3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(15):3003-3009. doi:10.7150/ijms.99233 This issue Cite
Short Research Communication
Clinical and Epidemiologic Features of Mycoplasma pneumoniae Infection Among Adults Hospitalized with Community-acquired Pneumonia
1. Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
2. California Department of Public Health, California, USA.
3. Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
4. Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Received 2024-6-4; Accepted 2024-8-21; Published 2024-11-11
Abstract
Background/Purpose: The burden and epidemiology of Mycoplasma pneumoniae (Mp) community-acquired pneumonia (CAP) among hospitalized U. S. adults (≥ 18 years) are poorly understood.
Methods: In the Etiology of Pneumonia in the Community (EPIC) study, we prospectively enrolled 2272 adults hospitalized with radiographically-confirmed pneumonia between January 2010—June 2012 and tested nasopharyngeal/oropharyngeal swabs for Mp by real-time polymerase chain reaction (PCR). Clinical and epidemiological features of Mp-PCR-positive and -negative adults were compared using logistic regression. Macrolide susceptibility was assessed by genotyping isolates.
Results: Among 2272 adults, 43 (1.8%) were Mp-PCR-positive (median age: 45 years); 52% were male, and 56% were non-Hispanic white. Only one patient had Mp macrolide resistance. Four (9%) were admitted to the intensive care unit (ICU). No in-hospital deaths were reported. Of the 9 (21%) who received an outpatient antibiotic ≤5 days pre-admission, 2 (22%) received an antibiotic with Mp activity. Variables significantly associated with higher odds of Mp detection included age {18-29 years [(adjusted odds ratio (aOR): 11.7 (95% confidence interval (CI): 5.1- 26.6) versus ≥50 years]} and radiographic lymphadenopathy [aOR: 3.5 (95% CI: 1.2- 9.3)].
Conclusions: M. pneumoniae, commonly known to cause “walking pneumonia”, was detected among hospitalized adults, with the highest prevalence among young adults. Although associated with clinically non-specific symptoms, approximately one out of every ten patients were admitted to the ICU. Increasing access to M. pneumoniae point-of-care testing could facilitate targeted treatment and avoid hospitalization.
Keywords: Mycoplasma pneumoniae, adults
Introduction
Mycoplasma pneumoniae is a known cause of adult community-acquired pneumonia (CAP) [1-3]. In addition to sporadic infection, M. pneumoniae can cause large community- and facility-based pneumonia outbreaks, especially in congregate settings such as universities, households, military installations, and healthcare facilities [1, 3-14]. M. pneumoniae also causes upper respiratory infections and, in rare instances, extra-pulmonary manifestations (e.g., encephalitis and pericarditis), usually after respiratory disease [2, 15-20]. Person-to-person aerosol spreads particularly close contact with and exposure to an infected person, which can result in the acquisition of the disease [2, 4, 21-25]. Long incubation periods (6-32 days) and the persistence of the organism in the respiratory tract after symptom resolution contribute to prolonged transmission [2, 4, 26, 27].
The Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study was a prospective multi-center and population-based study of the etiologies of hospitalized CAP among adults in the United States conducted from 2010 to 2012 [28]. In this study, M. pneumoniae was the second most common bacterial pathogen detected after Streptococcus pneumoniae among hospitalized adults (2%), with an estimated incidence of 0.5 (95% confidence interval (CI): 0.4-0.7) cases per 10,000 adults per year. [28]. Using this dataset, we describe the epidemiological and clinical characteristics of M. pneumoniae (Mp) among hospitalized adults with CAP in the EPIC study, comparing the characteristics of patients with and without M. pneumoniae.
Methods
Study population
The adult EPIC study details, published elsewhere, included five hospitals: three in Chicago, Illinois, and two in Nashville, Tennessee [28]. Adults (≥18 years old) with clinical and radiographically confirmed pneumonia were enrolled from January 2010 through June 2012 from the five hospitals [28]. Each site's and CDC's institutional review board approved the study protocol. Inclusion criteria were evidence of acute infection (reported fever or chills, documented fever or hypothermia, leukocytosis or leukopenia, or new altered mental status), evidence of acute respiratory illness (new cough or sputum production, chest pain, dyspnea, tachypnea, abnormal lung examination, or respiratory failure), and chest radiography findings consistent with pneumonia. Specific exclusion criteria included recent hospitalization, previous enrollment in the EPIC study, residence in an extended-care facility, an alternative diagnosis of a respiratory disorder or presence of a tracheostomy tube, cystic fibrosis, neutropenic cancer, recent solid-organ or hematopoietic stem-cell transplant, current graft-versus-host disease or bronchiolitis obliterans, or human immunodeficiency virus infection (with a CD4 cell count <200 per cubic millimeter). After informed consent was obtained, patients or their caregivers were interviewed, and medical charts were abstracted to collect clinical and epidemiological information. A dedicated study radiologist at each site reviewed all radiographic films to make a final determination of pneumonia. Radiographic evidence of pneumonia was defined as the presence of consolidation, other infiltrate, or pleural effusion [28]. A convenience sample of asymptomatic adults from the Nashville study site (n=238) who presented for non-acute care to a general medicine clinic was enrolled weekly from November 1, 2011, to June 30, 2012 (Supplementary material) [28].
