3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(15):3046-3057. doi:10.7150/ijms.100720 This issue Cite
Research Paper
Jing Si Herbal Tea Modulates Macrophage Polarization and Inflammatory Signaling in LPS-Induced Inflammation
1. Department of Chinese Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
2. Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
3. School of Medicine, Tzu Chi University, Hualien, Taiwan.
4. Department of Research, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
5. School of Chinese Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
*These authors have contributed equally to this work.
Received 2024-7-9; Accepted 2024-11-5; Published 2024-11-11
Abstract
Background: Sepsis is a lethal disease due to uncontrolled inflammatory responses. Macrophages play an important role in sepsis-associated inflammation. Jing Si Herbal Tea (JSHT) is a plant-based regimen with anti-inflammatory properties designed to treat respiratory diseases; however, its underlying therapeutic mechanism remains unclear. This study aimed to investigate the effects of JSHT on macrophage polarization and inflammatory signaling in lipopolysaccharide (LPS)-induced inflammation to provide therapeutic approaches for inflammatory diseases.
Methods: RAW264.7 cells were stimulated with LPS (1 µg/mL for 16 h) to induce inflammatory responses and treated by JSHT (0.0125% concentration for 4 h) in the experimental groups (Control, JSHT, LPS, Pre-JSHT, and Post-JSHT groups). We investigated the protein and cytokine expression levels using western blotting and enzyme-linked immunosorbent assay (ELISA). Macrophage morphology was observed using immunofluorescence staining. The polarization surface markers were detected by flow cytometry.
Results: In the LPS group, the expressions of the inflammatory signaling (pERK, pJNK, and nuclear NFκB) and the pro-inflammatory cytokine levels (TNF-α, IL-1β, and IL-6) were significantly increased, with M1 polarization (CD68+/CD80+) compared to the Control group. In the Pre-JSHT and Post-JSHT groups, the expressions of the inflammatory signaling and the pro-inflammatory cytokine levels were significantly decreased, with a higher M2 polarization ratio (CD163+/CD206+) compared to the LPS group. RAW264.7 cells exhibited filopodia protruding from the cell surface in the LPS group, which were inhibited in the Pre-JSHT and Post-JSHT groups.
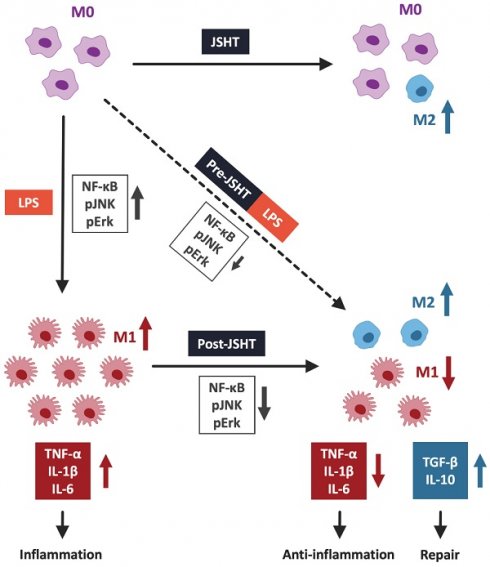
Conclusions: LPS induced M1 polarization with elevated inflammatory signaling and cytokine levels, while JSHT not only decreased M1 polarization but also promoted M2 polarization with decreased inflammatory responses. We propose JSHT as a potential anti-inflammatory agent against LPS-induced inflammation.
Keywords: herbal formula, Jing Si Herbal Tea, macrophage polarization, sepsis, systemic inflammatory response
Introduction
Sepsis, a condition characterized by dysregulated immune responses to bacterial, viral, and fungal infections, claims numerous lives in the world and poses a substantial health challenge worldwide.[1] Approximately 48.9 million incident cases of sepsis have been documented globally, with 11 million deaths attributed to sepsis.[1] This life-threatening condition, despite advancements in modern medicine, continues to have a high mortality rate, making it a crucial issue in critical care.[2] Prolonged stays in intensive care units and hospitals, coupled with the demand for extensive medical interventions, contribute significantly to the overall burden of sepsis on the healthcare system.[2] Consequently, sepsis not only poses a direct threat to life but also places a substantial strain on healthcare resources. Therefore, investigating the underlying mechanisms of sepsis and developing potential therapies to suppress excessive inflammatory responses in sepsis are critical issues.
Inflammation acts as a defense response against harmful stimuli from microbial pathogens, physical tissue damage, or toxic substances.[3] On the other hand, hyperinflammation can lead to cytokine storm and damage to local tissues.[3-5] Macrophages function as defensive effector cells against pathogens and play crucial regulatory roles in both innate and adaptive immune responses, including inflammation.[4, 6, 7] Macrophages can be divided into two functional subtypes: the classically activated macrophages (M1) and the alternatively activated macrophages (M2).[4, 8] M1-like macrophages, polarized by lipopolysaccharide (LPS), primarily engage in pro-inflammatory responses and produce pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6.[4, 8] In contrast, M2-like macrophages contribute to anti-inflammatory responses and tissue repair by producing anti-inflammatory cytokines such as IL-10 and transforming growth factor beta (TGF-β). [4, 8] Immune system dysregulation due to an imbalance in macrophage polarization has become an increasingly significant concern.[3, 8, 9] Addressing inflammation by modulating the balance of macrophage polarization has recently been recognized as a crucial and effective therapeutic strategy.
Jing Si Herbal Tea (JSHT) is an aqueous extract of a plant-based regimen developed by the Buddhist Tzu Chi Medical Foundation to regulate immunity and treat respiratory inflammatory diseases such as COVID-19. [10-12] JSHT comprises various herbal ingredients, including Artemisiae Argyi Folium (Artemisia argyi), Anisomeles indica (L.) Kuntze, Houttuyniae Herba (Houttuynia cordata Thunb.), Perillae Folium (Perilla frutescens), Glycyrrhizae Radix et Rhizoma (Glycyrrhiza glabra), Platycodonis Radix (Platycodon grandiflorus), Ophiopogonis Radix (Ophiopogon japonicus), and Chrysanthemi Flos (Chrysanthemum morifolium).[10-12] In a recent cohort study involving patients with mild-to-moderate COVID-19, the combination of JSHT with standard management demonstrated improvements in clinical inflammatory markers (C-reactive protein levels) and the severity of pulmonary infiltrate, as evaluated using the Brixia score.[10] Additionally, a reduced risk of intubation, critical conditions, and mortality has been observed in patients receiving the combined treatment.[10] Previous studies reported that JSHT shows anti-inflammatory effects.[10-13] However, the mechanisms underlying the therapeutic effects of JSHT are not fully understood.
