nonsteroidal anti-inflammatory drugs, and aspirin. The presence of these covariates was determined via demographic codes, ICD-9/ICD-10 diagnostic codes and ATC codes in the NHIRD of Taiwan. To affirm that the existing periods of these covariates in the current study are sufficient to impact the risk of keratopathy, only the covariates that had been established for more than one year before our index date were included in the current study. The people in the current study were followed until keratopathy occurred, they withdrew from the National Health Insurance System, or the end date of the Taiwan NHIRD study (set as December 31, 2020).
Statistical analysis
SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analyses in the current study. Descriptive analyses were performed to display the demography, systemic diseases and medications of the control group, mild CHD group and severe CHD group, and the standardized mean difference (SMD) was used to compare the distributions of initial characteristics. A SMD value greater than 0.1 was defined as a significant difference. Cox proportional hazard regression was then used to calculate adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for superficial keratopathy and infectious keratitis among the groups. The potential effects of demography, systemic disorders mentioned in the “confounding factors” section, and medications were adjusted in the Cox proportional hazard regression. A Kaplan‒Meier curve was generated to display the cumulative probability of superficial keratopathy and infectious keratitis between the two CHD groups, and the log-rank test was used to evaluate the cumulative probability between groups. With respect to subgroup analyses, CHD patients were stratified by age and sex, and Cox proportional hazard regression was used to compare the aHR and 95% CI of superficial keratopathy and infectious keratitis between the severe CHD population and the mild CHD population with different ages and sexes. Additionally, an interaction test was performed to explore the effects of CHD severity on keratopathy severity in different age and sex subgroups. Statistical significance was set as P < 0.05, and P values lower than 0.001 are indicate as such (P < 0.001).
Results
The initial characteristics of the three groups are available in Table 1. The sex ratio was identical among the groups due to the PSM procedure (both SMD = 0.000), and the age distributions of the people in the current study were grossly similar among the groups (both SMD = 0.001). In addition, the ratio of systemic diseases was not significantly different between the two groups (all SMDs < 0.1), and the percentage of patients receiving medication was also not significantly different between the mild CHD group and the severe CHD group (all SMDs < 0.1), except for the ratio of aspirin use (SMD: 0.105) (Table 1). In addition, the rates of hypertension, diabetes mellitus, hyperlipidemia, and aspirin use were significantly lower in the control group than in the mild CHD group (all SMD > 0.1) (Table 1).
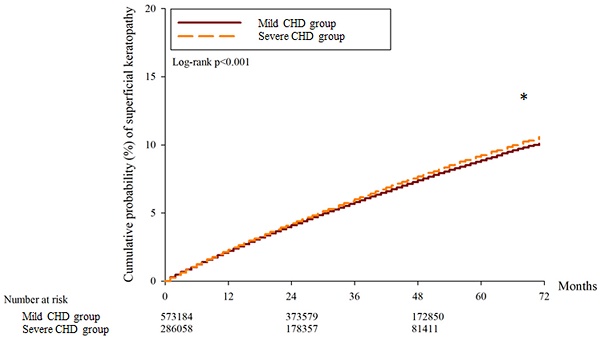
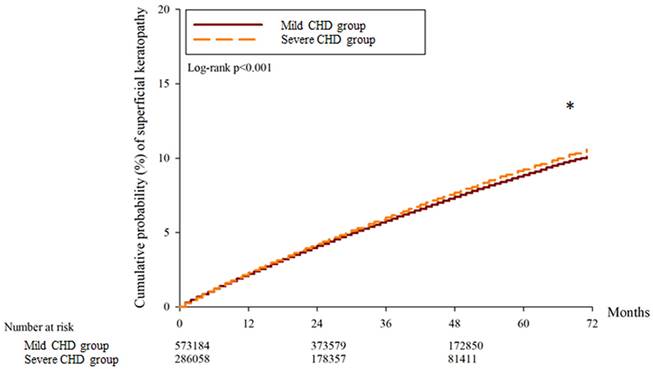
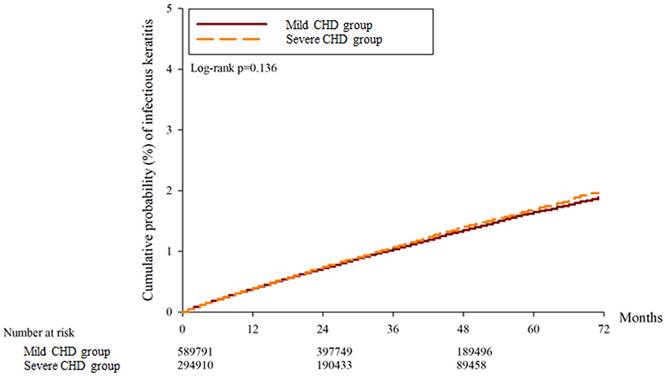