Specimen collection and laboratory testing
Blood, urine, and respiratory specimens were obtained for bacterial and viral pathogen testing. [28]. Nasopharyngeal/oropharyngeal (NP/OP) swabs were tested using CDC-developed real-time polymerase chain-reaction (PCR) assays. Mp-PCR-positive specimens were confirmed at CDC with multiplex PCR, culture, and molecular characterization, including macrolide susceptibility genotyping (Supplementary material) [29].
Case definitions
An enrolled patient with an Mp PCR-positive specimen at the study site was considered to have Mp CAP (Mp-PCR-positive). If Mp was not detected by PCR but another pathogen was detected, the patient was considered to have CAP without Mp (Mp-PCR-negative). Bacterial and viral pneumonia, and pneumonia without a pathogen detected, are defined in the Supplementary material. Co-detection was defined as detecting ≥1 other bacterial or viral pathogens. Additional definitions are provided in the Supplementary material.
Statistical analysis
We compared characteristics of adults with Mp-PCR-positive and -negative samples using Pearson chi-square or Fisher's exact tests for categorical variables and Mann-Whitney U and Wilcoxon signed-rank test for continuous variables, as appropriate. We assessed demographics, clinical features, and illness severity based on intensive care unit (ICU) admission, mechanical ventilation, death, and CURB-65 and Pneumonia Severity Index (PSI) scores [30, 31]. Stratified analyses were conducted based on whether a pathogen (bacterial or viral) was detected or not.
We used multivariable logistic regression to compare Mp-PCR-positive and negative patients to explore characteristics independently associated with Mp detection. Variables with a P-value <0.20 on bivariate analysis and those with known biological or epidemiological plausibility were considered candidates for multivariable models. We fitted models using all candidate variables and automated stepwise procedures and then fitted alternate models using only selected variables. We used the Akaike information criterion (AIC) to help select among alternate models - this statistic simultaneously accounts for the goodness of fit and complexity of the tentative models. To resolve collinearity between the study hospital and race in the final model, we only controlled for the study hospital. All statistical tests were interpreted in a 2-tailed fashion to estimate p-values and 95% confidence intervals (CI) and used SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Study population
From January 2010 to June 2012, 2488 (68%) of 3634 eligible adults were enrolled in the EPIC study; 2320 (93%) met the radiographic criteria of pneumonia. Of these 2320 adults, 2272 (98%) had Mp PCR testing performed onsite, and 43 (1.8%) were Mp-PCR-positive. A total of 810 (36%) were Mp-PCR-negative but had another pathogen detected, including rhinovirus (n=193, 24%), influenza A/B viruses (n=131, 16%), and S. pneumoniae (n=115, 14%) as the most detected pathogens.
Bivariate and stratified analyses
Below, we present the findings of the comparison between adults who were Mp-PCR-positive in comparison with those who were Mp-PCR-negative and had another pathogen detected, hereby designated as Mp-PCR-negative. The Supplementary Material provides the demographic characteristics of adults hospitalized for Mp CAP (Supplemental Table 1) and the unadjusted analyses of select epidemiologic features among adults hospitalized for CAP with Mp and those without Mp, including those in whom no pathogen was detected (Supplemental Table 2).
