3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2025; 22(1):101-109. doi:10.7150/ijms.98252 This issue Cite
Research Paper
Cadherin 23 is a prognostic marker of pancreatic cancer and promotes cell viability in floating culture conditions
1. Department of Surgery, The First Dongguan Affiliated Hospital, Guangdong Medical University. Dongguan, Guangdong 523710, China.
2. Department of General Surgery, Shenzhen University General Hospital/Shenzhen University Clinical Medical Academy, Shenzhen, Guangdong 518055, China.
3. Carson International Cancer Research Centre, Shenzhen University School of Medicine, Shenzhen, Guangdong 518055, China.
4. Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
5. Department of Clinical Application, Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan.
6. Department of Geriatrics, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, Guangdong 518000, China.
* These authors contributed equally to this article.
Received 2024-5-9; Accepted 2024-11-7; Published 2025-1-1
Abstract
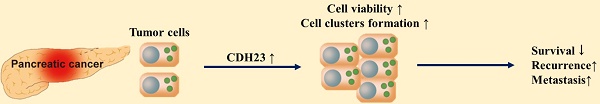
Purpose: Pancreatic cancer has the worst prognosis of all common cancers worldwide. Cadherin plays important roles in cancer cell invasion and metastasis. This study investigated the role and mechanism of Cadherin 23 (CDH23) action in the viability of pancreatic cancer cells.
Methods: We examined CDH23 expression in 70 surgical pancreatic cancer samples and examined relationships among the level of CDH23 expression, clinicopathological characteristics, and the prognosis of the pancreatic cancer patients. Furthermore, we silenced CDH23 expression in pancreatic cancer cell lines (Panc-1, SUIT-2, MIA PaCa-2, CFPAC-1, and Capan-2) and assessed the viability of these cells. CDH23 expression in pancreatic cancer patients and cell lines was examined using immunohistochemistry and western blotting.
Results: High levels of CDH23 in pancreatic cancer patients led to shorter overall survival and correlated with local recurrence and distance metastasis. The viability of pancreatic cancer cells in floating culture conditions decreased sharply when CDH23 was silenced. The viability and migration of pancreatic cancer cells in monolayer culture conditions did not change when CDH23 was silenced. The level of phosphorylated AKT was significantly decreased in the CDH23 knockdown cells in floating culture conditions.
Conclusion: High levels of CDH23 expression are correlated with a poor prognosis in pancreatic cancer and may serve as a novel prognostic marker.
Keywords: Pancreatic neoplasms, Cadherin 23, Prognostic marker, Tumor microenvironment
Introduction
Metastasis is a feature of cancer and the major cause of cancer related death [1]. Survival of cancer cells once they have detached from the primary tumor is important for the formation of metastases [2]; however, the circulation system is a hostile environment and only 0.01% of circulating tumor cells (CTCs) survive to achieve metastasis [3, 4]. Cell-cell adhesiveness of metastatic cells allows them to form multicellular clusters within the bloodstream, which increases the survival of such CTCs and contributes to the metastatic spread of cancer [5]. Studying the ability of tumor cells to aggregate and maintain their cohesion as they survive in the bloodstream may identify a novel and potentially targetable step in the blood borne dissemination of cancer [5].
Metastatic tumor cells often show changes in multiple cell surface molecules, including cadherins, such as E-cadherin and N-cadherin. These adhesion proteins play important roles in cancer cell invasion and metastasis processes [6-8]. Cadherin 23 (CDH23), a member of the cadherin superfamily, is an important constituent of the hair cell tip link. It regulates mechanoelectrical transduction and mutations in CDH23 cause deafness and age-related hearing loss [9]. Mutations in CDH23 are also detected in pituitary adenoma and play important roles in its pathogenesis [10]. CDH23 participates in cell adhesion and its expression is upregulated in breast cancer cells [11, 12], and the strong propensity of CDH23 for aggregation inhibits cell migration in lung adenocarcinoma cells [13]. In addition, methylated reduction of CDH23 represents poor outcome of diffuse large B-cell lymphoma [14]. Therefore, CDH23 may play a critical function in cancer progression; however, its specific role and mechanism of action are unclear.
In this study, we investigated a potential function for CDH23 in pancreatic tumors by assessing CDH expression in pancreatic ductal adenocarcinoma (PDAC) cell lines and tissue samples. CDH23 upregulated the viability of cancer cells in suspension culture, and this action may involve AKT pathway activation. Clinicopathological analysis indicated CDH23 to also be involved in metastasis and prognosis of PDAC patients.