A total of 30697, 32134 and 15977 superficial keratopathy episodes were recorded in the control group, mild CHD group, and severe CHD group, respectively. In addition, 6021, 6010 and 2982 infectious keratitis episodes were recorded in the control group, mild CHD group and severe CHD group, respectively. After multiple covariates were adjusted, the incidence of superficial keratopathy was significantly greater in the severe CHD group than in the mild CHD group (aHR: 1.026, 95% CI: 1.005-1.047, P = 0.037), and both groups presented a greater risk of developing superficial keratopathy than the control group did (both P < 0.05). On the other hand, the incidence of infectious keratitis in the severe CHD group was not significantly greater than that in the mild CHD group (aHR: 1.027, 95% CI: 0.979-1.077, P = 0.246), and both groups presented a similar risk of infectious keratitis to the control group (both P > 0.05) (Table 2). The cumulative incidence of superficial keratopathy was significantly greater in the severe CHD group than in the mild CHD group (P < 0.001) (Figure 1), and the cumulative incidence of infectious keratitis was similar between the severe CHD group and the mild CHD group (P = 0.136) (Figure 2).
Baseline characteristics of the groups
| Characteristic | Control group (N: 593100) | Mild CHD group (N: 593100) | Severe CHD group (N: 296550) | SMD1 | SMD2 |
|---|---|---|---|---|---|
| Sex | 0.000 | 0.000 | |||
| Male | 385840(65.05%) | 385840(65.05%) | 192920(65.05%) | ||
| Female | 207260(34.95%) | 207260(34.95%) | 103630(34.95%) | ||
| Age | 0.001 | 0.001 | |||
| <40 | 16844(2.84%) | 15836(2.67%) | 7799(2.63%) | ||
| 40-49 | 48338(8.15%) | 47745(8.05%) | 24762(8.35%) | ||
| 50-59 | 115477(19.47%) | 115061(19.40%) | 57501(19.39%) | ||
| 60-69 | 177515(29.93%) | 179176(30.21%) | 88431(29.82%) | ||
| >=70 | 234926(39.61%) | 235282(39.67%) | 118057(39.81%) | ||
| Comorbidity | |||||
| Hypertension | 199993(33.72%) | 388125(65.44%) | 220574(74.38%) | 0.156* | 0.025 |
| Diabetes mellitus | 106461(17.95%) | 183624(30.96%) | 124581(42.01%) | 0.189* | 0.049 |
| Hyperlipidemia | 99522(16.78%) | 252245(42.53%) | 170576(57.52%) | 0.362* | 0.035 |
| Ischemic stroke | 36416(6.14%) | 47448(8.00%) | 30011(10.12%) | 0.015 | 0.019 |
| Rheumatoid arthritis | 6821(1.15%) | 6702(1.13%) | 3796(1.28%) | 0.001 | 0.001 |
| Systemic lupus erythematosus | 831(0.14%) | 890(0.15%) | 860(0.29%) | 0.001 | 0.001 |
| Sjogren syndrome | 7473(1.26%) | 7354(1.24%) | 4181(1.41%) | 0.001 | 0.001 |
| Comedication | |||||
| NSAIDs | 310429(52.34%) | 344710(58.12%) | 178790(60.29%) | 0.006 | 0.002 |
| Corticosteroids | 114824(19.36%) | 104623(17.64%) | 64411(21.72%) | 0.002 | 0.004 |
| Aspirin | 122001(20.57%) | 225200(37.97%) | 178849(60.31%) | 0.143* | 0.105* |
CHD: coronary heart disease, SMD: standardized mean difference
SMD1: mild CHD group compared with the control group
SMD2: mild CHD group compared with the severe CHD group
* denotes a significant difference
The cumulative incidence of superficial keratopathy between the two groups. CHD: coronary heart disease. * Denotes a significant difference between the two groups.