Patient characteristics
Among the 43 Mp-PCR-positive patients, 52% were male, and 56% were non-Hispanic white (Table 2). In unadjusted analyses, Mp-PCR-positive patients were younger than Mp-PCR-negative (median 45 years vs. 57 years; P<0.01), had a longer duration of symptoms before hospitalization (median: 6.6 vs. 4.1 days; P<0.01), and were more likely to have a cough (100% vs. 91%; P=0.03), chest pain (63% vs. 43%, P=0.03), radiographic consolidation (79% vs. 64%, P = 0.04) or hilar lymphadenopathy (14% vs 6%, P=0.047). Mp-PCR-positive patients were less likely to have comorbidities (42% vs. 78%, P<0.01) or leukocytosis (28% vs. 49%, P <0.01) (Table 1). Overall, median hospital length of stay (LOS) was shorter (2 vs. 4 days, P<0.01) for Mp-PCR-positive versus Mp-PCR-negative patients.
Epidemiologic features among adults hospitalized for CAP with and without M. pneumoniae detection*, Etiology of Pneumonia in the Community (EPIC) study, January 2010—June 2012 (n=853)
| Characteristic | M. pneumoniae PCR-positive* (n=43) N (%) | M. pneumoniae PCR-negative with other detected pathogens* (n=810) N (%) | Unadjusted Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|
| Demographics Age in years 18-29 30-49 ≥50 | 14 (33) 12 (28) 17 (39) | 57 (7) 194 (24) 559 (69) | 8.1 (3.8 - 17.2) 2.0 (1.0 - 4.3) Reference | <0.01 0.3 |
| Male | 22 (51) | 378 (47) | 1.2 (0.6 - 2.2) | 0.6 |
| Race/ethnicity† | ||||
| Hispanic | 8 (19) | 95 (12) | 1.4 (0.6 - 3.1) | 0.5 |
| Non-Hispanic Black | 11 (25) | 301 (37) | 0.6 (0.3 - 1.2) | 0.1 |
| Non-Hispanic White | 24 (56) | 385 (48) | Reference | |
| Clinical Presentation | ||||
| Duration of symptoms prior to admission | ||||
| Median (IQR) in days | 6.6 (3.7- 8.8) | 4.1 (2.4- 7.5) | <0.01a | |
| Cough | 43 (100) | 735 (91) | NC | 0.03f |
| Fever/feverish | 37 (86) | 593 (73) | 2.3 (0.9 - 5.4) | 0.06 |
| Fatigue | 36 (84) | 645 (80) | 1.3 (0.6 - 3.0) | 0.5 |
| Chills | 33 (77) | 559 (69) | 1.5 (0.7 - 3.1) | 0.3 |
| Dyspnea | 27 (63) | 630 (78) | 0.5 (0.3 - 0.9) | 0.02 |
| Chest pain | 27 (63) | 375 (46) | 2.2 (1.03-3.7) | 0.03 |
| Headache | 27 (63) | 414 (51) | 1.6 (0.9 - 3.0) | 0.1 |
| Myalgia | 25 (58) | 370 (46) | 1.7 (0.9- 3.1) | 0.1 |
| Wheezing | 21 (49) | 382 (47) | 1.1 (0.6 - 2.0) | 0.8 |
| Nausea | 21 (49) | 310 (38) | 1.5 (0.8 - 2.8) | 0.2 |
| Sore throat | 12 (28) | 286 (35) | 0.7 (0.4 - 1.4) | 0.3 |
| Diarrhea | 11 (26) | 192 (24) | 1.1 (0.5 - 2.2) | 0.8 |
| Rhinorrhea | 9 (21) | 364 (45) | 0.3 (0.2 - 0.7) | <0.01 |
| Medical history | ||||
| Any comorbid condition | 33 (77) | 734 (91) | 0.3 (0.2 - 0.7) | 0.01 |
| Obesityb | 28 (67) | 516 (64) | 1.1 (0.6 - 2.1) | 0.8 |
| Coronary artery disease | 11 (26) | 229 (28) | 0.9 (0.4 - 1.8) | 0.7 |
| Diabetes mellitus | 5 (12) | 216 (27) | 0.4 (0.1 - 0.9) | 0.03 |
