3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(15):2992-3002. doi:10.7150/ijms.92419 This issue Cite
Research Paper
Arbutin overcomes tumor immune tolerance by inhibiting tumor programmed cell death-ligand 1 expression
1. Division of Cardiology, Department of Internal Medicine, Kaohsiung Armed Forces General Hospital, Kaohsiung, Taiwan.
2. Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 80424, Taiwan.
3. Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan.
4. Aerosol Science Research Center, National Sun Yat-sen University, Kaohsiung, Taiwan.
5. Department of Chemistry, National Sun Yat-sen University, Kaohsiung 80424, Taiwan.
6. College of semiconductor and advanced technology Research, National Sun Yat-sen University, Kaohsiung 80424, Taiwan.
7. Department of Medical Laboratory Science and Biotechnology, Kaohsiung Medical University, Kaohsiung 80708, Taiwan.
8. Department of Medical Research, China Medical University Hospital, China Medical University, Taichung, Taiwan.
# These authors contributed equally to this paper.
Received 2023-11-20; Accepted 2024-10-24; Published 2024-11-11
Abstract
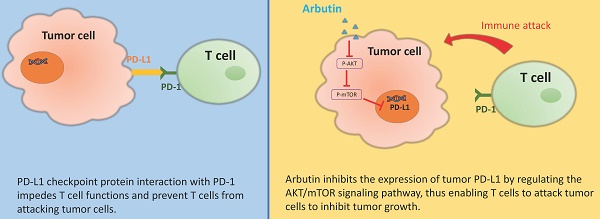
Arbutin, predominantly derived from the bearberry plant, exhibits promising immunomodulatory properties. Given its ability to influence the programmed cell death-ligand 1/ programmed cell death-1 (PD-L1/PD-1) pathway, it is emerging as a potential alternative treatment for cancer. A reduced expression of PD-L1, as seen after arbutin treatment, can bolster immune responses critical step in effective tumor immunotherapy. However, the molecular mechanism by which arbutin inhibits PD-L1 is still incompletely known. The expression of PD-L1 was decreased after tumor cells were treated with arbutin. Arbutin can downregulate the expression of PD-L1 on the cell surface via the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway. The findings suggest the protective role of arbutin and provide novel insights into immunotherapy, which involves inhibiting the AKT/mTOR signaling pathway. Arbutin might serve as a potential therapeutic agent alone or in combination with other treatments.
Keywords: Arbutin, programmed cell death protein ligand-1, tumor immune tolerance
Introduction
Arbutin is a natural hydroquinone glucoside predominantly sourced from the bearberry plant but has also been identified in at least 45 other plant families [1]. Functioning as a shield against free radical-mediated and enzymatic membrane cleavage in plants, arbutin has therapeutic significance in humans [2]. It is employed in cosmetics for skin whitening due to its strong tyrosinase inhibitory activity, which curbs melanin production [3]. Moreover, arbutin possesses other therapeutically significant properties: anti-inflammatory, anti-oxidative, anti-microbial, and potential antitumor capabilities [4, 5]. Studies have highlighted its cytotoxic nature against various tumor cell lines [6]. One mechanism through which arbutin achieves its anti-tumor effects is by inducing apoptosis, as confirmed in vitro [7]. Its impact on tumor-immune interactions remains to be fully explored.
Cancer, a genomic disorder, manifests through genomic instability and the accumulation of myriad point mutations during tumor evolution. This genomic diversity gives rise to tumor antigens, which the immune system perceives as foreign, initiating a cellular immune response [8]. Effective immune responses can potentially eliminate or impede malignant cells [9]. However, tumor cells have devised various strategies to circumvent immune surveillance, thereby neutralizing antitumor immune responses [10].
The past decades have witnessed significant strides in immunotherapy, a paradigm that bolsters the patient's immune system to target malignant cells [11]. Among the advances, immune checkpoint inhibitors, specifically, those targeting programmed cell death-1 (PD-1), programmed cell death-ligand 1 (PD-L1), indoleamine 2,3-dioxygenase (IDO), and cytotoxic T lymphocyte antigen 4 (CTLA-4) [12-14]. Their promising therapeutic efficacy has been demonstrated in various cancers, with some even gaining regulatory approval. Notably, PD-L1 inhibitors are associated with fewer immune-related side effects, positioning them as promising immunotherapeutic agents for diverse tumors [13, 14].
PD-L1, primarily expressed in activated immune cells and some epithelial cells, particularly under inflammation, plays a crucial role in immune tolerance. Its interaction with PD-1 impedes T cell functions, fostering tumor immune evasion [13]. Historical studies have also pointed to tumor-associated the role of PD-L1 in promoting immune suppression [15]. Beyond its immune checkpoint functions, PD-L1 also has intrinsic roles in cancer cells, such as promoting cell proliferation, survival, and metastasis. PD-L1 can activate the activated protein kinase B (AKT)/mammalian target of the rapamycin (mTOR) pathway within tumor cells, enhancing their survival and growth. PD-L1 has an intrinsic role in promoting tumor progression, independent of its well-established immunomodulatory effects. PD-L1 signaling influences various cellular processes, such as the transforming growth factor β (TGF-β) pathway and epithelial-mesenchymal transition [16], epidermal growth factor receptor (EGFR) signaling [17], and cellular metabolism [18]. By influencing this pathway, PD-L1 contributes to tumor progression independently of its immune-regulatory functions. Although direct studies on arbutin's effects on PD-L1 are limited, there is a possibility that arbutin may influence cancer pathways that involve PD-L1. Arbutin has been shown to have anti-proliferative effects on certain cancer cells by inhibiting oxidative stress and related pathways [19]. Given that PD-L1 can promote tumor proliferation, epithelial-mesenchymal transition (EMT), and immune evasion.
