3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(15):2981-2991. doi:10.7150/ijms.100845 This issue Cite
Research Paper
Construction for the predictive model of quality of life in patients after robot-assisted radical prostatectomy: a cohort study
1. Department of Urology, Institute of Urology and Center of Biomedical Big Data, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
2. West China Hospital of Sichuan University, No. 37, Guoxue Lane, Chengdu, 610041, Sichuan Province, China.
3. National Clinical Research Center of Geriatrics, the Center of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China.
4. College of Computer Science, Sichuan University, Chengdu 610065, China.
*These authors contributed equally to this work.
Received 2024-7-12; Accepted 2024-9-24; Published 2024-11-11
Abstract
Background: Urinary incontinence (UI) and erectile dysfunction (ED) often arise as frequent postoperative complications following robotic-assisted radical prostatectomy (RARP) for prostate cancer (PCa). These issues can significantly diminish patients' quality of life (QoL). The assessment of QoL is even more important because treatment decisions may be influenced by the expected QoL. Few studies have integrated the clinical profiles of patients with magnetic resonance imaging (MRI) metrics to assess postoperative UI and ED.
Methods: PCa patients treated with RARP between January 2018 and September 2022 were enrolled in this study. Preoperative clinical baseline characteristics and MRI parameters were retrospectively collected. The Expanded Prostate Cancer Index Composite Short Form (EPIC-26) questionnaire was completed to assess urinary continence and sexual function at regular postoperative follow-up. Preoperative baseline clinical characteristics and MRI parameters were subsequently used to screen for predictors of urinary continence and sexual function after RARP, and predictive models were constructed.
Results: A total of 627 patients with PCa who met the criteria were ultimately included in this study, with 1059 follow-up questionnaires. The predictive model for postoperative urinary continence was constructed with respect to age, history of transurethral resection of the prostate (TURP) surgery, clinical T stage (cT), Gleason score (GS), Charlson score, membranous urethral length (MUL), pubic symphysis-prostate apex length (PAL), urethral width, right anal sphincter thickness and anal levator muscle thickness (axial plane). Moreover, body mass index (BMI), cT, age, GS, Charlson score, internal obturator muscle thickness, urethral width and anal sphincter thickness were predictors of short-term and long-term postoperative sexual function. We were able to develop highly effective predictive models for postoperative urinary continence and sexual function in RARP patients by incorporating baseline clinical features and MRI parameters.
Conclusions: The predictive model enables the assessment of postoperative urinary continence and sexual function in patients after RARP and offers clinical guidance.
Keywords: robotic-assisted radical prostatectomy, urinary incontinence, quality of life, predictive model, sexual function.
Introduction
Prostate cancer (PCa) is globally recognized as the second most prevalent malignant neoplasm, with its mortality ranking fifth among all malignancies affecting males. China contributes to 8.2% of the global incidence of PCa annually and accounts for 13.6% of the worldwide mortality associated with this disease1. Although the overall incidence of PCa in China is lower than the global average, there has been a notable upwards trend in incidence in recent years2. Several studies have indicated that PCa imposes a substantial health burden on the Chinese population3. Therefore, it is imperative to implement effective strategies aimed at improving the quality of life of the populace.
Treatment options for PCa include radical prostatectomy (RP), radiotherapy with or without androgen deprivation therapy (ADT), or active surveillance (AS), which are selected on the basis of the risk of PCa recurrence and life expectancy4. In addition, RP is a pivotal therapeutic intervention for PCa. Robot-assisted radical prostatectomy (RARP) is widely acknowledged for its superior oncological and functional outcomes owing to its enhanced visual field and heightened precision5. Nevertheless, postoperative complications, including urinary incontinence (UI), erectile dysfunction (ED) and others, continue to be frequently observed. All these factors lead to a decrease in patient satisfaction with the surgical procedure and overall quality of life (QoL)6.
PCa is characterized by its relatively long natural history, and a significant proportion of patients experience mortality due to factors unrelated to the disease7. Therefore, QoL assessment becomes even more critical in situations where treatment decisions may be influenced by anticipated QoL outcomes8. The Expanded Prostate Cancer Index Composite (EPIC) is the most appropriate cancer-specific measure for assessing quality of life in urological patients9. The concise version of the EPIC-26, comprising 26 items, is the predominant self-assessment scale currently used. It assesses patients' QoL across five aspects: UI, symptoms related to urethral irritation and obstruction, intestinal function, sexual function, and hormone levels. This finding has also been corroborated in Chinese studies10.