The cumulative incidence of infectious keratitis between the two groups. CHD: coronary heart disease.
The risk of study events among the groups
| Event | Control | Mild CHD | Severe CHD |
|---|---|---|---|
| Superficial keratopathy | |||
| Person-months | 19802145 | 19778459 | 9457210 |
| Event | 30697 | 32134 | 15977 |
| aHR1 (95% CI) | Reference | 1.071(1.010-1.124)* | |
| aHR2 (95% CI) | Reference | 1.145(1.096-1.207)* | |
| aHR3 (95% CI) | Reference | 1.026(1.005-1.047)* | |
| Infectious keratitis | |||
| Person-months | 21198706 | 21024190 | 10077132 |
| Event | 6021 | 6010 | 2982 |
| aHR1 (95% CI) | Reference | 1.002(0.975-1.030) | |
| aHR2 (95% CI) | Reference | 1.035(0.982-1.088) | |
| aHR3 (95% CI) | Reference | 1.027(0.979-1.077) |
aHR: adjusted hazard ratio, CHD: coronary heart disease, CI: confidence interval
aHR1: mild CHD compared to control
aHR2: severe CHD patients compared with controls
aHR3: severe CHD compared with mild CHD
* denotes a significant difference
In the sex subgroup analyses, the incidence of superficial keratopathy was significantly greater in females with severe CHD than in females with mild CHD (aHR: 1.043, 95% CI: 1.011-1.076), whereas the incidence of infectious keratitis did not differ between the sex subgroups (Table 4). The correlations between CHD severity and keratopathy incidence in different severe CHD subgroups were not significant (both P > 0.05) (Table 4). With respect to the age subgroup analysis, the incidence of superficial keratopathy was significantly greater in severe CHD patients than in mild CHD patients older than 70 years (aHR: 1.067, 95% CI: 1.033-1.102) (Table 4), whereas the incidence of infectious keratitis was not significantly different across the severe CHD subgroups. The correlation between CHD severity and superficial keratopathy events was significantly greater in those older than 70 years (P = 0.002), and the correlations between CHD severity and infectious keratitis events were similar across all age subgroups (P = 0.752) (Table 4).
Subgroup analysis stratified by sex
| Event | aHR (95% CI) | P for interaction |
|---|---|---|
| Superficial keratopathy | 0.453 | |
| Male | 1.014(0.987-1.041) | |
| Female | 1.043(1.011-1.076)* | |
| Infectious keratitis | 0.426 | |
| Male | 1.042(0.983-1.105) | |
| Female | 1.012(0.935-1.095) |
aHR: adjusted hazard ratio, CI: confidence interval
* denotes a significant difference
Subgroup analysis stratified by age
| Event | aHR (95% CI) | P for interaction |
|---|---|---|
| Superficial keratopathy | 0.002* | |
| age<60 | 0.976(0.939-1.014) | |
| age 60-69 | 1.015(0.979-1.052) | |
| age ≥70 | 1.067(1.033-1.102)* | |
| Infectious keratitis | 0.752 | |
| age<60 | 1.040(0.960-1.126) | |
| age 60-69 | 1.007(0.924-1.099) | |
| age ≥70 | 1.019(0.942-1.103) |
aHR: adjusted hazard ratio, CI: confidence interval
* denotes a significant difference
Discussion
In the present study, severe CHD status was associated with a greater incidence of superficial keratopathy than mild CHD status. Moreover, this correlation was more prominent in patients older than 70 years than in the younger population. On the other hand, the associations between CHD severity and the incidence of infectious keratitis were not significant in the overall population.