This study aims to ascertain whether arbutin can modulate immune checkpoints and diminish tumor immune tolerance by suppressing PD-L1 expression. Our results indicate that arbutin regulates PD-L1 expression in tumor cells by targeting the AKT/mTOR pathway. Furthermore, animal models confirm the potential of arbutin in alleviating tumor immune tolerance in vivo. In essence, arbutin might pave the way for a novel mechanism in immune checkpoint inhibition. By modulating the AKT/mTOR pathway, arbutin may promote programmed cell death in cancer cells, thereby inhibiting tumor growth.
Materials and Methods
Reagents, Cell Lines, Plasmids, Salmonella and Mice
Arbutin, cisplatin and cobalt chloride (CoCl2) were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Deionized water was use to solubilize arbutin. Adherent cells murine melanoma cells (B16F10) [20] and murine lung carcinoma (LL2) [21] were cultured in Dulbecco's modified Eagle's medium (DMEM) added with 1% penicillin and 10% heat-inactivated fetal bovine serum at 37 ℃and 5% CO2 incubator. Suspension cells EL4 cell line (mouse T lymphocyte; (ATCC TIB-39™)) was cultured in Roswell Park Memorial Institute (RPMI) medium 1640 added with 1% penicillin, 0.05 mM 2-Mercaptoethanol and 10% heat-inactivated fetal bovine serum at 37 ℃and 5% CO2 incubator. The EL4 cells were derived from the C57BL/6 strain mice. The WEHI-3 cells are a murine leukemia cell line, specifically derived from the BALB/c strain mice. The WEHI-3 cells were cultured in Iscove's Modified Dulbecco's Medium (IMDM) added with 1% penicillin, 0.05 mM 2-Mercaptoethanol and 10% heat-inactivated fetal bovine serum at 37℃and 5% CO2 incubator. This WEHI-3 cells are extensively used as a model to study the immune response, particularly the mechanisms underlying the response of leukemic cells to various treatments. The EL4 mouse thymoma cells are used for a variety of research purposes, including studies on tumor immunology, cell signaling, apoptosis, necrosis and drug response. The origin of EL4 cells from murine thymoma makes them a relevant model for studying T-cell biology, providing insights into T cell development, and T cell function. The AKT plasmid that has constitutively active activity has been previously described [22]. A vaccine strain of Salmonella enterica serovar choleraesuis (S. Choleraesuis; S.C.) was obtained from Bioresources Collection and Research Center (Hsinchu, Taiwan) [21]. Animal experiments used C57BL/6 mice purchased from the National Laboratory Animal Center of Taiwan. The Laboratory Animal Care and Use Committee of the National Sun Yat-sen University approved the animal experimental protocol (permit number: 10829).
Cell viability assay
B16F10 and LL2 cells (5 × 104 cells/well) were plated in 96-well and incubated for one day, then cells were treated with arbutin (0.39, 0.78, 1.56 μM) in serum-free medium for 6 h, cisplatin (2 µg/ml) as positive control. Cell proliferation was evaluated by the WST-1 Cell Proliferation Assay Kit (Sigma-Aldrich) according to the manufacturer's instructions, and the absorbance was measured at the indicated times using the SPECTROstar Nano Microplate Reader. The cells were seeded in 96-well culture plates (104/well), then treated by arbutin (0.39-1.56 µM) for 6 h, cisplatin (16 µg/ml) as positive control. Cell proliferation was examined with the 5-bromo-2'-deoxyuridine (BrdU) Cell Proliferation Fluorescence Imaging Kit (AAT Bioquest, US) uses BrdU which was incorporated into cellular DNA during DNA synthesis. After fixing cells, the incorporated BrdU is labelled with iFluor® 488 MTA. The images were taken with the Olympus fluorescence microscopy [23].
Flow cytometry
B16F10 and LL2 cells were seeded in 6-well plates at a density of 5 × 105 cells per well and incubated for 24 h. Following this, the cells were treated with either PBS or arbutin at a concentration of 1.56 μM in a serum-free medium for 6 h. For PD-L1 detection, cells were harvested (Use gentle methods to detach cells, such as using cell scrapers instead of enzymatic treatments (e.g., trypsin) which can cleave surface proteins. Include protease inhibitors in the buffers during cell harvesting to protect surface proteins from degradation. Quickly process and fix the cells after harvesting to preserve surface marker expression), counted to ensure 5 × 105 cells, and then fixed in 70% ethanol at 4°C overnight. The fixed cells were then incubated with a PD-L1 primary antibody (Dilution: 1:2000) (GeneTex, Inc. Irvine, CA, USA) for 1 h at 4°C. This was followed by a 30-minute incubation with a fluorochrome-labeled goat anti-rabbit IgG secondary antibody (GeneTex Inc. Irvine, CA, USA) at the same temperature. The samples were subsequently analyzed using the Attune NxT Flow Cytometer (Life Technologies, Carlsbad, CA, USA).