Previous studies have demonstrated that advanced age, elevated body mass index (BMI), and comorbidities may be associated with an increased risk of UI11. Moreover, advanced age, comorbidities, nerve-sparing status, and preoperative erectile function are known factors associated with ED12. With the application of magnetic resonance imaging (MRI) technology, the correlation between patient anatomy and QoL has been further investigated. The findings of previous studies have indicated that the morphological characteristics of the prostatic apex and membranous urethral length (MUL) are factors associated with post-RARP UI within a one-year follow-up period13. However, limited research has integrated the clinical characteristics of patients with MRI parameters to investigate risk factors for ED.
Given the significance and research status of QoL after RARP, this study aims to explore related risk factors for postoperative urinary continence and sexual function in patients after RARP. The predictive models were constructed on the basis of patients' clinical baseline characteristics and preoperative MRI parameters.
Methods
Study population
The study enrolled a total of 627 patients with early prostate cancer who underwent RARP within the West China Prostate Cancer Cohort from January 2018 to September 202214. In addition, all patients included in the cohort underwent appropriate nerve sparing.
The inclusion criteria were as follows: (1) Patients who were diagnosed with PCa and underwent RARP performed by an exceptionally proficient surgeon. The exclusion criteria were as follows: (1) prostate MRI data were unavailable. (2) Relevant follow-up data were either unavailable or incomplete. Approval of the study protocols used was provided by the ethical committee, and the study was registered with the Chinese Clinical Trial Registry. This work is in line with the STROCSS criteria15.
Clinical characteristics
The clinical characteristics of the patients were collected through the hospital information system. Specifically, this information includes patient age, height, weight, comorbidities, preoperative prostate-specific antigen (PSA), history of transurethral resection of the prostate (TURP), Gleason score (GS), clinical T stage (cT), pathological needle biopsy results, etc., at the time of surgical intervention.
BMI was calculated by dividing their weight (in kg) by the square of their height (in m). The Charlson score was calculated on the basis of the patient's medical history and diagnosed comorbidities. The criterion is the sum of corresponding scores of each comorbidity: 1 point: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease (excluding hemiparesis), dementia, chronic lung disease, connective tissue disease, peptic ulcer, mild liver disease, and diabetes mellitus (without complications). 2 points: Diabetes mellitus with end-organ damage, hemiplegia (or paraplegia), moderate to severe renal abnormalities, nonmetastatic solid tumors, leukemia, lymphoma, and multiple myeloma. 3 points: moderate to severe liver function abnormalities. 6 points: metastatic solid tumor, and AIDS. After the cumulative scores were computed, patients were categorized into four groups on the basis of their scores: 0 points, 1-2 points, 3-4 points, and ≥5 points16.
MRI characteristics
Patients underwent MRI examination via a 3.0T device (GE Company, USA). Measurements of various MRI parameters were conducted on all T2-weighted images of patients who underwent preoperative prostate MRI examinations, with the participation of three observers, including the authors, under the supervision of experienced imaging physicians. The agreement among the three observers was evaluated via the intraclass correlation coefficient (ICC), and the results are presented in Supplementary Table 4. The main measurement indicators are as follows:
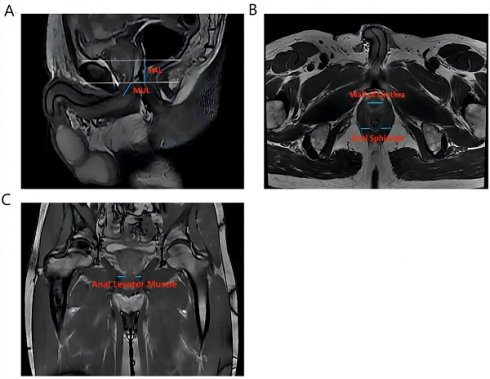
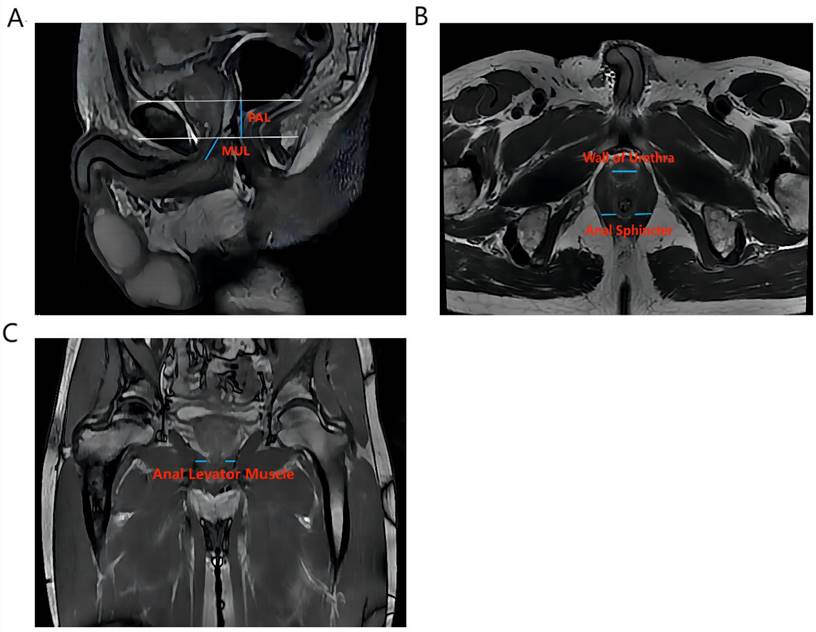
Vertical plane (Figure 1A): membrane urethra length (MUL), membrane urethra angle, prostate length, pubic symphysis-prostate apex length (PAL).