| Asthma | 4 (9) | 209 (26) | 0.3 (0.1 - 0.8) | 0.01 |
| Chronic obstructive pulmonary disease | 3 (7) | 186 (23) | 0.3 (0.1-0.8) | 0.01 |
| Renal disorder | 2 (5) | 120 (15) | 0.3 (0.1-1.2) | 0.06 |
| Cancer | 2 (5) | 165 (20) | 0.2 (0.1-0.8) | 0.01 |
| Smoker (current) | 9 (21) | 237 (29) | 0.6 (0.3 - 1.4) | 0.2 |
| Exam findings | ||||
| Rales | 22 (51) | 340 (42) | 1.4 (0.8 - 2.7) | 0.2 |
| Fever | 17 (40) | 248 (31) | 1.5 (0.8 - 2.8) | 0.2 |
| Tachypneac | 16 (37) | 322 (40) | 0.9 (0.5 - 1.7) | 0.7 |
| Wheeze | 11 (26) | 291 (36) | 0.6 (0.3 - 1.2) | 0.2 |
| Decreased breath sounds | 11 (26) | 240 (30) | 0.8 (0.4 - 1.6) | 0.6 |
| Rhonchi | 9 (21) | 236 (29) | 0.6 (0.3 - 1.4) | 0.2 |
| Hypoxiad | 5 (12) | 166 (20) | 0.5 (0.2 - 1.3) | 0.2 |
| Radiologic findingse | ||||
| Consolidation | 34 (79) | 517 (64) | 2.1 (1.01 - 4.5) | 0.04 |
| Single lobar infiltrate | 18 (42) | 259 (32) | 1.5 (0.8 - 2.9) | 0.2 |
| Multiple lobar infiltrate | 16 (37) | 233 (29) | 1.5 (0.8 - 2.8) | 0.2 |
| Air space/ interstitial diseases | 13 (30) | 307 (38) | 0.7 (0.4 - 1.4) | 0.3 |
| Pleural effusion | 9 (21) | 212 (26) | 0.7 (0.4 - 1.6) | 0.4 |
| Hilar lymphadenopathy | 6 (14) | 48 (6) | 2.6 (1.03 - 6.4) | 0.05f |
| Laboratory findings | ||||
| Hyponatremiag | 19 (45) | 264 (33) | 1.7 (0.9 - 3.1) | 0.1 |
| Leukocytosish | 12 (28) | 400 (49) | 0.4 (0.2- 0.8) | <0.01 |
| Severity | ||||
| Length of hospital stay (median, IQR in days) | 2 (1-4) | 4 (2-6) | <0.01 | |
| PSI Class Ii | 23 (54) | 151 (19) | Reference | |
| PSI Class IIi | 10 (23) | 211 (26) | 0.2 (0.07 -0.3) | <0.01 |
| PSI Class III-Vi | 10 (23) | 448 (55) | 0.3 (0.1 - 0.7) | <0.01 |
| ICU admission | 4 (9) | 210 (26) | 0.3 (0.1- 0.8) | 0.01 |
| Mechanical ventilation | 0 | 62 (29) | NC | 0.08f |
| Death | 0 | 16 (2) | NC | 0.7f |
| Antibiotics | ||||
| Receipt of an outpatient antibiotic | 10 (23) | 133 (16) | 1.5 (0.7 - 3.2) | 0.2 |
| Receipt of antibiotics within 5 days prior to admissionj | 9 (21) | 86 (11) | 2.2 (1.03 - 4.8) | 0.04f |
| Penicillinsk | 5 (56) | 15 (17) | Reference | |
| Macrolides | 1 (11) | 34 (40) | 0.1 (0.01 - 0.8) | 0.02f |
| Cephalosporin | 1 (11) | 3 (3) | 1.0 (0.08- 11.9) | 1.0 f |
| Quinolones | 1 (11) | 21 (24) | 0.1 (0.02 - 1.4) | 0.1 f |
Abbreviations: CI, Confidence interval; NC: Could not be calculated as one cell contains a zero
Note: Etiology of Pneumonia in the Community (EPIC) study, 2010-2012
*M. pneumoniae PCR-positive: A radiographically confirmed CAP patient enrolled in EPIC with a positive M. pneumoniae PCR. M. pneumoniae PCR-negative with another pathogen detected: A patient enrolled in EPIC with radiographically confirmed pneumonia who had a negative M. pneumoniae PCR result but had another pathogen detected, such as a bacterial or viral pathogen.