Western blot analysis
Arbutin influenced protein expression in B16F10 and LL2 cells. B16F10 and LL2 cells (5 × 105 cells/well) were placed into 6-well plates and incubated at 37 °C for 24 h. Then treatment with arbutin (0-1.56 μM) for 6 h, the expression of proteins was measured by Western blotting. To determine protein concentrations, we employed the Bicinchoninic Acid (BCA) Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA). The protein samples underwent separation via SDS-PAGE, after which the resolved proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Pall Life Science, Glen Cove, NY, USA). The membrane was then probed with the following primary antibodies: PD-L1 (Dilution: 1:1000) (GeneTex), phospho-AKT (Dilution: 1:1000) (Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA), AKT (Dilution: 1:1000) (Santa Cruz Biotechnology), phospho-mTOR (Dilution: 1:1000) (Cell Signaling, Danvers, MA, USA), mTOR (Dilution: 1:1000) (Cell Signaling), caspase 3 (Dilution: 1:1000) (GeneTex), LC3 (Dilution: 1:1000) (Novus Biologicals, Littleton, CO, USA) and a monoclonal antibody against β-actin (Dilution: 1:10000) (Sigma-Aldrich). We used either horseradish peroxidase-conjugated goat anti-mouse IgG or anti-rabbit IgG (Dilution: 1:20000) (both from Jackson ImmunoResearch Inc, West Grove, PA, USA) for secondary detection. The bound protein-antibody complexes were then visualized using the enhanced chemiluminescence system (T-Pro Biotechnology, New Taipei City, Taiwan). The resulting signals were quantified using the ImageJ software [24].
Co-culture system
B16F10 and LL2 cells (5 × 105 cells/well) were plated in 6 well-plates and treated with arbutin (1.56 μM) for 6 h. The control cells were treated with PBS. The supernatant was removed, and added the immune cells, murine EL4 lymphoblast cells, or murine WEHI-3 leukemia, which were mixed with an equal amount of serum-free medium. After 24 h, the immune cells were collected and the protein expression for Western blotting was detected and cell survival was assessed using the trypan blue exclusion assay [25].
Animal study
Six- to eight-week-old C57BL/6 mice were subcutaneously inoculated with 106 B16F10 or LL2 cells at day 0. The mice were intraperitoneally injected with arbutin (50 mg/kg) for 7 consecutive days from day 8 to day 14. The control mice were treated with PBS. Tumor size and body weight were measured every 3 days. The volume of palpable tumors was calculated using the following formula: (length of tumor) × (width of tumor)2 × 0.45. Five mice were sacrificed in each group on day 14 and the tumors were taken down for further analysis.
Statistical analysis
Experimental data were analyzed and reported as mean ± standard deviation (SD).
To evaluate statistically significant differences, the Student's t-test was utilized to evaluate statistically significant differences. The Student's t-test assumes that the data is approximately normally distributed. The primary purpose of the Student's t-test is to compare the means of two groups to determine if they are statistically different from each other. This is particularly useful in experiments where we want to assess the effect of a treatment or intervention compared to a control group. The p-value less than 0.05 was considered to be statistically significant.
Results
Ideal concentration of arbutin
Treatment options for metastatic tumor are severely limited, but recent immunotherapy approaches targeting immune checkpoints have shown great promise in various cancers, including melanoma and non-small cell lung cancer (NSCLC). In this study, we utilized an immunocompetent murine model of melanoma and NSCLC to investigate the effects of arbutin administration on tumor growth. In this investigation, we utilized murine melanoma B16F10 and murine lung carcinoma LL2 cells to examine the anti-tumor potential of arbutin. The cell viability assay, performed on both B16F10 and LL2 cells over 6 h with varying concentrations of arbutin, is shown in Figure 1. Our findings revealed that arbutin did not inhibit cell proliferation in B16F10 (Figure 1A) or LL2 cells (Figure 1B) across the tested doses. Cisplatin induced cell death as positive control (Figure 1 A and B). We hope to find a concentration of arbutin that has immunomodulatory effects without having direct cytotoxic effects on the cells. Previously, arbutin effectively induced apoptosis in the C6 glioma cells and the IC50 dose was obtained at 30 µM [26]. To monitor cell proliferation influence by arbutin in B16F10 and LL2 cells, we use BrdU fluorescence to investigate the range of 0-1.56 µM arbutin (Figure 1C). These cells treated with cisplatin as positive control. Figure 1D showed that arbutin did not significantly inhibited cell proliferation in these cells in a dose-dependent manner by quantification of BrdU-positive cells. Based on these observations, these dosages were subsequently chosen to assess the influence of arbutin on PD-L1 expression.
In vitro influence of arbutin on PD-L1 expression
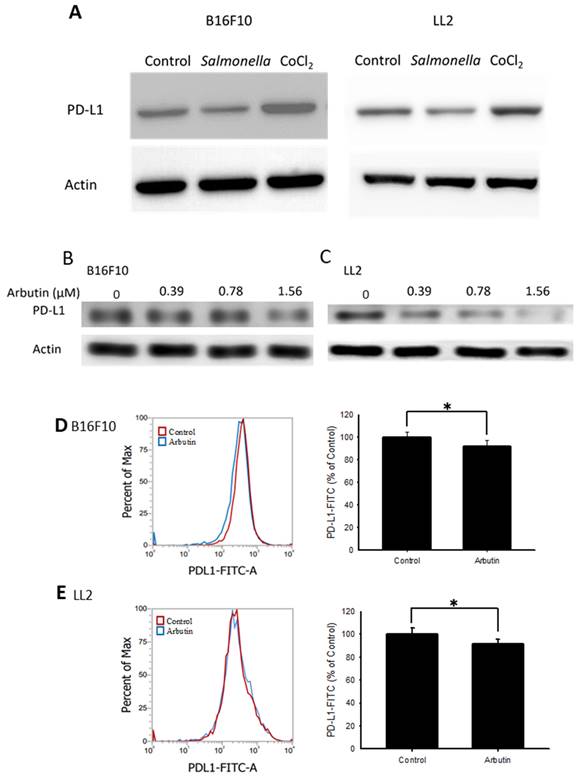
The role of the PD-L1-mediated immune checkpoint within the tumor microenvironment is critical for tumor immune evasion [27]. We examined the protein levels of PD-L1 in both B16F10 and LL2 cells. Previous studies indicated that Salmonella inhibit PD-L1 expression [13] and hypoxia (CoCl2 simulates anaerobic environment) enhanced PD-L1 expression [28]. Salmonella reduced PD-L1 expression and CoCl2 increased PD-L1 expression (Figure 2 A). Figure 2 B and C shows the PD-L1 levels in tumor cells following various arbutin treatments over 6 hr. Compared to the control, there was a noticeable decline in PD-L1 expression in both B16F10 (Figure 2 B) and LL2 cells (Figure 2 C) with incremental arbutin exposure. Flow cytometry analysis further revealed a significant reduction in cell surface PD-L1 expression post-treatment with 1.56 μM of arbutin in B16F10 (Figure 2 D) and LL2 cells (Figure 2 E). These experimental conditions do not trigger autophagy (Figure S1). These findings indicate that arbutin can dose-dependently suppress the surface expression of PD-L1 on tumor cells, without affecting their cell viability.