Materials and methods
Cells and culture conditions
The following five pancreatic cancer cell lines were used: CFPAC-1, SUIT-2, MIA PaCa-2 (Japanese Cancer Resource Bank, Tokyo, Japan), Panc-1 and Capan-2 (American Type Culture Collection, Manassas, USA). All cancer cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) at 37°C with humidified 90% air and 10% CO2. The human immortalized pancreatic ductal epithelial cell line, HPDE6-E6E7 clone 6, was cultured in keratinocyte serum-free media (Gibco) supplemented with epidermal growth factor (5 ng/ml), bovine pituitary extract (50 µg/ml), and penicillin-streptomycin (Gibco). Floating culture was performed using Ultra-Low attachment surface plates (Corning, USA).
Pancreatic tissues
We analyzed CDH23 expression in 70 tissue samples obtained from patients who underwent pancreatic resection for pancreatic cancer at our institution (Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University) between January 2005 and August 2012. We also obtained normal pancreatic tissue samples from intact pancreas resected during bile duct cancer surgery as control tissues. Survival was measured from the time of pancreatic resection, with death as the end point. Overall survival and disease-free survival analyses were performed in February 2016. The median observation time for overall survival and disease-free survival was 19 months (range 2-137 months) and 11 months (range 1-137 months), respectively. Forty-nine patients died during the follow-up. All surviving patients were followed up. Histological diagnosis of specimens was in accordance with the criteria of the updated World Health Organization classification [15]. Tumor stage was assessed according to The Union for International Cancer Controls (UICC) classification, 7th edition [16]. The study was approved by the Ethics Committee of the First Dongguan Affiliated Hospital, Guangdong Medical University and conducted according to the Ethical Guidelines for Human Genome/Gene Research enacted by the Japanese Government and the Helsinki Declaration.
Immunohistochemical procedures and evaluation
Immunohistochemistry was performed for CDH23 using a Histofine SAB-PO kit (Nichirei, Tokyo, Japan) as described previously [17]. Endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxidase. Antigen retrieval was performed by boiling in a microwave oven (in citrate buffer, pH 6.0). Sections were incubated with an anti-CDH23 antibody (H00064072-A01, Abnova, Taipei, Taiwan) overnight at 4°C. Carcinoma cells were identified according to morphology and counted in at least 20 fields per section at 200 × magnification. The distribution of CDH23 staining was evaluated as the percentage of stained cells, and was scored as follows: 0, no staining or less than 10%; 1, 11%-25%; 2, 26%-50%; 3, 51%-75%; and 4, 76%-100%. Cells were also scored for staining intensity, which was scored as 0, no staining; 1, weak; 2, moderate; or 3, strong. The multiplication product from these two scores (from 0-12) was used to assign patients into one of two groups according to CDH23 expression; a score of 0-4 represented low expression and a score of 6-12 represented high expression.
Silencing CDH23 using small interfering RNAs (siRNAs)
Gene silencing was performed using siRNA (Qiagen, MA, USA) directed against human CDH23. Target sequences were: siRNA-1 (5′-CCCAAATGTGTGCCCAGCTTA-3′); siRNA-2 (5′-CAGCGGAGTGCTGACCTTGAA-3′). Qiagen all-star siRNA was used as a negative control. Transfections were performed as described previously [18]. All cells were used in subsequent experiments 48 h after transfection.
Western blotting analysis
Western blotting was performed as described previously [17]. Cells were lysed in PRO-PREP (iNtRON Biotechnology, Seongnam, Korea) and proteins were separated on 4%-15% Mini-PROTEAN TGX Precast Gels (Bio-Rad Laboratories) and transferred to Trans-Blot Turbo Mini PVDF Transfer Packs (Bio-Rad Laboratories) using a Trans-Blot Turbo Transfer Starter System (Bio-Rad Laboratories). Antibodies used in this study were: anti-CDH23 (H00064072-A01, Abnova, Taipei, Taiwan), anti-E-cadherin (# 3195), anti-AKT (# 4691), anti-Phospho-AKT (# 4060), (Cell Signaling Technology, Danvers, MA, USA) and anti-GAPDH (ab8245; Abcam, Cambridge, MA, USA). Immunoblot signals were detected by enhanced chemiluminescence with ChemiDoc XRS (Bio-Rad Laboratories).
Migration assays
Migration of cultured cancer cells was assessed by counting the number of cells migrating through Transwell chambers (BD Biosciences, Franklin Lakes, NJ) as described previously [18]. Cells were maintained in 10% FBS/DMEM during these assays. Cells were transfected with siRNAs 48 h prior to the assay. Migration was determined after a 24 h period.
Floating culture system
Cells were seeded in a 96-well plate with an ultra-low attachment surface and round bottom (Product Number 7007; Corning Inc., Corning, NY, USA,). Photomicrographs of cells were taken using a BIOREVO BZ9000 microscope (Keyence, Osaka, Japan) and areas of spheroids were measured using a BZ Analyzer (Keyence).