Since the underlying mechanisms by which JSHT exerts its anti-inflammatory properties have not been fully elucidated, our study aimed to investigate the effects of JSHT on inflammatory signaling pathway and macrophage polarization in LPS-induced inflammation in RAW264.7 cells.
Materials and Methods
Reagents and cell Lines
The JSHT (Catalog No.: #4711393151427, a standardized manufactured vacuum-sealed portion) was provided by the Buddhist Tzu Chi Medical Foundation. LPS (#SI-L8274) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The mouse macrophage cell line, RAW264.7, was purchased from Taiwan's Bioresource Collection and Research Center. Dulbecco's Modified Eagle Medium (DMEM; 11965092) and fetal bovine serum (FBS; #A52567-01) were obtained from GIBCO (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Cell Counting Kit-8 (CCK-8; CK04) was purchased from Dojindo Laboratories (Kamimashiki-gun, Kumamoto, Japan). Mammalian cell PE LB (#786-180) was purchased from G-Bioscience (Billerica, MA, USA). Protein assay dye reagent (#500-0006) was obtained from Bio-Rad Laboratories, Inc. (Hercules, California, USA). Polyvinylidene fluoride (PVDF) membranes were purchased from Millipore. The blocking buffer (#37572) was purchased from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Enhanced chemiluminescence was detected using Cytiva software. Nuclear Extraction Kit (#2900) was purchased from Millipore (Billerica, Massachusetts, USA). Penicillin/streptomycin (#CC502-0100) was purchased from GeneDireX (Taipei, Taiwan). DAPI (#D1306) was purchased from Invitrogen (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Paraformaldehyde (#P6148) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies
The primary antibodies for phospho-JNK (pJNK), phospho-ERK 1/2 (pERK), and NFκB were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA). The primary antibody for β-actin was purchased from GeneTex (Irvine, California, USA). Horseradish peroxidase-hybridized secondary antibodies against goat anti-rabbit IgG and goat anti-mouse IgG were obtained from Invitrogen (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and Jackson ImmunoResearch (West Grove, Pennsylvania, USA), respectively. The PE anti-mouse CD163 and FITC anti-mouse CD80 antibodies used for fluorescence staining were obtained from BioLegend (San Diego, California, USA). APC anti-mouse CD68 and Per-CP/Cyaine 5.5 anti-mouse CD206 (MMR) antibodies were obtained from BioLegend.
Study protocol
The JSHT concentrate was diluted to a final concentration of 0.0125% in DMEM. LPS was prepared in DMEM at a final concentration of 1 μg/mL.[14] The cells were incubated at 37 °C in a 5% CO2 in DMEM enhanced with 10% FBS and 1% penicillin/streptomycin.
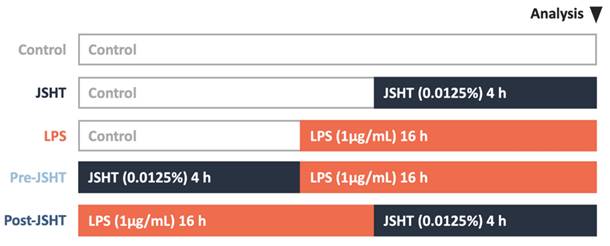
The study protocol is presented in Figure 1. There were five experimental groups in this study: Control, JSHT (0.0125% concentration for 4 h), LPS (1 µg/mL for 16 h), Pre-JSHT (induction of JSHT for 4 h followed by LPS for 16 h), and Post-JSHT (induction of LPS for 16 h followed by JSHT for 4 h) groups. The experimental conditions were determined according to the dose titration and time-course analysis (Figure 2). After culturing under the conditions in the experimental groups for 20 h, the RAW264.7 cells were harvested, extracted, and subjected to the following experiments.
Measurement of pERK and pJNK expressions
Whole-cell protein was extracted using Mammalian Cell PE LB, and the protein concentration was quantified using a protein assay dye reagent, according to the manufacturer's protocols. Approximately 50 µg of total protein was applied to each lane and separated by 12% Tris-glycine SDS-PAGE at 140V for 1 h (depending on the molecular weight of the target protein) and then transferred to a PVDF membrane at 200 mA for 2 h.
Primary antibodies were diluted with commercial protein-free blocking buffer according to the manufacturer's instructions. The membrane was soaked for 1h in protein-free blocking buffer, then probed primary antibody at 4℃ overnight. Secondary antibodies were also diluted according to the manufacturer's instructions, hybridized for 1 h at room temperature, and detected using enhanced chemiluminescence via Cytiva software.[15-17]
Measurement of NFκB expression
Nuclear proteins from each experimental group were extracted using a Nuclear Extraction Kit according to the manufacturer's protocol. Well-extracted nuclear proteins were quantified and analyzed by western blotting as described above.[14]
Morphology of RAW264.7 cells
RAW264.7 were cultured in 6-well plates at a density of 104 cells/well and treated under different conditions, as described in Figure 1. After fixing with 4% formaldehyde for 30 min, RAW264.7 cells were stained with phalloidin for 20-90 min and DAPI for 5 min, and then sealed. The cell morphology was observed using a confocal microscope.[18]
Measurement of the macrophage polarization surface markers
RAW264.7 cells were treated as described above and harvested. A total of 106 cells were stained with APC anti-mouse CD68, FITC anti-mouse CD80, PE anti-mouse CD163, and Per-CP/Cyaine 5.5 anti-mouse CD206 (MMR) antibodies for 30 min. The cells were then resuspended in 0.5% paraformaldehyde and flow cytometric analysis was performed.[8, 9, 19, 20]
Study protocol. Five experimental groups were used in this study: Control, JSHT (0.0125% concentration for 4 h), LPS (1 µg/mL for 16 h), Pre-JSHT (induction of JSHT for 4 h followed by LPS for 16 h), and Post-JSHT (induction of LPS for 16 h followed by JSHT for 4 h) groups.