†The race/ethnicity of the 29 Mp-PCR negative not presented are Non-Hispanic Asian (n=15), Non-Hispanic Hawaiian/Pacific Islander (n=1), Non-Hispanic American Indian/American Native (n=6), multiracial (n=3), and other (n=4)
a Wilcoxon Two-sample test
b Body mass index (BMI) was calculated as weight (kg)/height (m)2; categories included underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), and obese (≥ 25 kg/m2). BMI was missing for one Mp-PCR-positive and seven Mp-PCR-negative patients
cTachypnea: >20 breaths/min were considered as abnormal
dHypoxia: Oxygen saturation rate (SpO2) <90% on admission using pulse oximetry on room air or a fraction of inspired oxygen (FiO2) of >0 L or >21% at presentation
e The radiographic findings are not mutually exclusive and could overlap
f Fisher's exact
g Serum sodium <135 U/L. For Mp-PCR-positive the denominator is 42 and for Mp-PCR-negative the denominator is 799
h WBC >11,000/mm3 was considered abnormal. For Mp-PCR-negative, the denominator is 797
iThe categories were Class 1 (PSI score 0-50 points), Class II (PSI score 51-70 points), Class III (PSI 71 - 90 points), Class, IV (PSI score 91 - 130 points), and Class V (PSI score 131 - 395 points).
jThe percentages are based on those who received an antibiotic within 5 days prior to admission
kPenicillins included amoxicillin, penicillin, ampicillin-sulbactam, amoxicillin, and amoxicillin-clavulanate
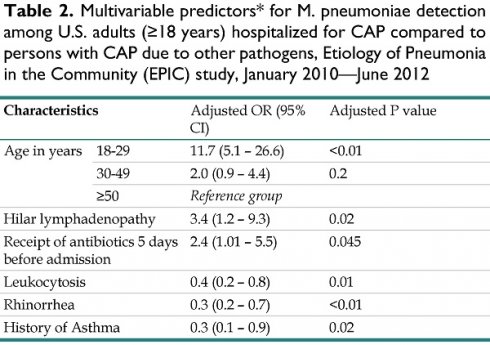
Multivariable predictors* for M. pneumoniae detection among U.S. adults (≥18 years) hospitalized for CAP compared to persons with CAP due to other pathogens, Etiology of Pneumonia in the Community (EPIC) study, January 2010—June 2012
| Characteristics | Adjusted OR (95% CI) | Adjusted P value | |
|---|---|---|---|
| Age in years | 18-29 | 11.7 (5.1 - 26.6) | <0.01 |
| 30-49 | 2.0 (0.9 - 4.4) | 0.2 | |
| ≥50 | Reference group | ||
| Hilar lymphadenopathy | 3.4 (1.2 - 9.3) | 0.02 | |
| Receipt of antibiotics 5 days before admission | 2.4 (1.01 - 5.5) | 0.045 | |
| Leukocytosis | 0.4 (0.2 - 0.8) | 0.01 | |
| Rhinorrhea | 0.3 (0.2 - 0.7) | <0.01 | |
| History of Asthma | 0.3 (0.1 - 0.9) | 0.02 | |
Abbreviations: CI, Confidence interval
Note: Etiology of Pneumonia in the Community (EPIC) study, 2010-2012
*Variables that were tested in the model but did not reach significance: hospital site, sex; clinical presentation of fever/feverish, dyspnea, chest pain; leukocytosis, radiographic findings: consolidation; history of chronic obstructive pulmonary disease, renal disease, cancer; receipt of antibiotics within 5 days prior to admission; duration of symptoms prior to admission; household size; interaction terms: age and renal disease, age and diabetes mellitus, age and cancer, hospital site and receipt of antibiotics 5 days prior to admission
Severity
No deaths were reported among Mp-PCR-positive patients during hospitalization, while 16 (2%) of Mp-PCR-negative patients died. Mp-PCR-positive patients were less likely to have ICU admission (9% (4) vs. 26% (210), P<0.01), and none underwent mechanical ventilation. The Mp-PCR-positive patients admitted to the ICU (n=4) had ≥1 co-morbidity. Among the Mp-PCR-negative patients admitted to the ICU, 62 (29%) required mechanical ventilation, and 15 (7%) died. For Mp-PCR-positive patients in the ICU, the median LOS was shorter (5 vs. 7.5 days, P=0.3) when compared with Mp-PCR-negative. No significant difference in CURB-65 scores between the groups was found, but Mp-PCR-positive patients were significantly more likely to be in lower PSI classes than Mp-PCR-negative (Table 1).
Antibiotic treatment
A higher proportion of Mp-PCR-positive patients received outpatient antibiotics within 5 days prior to admission compared with Mp-PCR-negative patients (21% vs. 11%; P=0.04) (Table 1); penicillins were more commonly administered among Mp-PCR-positive than Mp-PCR-negative (56% vs. 17%, P=0.01) patients. Of nine Mp-PCR-positive patients who received an outpatient antibiotic before admission, only two received an antibiotic with activity against Mp.