Cell Viability Assay
Pancreatic cells (1.5 × 103 cells/well) were seeded in 96-well plates (Greiner Bio-One, Frickenhausen, Germany) or Ultra-Low attachment surface 96-well plates (#7007, Corning, USA) 24 h after transfection with siRNA. Cell viability was examined using the CellTiter-Glo Luminescent Cell Viability Assay Kit (G7570, Promega) following the manufacturer's instructions. Background was subtracted using values from wells containing only culture medium.
Caspase-3 activity assay
SUIT-2 cells (2.0 × 104 cells/well) were seeded in 6-well culture plate or Ultra-Low attachment surface 6-well plates (#3471, Corning, USA) 24 h after transfection with siRNA. Protein extracts were prepared following manufacturer's instructions by using Bradford Protein Assay kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China). Caspase-3 activity was measured using Caspase-3 Activity Assay kit (Beyotime Institute of Biotechnology, Nantong, Jiangsu, China) in which cell extracts were mixed with Ac-DEVD-pNA substrate for 2 h at 37°C in 96-well plates prior to colorimetric measurement of p-nitroanilide product at 405 nm [19].
Statistical analysis
A χ2-test was used to analyze the correlation between CDH23 expression and clinicopathological characteristics. Survival analysis was performed using Kaplan-Meier analysis and curves were compared using the log-rank test. For in vitro experiments, values are expressed as the mean ± standard deviation. Comparison between two groups was performed using Student's t-test. Statistical significance was defined as P < 0.05. All statistical analyses were performed using JMP 13 software (SAS Institute, NC, USA).
Results
High CDH23 expression is significantly associated with poor prognosis
We analyzed general data and prognostic factors of 70 patients undergoing pancreatic cancer surgery. Their clinicopathological characteristics are listed in Table 1. The results of univariate analysis showed that better 5-year overall survival of patients was correlated with low CDH23 expression, early tumor stage, no residual tumor, early histological grade and negative vascular invasion (P < 0.05) (Table 2). The results of multivariate analysis showed that CDH23 expression and residual tumor were adverse factors for overall survival (Table 3). These results indicated that high CDH23 expression was associated with poor prognosis of pancreatic cancer.
CDH23 expression promotes pancreatic cancer metastasis and recurrence
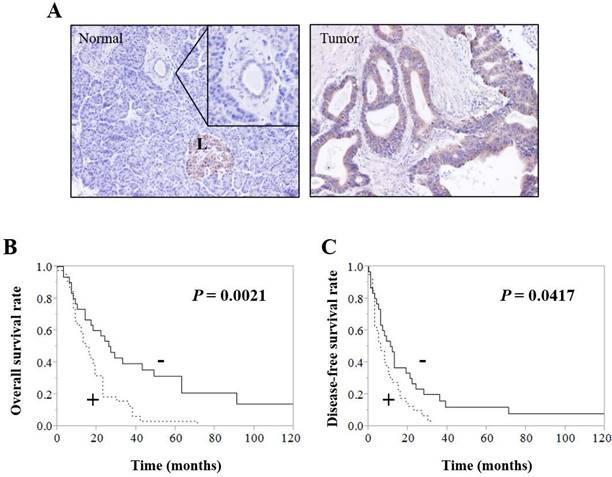
Immunohistochemistry showed that CDH23 was expressed in normal pancreatic tissues of control patients and tumor tissues of pancreatic cancer patients; however, the level of CDH23 expression in pancreatic cancer patient tumor tissues was significantly higher compared with normal pancreatic tissues of control patients (Fig. 1A). CDH23 expression was strongly associated with local recurrence and distant metastasis (Table 4). Moreover, Kaplan-Meier analysis indicated that pancreatic cancer patients with high levels of CDH23 expression suffered from poor survival (Fig. 1B/C).