Axial plane (Figure 1B): Thickness of the left/right musculus obturator internus, prostate height, prostate width, distance between the outer/inner edges of the anal levator muscle, transverse membranous urethra thickness, left/right anal sphincter thickness, and thickness of the urethral wall.
Coronal plane (Figure 1C): left/right anal levator muscle thickness
Calculation: Anal levator muscle thickness (axial plane) = (outer edge spacing of the anal levator muscle - inner edge spacing of the anal levator muscle)/2. The surface area of the membrane urethra section = (1/2 × thickness of transverse membrane urethra) × (1/2 × thickness of anterior and posterior membrane urethra) × π. Prostate volume = prostate height × prostate length × prostate width ×π/6. Membrane urethra volume = (transverse membrane urethral thickness × 1/2) × (anterior and posterior membrane urethral thickness × 1/2) ×π× membrane urethral length.
Follow-up data
The assessment of patient QoL and completion of the EPIC-26 scale were implemented during the follow-up period through a combination of outpatient visits and telephone consultations. The scale comprises five dimensions, namely, the urinary incontinence dimension (4 items), the symptoms of urethral stimulation and obstruction dimension (4 items), the intestinal function dimension (6 items), the sexual function dimension (5 items), and the hormone level dimension (5 items). The quality of life in each dimension was assessed on a scale ranging from 0 to 100, with higher scores indicating superior levels10.
Flow chart of inclusion and exclusion criteria in this study.
The main measurement indicators of anatomical structure on all T2-weighted images of patients. Figure 1A shows an example of a sagittal parameter on preoperative MR image; Figure 1B shows an example of a preoperative axial plane parameter; Figure 1C shows an example of a preoperative coronal parameter. PAL: pubic symphysis‒prostate apex length; MUL: membranous urethral length
Construction of the predictive model
Continuous variables are expressed as the means ± standard deviations (SDs), whereas categorical variables are presented as rates and percentages. P values were obtained via t tests to detect differences among continuous variables and chi-square tests for differences among categorical variables. Univariate analysis was used to assess the correlation between various variables and urinary continence and sexual function outcomes in PCa patients separately, and multivariate analysis was also performed. The results are presented as odds ratios (ORs) and 95% confidence intervals (CIs), and two-sided P values < 0.05 were considered to indicate statistical significance. In addition, predictive factors were also screened by least absolute shrinkage and selection operator (LASSO) regression analysis.
We developed a logistic regression prediction model for urinary continence and linear regression prediction models for sexual function by integrating potential predictors identified through univariate and multivariate analyses, as well as LASSO regression analysis. When the predictive model was constructed, a multivariate score polynomial check and transformation were performed on the continuous variables. The multiple-score polynomial model that best predicts outcome was selected, pairwise interaction terms among independent variables were screened for, and bootstrap resampling was conducted for internal validation (100 resamples).