Arbutin modulates PD-L1 expression in tumor cells via the AKT/mTOR signaling pathway
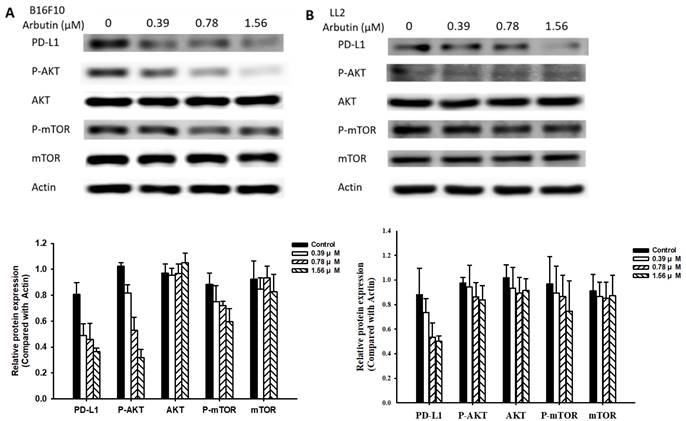
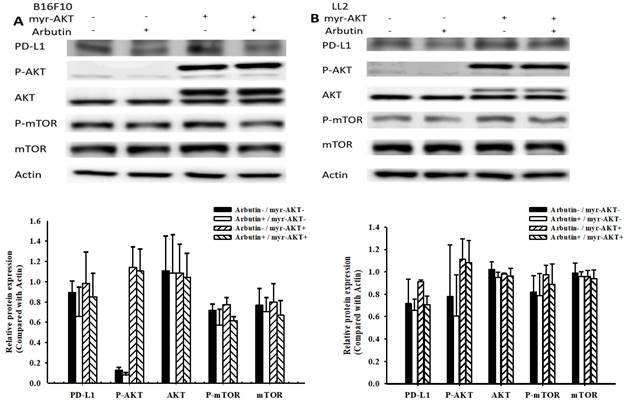
In this study, we delved deeper into the mechanistic influence of arbutin on tumors. Activation of the PI3K/AKT signaling pathway plays a pivotal role in tumor cell survival, proliferation, and motility. Past studies have posited that PD-L1 expression is governed by the AKT/mTOR signaling pathway [13]. Moreover, the activation of this signaling pathway has been associated with enhanced PD-L1 expression. With this background, we evaluated the expression levels of PD-L1, phosphorylated AKT, and phosphorylated mTOR, as shown in Figure 3. Arbutin treatment diminished the phosphorylation levels of AKT and mTOR, pointing towards its inhibitory effect on the AKT/mTOR pathway in B16F10 (Figure 3A) and LL2 cells (Figure 3B). To further substantiate the role of arbutin in modulating PD-L1 expression through AKT phosphorylation, we employed a constitutively active AKT plasmid to restore AKT/mTOR signaling. Figure 4 indicates that post-transfection with the active AKT plasmid, there was a noticeable elevation in PD-L1 expression, accompanied by an increase in AKT/mTOR phosphorylation. Relative to the control transfection, the active AKT plasmid-transfected cells showed augmented PD-L1 expression following arbutin exposure, observed in B16F10 (Figure 4A) and LL2 cells (Figure 4B). Our results underscore the importance of arbutin's downregulation of AKT phosphorylation in diminishing PD-L1 expression in B16F10 and LL2 cells. Arbutin essentially hinders tumor PD-L1 expression by orchestrating changes in the AKT/mTOR signaling pathway.
Effect of Arbutin on cell viability in B16F10 and LL2 cell. The B16F10 and LL2 cells (5 × 105 cells/well) were placed into 96-well plates and incubated at 37℃ for 24 h. The B16F10 (A) and LL2 (B) cells were treated with indicated concentrations of arbutin or cisplatin (2 µg/ml) for 6 h. Cell viability was evaluated by WST-1 cell proliferation assay. (mean ± SD, n=5). The B16F10 and LL2 cells (5 × 104 cells/well) were placed into 96-well plates and incubated at 37℃ for 24 h. The B16F10 and LL2 cells were treated with indicated concentrations of arbutin for 6 h. (C) Then the cells were fixed and stained for BrdU (green) and nuclei were counterstained with Hoechst 33342 (blue). (D) The cells were counted under a fluorescence microscope (mean ± SD, n=3; ***, p < 0.001).