Clinicopathological characteristics of patients (n=70)
| Median age | 67 years (Range 36-85 years) |
|---|---|
| Sex (Male/Female) | 46(65.7%) / 24 (34.3 %) |
| pT category | |
| T1 | 3(4.3%) |
| T2 | 1(1.4%) |
| T3 | 64(91.4%) |
| T4 | 2(2.9%) |
| pN category | |
| pN0 | 17 (24.3%) |
| pN1 | 53(75.7%) |
| UICC stage | |
| I | 4(5.7%) |
| II | 62(88.6%) |
| III | 1(1.4%) |
| IV | 3(4.3%) |
| Residual tumor category | |
| R0 | 52(74.3%) |
| R1 | 18(25.7%) |
| Histologic grade | |
| Grade 1 | 15(21.4%) |
| Grade 2 | 31(44.3%) |
| Grade 3 | 24(34.3%) |
| Vascular invasion | |
| Negative | 26(37.1 %) |
| Positive | 44(62.9 %) |
| Perineural invasion | |
| Negative | 11(15.7%) |
| Positive | 59(84.3%) |
| Lymphatic invasion | |
| Negative | 14(20.0%) |
| Positive | 56(80.0%) |
Inhibition of CDH23 expression does not affect migration or viability of pancreatic cancer cells in monolayer culture conditions
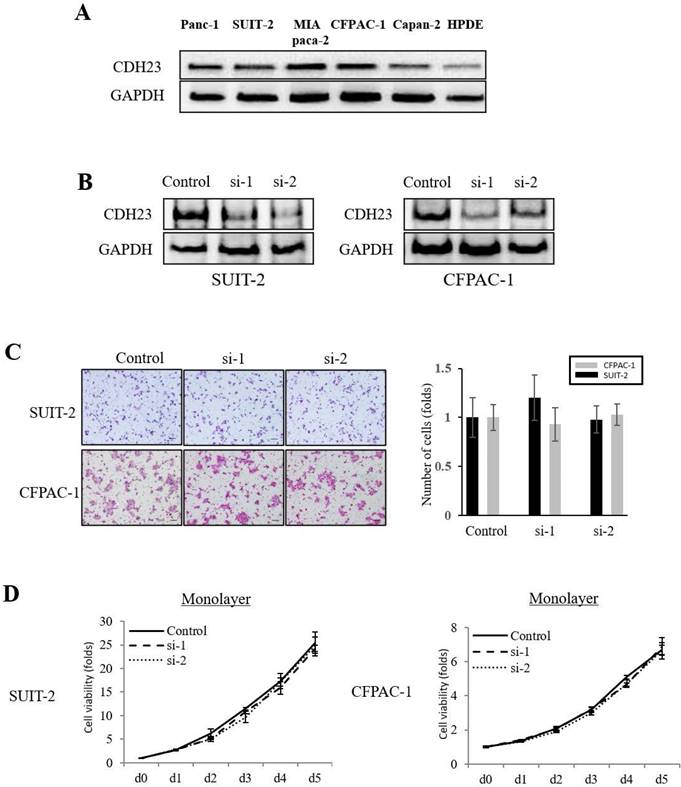
The expression of CDH23 in pancreatic cancer cell lines (Panc-1, SUIT-2, MIA PaCa-2, CFPAC-1, Capan-2) was higher than that in normal pancreatic epithelial cells (HPDE cells) (Fig. 2A). To investigate the effect of CDH23 expression in pancreatic cancer cells, we stably downregulated CDH23 expression by siRNA in SUIT-2 and CFPAC-1 cells and confirmed downregulation at both mRNA and protein levels (Fig. 2B). Furthermore, Transwell chamber assays showed that CDH23 silencing did not inhibit migration or cell viability of pancreatic cancer cells in monolayer culture conditions (Fig. 2C/D).
CDH23 promotes viability of pancreatic cancer cells in floating culture conditions
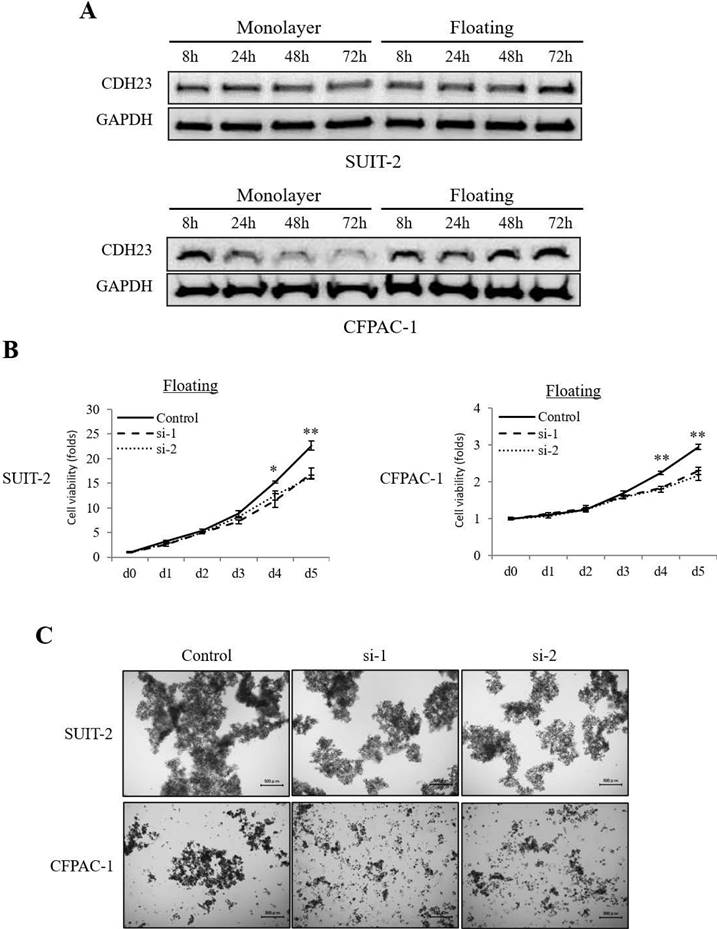
Interestingly, the expression of CDH23 was higher in floating culture conditions than in monolayer culture conditions (Fig. 3A). When CDH23 expression was downregulated in pancreatic cancer cells by siRNA, their viability in floating culture conditions decreased sharply (Fig. 3B), and high CDH23 expression seems more likely to form cell clusters (Fig. 3C).