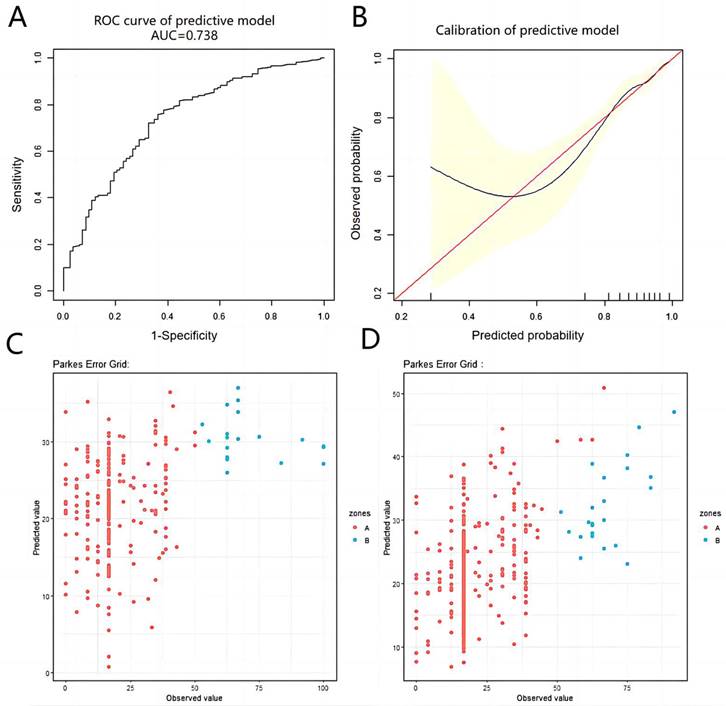
For the predictive model of urinary continence, we constructed a receiver operating characteristic (ROC) curve on the basis of the results of logistic regression analysis, and the area under the ROC curve (AUC) was used to quantify the discriminative capacity of the model. Moreover, a model calibration curve was employed to evaluate its calibration ability. In addition, for the prediction model of sexual function, the results of linear regression analysis were used to conduct error grid analysis (EGA), and the Parkes EGA was graphed to illustrate the concordance between the predicted values derived from the predictive model and the actual observed values.
The EGA represents the level of error between the predicted and actual values in regions A to E. The results from region A indicate that the predicted and observed values are within a 20% margin, which does not impact clinical decision making. The level of error in Region B exceeds 20%, yet it does not significantly impact clinical decision-making. The discrepancy between the predicted and observed values in region C suggests an overestimation, whereas in region E, it indicates an underestimation. Both types of discrepancy can significantly impact clinical decision-making. Region D indicates undiscovered observations that may influence clinical decision-making17-19. The model is considered to have great predictive power when 99% of the predicted values fall within Regions A and B20.
Statistical software
The statistical analyses in this study were conducted via EmpowerStats software (http://www. empowerstats.com, X&Y Solutions, Inc., Boston, MA) and the R programming language. The statistical significance of differences was determined by P values, with a threshold set at P <0.05.
Results
Construction of a predictive model for urinary continence
On the basis of the inclusion criteria and exclusion criteria, 627 individuals were enrolled in this study. Among these patients, 544 individuals achieved recovery from urinary continence, whereas 83 individuals still used urinal pads. The median follow-up durations were 20.29 months and 25.38 months in the respective populations. The mean age of patients who achieved recovery from urinary continence was 66.87 years, whereas the mean age of patients who did not regain urinary continence was 69.96 years. Compared with individuals with persistent UI, those with restored urinary control presented a relatively younger age profile (66.87±7.44 vs 69.96±7.33, P<0.001), a longer MUL (1.07±0.35 vs 0.97±0.31, P=0.016), a shorter PAL (3.07±0.66 vs 3.28±0.70, P=0.008), and a thinner right anal sphincter. The proportion of individuals without a history of TURP surgery who achieved urinary control recovery was significantly greater than that of those who did not (94.67% vs 85.54%, P=0.005) (Table 1).
The variables in Table 1 were first subjected to univariate analysis. The results demonstrated that age (OR 0.94, 95% CI 0.91-0.97, P<0.001), PAL (OR 0.62, 95% CI 0.44-0.89, P=0.008), right anal sphincter thickness (OR 0.27, 95% CI 0.09-0.89, P=0.031), history of TURP surgery=1 time (OR 0.32, 95% CI 0.16-0.66, P=0.002) and GS=9 score (OR 0.27, 95% CI 0.10-0.72, P=0.009) were significantly associated with recovery from urinary continence in the entire study population. Moreover, MUL was an independent risk factor for UI after RARP (OR 2.52, 95% CI 1.19-5.36; P=0.016). After further multivariate analysis, recovery from urinary continence was not significantly correlated with PAL, whereas the significance of other variables remained unchanged (Table 2 and Supplementary Table 1).