Impact of arbutin treatment on immune cell protein expression
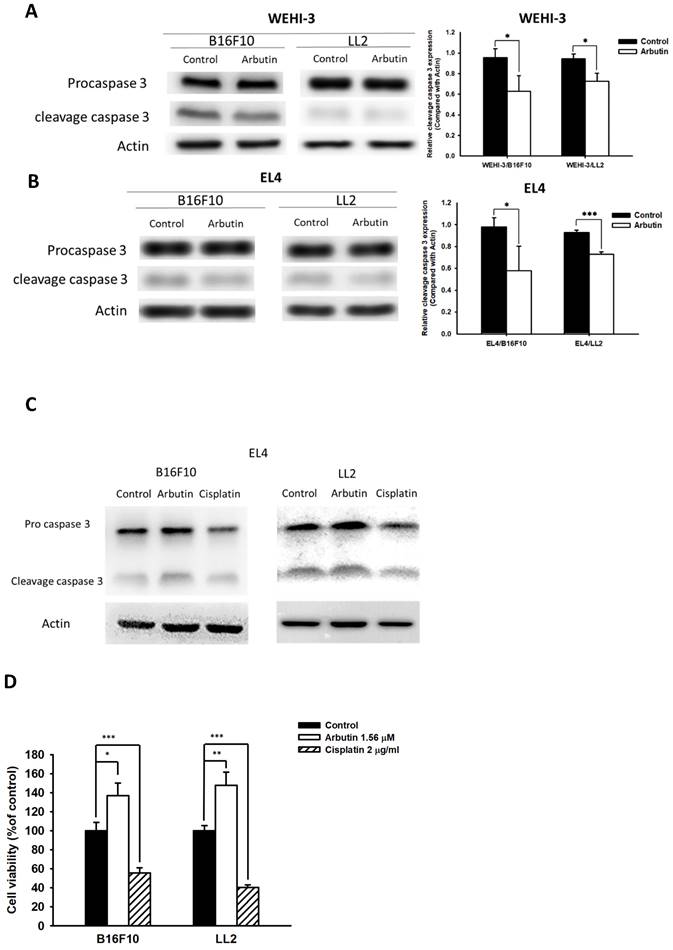
Previous research has illuminated that PD-L1 is abundantly expressed in human lung cancer and melanoma, with high levels of PD-L1 in tumors potentially triggering T-cell apoptosis [13]. In light of this, we sought to discern whether the observed decrease in PD-L1 after co-culturing immune cells with tumor cells played a pivotal role in the effects elicited by arbutin on immune cell activity. For this, we chose mouse EL4 lymphocytes and WEHI-3 leukemia cells to co-culture with B16F10 and LL2 tumor cells treated with 1.56 μM arbutin. Results from the Western blot analysis highlighted a marked decrease in cleaved caspase 3 in WEHI-3 (Figure 5 A) and EL4 (Figure 5 B and C) cells when co-cultured with arbutin-treated tumor cells. In Figure 5 C, cisplatin induced cell apoptosis as positive control. Furthermore, the EL4 cell viability was higher after the co-cultured with arbutin-treated tumor cells than that derived from the tumor cells treated with PBS (Figure 5 D). These findings attest to the inhibitory effect of arbutin on the production and functionality of PD-L1 in tumor cells.
Arbutin inhibited PD-L1 expression in B16F10 and LL2 cells. Arbutin reduced PD-L1 expression in B16F10 and LL2 cells. (A) B16F10 and LL2 cells (5 × 105 cells/well) were placed into 6-well plates and incubated at 37 °C for 24 h. Then treatment with Salmonella (5 × 107 cells/well) for 1.5h or CoCl2 (200 μM) for 6 h, the expression of PD-L1 was measured by Western blotting. B16F10 (B) and LL2 (C) cells (5 × 105 cells/well) were placed into 6-well plates and incubated at 37 °C for 24 h. Then treatment with arbutin (0-1.56 μM) for 6 h, the expression of PD-L1 was measured by Western blotting. Arbutin reduced PD-L1 expression on the surface of cells. After treatment with arbutin (1.56 μM) for 6 h, the expression of PD-L1 on the surface of B16F10 (C) and LL2 (D) cells was measured by flow cytometry. (mean ± SD, n=5; *, p < 0.05).
Arbutin-mediated PD-L1 protein expression. B16F10 (A) and LL2 (B) cells (5 × 105 cells/well) were placed into 6-well plates and incubated at 37 °C for 24 h. Then treatment with arbutin (0-1.56 μM) for 6 h, the expression of PD-L1 and phosphorylation-AKT/mTOR were measured by Western blotting. Quantification histograms are presented beneath each Western blotting plot. Data are expressed as the mean ± SD of three-time repeated determinations. Each experiment was repeated three times with similar results.
Arbutin reduces PD-L1 expression through the AKT/mTOR pathway. B16F10 (A) and LL2 (B) cells (5 × 105 cells/well) were placed into 6-well plates and incubated at 37 °C for 24 h. Transfect the cells with control or constitutively active AKT plasmid (5ug) at 37°C for 6 h, then treat with arbutin (1.56 μM) for 6 h. Analyze the expression levels of the AKT/mTOR proteins and PD-L1 in the cells using Western blotting. Quantification histograms are presented beneath each Western blotting plot. Data are expressed as the mean ± SD of three-time repeated determinations. Each experiment was repeated three times with similar results.