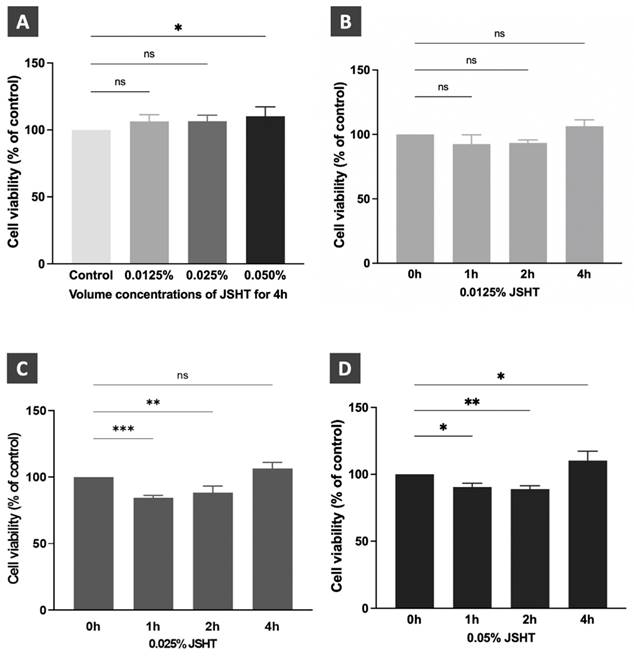
Dose titration and time course analysis of JSHT in RAW264.7 cells. (A) Cell viability after JSHT induction at specified concentrations for 4 h. Time course analysis results of JSHT induction at (B) 0.0125% concentration; (C) 0.025% concentration; (D) 0.05% concentration. All data are presented as the mean ± standard deviation (SD). *p < 0.05; **p < 0.01; ***p < 0.001; ns: not significant. The experiment was performed thrice in duplicate.
Enzyme-linked immunosorbent assay (ELISA)
The ELISA kit for mouse IL-1β, IL-6, and TNF-α were purchased from Abclonal (Woburn, Massachusetts, USA), and the ELISA kit for mouse TGF-β and mouse IL-10 were obtained from Invitrogen. The culture medium was collected and the protein concentration was quantified using an ELISA kit according to the manufacturer's protocols.[13, 14, 16, 21]
Statistical analysis
Statistical analyses were performed using GraphPad Prism 10 for macOS (Version 10.0.1 (170), GraphPad Software, San Diego, CA, USA, www.graphpad.com). Differences between the five groups (Control, JSHT, LPS, Pre-JSHT, and Post-JSHT) were evaluated using analysis of variance (ANOVA). Post-hoc comparisons were performed using the Least Significant Difference (LSD) method to identify specific group differences. Statistical significance was set at p < 0.05.
Results
Dose titration and time course analysis of JSHT in RAW264.7 cells
We performed dose titration and time-course analyses of JSHT using CCK-8 cell viability assay in RAW264.7 cells.[15, 16] The results are shown in Figure 2. After treatment for 4 h, JSHT at 0.0125% and 0.025% concentrations showed no statistical differences compared to the control condition. JSHT at 0.05% concentration significantly increased the relative cell viability compared to the control condition (p < 0.05). Treated with JSHT at 0.0125% concentration for 1, 2, and 4 h showed no statistical differences compared to the control condition (p > 0.05; Figure 2B). Treated with 0.025% concentration for 1 and 2 h significantly decreased the relative cell viability (p < 0.01), whereas treatment with 0.025% concentration for 4 h showed no statistical differences compared to the control condition (p > 0.05; Figure 2C). Treated with JSHT at 0.05% concentration for 1, 2, and 4 h showed statistically significant differences compared to the control condition (p < 0.05; Figure 2D). Based on the obtained results, we chose JSHT at a concentration of 0.0125% for 4 h in the subsequent experiments.
Regulation of pERK, pJNK, and nuclear NFκB expression after LPS and JSHT treatment in RAW264.7 cells
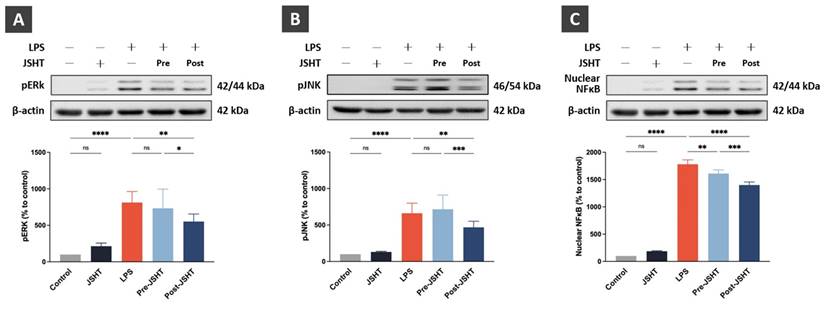
To evaluate the effect of JSHT on the inflammatory signaling pathway in LPS-stimulated RAW264.7 cells, we analyzed the expressions of pERK, pJNK, and nuclear NFκB using western blotting analysis (Figure 3).
Compared to the Control group, the expression of pERK showed no statistical differences in the JSHT group (p > 0.05), whereas pERK expression was significantly increased in the LPS group (p < 0.0001; Figure 3A). Compared to the LPS group, pERK expression showed no statistical differences in the Pre-JSHT group(p > 0.05), whereas pERK expression was significantly decreased in the Post-JSHT group (p < 0.01; Figure 3A). The expression of pERK was significantly lower in the Post-JSHT group than in the Pre-JSHT group (p < 0.05; Figure 3A).
Compared to the Control group, the expression of pJNK showed no statistical differences in the JSHT group (p > 0.05), whereas the expression of pJNK was significantly increased in the LPS group (p < 0.0001; Figure 3B). Compared to the LPS group, the expression of pJNK showed no statistical differences in the Pre-JSHT group (p > 0.05), whereas the expression of pJNK was significantly decreased in the Post-JSHT group (p < 0.01; Figure 3B). The expression of pJNK was significantly lower in the Post-JSHT group than in the Pre-JSHT group (p < 0.001; Figure 3B).
Compared to the Control group, the expression of nuclear NFκB showed no statistical differences in the JSHT group (p > 0.05), while the expression of nuclear NFκB significantly increased in the LPS group (p < 0.0001; Figure 3C). Compared to the LPS group, the expression of nuclear NFκB showed a significant decrease in the Pre-JSHT and Post-JSHT groups (p < 0.01; Figure 3C). The expression of nuclear NFκB was significantly lower in the Post-JSHT group compared to the Pre-JSHT group (p < 0.001; Figure 3C). These results indicated that JSHT inhibited LPS-induced inflammatory signaling activation in RAW 264.7 cells.