During hospitalization, all Mp-PCR-positive patients received an antibiotic with activity against Mp: 14 (33%) received a macrolide only, 14 (33%) received a macrolide and a fluoroquinolone, 8 (19%) received a fluoroquinolone only, 3 (7%) received a fluoroquinolone and a tetracycline, 2 (5%) received a macrolide and tetracycline, and 2 (5%) received a tetracycline only.
A single patient had a macrolide-resistant Mp strain, as determined by sensitivity testing at the CDC laboratory, without a history of macrolide receipt prior to admission (Supplementary material). Upon hospital admission, the patient received azithromycin (day 1) and ceftriaxone (day 2) and was discharged on levofloxacin.
Co-detections
The prevalence of co-detections between Mp-PCR-positive and Mp-PCR-negative patients was comparable (6 [14%] vs. 104 [13%], P=0.8). Among the six Mp-PCR-positive patients with a co-detected pathogen, a single co-pathogen was identified in five [human metapneumovirus (HMPV), parainfluenza virus 2, influenza A virus, rhinovirus, and methicillin-sensitive Staphylococcus aureus] and both respiratory syncytial virus and rhinovirus were detected in one Mp-PCR-positive patient.
Multivariable analyses
In multivariable analyses, Mp-PCR-positive patients were more likely to be younger (18-29 years versus ≥50 years); adjusted odds ratio (aOR): 11.7, 95% CI: 5.1- 26.6, but were clinically indistinct from Mp-PCR-negative patients (Table 2). Mp-PCR-positive patients were more likely to have radiographic evidence of lymphadenopathy (aOR: 3.5, 95% CI: 1.2- 9.3) and less likely to have leukocytosis (aOR: 0.4, 95% CI: 0.2- 0.8), rhinorrhea (aOR 0.3, 95% CI: 0.1 - 0.7), or asthma (aOR 0.3, 95% CI: 0.1 - 0.9) (Table 2).
Discussion
In this large multi-center active surveillance study with prospective enrollment and systematic microbiological testing, Mp was detected among approximately 2% of adults hospitalized with CAP and was more prevalent among younger adults aged 18-49 years, which is different from the age distribution for other types of pneumonia [28]. Among Mp-PCR-positive patients, illness was not severe, and the median LOS was 2 days. Only four adults with Mp were admitted to the ICU; all had comorbidities. No adult required invasive mechanical ventilation or died. Symptoms and clinical features were not sufficiently distinct in adults to differentiate CAP due to Mp from other etiologies.
This prospective population-based study uniquely uses PCR as the diagnostic test of choice for M. pneumoniae. PCR is the current gold standard test for Mp detection due to its higher specificity compared to traditional methods such as serology [32,33]. Serology also lacks timeliness due to the need to obtain a convalescent sample to confirm infection. Mycoplasma is fastidious and difficult to grow, so culture is not routinely available; it is also a resource- and labor-intensive method, resulting in this methodology's discontinuation in clinical laboratories [34]. Although Food and Drug Administration (FDA)-approved and validated laboratory-developed assays (mostly multiplex assays) are now available for Mp detection, usage remains disappointingly low [32, 35]. The role of Mp in adults is likely underestimated clinically, and improving access to PCR testing could lead to opportunities to tailor antibiotic therapy to treat Mp infection, as co-detection of other bacteria was rare.
Macrolide antibiotics, such as azithromycin, are recommended first-line therapy for Mp CAP in adults; other options include fluoroquinolones and tetracyclines [36]. Macrolide resistance was only detected in one isolate. During hospitalization, all Mp-PCR-positive patients received antibiotics with activity against Mp. However, the role of antibiotics in the treatment of Mp CAP remains unclear; our study was not designed to address this question and had an inadequate sample size to determine the role of antibiotics in general or, specifically, the role of macrolides in Mp treatment. However, Mp-PCR-positive patients who apparently failed outpatient antibiotic treatment had more commonly received penicillins, suggesting that the diagnosis of mycoplasma by PCR would facilitate appropriate treatment if Mp infection was confirmed.