Univariate survival analysis of conventional prognostic factors and CDH23 expression in pancreatic cancer patients resection (n = 70)
| Characteristics | No. of cases | Median Survival time (Mo) | 5 -Year Survival Rate (%) | P value |
|---|---|---|---|---|
| CDH23 expression | 0.0021 | |||
| Low | 30 | 26.5 | 28.4 | |
| High | 40 | 16 | 4.4 | |
| Age | 0.6863 | |||
| < 65 | 28 | 19 | 12.1 | |
| ≥ 65 | 42 | 17 | 15.8 | |
| Sex | ||||
| Male | 46 | 23 | 12.5 | 0.3658 |
| Female | 24 | 22 | 17.3 | |
| pT category | 0.3309 | |||
| pT1/ pT2 | 4 | 35.5 | 37.1 | |
| pT3/ pT4 | 66 | 18 | 12.1 | |
| pN category | 0.0762 | |||
| pN0 | 17 | 24 | 31.5 | |
| pN1 | 53 | 16 | 8.8 | |
| UICC stage | 0.0091 | |||
| I / II | 66 | 19 | 15.4 | |
| III / IV | 4 | 8.5 | 0.18 | |
| Residual tumor | 0.0002 | |||
| R0 | 52 | 23 | 18.9 | |
| R1 | 18 | 9 | 1.6 | |
| Histologic Grade | 0.0406 | |||
| Grade 1 | 15 | 31 | 33.1 | |
| Grade 2 | 31 | 19 | 12.0 | |
| Grade 3 | 24 | 9.5 | 4.9 | |
| Lymphatic invasion(ly) | 0.5055 | |||
| Negative | 14 | 20 | 19.4 | |
| Positive | 56 | 18 | 12.6 | |
| Vascular invasion (v) | 0.0122 | |||
| Negative | 26 | 27 | 26.9 | |
| Positive | 44 | 13 | 6.9 | |
| Perineural invasion (ne) | 0.1621 | |||
| Negative | 11 | 30 | 28.6 | |
| Positive | 51 | 17 | 11.0 |
Multivariate analysis of conventional prognostic factors and CDH23 expression in pancreatic cancer patients
| Characteristics | Relative Risk | 95%Confidence Interval | P value | |
|---|---|---|---|---|
| CDH23 expression | 2.321 | 1.307-4.228 | 0.0038 | |
| Residual tumor(R1) | 2.568 | 1.240-5.192 | 0.0119 | |
| UICC stage | 1.412 | 0.386-4.073 | 0.5695 | |
| Histologic Grade | - | - | 0.6193 | |
| Vascular invasion | 1.237 | 0.652-2.379 | 0.5165 | |
The relative risks of UICC stage and Histological grade are not shown because of the two parameters involved
Relationship between CDH23 expression and various clinicopathological factors in patients with pancreatic ductal adenocarcinoma (n = 70)
| Characteristics | Low expression group N = 30(42.9%) | High expression group N = 40(57.1%) | P value |
|---|---|---|---|
| Age | 0.0545 | ||
| < 65 | 8(26.67) | 20(50.00) | |
| ≥ 65 | 22(73.33) | 20(50.00) | |
| Sex | 0.7166 | ||
| Female | 11(36.67) | 13(32.50) | |
| Male | 19(63.33) | 27(67.50) | |
| pT category | 0.7673 | ||
| pT1 / pT2 | 2(6.67) | 2(5.00) | |
| pT3 / pT4 | 28(93.33) | 38(95.00) | |
| pN category | 0.6881 | ||
| pNo | 8(26.67) | 9(22.50) | |
| pN1 | 22(73.33) | 31(73.50) | |
| UICC staging | 0.4443 | ||
| I / II | 29(96.67) | 37(92.50) | |
| III / IV | 1(3.33) | 3(7.50) | |
| Residual tumor | 0.6922 | ||
| R0 | 23(76.67) | 29(72.50) | |
| R1 | 7(23.33) | 11(27.50) | |
| Histologic grade | 0.6190 | ||