Morphology alteration of RAW264.7 cells after LPS and JSHT treatment
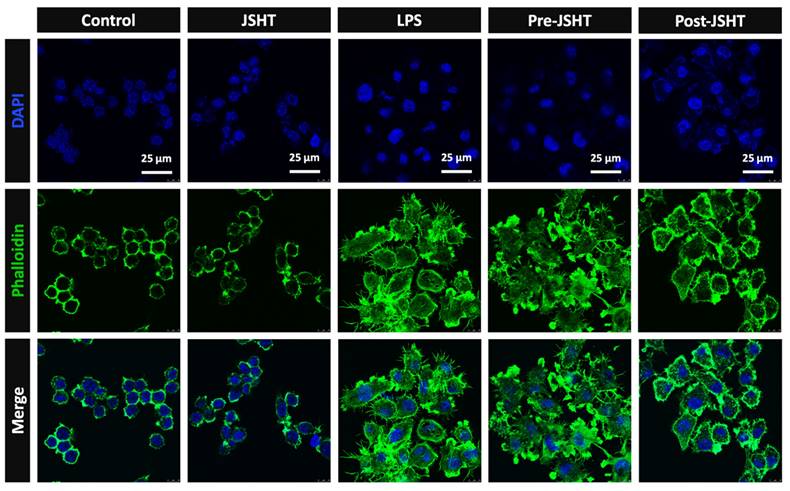
To evaluate the morphology of RAW264.7 cells after LPS and JSHT treatment, we performed immunofluorescent staining of phalloidin. The results are presented in Figure 4. The morphology of RAW264.7 cells was circular with smooth surface and few filopodia in the Control group. The morphology of the JSHT group was similar to that of the Control group. In the LPS group, RAW264.7 cells exhibited relatively high cellular volume and obvious filopodia protruding from the cell surface. In the Pre- and Post-JSHT groups, there were morphological alterations with lower cellular volume and fewer filopodia stretching out of the surface compared to the LPS group. The results revealed that JSHT significantly attenuated the LPS-induced morphological alterations in RAW264.7 cells.
JSHT inhibited the LPS-induced pro-inflammatory signaling activation in RAW 264.7 cells. The Western blotting analysis results of (A) pERK; (B) pJNK; and (C) nuclear NFκB. All data are presented as the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; ns: not significant. The experiment was performed six times in duplicate for pERK and pJNK, and three times in duplicate for nuclear NFκB.
RAW264.7 morphology alteration in the experimental groups. Microphotographs of the IF staining results (630X). Blue: nuclei stained with DAPI. Green: cell skeleton and filopodia stained with phalloidin.
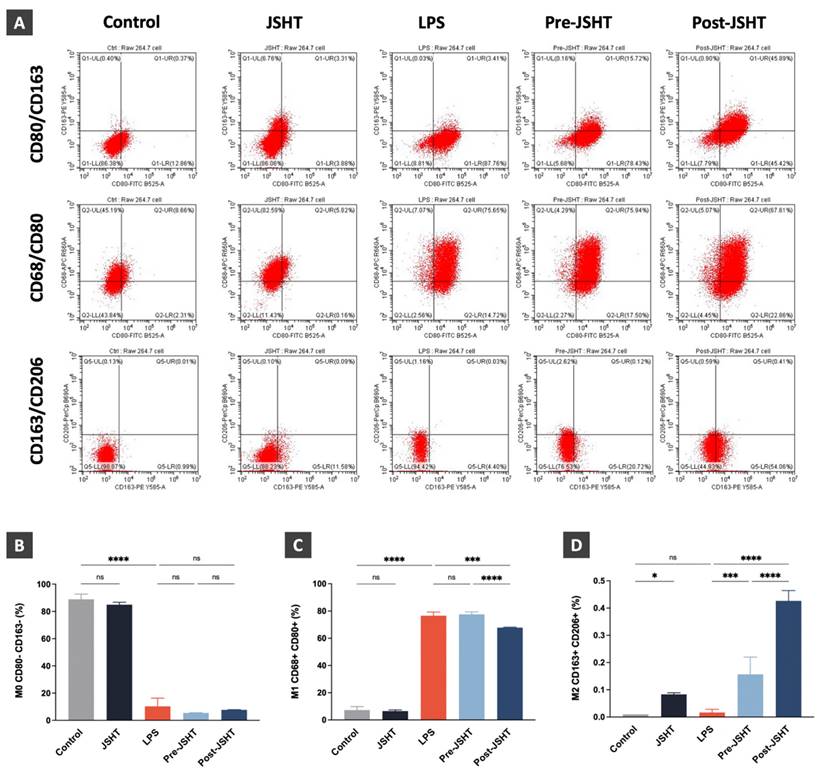
Regulation of macrophage polarization after LPS and JSHT treatment in RAW264.7 cell
To investigate LPS- and JSHT- induced macrophage polarization in RAW 264.7 cells, we performed flow cytometry analysis focusing on the expression of macrophage surface markers in the experimental groups. CD68 and CD80 are surface markers of M1 macrophages, while CD163 and CD206 are surface markers of M2 macrophages.[8, 19, 20, 22] In this study, RAW264.7 cells with CD80- and CD163- markers were identified as M0 macrophage. RAW264.7 cells expressing both CD68+ and CD80+ markers were identified as M1 macrophages. RAW264.7 cells expressing both CD163+ and CD206+ markers were identified as M2 macrophages. The flow cytometry results are presented in Figure 5.
The M0 macrophage expression ratios showed no significant difference in JSHT group (p > 0.05) and a significant decrease in the LPS group compared to the Control group (p < 0.0001; Figure 5B). There were no significant differences in the Pre-JSHT and Post-JSHT groups compared to the LPS group ((p > 0.05; Figure 5B). Regarding the M1 macrophage expression ratios, the results showed no significant change in the JSHT group (p > 0.05) and a significant increase in the LPS group compared to the Control group (p < 0.0001; Figure 5C). The results showed no significant difference in the Pre-JSHT group (p > 0.05) and a significant decrease in the Post-JSHT group compared to the LPS group (p < 0.001; Figure 5C). Regarding the M2 macrophage expression ratios, the results showed a significant increase in the JSHT group (p < 0.05) and no significant difference in the LPS group compared to the Control group (p > 0.05; Figure 5D). There were significant increases in the Pre-JSHT and Post-JSHT groups compared to the LPS group (p < 0.001 in Pre-JSHT group and p < 0.0001 in Post-JSHT group; Figure 5D). These results revealed that JSHT activated M2 polarization and inhibited LPS-induced M1 polarization.