Arbutin suppresses PD-L1 expression and tumor growth in vivo
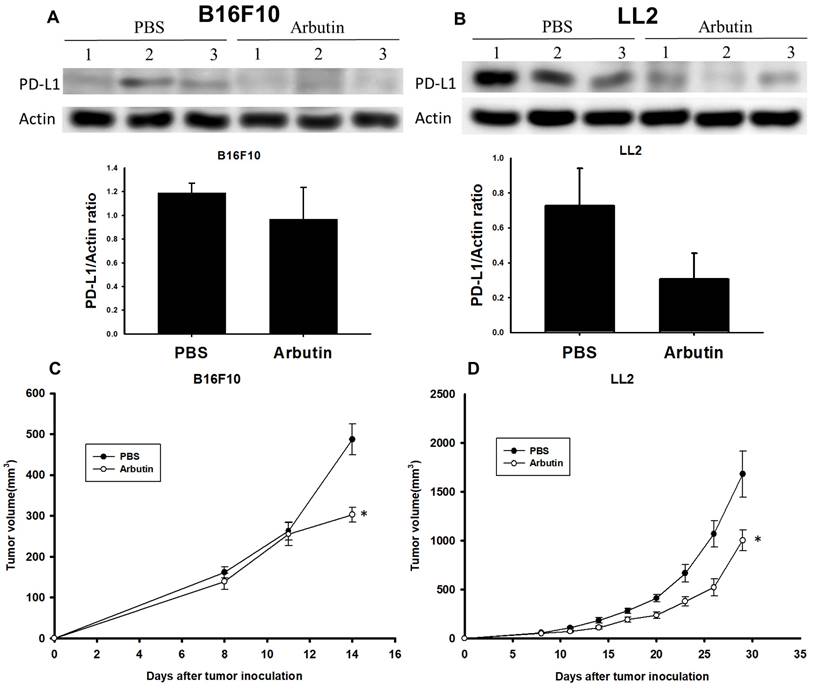
To validate the inhibitory effect of arbutin on tumor growth in a live model, C57BL/6 mice were subcutaneously injected with either B16F10 or LL2 cells (106) on day 0. Starting on day 7, the mice received intraperitoneal injections of arbutin (50 mg/kg) or PBS daily for consecutive seven days. After this treatment duration, tumors were harvested, processed, and analyzed for PD-L1 expression levels. Figure 6A reveals that PD-L1 expression was notably suppressed in the arbutin-treated B16F10-bearing mice compared to the PBS control group. A similar trend was observed in LL2-bearing mice, as illustrated in Figure 6B. Furthermore, the tumor volume was monitored every three days starting from day 7. The data demonstrated that the arbutin-treated group experienced a significant reduction in tumor growth volume compared to the PBS control group in both tumor models (Figure 6 C and D). This study conclusively showed that murine melanoma and lung tumor cells exhibited markedly decreased growth following arbutin treatment.
Arbutin affected apoptosis in immune cells. B16F10 and LL2 cells (5 × 105 cells/well) were placed into 6-well plates, incubated at 37 °C for 24 h, and then treated with arbutin (1.56 μM) for 6 h. WEHI-3 (A) and EL4 (B) cells were co-cultured with arbutin-treated B16F10 and LL2 cells before harvesting. The protein expression was analyzed by Western blotting. (C) EL4 cells were co-cultured with arbutin-treated B16F10 and LL2 cells or treated with cisplatin (2 µg/ml) before harvesting. The protein expression was analyzed by Western blotting. (D) The EL4 cell number were measured by staining with trypan blue. (n = 6, data are mean± SD; * p < 0.05; **, p < 0.01; *** , p < 0.001). Each experiment was repeated three times with similar results.
Arbutin inhibits tumor growth and PD-L1 expression in vivo. B16F10 and LL2 tumor cells were subcutaneously injected into C57BL/6 mice on day 0. Tumor growth was allowed for seven days, and arbutin (50 mg/kg) was administered via intraperitoneal injection for seven consecutive days from day 8. Tumor volumes were measured every three days. On Day 15, tumors were extracted using a lysis buffer. The supernatant was collected, and PD-L1 expression levels were analyzed using Western blotting for (A) B16F10 (n=3) and (B) LL2 (n=3) tumors. Tumor volumes were compared between the control group and the arbutin-treated group for (C) B16F10 (n=10) and (D) LL2 (n=9) tumors. (mean ± SEM; *, p < 0.05).
Discussion
Immune checkpoint inhibitors are immunotherapies that block immune checkpoints, thereby promoting anti-tumor immune responses. Prominent among these are the CTLA-4 and PD-1/PD-L1 pathways. By inhibiting checkpoint proteins, these therapies can rejuvenate T-cell activity and contribute to tumor regression [27]. However, expanding the use of immune checkpoint inhibitors in clinical settings has exposed significant challenges, including the risk of collateral effects on the immune system [29]. Arbutin stands out due to its range of properties: it has a whitening effect, is anti-inflammatory, and possesses antioxidant properties that impede melanin formation. Prior research has highlighted its capability to down-regulate proteins related to tumor growth [30]. Our investigations indicate that arbutin exerts its effects primarily through immunomodulation, rather than direct cytotoxicity to tumor cells (Figure 1 and 2). Its treatment reduces PD-L1 expression at both surface and protein levels, underscoring its potential as an immunotherapeutic agent. Arbutin bolsters host immunity by attenuating effector T cell apoptosis through decreased PD-L1 expression.
Arbutin indeed reduced the expression of PD-L1 in this study. The reduction in surface PD-L1 by arbutin (Figure 2 D and E) was not as significant as the reduction observed in total PD-L1 (Figure 2 B and C). Arbutin may indeed reduce the intrinsic PD-L1 levels in tumor cells, thereby affecting tumor malignancy. Because arbutin reduces a small amount of surface PD-L1 but significantly inhibits the expression of intrinsic PD-L1, it is expected to have a synergistic effect when combined with anti- CTLA-4 therapy. These findings support the notion that reducing intrinsic PD-L1 expression can synergize with immune checkpoint blockade (ICB) therapy, potentially leading to better clinical outcomes in cancer patients [29].