CHD can damage coronary arterial vessels to a large extent and can also cause damage to other human tissues [8, 23, 24]. CHD is highly related to the development of systemic inflammatory diseases, including ankylosing spondylitis and inflammatory bowel disease [25, 26]. Inflammatory status is common in people with CHD, and the neutrophil-to-lymphocyte ratio is a crucial inflammatory marker that can predict the development of subsequent CHD [27]. Additionally, the plasma concentrations of the cytokines interleukin and C-reactive protein are significantly greater in people diagnosed with CHD [28]. In addition to inflammation, hyperlipidemia is another CHD pathophysiology that is significantly associated with the presence of atherosclerotic plaques and subsequent coronary artery stenosis [29]. Atherosclerotic plaque and subsequent CHD could result from high triglyceride expression [30], and higher LDL expression is also a well-established predisposing factor for CHD formation [30]. In addition to hyperlipidemia and inflammation, high oxidative stress is another mechanism for CHD that is significantly elevated in those with CHD and acute cardiovascular events [31]. With respect to the corneal aspect, oxidative stress contributes to ocular surface damage, and erosion of the corneal surface may occur [32]. Moreover, the inflammatory reaction is a critical factor in the development of dry eye disease [33], and severe dry eye disease can cause corneal lesions [34]. Infectious keratitis is caused mainly by microorganisms, and a prominent inflammatory response is also found in patients with infectious keratitis [17]. Since both CHD and keratopathy have similar mechanisms involving inflammation and oxidative stress [17, 23, 31, 32], we speculate that the severity of CHD may influence the development of subsequent corneal disorders of different forms. The hypothesis was supported by the outcomes of the current study, at least to some degree.
Severe CHD was associated with a greater possibility of subsequent superficial keratopathy in the current study. In previous studies, keratopathy has been reported in patients with systemic inflammatory diseases such as psoriasis [35]. Nevertheless, the potential correlation between CHD severity and the risk of developing keratopathy with different severities was not reported in a previous study. To our knowledge, this may be a preliminary experience that reveals a positive correlation between CHD severity and subsequent superficial keratopathy occurrence. Furthermore, only superficial keratopathy episodes that occurred 6 months after CHD diagnosis were counted in the current study; thus, the time sequence between severe CHD and superficial keratopathy could be established. On the other hand, some predisposing factors for corneal disease, including rheumatic arthritis and Sjogren syndrome, were included in the Cox proportional hazard regression to adjust their effect on the development of keratopathy [36]. Consequently, severe CHD may be an independent and crucial risk factor for subsequent development of superficial keratopathy. Although the number of keratopathies in the severe CHD group was greater than half of that in the mild CHD group, the mild CHD group had 2-fold more participants and 2.1-fold longer follow-up than did the severe CHD group. Thus, the incidence of keratopathy per person during a fixed interval was significantly greater in the severe CHD group according to the Cox proportional hazard regression models, which were adjusted for the effects of several keratopathy-related risk factors. We believe that this result could present the real risk degree rather than the direct comparison of numerical values. In addition, the cumulative probability of superficial keratopathy was significantly greater in the severe CHD population than in the mild CHD population. This result may further imply the influence of severe CHD on corneal health and that a long-term severe CHD course results in additional damage to the cornea. In contrast, the incidence and cumulative probability of infectious keratitis did not increase in the severe CHD population compared with the mild CHD population. A possible explanation is that microorganism invasion still accounts for the major and prominent pathophysiology of infectious keratitis [17]; thus, the effects of inflammation and oxidative stress in infectious keratitis may not be significant.