Regulation of cytokine expression levels after LPS and JSHT treatment in RAW264.7 cells
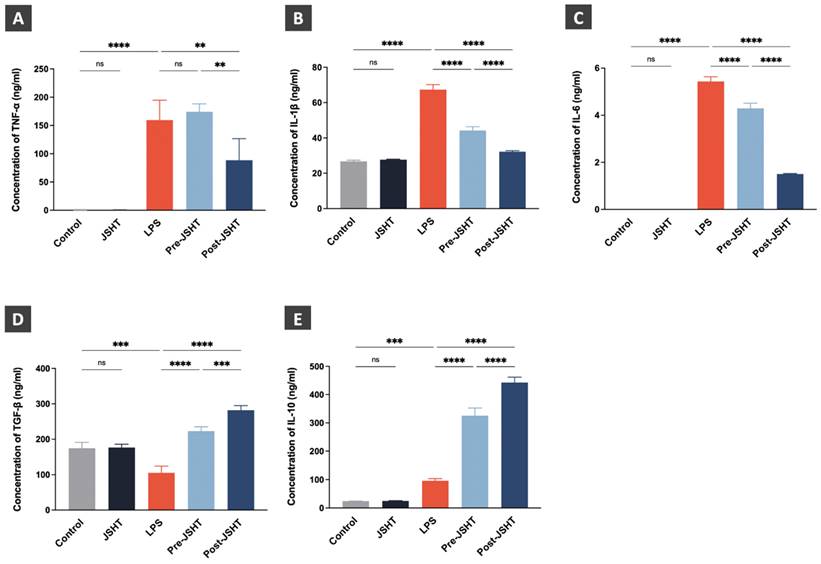
The effects of LPS and JSHT treatment on cytokine expression levels are presented in Figure 6. The expression levels of the pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) showed no statistical differences in the JSHT group compared to the Control group (p > 0.05; Figure 6A-C). The expression levels of TNF-α, IL-1β, and IL-6 were significantly increased in the LPS group compared to the Control group (p < 0.0001; Figure 6A-C). The expression levels of IL-1β and IL-6 were significantly decreased in the Pre-JSHT group compared to the LPS group (p < 0.0001; Figure 6B, C). The expression levels of TNF-α, IL-1β, and IL-6 were significantly decreased in the Post-JSHT group compared to the LPS group (p < 0.0001; Figure 6B, C). Notably, the expression levels of TNF-α, IL-1β, and IL-6 were significantly lower in the Post-JSHT compared to the Pre-JSHT group (p < 0.01; Figure 6A-C). These results indicated that JSHT inhibited LPS-induced inflammation in RAW264.7 cells.
The expression levels of the anti-inflammatory cytokines (TGF-β and IL-10) showed no statistical differences in the JSHT group compared to the Control group (p > 0.05; Figure 6D, E). In the LPS group, the expression level of TGF-β were significantly decreased (p < 0.001; Figure 6D), and the expression level of IL-10 were significantly increased compared to the Control group (p < 0.001; Figure 6E). The expression levels of TGF-β and IL-10 showed a significant increase in the Pre-JSHT group and Post-JSHT groups compared to the LPS group (p < 0.0001; Figure 6D, E). Interestingly, the expression levels of TGF-β and IL-10 were significantly higher in the Post-JSHT compared to the Pre-JSHT group (p < 0.001; Figure 6D, E). These results revealed that JSHT promoted anti-inflammatory effects during LPS stimulation in RAW264.7 cells.
Expressions of macrophage polarization surface markers in RAW 264.7 cells. (A) FLOW cytometry analysis results of the expressions of macrophage polarization markers in the RAW 264.7 cells. (B) The expression ratios of M0 macrophage (CD80-/CD163-); (C) The expression ratios of M1 macrophage (CD68+/CD80+); (D) The expression ratios of M2 macrophage (CD163+/CD206+) in the experimental groups. All data are presented as the mean ± SD. *p < 0.05; ***p < 0.001; ****p < 0.0001; ns: not significant. The experiment was performed three times in duplicate.
Cytokine expression levels after LPS and JSHT treatment in the experimental groups. Concentrations of (A) TNF-α; (B) IL-1β; (C) IL-6; (D) TGF-β; (E) IL-10. All data are presented as the mean ± SD. **p < 0.01; ***p < 0.001; ****p < 0.0001; ns: not significant. The experiments were performed thrice in duplicate.
Discussion
This study demonstrated that JSHT inhibited LPS-induced inflammation, in aspects of increased expression of pERK, pJNK, and nuclear NFκB, increased pro-inflammatory cytokine levels (TNF-α, IL-1β, and IL-6), obvious filopodia protrusion, and a higher ratio of M1 macrophages (CD80+/CD68+) in RAW264.7 cells. Additionally, both Pre- and Post-JSHT groups showed an increase in anti-inflammatory cytokines (TGF-β and IL-10) and a higher ratio of M2 macrophages (CD163+/CD206+). Stronger anti-inflammatory effects and M2 polarization were observed in the Post-JSHT group compared to the Pre-JSHT group. The results indicated that JSHT acts as an anti-inflammatory agent against LPS-induced inflammation with respect to macrophage polarization, inflammatory signaling, and pro- and anti-inflammatory cytokine levels (Figure 7).
In this study, we found that LPS-stimulated macrophage polarization to M1, and MAPK signaling pathways (pERK and pJNK), NFκB, and pro-inflammatory cytokines.[23] LPS serves as the primary structural component of the outer membrane of Gram-negative bacteria and has robust activating effects on macrophages.[24] The morphological and physiological changes in activated macrophages after LPS stimulation include filopodia protrusion and transformation into M1 macrophages.[9, 20, 25, 26] During macrophage activation, LPS induced a cascade of events, leading to NFκB nuclear translocation and phosphorylation of ERK and JNK.[27] Activated NFκB and MAPK pathways up-regulate pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.[25, 27] Following stimulation with LPS, macrophages undergo these processes, leading to their polarization into the M1 phenotype.[8, 26]
We found that JSHT exerted immune modulatory effects, mitigating the activation of macrophages by dampening the transformation of M1 macrophages and downstream pro-inflammatory responses induced by LPS. JSHT is composed of many components that exhibit anti-inflammatory activity. Houttuyniae Herba has been explored as a potential therapeutic agent because of its anti-inflammatory properties.[10, 12, 28, 29] It reduces leukocytosis, lowers pro-inflammatory cytokine levels, and downregulates MAPK and NFκB pathways.[28, 29] Perillae Folium effectively inhibits the inflammatory response by deactivating the HMGB1 signaling pathway and significantly suppresses the production of cytokines such as TNF-α and/or IL-6.[30, 31]. Glycyrrhizae Radix has anti-inflammatory and immunomodulatory activities, involving NFκB regulation to alleviate inflammatory responses induced by LPS.[32] Anisomeles indica exhibits anti-inflammatory activities, demonstrated by its ability to mitigate NFκB phosphorylation and enhance SIRT1 expression in the ischemia-reperfusion rat model.[33] In a murine model of LPS-induced acute lung injury, iso-seco-tanapartholide, a compound found in Artemisia argyi, inhibited the expression of pro-inflammatory factors TNF-α, IL-1β, and IL-6 induced by LPS.[34] Platycodi Radix displays anti-inflammatory properties that markedly inhibit the expression of NFκB, TNF-α, IL-6, and caspase-3.[35] Ophiopogonis Radix displays anti-inflammatory activities, resulting in decreased activation of p65 and phosphorylated IκB in the NFκB pathway, and a substantial reduction in the expression of pro-inflammatory cytokines.[36-39] Chrysanthemi Flos also has anti-inflammatory activity that notably suppresses the NFκB pathways, pro-inflammatory cytokines, and C-reactive protein levels, while mitigating increased leukocytes.[40-43] Luteolin, a natural anti-inflammatory agent, exerts effects against cytokine storms; luteolin treatment significantly reduced the serum levels of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α.[42, 44, 45] In a recent study, JSHT also demonstrated a protective effect against cytokine-induced injury in normal human lung fibroblasts.[11]
JSHT alleviated LPS-induced inflammation in RAW 264.7 cells by modulating macrophage polarization. LPS induction results in the nuclear translocation of NFκB and activates the pERK/pJNK signaling pathway. It promotes macrophages to pro-inflammatory M1 polarization and increases the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. JSHT-administration inhibits LPS-induced nuclear translocation of NFκB and downregulates the pERK/pJNK signaling pathway. It raises tendency of macrophages to anti-inflammatory M2 polarization and decreases the LPS-induced secretion of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), while increases repair cytokines (TGF-β and IL-10).