| Grade 1 | 8(26.67) | 7(17.50) | |
| Grade 2 | 13(43.33) | 18(45.00) | |
| Grade 3 | 9(30.00) | 15(37.50) | |
| Lymphatic invasion(ly) | 0.5475 | ||
| Negative | 7(23.33) | 7(17.50) | |
| Positive | 23(76.67) | 33(82.50) | |
| Vascular invasion(v) | 0.3540 | ||
| Negative | 13(43.33) | 13(32.50) | |
| Positive | 17(56.67) | 27(67.50) | |
| Perineural invasion(ne) | 0.8499 | ||
| Negative | 5(16.67) | 6(15.00) | |
| Positive | 25(83.33) | 34(85.00) | |
| Local recurrence | 0.0082 | ||
| Negative | 17(56.67) | 34(85.00) | |
| Positive | 13(43.33) | 6(15.00) | |
| Distance metastasis | 0.0307 | ||
| Negative | 15(50.00) | 10(25.00) | |
| Positive | 15(50.00) | 30(75.00) |
CDH23 promotes AKT phosphorylation in pancreatic cancer cells in floating culture conditions
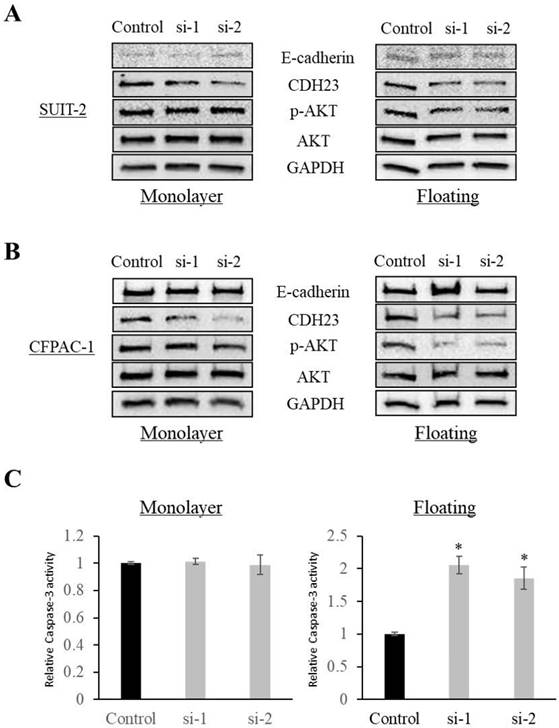
Western blotting showed that CDH23 knockdown increased the expression of E-cadherin and decreased the phosphorylation of AKT in cells in floating culture conditions (P < 0.05), while there was no significant difference in monolayer culture conditions (Fig. 4A, B). In addition, knockdown of CDH23 increased Caspase-3 activity under floating culture conditions, but CDH23 knockdown does not affect the pro-apoptotic protein Caspases-3 under monolayer culture conditions (Fig. 4C).
CDH23 expression correlates with poor outcome in pancreatic ductal adenocarcinoma. (A) Immunohistochemical analysis of CDH23 in human pancreatic ductal adenocarcinoma (PDAC) specimens. A normal pancreatic specimen showing weak-to-no staining in pancreatic ductal epithelial cells. (B) And (C) Kaplan-Meier survival analysis of CDH23 expression in PDAC cancer cells. High CDH23 expression was associated with shorter overall survival (B) and disease-free survival (C).
Knockdown of CDH23 does not affect migration or viability of pancreatic cancer cells in monolayer culture conditions. (A) Representative western blot analysis of CDH23 in distinct pancreatic cancer cells. GAPDH abundance is used as an internal control. Cell types, protein masses, and siRNA treatment are indicated. (B) A stable cell line with CDH23 knocked-down was generated using siRNA. Down-regulation of CHD23 was confirmed by western blotting. GAPDH was used as a control. (C) Evaluation of SUIT-2 and CFPAC-1 cell migration following transfection with control or CDH23-specific siRNAs. Representative photomicrographs are shown in the panels on the left-hand side. Bar charts summarize the migration of cells in each siRNA treatment group. (D) Evaluation of SUIT-2 and CFPAC-1 cell viability following transfection with control or CDH23-specific siRNAs in monolayer culture conditions. Line charts show the viability of cells in each siRNA treatment group in monolayer culture conditions.