LASSO regression analysis was subsequently conducted on all the variables. When determining the optimal λ value that minimizes average cross-validation error, the identified predictive factors include age, history of TURP surgery=1 time, cT=T2c, cT=T4, GS=6 points, GS=9 points, Charlson score=3--4, MUL, PAL, urethral width, right anal sphincter thickness and anal levator muscle thickness (axial plane).
The predictive variables obtained through the aforementioned methods were subjected to logistic regression analysis to construct a predictive model. The final formula was 9.34575 - 0.74435* (history of TURP surgery=1 time) + 0.04793*(cT<T2c) + 0.44740*(cT=T2c) + 0.22512*(cT=T3b) - 0.07319*Age(year) + 0.47809*(GS=6 points) - 0.21700*(GS=8 points) - 0.84119*(GS=9 points) + 0.09937*(Charlson score=0) + 0.57344*(Charlson score=3-4 points) + 0.98297*MUL(cm) - 0.13776*PAL(cm) - 0.80022*Urethral width(cm) - 1.61330*Right anal sphincter thickness(cm) - 1.21959*Anal levator muscle thickness (axial plane)(cm). The AUC of the ROC curve for the obtained model was 0.738 (>0.7), indicating excellent predictive capacity (Figure 2A). However, in the calibration curve, some of the predicted values are underestimated compared with the actual values; nevertheless, the overall calibration capability remains reasonable (Figure 2B).
Construction of a predictive model for sexual function
The entire study population was enrolled, 266 of which were short-term follow-up patients, while the other 437 were long-term follow-up patients, with a cut-off of 12 months after surgery. The mean age of the short-term follow-up population was 66.92 years, with a mean BMI of 24.02 and an average follow-up duration of 6.34 months, and it was 67.51 years in the long-term follow-up population, with a mean BMI of 24.17 and an average follow-up duration of 32.13 months. Details of the various indicators of the patients are shown in Supplementary Table 2.
In the short-term follow-up population, univariate analysis revealed significant differences in age, BMI, cT=T4, GS=9 points, left internal obturator muscle thickness and urethral width. However, only age (β=-0.45, 95% CI=-0.73--0.18, P=0.001) and cT=T4 (β=-10.31, 95% CI=-19.90--0.72, P=0.036) were found to be statistically significant in the multivariate analysis, suggesting that age and cT=T4 were potential predictors of sexual function in the short term following RARP (Table 3 and Supplementary Table 3). A 1-year increase in age was associated with a 0.45-point decrease in the sexual function score. All variables were subsequently subjected to LASSO regression analysis, which revealed that the predictive factors for sexual function included age, BMI, history of TURP surgery=1 time, cT=T2c, cT=T3a, cT=T4, GS=6, GS=9, Charlson score=0, left internal obturator muscle thickness, urethral width and right anal sphincter thickness. By utilizing potential predictor variables obtained through the aforementioned approaches, we have derived final predictive model formulas. In the short-term follow-up population, the formula was 8.51449 + 0.59933 * BMI (kg/m2) - 3.64065 * (history of TURP surgery = 1 time) + 7.27015 * (cT <T2c) + 10.59556 * (cT=T2c) + 3.20239 * (cT=T3b) - 3.46238 * (cT=T4) - 0.29899 * Age (years) + 0.04724 * (GS=6 scores) - 2.99144 * (GS=7 scores) - 5.62285 * (GS=9 scores) + 3. 15437 * (Charlson score=0 scores) - 1.70589 * (Charlson score=3-4 scores) + 6.21213 * Left internal obturator muscle thickness (cm) + 5.33641 * Urethral width (cm) - 6.38418 * Right anal sphincter thickness (cm). The EGA of the prediction model is illustrated in Figure 2C. The results indicated that all the predicted values were situated within either region A or B, with 92.9% falling within region A and 7.1% falling within region B.
The predictive and calibrated capacity of the prediction models in this study. Figure 2A shows the ROC curve of the prediction model of urinary continence after RARP. Figure 2B shows the calibration curve of the prediction model of urinary continence after RARP. Figure 2C shows the EGA of the predictive model of short-term sexual function score after RARP. Figure 2D shows the EGA of the predictive model of short-term sexual function score after RARP.