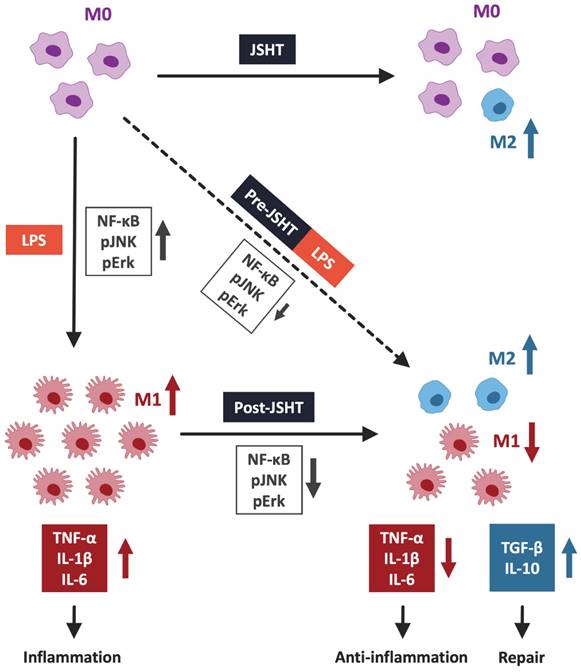
The observed elevation in M2 and related cytokines, TGF-β and IL-10 following JSHT administration implies potential anti-inflammatory effects and reparative properties. TGF-β plays a role in repairing substantial DNA damage by facilitating the interaction and localization of repair protein complexes involved in the incision step of nucleotide excision repair.[46] IL-10 enhances endothelial progenitor cell infiltration and promotes healing after injury.[47] Few studies have addressed the therapeutic mechanisms of the herbs involved in M2 macrophages polarization and their repair. One report indicated that compounds from Perilla frutescens not only inhibited pro-inflammatory cytokines but also enhanced the anti-inflammatory cytokine IL-10.[48-51] Platycodonis Radix inhibits M1 polarization and promotes M2 polarization, leading to an increase in IL-10 and a reduction in TNF-α, IL-6, and IL-1β.[35, 52]
Clinical implication
The clinical implications of the findings in this study on JSHT are significant. This herbal tea was approved by the Ministry of Health and Welfare of Taiwan (registration number MOHW-PM-060 635).[10-13] The ability of JSHT to modulate macrophage polarization and regulate inflammatory responses, particularly in the context of LPS-induced inflammation, suggests its potential therapeutic application in conditions characterized by dysregulated immune responses. The observed inhibition of pro-inflammatory signaling pathways and promotion of anti-inflammatory properties in macrophages following JSHT treatment present promising avenues for interventions aimed at mitigating inflammation-related disorders. Further exploration of JSHT in clinical settings could reveal novel strategies for managing conditions in which macrophage polarization and immune responses play pivotal roles, thereby contributing to the development of effective therapeutic approaches.
Limitations
This study has several limitations. First, this study was conducted at the cellular level, subsequent investigations involving animals and clinical trials are required to confirm the clinical effects. Secondly, this study focused on the initial phase of LPS-induced inflammation. Further research is imperative to explore the long-term effects of JSHT. Finally, we focused on macrophage polarization, MAPK signaling, and cytokine pathways, which are crucial for inflammation. A more comprehensive exploration of the diverse mechanisms will enhance our understanding of the mechanisms underlying the attenuation of inflammation by JSHT.
Conclusions
This study highlights the effects of JSHT in modulating macrophage polarization, inhibiting inflammatory responses by suppressing pro-inflammatory cytokines, and enhancing anti-inflammatory cytokines. In the context of LPS-induced inflammation, which is characterized by elevated pERK and pJNK levels, increased pro-inflammatory cytokine levels, and macrophage activation with M1 polarization, while JSHT significantly mitigated these effects. Moreover, JSHT demonstrated positive effects by increasing anti-inflammatory cytokine levels and promoting M2 polarization.
Abbreviations
IL: interleukin
JSHT: Jing Si Herbal Tea
LPS: lipopolysaccharide
NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells
pERK: phospho-ERK, extracellular signal-regulated kinases
pJNK: phosphor-JNK, c-Jun N-terminal kinase
TGF-β: transforming growth factor
TNF-α: Tumor necrosis factor
Acknowledgements
This research was conducted in the Central Laboratory of the Research Department at Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
Funding
Funding for this study was provided by grants from Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-111-RT-3(3/3) and TCRD-TPE-112-10), and Buddhist Tzu Chi Medical Foundation (TCMF-JCT 112-09 and TCMF-JCT 113-07).
Author contributions
The authors confirmed their contribution as follows: Meng-Jiun Wei: formal analysis, supervision, writing of original draft, visualization, review and editing manuscript; Kuo-Liang Huang: formal analysis, supervision, and review manuscript; Hsiu-Fan Kang: data collection and curation, investigation, methodology, writing original draft; Guan-Ting Liu: data collection and curation, investigation, and methodology; Chan-Yen Kuo: formal analysis, investigation, methodology and supervision; I-Shiang Tzeng: formal analysis, investigation, and supervision; Po-Chun Hsieh: conceptualization and study design, formal analysis, funding acquisition, visualization, project administration, supervision, review and editing manuscript; Chou-Chin Lan: conceptualization and study design, formal analysis, funding acquisition, visualization, project administration, supervision, writing original draft, review and editing manuscript. All the authors reviewed the results and approved the final version of the manuscript.