In the subgroup analyses, females with severe CHD presented with a significantly greater risk of developing superficial keratopathy than women diagnosed with mild CHD did. On the other hand, the male population with severe CHD did not have a significantly greater risk of developing superficial keratopathy than men diagnosed with mild CHD did. A possible explanation for our finding might be that the actual CHD severity in the female population might be greater than that in the male population [37]. The criteria for severe CHD are based on the PCI approach, but the patients who underwent PCI did not have the exact same CHD severity. As a consequence, the actual CHD severity in the female population may be greater; thus, elevated inflammatory and oxidative stress leads to a greater incidence of subsequent superficial keratopathy. However, the interaction test revealed that the correlations between superficial keratopathy and severe CHD were similar in different sexes, which further implies that female sex may be a nonprominent risk factor for the development of superficial keratopathy in the severe CHD population. On the other hand, the severe CHD population older than 70 years presented a significantly greater risk of developing subsequent superficial keratopathy development than did mild CHD patients of the same age. Moreover, the correlation between CHD severity and the risk of developing superficial keratopathy in patients older than 70 years was significantly greater than that in their younger counterparts. Few studies have investigated this association. Age is a prominent predisposing factor for the development of corneal disease [38]; thus, our findings support the established concept. Severe CHD was not strongly related to keratopathy in any of the subgroup analyses, which is consistent with the results of the whole-population analysis.
With respect to the epidemiology of CHD, CHD is a major vascular disease that is found in approximately 5-6% of the Caucasian population [39, 40], and the prevalence of CHD may reach 25% in specific regions of East Asia [41]. Although the prevalence of CHD has gradually decreased in recent decades, severe CHD that presents with coronary arterial occlusion is still diagnosed in more than 30% of all CHD patients [42], and prominent CHD can result in a noticeable mortality rate in the European population according to earlier publications [43]. On the other hand, keratopathy is not an uncommon ocular disease, as it is found in more than 1% of people worldwide [19, 44]. Although superficial keratopathy does not usually cause visual impairment, such as that observed in patients with infectious keratitis [17], ocular symptoms in those with superficial keratopathy may reduce quality of life. Since CHD and keratopathy affect many people and can lead to substantial burdens in daily life, the associations between these two diseases should be emphasized.
There are several limitations in the current research. First, we consumed the claimed data, not the real medical documents, for all the analyses of the current study. Multiple critical parameters, including the complete blood count results, lipid profile results, echocardiogram results, cardiac angiography results, details of PCI, postoperative status of PCI, recurrence of coronary arterial occlusion, external eye picture of keratopathy, site and pattern of keratopathy, cause of keratopathy, species of microorganism involved in infectious keratitis, and outcome of medical therapies for CHD and keratopathy, were not evaluated. In addition, the retrospective design of the current study could reduce the homogeneity of the study population, although we applied the PSM procedure to increase the degree of homogeneity. Moreover, the criteria for the PCI indication or the diagnosis of corneal diseases may differ across physicians. Thus, the diagnostic standards for severe CHD, superficial keratopathy, and infectious keratitis may be different in this study, thus reducing the reliability of our results. Additionally, the effect of eyelid dysfunction on keratopathy was not analyzed because many of them were only used in self-paid cosmetic surgeries, and lifestyle risk factors (e.g., cigarette smoking, length of cellphone usage) for keratopathy are nearly unavailable in the NHIRD and thus could not be evaluated. Finally, nearly all the people in the current study were Han Taiwanese, and the external validity of the current study may be diminished.
In conclusion, compared with mild CHD, severe CHD is correlated with the risk of developing superficial keratopathy, especially in people older than 70 years. Furthermore, the risk of developing superficial keratopathy attendance is positively related to the disease duration of severe CHD. Consequently, a periodic ophthalmic exam may be recommended for people with severe CHD to manage underlying keratopathy if it is present. Further large-scale prospective studies to evaluate the relationship between CHD extent and the therapeutic outcome of keratopathy are needed.
Abbreviations
CHD: coronary heart disease; PCI: percutaneous coronary intervention; NHIRD: National Health Insurance Research Database; ICD-9: International Classification of Diseases Ninth Revision; ICD-10: International Classification of Diseases Tenth Revision; PSM: propensity score matching; SMD: standardized mean difference; aHR: adjusted hazard ratio; CI: confidence interval; N: number.
Competing Interests
The authors have no proprietary or commercial interest in any materials mentioned in this article.