Baseline demographic, clinical and anatomical characteristics of the study patients.
| Characteristics | Urinary continence | Urinary incontinence | P value |
|---|---|---|---|
| Size | 83 | 544 | |
| Follow-up time (month) | 20.29 ± 18.37 | 25.38 ± 16.08 | 0.009 |
| Age (year) | 69.96 ± 7.33 | 66.87 ± 7.44 | <0.001 |
| BMI (kg/m2) | 24.33 ± 2.34 | 24.11 ± 2.70 | 0.483 |
| PSA (ng/mL) | 26.22 ± 48.51 | 23.07 ± 40.62 | 0.522 |
| Urethral width (cm) | 1.11 ± 0.29 | 1.07 ± 0.20 | 0.181 |
| Urethral wall thickness (cm) | 1.08 ± 0.16 | 1.07 ± 0.17 | 0.804 |
| Membranous urethral length (cm) | 0.97 ± 0.31 | 1.07 ± 0.35 | 0.016 |
| Membranous urethral angle (°) | 122.42 ± 11.72 | 120.83 ± 10.48 | 0.207 |
| Transverse width of the membranous urethra(cm) | 1.12 ± 0.18 | 1.10 ± 0.22 | 0.426 |
| fore-and-aft width of the membranous urethra(cm) | 1.11 ± 0.22 | 1.09 ± 0.22 | 0.598 |
| Sectional area of membranous urethra (cm2) | 0.99 ± 0.33 | 0.97 ± 0.37 | 0.557 |
| Membranous urethra volume (cm3) | 0.94 ± 0.39 | 1.02 ± 0.47 | 0.169 |
| Left internal obturator muscle thickness (cm) | 1.81 ± 0.30 | 1.84 ± 0.31 | 0.402 |
| Right internal obturator muscle thickness (cm) | 1.82 ± 0.31 | 1.87 ± 0.32 | 0.192 |
| Left anal levator muscle thickness (coronal plane) (cm) | 0.96 ± 0.24 | 0.93 ± 0.23 | 0.411 |
| Right anal levator muscle thickness (coronal plane) (cm) | 0.97 ± 0.23 | 0.95 ± 0.23 | 0.454 |
| Anal levator muscle thickness (axial plane) (cm) | 1.09 ± 0.20 | 1.05 ± 0.22 | 0.078 |
| Left anal sphincter thickness(cm) | 0.54 ± 0.14 | 0.54 ± 0.17 | 0.946 |
| Right anal sphincter thickness (cm) | 0.58 ± 0.32 | 0.53 ± 0.15 | 0.011 |
| Pubic symphysis-prostate apex length (cm) | 3.28 ± 0.70 | 3.07 ± 0.66 | 0.008 |
| Prostate volume (cm3) | 31.55 ± 14.99 | 32.26 ± 18.46 | 0.740 |
| No. preoperative cT stage (%) | 0.545 | ||
| <T2c | 50 (60.24%) | 318 (58.46%) | |
| T2c | 16 (19.28%) | 138 (25.37%) | |
| T3a | 5 (6.02%) | 25 (4.60%) | |
| T3b | 6 (7.23%) | 41 (7.54%) | |
| T4 | 6 (7.23%) | 22 (4.04%) | |
| No. pathological GS (%) | 0.069 | ||
| 6 | 6 (7.23%) | 82 (15.07%) | |
| 7 | 45 (54.22%) | 319 (58.64%) | |
| 8 | 14 (16.87%) | 75 (13.79%) | |
| 9 | 18 (21.69%) | 67 (12.32%) | |
| 10 | 0 (0.00%) | 1 (0.18%) | |
| No. Charlson score (%) | 0.482 | ||
| 0 | 34 (40.96%) | 220 (40.44%) | |
| 1-2 | 40 (48.19%) | 255 (46.88%) | |
| 3-4 | 6 (7.23%) | 60 (11.03%) | |
| ≥5 | 3 (3.61%) | 9 (1.65%) | |
| No. history of TURP surgery (%) | 0.005 | ||
| 0 | 71 (85.54%) | 515 (94.67%) | |
| 1 | 12 (14.46%) | 28 (5.15%) | |
| 3 | 0 (0.00%) | 1 (0.18%) |
Note: The results in the table are Middle + SD/N (%)
In the long-term follow-up population, univariate analysis revealed that age, baseline PSA, cT=T2c, cT=T3b, GS=7,8,9 points, Charlson score=1-2 points, PAL, left/right internal obturator muscle thickness, urethral width and left anal sphincter thickness were associated with sexual function scores in long-term QoL following RARP. A multivariate analysis revealed that age, cT=T2c, GS=7,8,9, Charlson score=1-2, Charlson score ≥ 5 and left anal sphincter thickness were significantly correlated with sexual function scores (Table 3 and Supplementary Table 3). All variables were subsequently subjected to LASSO regression analysis, which revealed that age, BMI, GS=6 points, GS=8 points, baseline PSA, cT=T2c, cT=T3b, history of TURP surgery=0, Charlson score=1-2 points, PAL, left internal obturator muscle thickness, urethral width, left/right anal sphincter thickness, urethral wall thickness, left anal levator muscle thickness (coronal plane), prostate volume and membranous urethra volume were potential predictors of sexual function scores. By utilizing potential predictor variables obtained through the aforementioned approaches, we derived a final predictive model formula: 10.09756 + 0.17601*BMI (kg/m2) - 0.00928*Baseline PSA (ng/ml) -1.23761*(cT <T2c) - 4.44128*(cT=T2c) + 0.02478*(cT=T3a) - 4.81480*(cT=T3b) + 8.34815*((age (year)/100) ^ 2) + 6.33283*(GS=6 points) + 0.29983*(GS=8 points) - 4.81519*(GS=9 points) + 3.39984*(Charlson score=0 points) + 0.55016*(Charlson score=3-4 points) - 1.27232*PAL (cm) + 2.85673*Left internal obturator muscle thickness (cm) -1.79791 * Right internal obturator muscle thickness (cm) + 4.91769*urethral width (cm) - 12.37772*Left anal sphincter thickness (cm) -2. 76090*right anal sphincter thickness (cm) - 4.16998*urethral wall thickness (cm) + 0.92614 * left anal levator muscle thickness (coronal plane) (cm) ^ -2) - 0.03124 * prostate volume (cm3) - 1.34870*membranous urethra volume (cm3). The EGA of the model revealed that the predicted values are distributed within regions A and B, with 94.5% falling within region A and the remaining 5.5% falling within region B (Figure 2D). The constructed prediction model exhibited excellent capacity for accurate predictions.