Data availability
The primary data for this study are presented in this article. For additional information, please contact the corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR. et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-11
2. Guarino M, Perna B, Cesaro AE, Maritati M, Spampinato MD, Contini C. et al. 2023 Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. Journal of clinical medicine. 2023;12:3188
3. Nedeva C, Menassa J, Puthalakath H. Sepsis: Inflammation Is a Necessary Evil. Front Cell Dev Biol. 2019;7:108
4. Lee H, Han JH, An K, Kang YJ, Hwangbo H, Heo JH. et al. Recombinant human KAI1/CD82 attenuates M1 macrophage polarization on LPS-stimulated RAW264.7 cells via blocking TLR4/JNK/NF-κB signal pathway. BMB reports. 2023;56:359-64
5. Chen J, Wei H. Immune Intervention in Sepsis. Front Pharmacol. 2021;12:718089
6. Luo R, Li X, Wang D. Reprogramming Macrophage Metabolism and its Effect on NLRP3 Inflammasome Activation in Sepsis. Front Mol Biosci. 2022;9:917818
7. Qiu P, Liu Y, Zhang J. Review: the Role and Mechanisms of Macrophage Autophagy in Sepsis. Inflammation. 2019;42:6-19
8. Chen X, Liu Y, Gao Y, Shou S, Chai Y. The roles of macrophage polarization in the host immune response to sepsis. Int Immunopharmacol. 2021;96:107791
9. Wang S, Cao M, Xu S, Shi J, Mao X, Yao X. et al. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. Inflammation. 2020;43:95-108
10. Hsieh PC, Chao YC, Tsai KW, Li CH, Tzeng IS, Wu YK. et al. Efficacy and Safety of Complementary Therapy With Jing Si Herbal Tea in Patients With Mild-To-Moderate COVID-19: A Prospective Cohort Study. Front Nutr. 2022;9:832321
11. Wang CH, Yang JS, Chen CJ, Su SH, Yu HY, Juan YN. et al. Protective effects of Jing-Si-herbal-tea in inflammatory cytokines-induced cell injury on normal human lung fibroblast via multiomic platform analysis. Tzu Chi Med J. 2024;36:152-65
12. Lu P-H, Tseng C-W, Lee J-L, Lee E-Y, Lin Y-P, Lin IH. et al. Jing Si Herbal Drink as a prospective adjunctive therapy for COVID-19 treatment: Molecular evidence and mechanisms. Pharmacological Research - Modern Chinese Medicine. 2022;2:100024
13. Chiang CY, Lin YJ, Weng WT, Lin HD, Lu CY, Chen WJ. et al. Recuperative herbal formula Jing Si maintains vasculature permeability balance, regulates inflammation and assuages concomitants of "Long-Covid". Biomed Pharmacother. 2023;163:114752
14. Shin J, Choi LS, Jeon HJ, Lee HM, Kim SH, Kim KW. et al. Synthetic Glabridin Derivatives Inhibit LPS-Induced Inflammation via MAPKs and NF-κB Pathways in RAW264.7 Macrophages. Molecules. 2023;28:2135
15. Hsieh PC, Peng CK, Liu GT, Kuo CY, Tzeng IS, Wang MC. et al. Aqueous Extract of Descuraniae Semen Attenuates Lipopolysaccharide-Induced Inflammation and Apoptosis by Regulating the Proteasomal Degradation and IRE1α-Dependent Unfolded Protein Response in A549 Cells. Front Immunol. 2022;13:916102
16. Hsieh PC, Huang KL, Peng CK, Wu YK, Liu GT, Kuo CY. et al. Aqueous extract of Descuraniae Semen attenuates lipopolysaccharide-induced inflammation by inhibiting ER stress and WNK4-SPAK-NKCC1 pathway. J Cell Mol Med. 2024;28:e18589
17. Park MY, Ha SE, Kim HH, Bhosale PB, Abusaliya A, Jeong SH. et al. Scutellarein Inhibits LPS-Induced Inflammation through NF-κB/MAPKs Signaling Pathway in RAW264.7 Cells. Molecules. 2022;27:3782
18. Romani M, Auwerx J. Phalloidin Staining of Actin Filaments for Visualization of Muscle Fibers in Caenorhabditis elegans. Bio Protoc. 2021;11:e4183
19. Xia T, Zhang M, Lei W, Yang R, Fu S, Fan Z. et al. Advances in the role of STAT3 in macrophage polarization. Front Immunol. 2023;14:1160719
20. Bassiouni M, Arens P, Zabaneh SI, Olze H, Horst D, Roßner F. The Relationship between the M1/M2 Macrophage Polarization and the Degree of Ossicular Erosion in Human Acquired Cholesteatoma: An Immunohistochemical Study. J Clin Med. 2022;11:4826
21. Xu R, Ma L, Chen T, Wang J. Sophorolipid Suppresses LPS-Induced Inflammation in RAW264.7 Cells through the NF-κB Signaling Pathway. Molecules. 2022;27:5037
22. Liu C, Xiao K, Xie L. Advances in the Regulation of Macrophage Polarization by Mesenchymal Stem Cells and Implications for ALI/ARDS Treatment. Front Immunol. 2022;13:928134
23. Tong W, Chen X, Song X, Chen Y, Jia R, Zou Y. et al. Resveratrol inhibits LPS-induced inflammation through suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. Exp Ther Med. 2020;19:1824-34
24. Jackie J, Lau WK, Feng HT, Li SFY. Detection of Endotoxins: From Inferring the Responses of Biological Hosts to the Direct Chemical Analysis of Lipopolysaccharides. Critical reviews in analytical chemistry. 2019;49:126-37
25. Singh R, Dubey V, Wolfson D, Ahmad A, Butola A, Acharya G. et al. Quantitative assessment of morphology and sub-cellular changes in macrophages and trophoblasts during inflammation. Biomedical optics express. 2020;11:3733-52
26. Gabarin RS, Li M, Zimmel PA, Marshall JC, Li Y, Zhang H. Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: Avenues for Novel Therapeutic Strategies. J Innate Immun. 2021;13:323-32
27. Li W, Cai Z, Schindler F, Bahiraii S, Brenner M, Heiss EH. et al. Norbergenin prevents LPS-induced inflammatory responses in macrophages through inhibiting NFκB, MAPK and STAT3 activation and blocking metabolic reprogramming. Front Immunol. 2023;14:1117638
28. Das SK, Mahanta S, Tanti B, Tag H, Hui PK. Identification of phytocompounds from Houttuynia cordata Thunb. as potential inhibitors for SARS-CoV-2 replication proteins through GC-MS/LC-MS characterization, molecular docking and molecular dynamics simulation. Molecular diversity. 2022;26:365-88