References
1. Sarwar N, Thompson AJ, Di Angelantonio E. Markers of inflammation and risk of coronary heart disease. Dis Markers. 2009;26:217-25
2. Almeida SO, Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med. 2019;29:451-5
3. Al-Lamee RK, Nowbar AN, Francis DP. Percutaneous coronary intervention for stable coronary artery disease. Heart. 2019;105:11-9
4. Xie Q, Huang J, Zhu K, Chen Q. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with coronary heart disease and type 2 diabetes mellitus: Cumulative meta-analysis. Clin Cardiol. 2021;44:899-906
5. South T. Coronary artery bypass surgery. Crit Care Nurs Clin North Am. 2011;23:573-85
6. Lorenzen US, Buggeskov KB, Nielsen EE, Sethi NJ, Carranza CL, Gluud C. et al. Coronary artery bypass surgery plus medical therapy versus medical therapy alone for ischaemic heart disease: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8:246
7. Zhong P, Li Z, Lin Y, Peng Q, Huang M, Jiang L. et al. Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta Ophthalmol. 2022;100:225-33
8. Wirtz PH, von Känel R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr Cardiol Rep. 2017;19:111
9. Wu XF, Huang JY, Chiou JY, Chen HH, Wei JC, Dong LL. Increased risk of coronary heart disease among patients with primary Sjögren's syndrome: a nationwide population-based cohort study. Sci Rep. 2018;8:2209
10. Priyamvara A, Dey AK, Bandyopadhyay D, Katikineni V, Zaghlol R, Basyal B. et al. Periodontal Inflammation and the Risk of Cardiovascular Disease. Curr Atheroscler Rep. 2020;22:28
11. Vural U, Kizilay M, Aglar AA. Coronary Involvement in Behçet's Disease: what are its Risks and Prognosis? (Rare Cases and Literature Review). Braz J Cardiovasc Surg. 2019;34:749-58
12. Chen H, Zhang Y, Li C, Wu W, Liu J, Zhang F. et al. Coronary involvement in patients with Behçet's disease. Clin Rheumatol. 2019;38:2835-41
13. Escobar E. Hypertension and coronary heart disease. J Hum Hypertens. 2002;16(Suppl 1):S61-3
14. Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJ. Cardiovascular metabolic syndrome - an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9:218-32
15. Girkin CA, Kannel WB, Friedman DS, Weinreb RN. Glaucoma risk factor assessment and prevention: lessons from coronary heart disease. Am J Ophthalmol. 2004;138:S11-8
16. Sharma S. Keratitis. Biosci Rep. 2001;21:419-44
17. Cabrera-Aguas M, Khoo P, Watson SL. Infectious keratitis: A review. Clin Exp Ophthalmol. 2022;50:543-62
18. Courrier E, Lépine T, Hor G, Fournier C, He Z, Chikh M. et al. Size of the Lesions of Superficial Punctate Keratitis in Dry Eye Syndrome Observed With a Slit Lamp. Cornea. 2016;35:1004-7
19. Hogan MJ, Crawford JW. Epidemic Keratoconjunctivitis: (Superficial Punctate Keratitis, Keratitis Subepithelialis, Keratitis Maculosa, Keratitis Nummularis) With a Review of the Literature and a Report of 125 Cases. Am J Ophthalmol. 2018;190:xxix-xlii
20. Moshirfar M, Peterson T, Ungricht E, McCabe S, Ronquillo YC, Brooks B. et al. Thygeson Superficial Punctate Keratitis: A Clinical and Immunologic Review. Eye Contact Lens. 2022;48:232-8
21. Ting DSJ, Ho CS, Deshmukh R, Said DG, Dua HS. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye (Lond). 2021;35:1084-101
22. Lee CY, Yang SF, Huang JY, Chang CK. The Extents of Coronary Heart Disease and the Severity of Newly Developed Dry Eye Disease: A Nationwide Cohort Study. Diagnostics (Basel). 2024;14:586
23. Harrington RA. Targeting Inflammation in Coronary Artery Disease. N Engl J Med. 2017;377:1197-8
24. Yang J, Feng L, Ren J, Wu G, Chen S, Zhou Q. et al. Correlation between the severity of periodontitis and coronary artery stenosis in a Chinese population. Aust Dent J. 2013;58:333-8