Discussion
The present study aimed to investigate postoperative urinary continence and sexual function outcomes following RARP and subsequently developed and validated predictive models. The results indicated that age, MUL, and PAL were significant predictors of urinary continence recovery after RARP. The probability of postoperative urinary continence recovery is inversely associated with age and PAL, whereas the magnitude of MUL is directly proportional. A prospective study conducted by Stanford et al. involving over 1200 men who underwent radical prostatectomy (RP) demonstrated a significant association between age and the rate of recovery from urinary continence. Specifically, patients younger than 60 years of age are more likely to recover from UI21. Multiple studies have incorporated MUL into predictive models for UI22-24, demonstrating an inverse relationship between the length of MUL and the duration of postoperative recovery required for urinary function24. Notably, patients who achieved urinary continence at 3 months postsurgery had shorter PAL lengths than did those who experienced continence issues (26.0 vs 28.1 mm, P<0.05)25. The results of these studies are in line with the findings of this study.
The present study also revealed that GS, anal sphincter thickness and history of TURP surgery were significant predictive factors for recovery from urinary continence following RARP. These factors have been documented in the literature, and the findings are consistent with those of this study. Tienza et al. reported that a patient's surgical history of TURP was an independent prognostic factor for postoperative UI (OR 6.13, CI 95% 1.86-20.18, P=0.003)26. Gupta et al. also reported a greater prevalence of UI among patients who underwent TURP than among those without prior TURP surgery27. However, with increasing age, the risk of both benign prostatic hyperplasia (BPH) and prostate cancer (PCa) increases. Concurrently, there is a growing trend in the number of patients with a history of TURP who undergo RARP27. Consequently, delineating the precise association between TURP surgery history and urinary control poses a significant challenge. Additionally, a retrospective study conducted by Palisaar et al., involving 4,028 patients, revealed that both cT and GS emerged as independent predictors of UI following RARP28.
Variable selection is a crucial issue in the development of prediction models, as the predictive performance of models is partially influenced by the variables incorporated in the models. In this study, we employed LASSO regression, a machine learning technique widely used for variable selection in academic research29. It imposes a penalty on the absolute size of regression coefficients by considering the tuning parameter λ. Consequently, LASSO regression effectively drives the coefficients of irrelevant variables toward zero, thereby facilitating automatic variable selection and promoting the interpretability of the model. Moreover, LASSO regression frequently avoids overfitting and results in better prediction performance than the random forest algorithm in sparse datasets30.
Univariate and multivariate analyses of anatomical factors considered in the predictive model of urinary continence.
| Characteristics | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| Membranous urethral length (cm) | 2.52 (1.19, 5.36) | 0.016 | 2.73 (1.18, 6.36) | 0.020 |
| Pubic symphysis-prostate apex length (cm) | 0.62 (0.44, 0.89) | 0.008 | 0.88 (0.59, 1.32) | 0.547 |
| Right anal sphincter thickness(cm) | 0.27 (0.09, 0.89) | 0.031 | 0.15 (0.04, 0.53) | 0.004 |
Note: The results in the table are ORs (95% CIs) and P values.