29. Yang Y, Lai Q, Wang C, Zhou G. Protective Effects of Herba Houttuyniae Aqueous Extract against OVA-Induced Airway Hyperresponsiveness and Inflammation in Asthmatic Mice. Evidence-based complementary and alternative medicine: eCAM. 2022;2022:7609785
30. Wang XF, Li H, Jiang K, Wang QQ, Zheng YH, Tang W. et al. Anti-inflammatory constituents from Perilla frutescens on lipopolysaccharide-stimulated RAW264.7 cells. Fitoterapia. 2018;130:61-5
31. Wu X, Dong S, Chen H, Guo M, Sun Z, Luo H. Perilla frutescens: A traditional medicine and food homologous plant. Chin Herb Med. 2023;15:369-75
32. Sun Z, He G, Huang N, Thilakavathy K, Lim JCW, Kumar SS. et al. Glycyrrhizic Acid: A Natural Plant Ingredient as a Drug Candidate to Treat COVID-19. Frontiers in pharmacology. 2021;12:707205
33. Hu C, Zhang S, Chen Q, Wang R. Ovatodiolide protects ischemia-reperfusion-induced neuronal injury via microglial neuroinflammation via mediating SIRT1/NF-κB pathway. Brain research bulletin. 2022;180:97-107
34. Kong M, Zhu D, Dong J, Kong L, Luo J. Iso-seco-tanapartholide from Artemisia argyi inhibits the PFKFB3-mediated glycolytic pathway to attenuate airway inflammation in lipopolysaccharide-induced acute lung injury mice. J Ethnopharmacol. 2023;301:115781
35. Zhang S, Chai X, Hou G, Zhao F, Meng Q. Platycodon grandiflorum (Jacq.) A. DC.: A review of phytochemistry, pharmacology, toxicology and traditional use. Phytomedicine. 2022;106:154422
36. Mao D, Tian XY, Mao D, Hung SW, Wang CC, Lau CBS. et al. A polysaccharide extract from the medicinal plant Maidong inhibits the IKK-NF-κB pathway and IL-1β-induced islet inflammation and increases insulin secretion. J Biol Chem. 2020;295:12573-87
37. Yao QW, Wang XY, Li JC, Zhang J. Ophiopogon japonicus inhibits radiation-induced pulmonary inflammation in mice. Ann Transl Med. 2019;7:622
38. Wu Y, Yu X, Wang Y, Huang Y, Tang J, Gong S. et al. Ruscogenin alleviates LPS-triggered pulmonary endothelial barrier dysfunction through targeting NMMHC IIA to modulate TLR4 signaling. Acta Pharm Sin B. 2022;12:1198-212
39. Qiao Y, Jiao H, Wang F, Niu H. Ophiopogonin D of Ophiopogon japonicus ameliorates renal function by suppressing oxidative stress and inflammatory response in streptozotocin-induced diabetic nephropathy rats. Braz J Med Biol Res. 2020;53:e9628
40. Wan-Li L, Dan G, Wei Kevin Z. The Composition of chrysanthemum extracts and their pharmacological functions. STEMedicine. 2020 2
41. Li J, Qiu C, Xu P, Lu Y, Chen R. Casticin Improves Respiratory Dysfunction and Attenuates Oxidative Stress and Inflammation via Inhibition of NF-ĸB in a Chronic Obstructive Pulmonary Disease Model of Chronic Cigarette Smoke-Exposed Rats. Drug design, development and therapy. 2020;14:5019-27
42. Zhou H, Wei Q, Yang L, Gao Y. Medication Rules and Mechanism of Topical Traditional Chinese Medicine for Meibomian Gland Dysfunction-Related Dry Eye Disease. Altern Ther Health Med. 2023;29:126-32
43. Liu Y, Lu C, Zhou J, Zhou F, Gui A, Chu H. et al. Chrysanthemum morifolium as a traditional herb: A review of historical development, classification, phytochemistry, pharmacology and application. J Ethnopharmacol. 2024;330:118198
44. Gour A, Manhas D, Bag S, Gorain B, Nandi U. Flavonoids as potential phytotherapeutics to combat cytokine storm in SARS-CoV-2. Phytotherapy research: PTR. 2021;35:4258-83
45. Lodhi S, Vadnere GP, Patil KD, Patil TP. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. Journal of traditional and complementary medicine. 2020;10:60-9
46. Zheng H, Jarvis IWH, Bottai M, Dreij K, Stenius U. TGF beta promotes repair of bulky DNA damage through increased ERCC1/XPF and ERCC1/XPA interaction. Carcinogenesis. 2019;40:580-91
47. Short WD, Steen E, Kaul A, Wang X, Olutoye OO 2nd, Vangapandu HV. et al. IL-10 promotes endothelial progenitor cell infiltration and wound healing via STAT3. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2022;36:e22298
48. Hou T, Netala VR, Zhang H, Xing Y, Li H, Zhang Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules. 2022;27:3578
49. Youn I, Han S, Jung HJ, Noh SG, Chung HY, Koo YK. et al. Anti-Inflammatory Activity of the Constituents from the Leaves of Perilla frutescens var. acuta. Pharmaceuticals (Basel). 2023;16:1655
50. Zi Y, Yao M, Lu Z, Lu F, Bie X, Zhang C. et al. Glycoglycerolipids from the leaves of Perilla frutescens (L.) Britton (Labiatae) and their anti-inflammatory activities in lipopolysaccharide-stimulated RAW264.7 cells. Phytochemistry. 2021;184:112679
51. Tantipaiboonwong P, Pintha K, Chaiwangyen W, Suttajit M, Khanaree C, Khantamat O. Bioefficacy of Nga-Mon (Perilla frutescens) Fresh and Dry Leaf: Assessment of Antioxidant, Antimutagenicity, and Anti-Inflammatory Potential. Plants (Basel). 2023;12:2210
52. Yang Z, Lin S, Feng W, Liu Y, Song Z, Pan G. et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization. Frontiers in pharmacology. 2022;13:999179
Author contact
![]() Corresponding author: Chou-Chin Lan, Prof., MD, PhD, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; School of Medicine, Tzu Chi University, Hualien, Taiwan; Address: No.289, Jianguo Rd., Xindian Dist., New Taipei City 23142, Taiwan; Tel.: 886-2-6628-9779; Fax: 886-2-6628-9009; E-mail: bluescopycom.tw.
Corresponding author: Chou-Chin Lan, Prof., MD, PhD, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan; School of Medicine, Tzu Chi University, Hualien, Taiwan; Address: No.289, Jianguo Rd., Xindian Dist., New Taipei City 23142, Taiwan; Tel.: 886-2-6628-9779; Fax: 886-2-6628-9009; E-mail: bluescopycom.tw.

 Global reach, higher impact
Global reach, higher impact