Our study has several limitations. The study was completed several years ago when the availability of multiplex PCR was less common, but PCR was the standard method for diagnosis in this study. To reduce misclassification, we excluded patients with no detected pathogens from the analyses. It is possible that these patients may have been in their convalescent phase or that available diagnostic testing did not detect Mp [28]. The unavailability of all specimen types among the enrolled individuals could have been another factor. Invasive procedures to obtain specimens directly from the lung were not usually performed, and results were only available as part of routine clinical care [28]. Another limitation was the difficulty in assessing the role of each pathogen in causing pneumonia when co-detections occurred. No Mp detections were found among a convenience sample of healthy adult controls without respiratory symptoms obtained from an outpatient population (Supplementary Material), suggesting that asymptomatic infection is rare. In the EPIC study, patients 65 years or older and those undergoing invasive mechanical ventilation were less likely to be enrolled [28]. Also, overall, the enrolled patients in the EPIC study were probably less sick than the non-enrolled patients based on the exclusion criteria. Finally, our findings may not be representative of other settings because all study sites were located in urban U.S. medical centers.
Conclusions
Mycoplasma pneumoniae was the second most common bacteria detected among adults hospitalized with CAP, with a higher prevalence among younger adults [28]. The illness was not severe, and clinical signs and symptoms were not distinct between Mp and other respiratory pathogens. Improved access to and validation of point-of-care diagnostic testing using PCR for Mp detection may facilitate prompt and appropriate clinical triage and antibiotic therapy, particularly in younger adults who present with CAP.
Supplementary Material
Supplementary information and tables.
Acknowledgements
We thank the participants who graciously consented to participate in the Etiology of Pneumonia in the Community (EPIC) study.
Financial support
The CDC's Influenza Division at the National Center for Immunizations and Respiratory Diseases supported this work through cooperative agreements with each study site. It was based on a competitive research funding opportunity.
Data availability statement
Restrictions apply to the availability of these data, which were used under agreement for this study.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Competing Interests
Dr. Edwards reports grants from CDC, during the conduct of the study; grants from NIH, other from Bionet, other from IBM, other from Sanofi, other from X-4 Pharma, other from Sequirus, other from Moderna, other from Pfizer, other from Merck, outside the submitted work. Dr. Grijalva reports personal fees from Sanofi, personal fees from Merck, personal fees from Pfizer, grants from Sanofi, grants from Campbell Alliance, grants from Centers for Disease Control and Prevention, grants from the National Institutes of Health, grants from Agency for HealthCare Research and Quality, other from Food and Drug Administration, outside the submitted work. Dr. Zhu reports grants from CDC, during the conduct of the study. Dr. Self reports grants from the Centers for Disease Control and Prevention during the conduct of the study, grants and personal fees from Merck & Co., grants from Pfizer, and personal fees from Aerpio Pharmaceuticals outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
1. Hammerschlag MR. Mycoplasma pneumoniae infections. Curr Opin Infect Dis. 2001;14(2):181-6
2. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17(4):697-728 table of contents
3. Sopena N, Sabria M, Pedro-Botet ML. et al. Prospective study of community-acquired pneumonia of bacterial etiology in adults. Eur J Clin Microbiol Infect Dis: official publication of the European Society of Clinical Microbiology. 1999;18(12):852-8
4. Foy HM, Grayston JT, Kenny GE, Alexander ER, McMahan R. Epidemiology of Mycoplasma pneumoniae infection in families. JAMA. 1966;197(11):859-66
5. Foy HM, Kenny GE, McMahan R, Kaiser G, Grayston JT. Mycoplasma pneumoniae in the community. Am J Epidemiol. 1971;93(1):55-67
6. Waites KB, Atkinson TP. The role of Mycoplasma in upper respiratory infections. Curr Infect Dis Rep. 2009;11(3):198-206
7. Feikin DR, Moroney JF, Talkington DF. et al. An outbreak of acute respiratory disease caused by Mycoplasma pneumoniae and adenovirus at a federal service training academy: new implications from an old scenario. Clin Infect Dis. 1999;29(6):1545-50