Discussion
Pancreatic cancer is a malignant digestive system tumor with insidious onset, invasion and early-stage metastasis. Although the existing treatment methods continue to advance, the overall prognosis of patients is still very poor. In recent years, the incidence of pancreatic cancer has increased worldwide. High rates of invasion and metastasis are independent risk factors affecting the prognosis of patients with pancreatic cancer. Therefore, it is important to further clarify the molecular mechanisms associated with invasion and metastasis of pancreatic cancer and to find marker genes that can guide early diagnosis and treatment of patients.
CDH23 is a cadherin that is widely distributed on the cell membrane surface of normal cells. It is also present in many tumors, including breast, colorectal, and renal cancer tissues [9, 20, 21], and upregulation of CDH23 expression corresponds to a decrease of overall survival in patients with acute myeloid leukemia [22]. Our clinical data showed a poorer prognosis for pancreatic cancer patients with high levels of CDH23 expression, and CDH23 expression was associated with local recurrence and distant metastasis. These data suggest that high CHD23 expression in pancreatic cancer tissues is related to progression of the disease and may be a marker for the prognosis of pancreatic cancer.
CDH23 expression promotes viability of pancreatic cancer cells in floating culture conditions. (A) Western blotting showing CDH23 protein levels in SUIT-2 and CFPAC-1 pancreatic cancer cells at 8, 24, 48 and 72 h in monolayer and floating culture conditions. (B) Evaluation of the viability of SUIT-2 and CFPAC-1 cells following transfection with control or CDH23-specific siRNAs in floating culture conditions. Line charts show the viability of cells in each siRNA treatment group in floating culture conditions. (* P < 0.05; ** P < 0.01) (C) Representative images of SUIT-2 and CFPAC-1 cell cluster following transfection with control or CDH23-specific siRNAs in floating culture conditions.
CDH23 expression promotes AKT phosphorylation in floating culture conditions. (A) Phosphorylation of AKT was reduced after knockdown of CDH23 in SUIT-2 cells in floating culture conditions. No remarkable change was observed in monolayer culture conditions. (B) Phosphorylation of AKT was reduced after knockdown of CDH23 in CFPAC-1 cells in floating culture conditions. No remarkable change was observed in monolayer culture conditions. (C) Caspase-3 activities was increased after knockdown of CDH23 in floating culture conditions. No remarkable change was observed in monolayer culture conditions. (* P < 0.05)
The metastatic ability of tumor cells is an important feature of malignant tumors that affects the recurrence and metastasis of tumors. Moreover, circulating tumor cell (CTC) clusters contribute to metastatic propensity, especially when they affect the ability of epithelial tumor cells to survive the loss of cell adherence and shear forces in the blood stream [23]. In this context, either mesenchymal transformation, stromal-derived factors, or persistent interepithelial cell junctions may provide survival signals that attenuate this apoptotic outcome [23]. CTC clusters are more likely to resist initial cell death after lodging in distant organs, thereby promoting metastasis [24]. In this study, we found that CHD23 promotes pancreatic cancer cells viability under the floating culture conditions but not under the monolayer culture conditions, and high CDH23 expression is more likely to promote the formation of cell clusters. CDH23 is also upregulated in breast cancer cells, and it participates in cell adhesion and play a role in the early stages of metastasis [11]. Therefore, CDH23 may affect the viability of floating cells by regulating cell adhesion, thus promoting the metastasis and progression of pancreatic cancer.
This study also analyzed the molecular mechanism of CDH23 action on pancreatic cancer cell lines. Knockdown of CDH23 significantly decreased the level of Akt activation, while it increased the activity of pro-apoptotic factor Caspase-3, both are in the floating culture conditions. The Akt signaling regulates cell proliferation and survival, however, Akt regulation of cell survival involves direct inhibition of pro-apoptotic signals [25]. Therefore, CDH23 may promote the viability of pancreatic cancer cells through Akt signal. Pancreatic cancer cells survive after leaving the extracellular matrix under floating culture conditions, partly by inhibiting cell apoptosis. The specific molecular mechanism needs to be further clarified.
Conclusion
In this study, we detected the expression of CDH23 in pancreatic cancer and explored the impact of CDH23 on tumor development. We found that the expression of CDH23 affects the progression and prognosis of pancreatic cancer. In addition, the CDH23 signaling pathway regulates the phosphorylation of Akt and affects cell viability under floating culture conditions. Therefore, these data provide new insights into the pathogenesis of pancreatic cancer, and CDH23-mediated signal transduction may be a new therapeutic target.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (82174137), The Science, Technology and Innovation Committee of Shenzhen (20200814071107001), Talent Development Foundation of The First Dongguan Affiliated Hospital of Guangdong Medical University (GCC2023016, GCC2023019) and Dongguan Science and Technology of Social Development Program (20231800940202, 20231800937512). We thank Jeremy Allen, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing a draft of this manuscript.