25. Feng KM, Chien WC, Chen YH, Sun CA, Chung CH, Chen JT. et al. Increased Risk of Acute Coronary Syndrome in Ankylosing Spondylitis Patients With Uveitis: A Population-Based Cohort Study. Front Immunol. 2022;13:890543
26. Masood F, Ehrenpreis ED, Rubin G, Russell J, Guru S, Luzzi P. State of the art review: coronary artery disease in patients with inflammatory bowel disease: mechanisms, prevalence, and outcomes. Acta Cardiol. 2022;77:297-306
27. Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G. et al. Systemic immune-inflammation index predicts the severity of coronary stenosis in patients with coronary heart disease. Coron Artery Dis. 2021;32:715-20
28. Li H, Sun K, Zhao R, Hu J, Hao Z, Wang F. et al. Inflammatory biomarkers of coronary heart disease. Front Biosci (Schol Ed). 2018;10:185-96
29. Kopin L, Lowenstein C. Dyslipidemia. Ann Intern Med. 2017;167:Itc81-itc96
30. Shaya GE, Leucker TM, Jones SR, Martin SS, Toth PP. Coronary heart disease risk: Low-density lipoprotein and beyond. Trends Cardiovasc Med. 2022;32:181-94
31. Vassalle C, Bianchi S, Bianchi F, Landi P, Battaglia D, Carpeggiani C. Oxidative stress as a predictor of cardiovascular events in coronary artery disease patients. Clin Chem Lab Med. 2012;50:1463-8
32. Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Free Radic Biol Med. 2008;45:1047-55
33. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112:71-81 quiz 2
34. Zhang X, M VJ, Qu Y, He X, Ou S, Bu J. et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int J Mol Sci. 2017;18:1398
35. Lee CY, Chen HC, Lin HW, Huang JY, Lin TL, Yang CH. et al. Increased risk of keratopathy after psoriasis: A nationwide population-based study. PLoS One. 2018;13:e0201285
36. Yu K, Bunya V, Maguire M, Asbell P, Ying GS. Systemic Conditions Associated with Severity of Dry Eye Signs and Symptoms in the Dry Eye Assessment and Management Study. Ophthalmology. 2021;128:1384-92
37. Khamis RY, Ammari T, Mikhail GW. Gender differences in coronary heart disease. Heart. 2016;102:1142-9
38. Gipson IK. Age-Related Changes and Diseases of the Ocular Surface and Cornea. Investigative Ophthalmology & Visual Science. 2013;54:ORSF48-ORSF53
39. Lee YH, Fang J, Schieb L, Park S, Casper M, Gillespie C. Prevalence and Trends of Coronary Heart Disease in the United States, 2011 to 2018. JAMA Cardiol. 2022;7:459-62
40. Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart. 2016;102:1945-52
41. Zhu KF, Wang YM, Zhu JZ, Zhou QY, Wang NF. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur J Prev Cardiol. 2016;23:530-43
42. Dalen JE, Alpert JS, Goldberg RJ, Weinstein RS. The epidemic of the 20(th) century: coronary heart disease. Am J Med. 2014;127:807-12
43. Voutilainen A, Brester C, Kolehmainen M, Tuomainen TP. Epidemiological analysis of coronary heart disease and its main risk factors: are their associations multiplicative, additive, or interactive? Ann Med. 2022;54:1500-10
44. Balal S, Ansari AS, Sim PY, Juwale H, Ismailjee MA, Hussain R. et al. The incidence and prevalence of recurrent corneal erosion syndrome in London, UK. Eye (Lond). 2023;37:3213-6
Author contact
![]() Corresponding author: Chao-Kai Chang, MD, PhD, Nobel Eye Institute, Address: No. 13-5, Gongyuan Rd., Zhongzheng Dist., Taipei 100008, Taiwan; Tel.: +886-2-2370-5666; Fax: +886-2-2375-4509; E-mail: chaokaihinet.net.
Corresponding author: Chao-Kai Chang, MD, PhD, Nobel Eye Institute, Address: No. 13-5, Gongyuan Rd., Zhongzheng Dist., Taipei 100008, Taiwan; Tel.: +886-2-2370-5666; Fax: +886-2-2375-4509; E-mail: chaokaihinet.net.

 Global reach, higher impact
Global reach, higher impact