8. Amundson DE, Weiss PJ. Pneumonia in military recruits. Mil Med. 1994;159(10):629-31
9. Fisher B, Yu B, Armstrong D, Magill J. Outbreak of Mycoplasma pneumoniae infection among hospital personnel. Am J med Sci. 1978;276(2):205-9
10. Kleemola M, Jokinen C. Outbreak of Mycoplasma pneumoniae infection among hospital personnel studied by a nucleic acid hybridization test. J Hosp Infect. 1992;21(3):213-21
11. Mogabgab WJ. Mycoplasma pneumoniae and adenovirus respiratory illnesses in military and university personnel, 1959-1966. Am Rev Respir Dis. 1968;97(3):345-58
12. Hastings DL, Harrington KJ, Kutty PK. et al. Mycoplasma pneumoniae Outbreak in a Long-Term Care Facility - Nebraska, 2014. MMWR Morbidity and mortality weekly report. 2015;64(11):296-9
13. Waller JL, Diaz MH, Petrone BL. et al. Detection and characterization of Mycoplasma pneumoniae during an outbreak of respiratory illness at a university. J Clin Microbiol. 2014;52(3):849-53
14. Evans AS, Allen V, Sueltmann S. Mycoplasma pneumoniae infections in University of Wisconsin students. Am Rev Respir Dis. 1967;96(2):237-44
15. Marston BJ, Plouffe JF, File TM Jr. et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance Study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med. 1997;157(15):1709-18
16. Mansel JK, Rosenow EC 3rd, Smith TF, Martin JW Jr. Mycoplasma pneumoniae pneumonia. Chest. 1989;95(3):639-46
17. Chan ED, Welsh CH. Fulminant Mycoplasma pneumoniae pneumonia. West J Med. 1995;162(2):133-42
18. Sterner G, Biberfeld G. Central nervous system complications of Mycoplasma pneumoniae infection. Scand J Infect Dis. 1969;1(3):203-8
19. Farraj RS, McCully RB, Oh JK, Smith TF. Mycoplasma-associated pericarditis. Mayo Clinic proceedings Mayo Clinic. 1997;72(1):33-6
20. Dionisio D, Valassina M, Mata S. et al. Encephalitis caused directly by Mycoplasma pneumoniae. Scand J Infect Dis. 1999;31(5):506-9
21. Miyashita N, Akaike H, Teranishi H, Ouchi K, Okimoto N. Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrob Agents Chemother. 2013;57(10):5181-5
22. Tsai V, Pritzker BB, Diaz MH. et al. Cluster of macrolide-resistant Mycoplasma pneumoniae infections in Illinois in 2012. J Clin Microbiol. 2013;51(11):3889-92
23. Biberfeld G, Sterner G. A study of Mycoplasma pneumoniae infections in families. Scand J Infect Dis. 1969;1(1):39-46
24. Dorigo-Zetsma JW, Wilbrink B, van der Nat H, Bartelds AI, Heijnen ML, Dankert J. Results of molecular detection of Mycoplasma pneumoniae among patients with acute respiratory infection and in their household contacts reveals children as human reservoirs. J Infect Dis. 2001;183(4):675-8
25. Balassanian N, Robbins FC. Mycoplasma pneumoniae infection in families. N Engl J Med. 1967;277(14):719-25
26. Lin WC, Lee PI, Lu CY. et al. Mycoplasma pneumoniae encephalitis in childhood. J Microbiol Immunol Infect. 2002;35(3):173-8
27. Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008;8:93
28. Jain S, Self WH, Wunderink RG. et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415-27
29. Diaz MH, Benitez AJ, Cross KE. et al. Molecular detection and characterization of Mycoplasma pneumoniae among patients hospitalized with community-acquired pneumonia in the United States. Open Forum Infect Dis. 2015;2(3):ofv106
30. Lim WS, van der Eerden MM, Laing R. et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-8231
31. Fine MJ, Auble TE, Yealy DM. et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243-50
32. Food and Drug Administration. Nucleic Acid Based Tests. https://www.fda.gov/medical-devices/in-vitro-diagnostics/nucleic-acid-based-tests
33. Loens K, Ieven M. Mycoplasma pneumoniae: Current knowledge on nucleic acid amplification techniques and serological diagnostics. Front Microbiol. 2016;7:448
34. She RC, Thurber A, Hymas WC. et al. Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol. 2010;48(9):3380-2
35. Winchell JM, Mitchell SL. Detection of Mycoplasma pneumoniae by real-time PCR. Methods Mol Biol. 2013;943:149-58
36. Mandell LA, Bartlett JG, Dowell SF. et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin Infect Dis. 2003;37(11):1405-33
Author contact
![]() Corresponding author: Preeta K. Kutty, MD, MPH [ORCiD: 0000-0001-9813-8048], Centers for Disease Control and Prevention, 1600 Clifton Rd NE, MS A-31, Atlanta, GA 30329 USA, Email: pkuttygov.
Corresponding author: Preeta K. Kutty, MD, MPH [ORCiD: 0000-0001-9813-8048], Centers for Disease Control and Prevention, 1600 Clifton Rd NE, MS A-31, Atlanta, GA 30329 USA, Email: pkuttygov.

 Global reach, higher impact
Global reach, higher impact