Author contributions
BZ and GY conceptualized the idea, prepared the design; BZ and CZ wrote the manuscript. BZ, CZ, PS, RL, SF, XC, QL and JQ performed the experiments. BZ, GY and RL interpreted the data and produced the main document. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology. 1999;117(6):1464-84
2. Saias L, Gomes A, Cazales M. et al. Cell-Cell Adhesion and Cytoskeleton Tension Oppose Each Other in Regulating Tumor Cell Aggregation. Cancer Res. 2015;75:2426-33
3. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453-8
4. Pereira-Veiga T, Schneegans S, Pantel K. et al. Circulating tumor cell-blood cell crosstalk: Biology and clinical relevance. Cell Rep. 2022;40:111298
5. Aceto N, Bardia A, Miyamoto DT. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110-22
6. Stemmler MP. Cadherins in development and cancer. Mol Biosyst. 2008;4:835-15
7. Santarosa M, Maestro R. The Autophagic Route of E-Cadherin and Cell Adhesion Molecules in Cancer Progression. Cancers (Basel). 2021;13(24):6328
8. Sommariva M, Gagliano N. E-Cadherin in Pancreatic Ductal Adenocarcinoma: A Multifaceted Actor during EMT. Cells. 2020;9(4):1040
9. Vanniya SP, Srisailapathy C, Kunka MR. The tip link protein Cadherin-23: From Hearing Loss to Cancer. Pharmacol Res. 2018;130:25-35
10. Zhang Q, Peng C, Song J. et al. Germline Mutations in CDH23, Encoding Cadherin-Related 23, Are Associated with Both Familial and Sporadic Pituitary Adenomas. Am J Hum Genet. 2017;100(5):817-23
11. Apostolopoulou M, Ligon L. Cadherin-23 mediates heterotypic cell-cell adhesion between breast cancer epithelial cells and fibroblasts. Plos One. 2012;7:e33289
12. Padmanaban V, Krol I, Suhail Y. et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573(7774):439-44
13. Sannigrahi MK, Srinivas CS, Deokate N. et al. The strong propensity of Cadherin-23 for aggregation inhibits cell migration. Mol Oncol. 2019;13(5):1092-1109
14. Cao B, Guo X, Huang L. et al. Methylation silencing CDH23 is a poor prognostic marker in diffuse large B-cell lymphoma. Aging. 2021;13(13):17768-88
15. Bosman FT, Carneiro F, Hruban RH. et al. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press. 2010
16. Edge SB, Byrd DR, Compton CC. et al. Exocrine and Endocrine Pancreas, AJCC Cancer Staging Manual. 7th edition. New York, USA: Springer. 2010
17. Zheng B, Ohuchida K, Chijiiwa Y. et al. CD146 attenuation in cancer-associated fibroblasts promotes pancreatic cancer progression. Mol Carcinog. 2016;55(11):1560-72
18. Zheng B, Ohuchida K, Cui L. et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int J Oncol. 2015;47(2):490-8
19. Wang Y, Gao J, Zhang D. et al. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53(1):132-44
20. Singaraju GS, Sagar A, Kumar A. Structural basis of the strong cell-cell junction formed by cadherin-23. FEBS J. 2019;287(11):2328-47
21. Onder TT, Gupta PB, Mani SA. et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68(10):3645-54
22. Yang J, Lu F, Ma G. et al. Role of CDH23 as a prognostic biomarker and its relationship with immune infiltration in acute myeloid leukemia. BMC Cancer. 2022;22(1):568
23. Aceto N, Bardia A, Miyamoto DT. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110-22
24. Min Yu. Metastasis Stemming from Circulating Tumor Cell Clusters. Trends Cell Biol. 2019;29(4):275-6
25. Zhang X, Tang N, Hadden TJ. et al. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978-86
Author contact
![]() Corresponding authors: Biao Zheng, Department of Surgery, The First Dongguan Affiliated Hospital, Guangdong Medical University. Dongguan, Guangdong 523710, China; E-mail: zhengbiaoedu.cn. Guojun Yao, Department of Geriatrics, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, Guangdong 518000, China; E-mail: winnieyaocom.
Corresponding authors: Biao Zheng, Department of Surgery, The First Dongguan Affiliated Hospital, Guangdong Medical University. Dongguan, Guangdong 523710, China; E-mail: zhengbiaoedu.cn. Guojun Yao, Department of Geriatrics, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People's Hospital, Shenzhen, Guangdong 518000, China; E-mail: winnieyaocom.

 Global reach, higher impact
Global reach, higher impact