Though in vivo studies have substantiated arbutin's tumor-inhibiting capabilities, additional evidence remains crucial. Notably, PD-L1 in tumor cells promotes immune suppression by increasing IL-10 production in peripheral regulatory T cells [30]. The PD-1/PD-L1 interaction inhibits tumor-infiltrating CD4+/CD8+ T cells, reducing cytokine secretion and facilitating tumor immune evasion [13]. Hence, a deeper investigation into arbutin's impact on T cell function is warranted [31-32]. Unlike many inhibitors, distinct advantage of arbutin lies in its widespread presence in various plants, allowing easy acquisition through diverse dietary sources. More significantly, its consumption poses no harm to humans. Arbutin has been shown to suppress the proliferation of certain cancer cells by downregulating the AKT/mTOR signaling pathway. This inhibition can lead to reduced tumor cell growth and reduction of inflammation [26]. In this study, arbutin may influence the intrinsic functions of PD-L1 by modulating the AKT/mTOR signaling pathway, potentially leading to decreased T/tumor cell proliferation and enhanced apoptosis. This suggests a promising avenue for cancer therapy research, combining arbutin's anti-cancer properties with its effects on crucial signaling pathways involved in tumor progression.
Arbutin, a natural hydroxyquinone glucoside, exists in two configurations: α and β-quercetin glycosides. Current findings highlight that α-glycosides exhibit more significant activity than their β counterparts, leading to α-arbutin's heightened efficacy against tyrosinase. The inhibitory effect of α-arbutin on tyrosinase surpasses that of β-arbutin tenfold [33]. This study, however, utilized β-arbutin. A deeper exploration into α-arbutin's effects on tumor cells might offer insights beneficial for drug development or adjuvant therapies. By comprehensively understanding the mechanisms of natural compounds, there's potential for harnessing their anticancer properties, paving the way for innovative cancer treatments.
Supplementary Material
Supplementary figure.
Acknowledgements
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Science and Technology, Taiwan (MOST 109-2326-B-110-001-MY3; NSTC 113-2811-B110-008), Higher Education Sprout Project, and Kaohsiung Armed Forces General Hospital, Taiwan (KAFGH_A_111001).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
Ching-Han Liu: Validation, Investigation, Formal analysis, Writing - original draft. Rui-Yang Song: Validation, Investigation, Formal Analysis, Writing - initial draft. Li-Hsien Wu: Data curation, Resources. Jing-Ru Weng, Li-Hsien Wu, Ming-Der Huang and Xin-He Wu: Data curation, Resources. Chia C. Wang, Che-Hsin Lee: Supervision, Project administration, Writing - review & editing, Funding acquisition.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Man X, Yang L, Liu S, Yang L, Li M, Fu Q. Arbutin promotes MC3T3-E1 mouse osteoblast precursor cell proliferation and differentiation via the Wnt/β-catenin signaling pathway. Mol Med Rep. 2019;19(6):4637-4644
2. Oliver AE, Crowe LM, de Araujo PS, Fisk E, Crowe JH. Arbutin inhibits PLA2 in partially hydrated model systems. Biochim Biophys Acta. 1996;1302(1):69-78
3. Chen YC, Liu YY, Chen L, Tang DM, Zhao Y, Luo XD. Antimelanogenic Effect of Isoquinoline Alkaloids from Plumula Nelumbinis. J Agric Food Chem. 2023;71(43):16090-16101
4. Xu KX, Xue MG, Li Z, Ye BC, Zhang B. Recent Progress on Feasible Strategies for Arbutin Production. Front Bioeng Biotechnol. 2022;10:914280
5. Mirzaei S, Zarrabi A, Asnaf SE, Hashemi F, Zabolian A, Hushmandi K, Raei M, Goharrizi MASB, Makvandi P, Samarghandian S, Najafi M, Ashrafizadeh M, Aref AR, Hamblin MR. The role of microRNA-338-3p in cancer: growth, invasion, chemoresistance, and mediators. Life Sci. 2021;268:119005
6. Hazman Ö, Sarıova A, Bozkurt MF, Ciğerci İH. The anticarcinogen activity of β-arbutin on MCF-7 cells: Stimulation of apoptosis through estrogen receptor-α signal pathway, inflammation and genotoxicity. Mol Cell Biochem. 2021;476(1):349-360
7. Ebadollahi SH, Pouramir M, Zabihi E, Golpour M, Aghajanpour-Mir M. The Effect of Arbutin on The Expression of Tumor Suppressor P53, BAX/BCL-2 Ratio and Oxidative Stress Induced by Tert-Butyl Hydroperoxide in Fibroblast and LNcap Cell Lines. Cell J. 2021;22(4):532-541
8. Gubin MM, Schreiber RD. CANCER. The odds of immunotherapy success. Science. 2015;350(6257):158-159
9. Moriya T, Hashimoto M, Matsushita H, Masuyama S, Yoshida R, Okada R, Furusawa A, Fujimura D, Wakiyama H, Kato T, Choyke PL, Kusumoto Y, Chtanova T, Kobayashi H, Tomura M. Near-infrared photoimmunotherapy induced tumor cell death enhances tumor dendritic cell migration. Cancer Immunol Immunother. 2022;71(12):3099-3106
10. Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3(11):999-1005
11. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11
12. Pangilinan CR, Lee CH. Highlights of Immunomodulation in Salmonella-Based Cancer Therapy. Biomedicines. 202; 9(11):1566.
13. Chen MC, Pangilinan CR, Lee CH. Salmonella Breaks Tumor Immune Tolerance by Downregulating Tumor Programmed Death-Ligand 1 Expression. Cancers (Basel). 2019;12(1):57
14. Chang HL, Kuo YH, Wu LH, Chang CM, Cheng KJ, Tyan YC, Lee CH. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int J Med Sci. 2020;17(7):939-945
15. Chuan D, Fan R, Chen B, Ren Y, Mu M, Chen H, Zou B, Dong H, Tong A, Guo G. Lipid-Polymer Hybrid Nanoparticles with Both PD-L1 Knockdown and Mild Photothermal Effect for Tumor Photothermal Immunotherapy. ACS Appl Mater Interfaces. 2023;15(36):42209-42226
16. Lems CM, Burger GA, Beltman JB. Tumor-mediated immunosuppression and cytokine spreading affects the relation between EMT and PD-L1 status. Front Immunol. 2023;14:1219669
17. Lin HH, Chang CW, Liao YT, Yeh SD, Lin HP, Ho HM, Cheung CH, Juan HF, Chen YR, Su YW, Chen LM, Tan TH, Lin WJ. DUSP22 inhibits lung tumorigenesis by suppression of EGFR/c-Met signaling. Cell Death Discov. 2024;10(1):285
18. Hodgins JJ, Abou-Hamad J, O'Dwyer CE, Hagerman A, Yakubovich E, Tanese de Souza C, Marotel M, Buchler A, Fadel S, Park MM, Fong-McMaster C, Crupi MF, Makinson OJ, Kurdieh R, Rezaei R, Dhillon HS, Ilkow CS, Bell JC, Harper ME, Rotstein BH, Auer RC, Vanderhyden BC, Sabourin LA, Bourgeois-Daigneault MC, Cook DP, Ardolino M. PD-L1 promotes oncolytic virus infection via a metabolic shift that inhibits the type I IFN pathway. J Exp Med. 2024;221(7):e20221721
19. Wang CQ, Wang XM, Li BL, Zhang YM, Wang L. Arbutin suppresses osteosarcoma progression via miR-338-3p/MTHFD1L and inactivation of the AKT/mTOR pathway. FEBS Open Bio. 2021;11(1):289-299
20. Lin CY, Wu CY, Wang CC, Lee CH. Exposure to phenols reduces melanogenesis in B16F10 cells and zebrafish. Aquat Toxicol. 2024;266:106806
21. Wu LH, Huang YT, Lin CY, Lee CH. Salmonella-induced inhibition of β3-adrenoceptor expression in tumors and reduces tumor metastasis. J Cancer. 2024;15(5):1203-1212
22. Yen WC, Li QZ, Wu LH, Lee WY, Chang WW, Chien PJ, Lee CH. Salmonella inhibits tumor metastasis by downregulating epithelial cell adhesion molecules through the protein kinase-B/mammalian target of rapamycin signaling pathway. Int J Biol Macromol. 2023;253(Pt 3):126913
23. Wu LH, Pangilinan CR, Lee CH. Downregulation of AKT/mTOR signaling pathway for Salmonella-mediated autophagy in human anaplastic thyroid cancer. J Cancer. 2022;13(11):3268-3279
24. Ni YJ, Huang ZN, Li HY, Lee CC, Tyan YC, Yang MH, Pangilinan CR, Wu LH, Chiang YC, Lee CH. Hinokitiol impedes tumor drug resistance by suppressing protein kinase B/mammalian targets of rapamycin axis. J Cancer. 2022;13(6):1725-1733
25. Lin HC, Yang CJ, Kuan YD, Wang WK, Chang WW, Lee CH. he inhibition of indoleamine 2, 3-dioxygenase 1 by connexin 43. Int Med Sci. 2017;14(12):1181-1188
26. Yang Z, Shi H, Chinnathambi A, Salmen SH, Alharbi SA, Veeraraghavan VP, Surapaneni KM, Arulselvan P. Arbutin exerts anticancer activity against rat C6 glioma cells by inducing apoptosis and inhibiting the inflammatory markers and P13/Akt/mTOR cascade. J Biochem Mol Toxicol. 2021;35(9):e22857
27. Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24(1):26
28. Zhu Y, Zang Y, Zhao F, Li Z, Zhang J, Fang L, Li M, Xing L, Xu Z, Yu J. Inhibition of HIF-1alpha by PX-478 suppresses tumor growth of esophageal squamous cell cancer in vitro and in vivo. Am J Cancer Res. 2017;7(5):1198-1212
29. Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33(17):1974-82
30. Safari H, Zabihi E, Pouramir M, Morakabati P, Abedian Z, Karkhah A, Nouri HR. Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1β and TNF-α in LNCaP; prostate cancer. J Food Biochem. 2020;44(9):e13360
31. Qin G, Bai F, Hu H, Zhang J, Zhan W, Wu Z, Li J, Fu Y, Deng Y. Targeting the NAT10/NPM1 axis abrogates PD-L1 expression and improves the response to immune checkpoint blockade therapy. Mol Med. 2024;30(1):13
32. Dong Y, Han Y, Huang Y, Jiang S, Huang Z, Chen R, Yu Z, Yu K, Zhang S. PD-L1 Is Expressed and Promotes the Expansion of Regulatory T Cells in Acute Myeloid Leukemia. Front Immunol. 2020;11:1710
33. Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of α- and β-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem. 1995;59(1):143-4
Author contact
![]() Corresponding authors: Dr. Che-Hsin Lee, Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan, 70 Lienhai Rd. Kaohsiung 80424, Taiwan. E-mail: chleensysu.edu.tw.
Corresponding authors: Dr. Che-Hsin Lee, Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan, 70 Lienhai Rd. Kaohsiung 80424, Taiwan. E-mail: chleensysu.edu.tw.

 Global reach, higher impact
Global reach, higher impact