Univariate and multivariate analyses of anatomical factors considered in the predictive model of sexual function.
| Characteristics | Univariate analysis | P value | Multivariate analysis | P value |
|---|---|---|---|---|
| Short-term after RARP (≤12 months) | ||||
| Left internal obturator muscle thickness(cm) | 11.28 (4.18, 18.38) | 0.002 | 5.29 (-2.57, 13.16) | 0.188 |
| Urethral width (cm) | 11.62 (2.41, 20.83) | 0.014 | 6.54 (-3.03, 16.12) | 0.182 |
| Long-term after RARP (>12 months) | ||||
| Urethral width (cm) | 6.40 (0.10, 12.70) | 0.047 | 0.90 (-5.07, 6.86) | 0.769 |
| Left internal obturator muscle thickness(cm) | 6.27 (1.92, 10.63) | 0.005 | 3.03 (-2.39, 8.45) | 0.273 |
| Right internal obturator muscle thickness(cm) | 4.13 (0.03, 8.22) | 0.049 | -2.99 (-8.17, 2.18) | 0.258 |
| Left anal sphincter thickness(cm) | -9.39 (-17.41, -1.36) | 0.022 | -13.07 (-20.53, -5.62) | <0.001 |
| Pubic symphysis-prostate apex length (cm) | -2.49 (-4.45, -0.54) | 0.013 | -0.94 (-2.86, 0.98) | 0.340 |
Note: The results in the table are OR (95% CI) P values. The population was considered a short-term follow-up population and long-term follow-up population on the basis of a cut-off of 12 months after RARP.
For the constructed predictive model of urinary continence, this study integrated preoperative clinical baseline characteristics and MRI features. The AUC of the predictive model exceeded 0.7, indicating excellent predictive ability. Furthermore, the calibration curve demonstrated high levels of calibration and discrimination in the model. By incorporating preoperative clinical parameters, including the American Society of Anesthesiologists (ASA) grade and surgical experience, Collette et al. developed a predictive model for urinary continence31. Although it encompasses numerous preoperative clinical parameters, the model's AUC merely reached 0.61 or 0.60, with no consideration given to the impact of MRI features. The AUCs of the calibrated models developed by Pinkhasov et al. at 6, 12, and 24 months after RARP were 0.52, 0.52, and 0.76, respectively. Notably, the final models exhibited superior predictive power compared with any individual clinical variable32. None of the models constructed by these studies incorporated MRI parameters and had relatively low AUCs. Honda et al. attempted to develop a novel prediction model for urinary continence33, incorporating MUL and lift muscle thickness while disregarding the influence of clinical baseline features despite their inclusion of MRI characteristics. Additionally, a predictive model developed by Miyake et al. incorporated only the bladder neck angle and MUL and exhibited unsatisfactory predictive performance34. In this context, the present study effectively integrates clinical and MRI features to successfully develop and validate a predictive model for postoperative urinary control, thereby conferring significant advantages.
In terms of sexual function, among the variables ultimately used to construct the predictive model by screening relevant variables of the sexual function dimension score, BMI, cT, age, GS, Charlson score, obturator muscle thickness, urethral width, and anal sphincter thickness were common predictors of postoperative short-term and long-term QoL models. Haskins et al. developed a predictive model for postoperative sexual function and reported that men under the age of 60 years, with a normal BMI, without diabetes or hypertension, with nerve-sparing surgery, and with no history of smoking presented an increased likelihood of achieving functional erections following surgery35. A study conducted by Neumaier et al. also demonstrated a significant association between BMI> 30 kg/m2 (r <0.001) and age (r=0.011) and decreased sexual function after surgery36. In this study, univariate analysis revealed a negative correlation between age and short-term as well as long-term sexual function scores, with each one-year increase in age resulting in decreases of 0.5 (r=0.001) and 0.6 (r<0.001) points, respectively, while the multivariate results were 0.4 (r=0.023) and 0.6 (r<0.001) points, respectively. The observed differences, although small in magnitude, were statistically significant. Therefore, the significance of age and BMI in predicting sexual function is evident. In a study involving 643 patients aimed at constructing a nomogram for predicting sexual function one year after RARP, the authors reported a negative correlation between the Gleason score (r=0.0002) and the Charlson score (r=0.02) with sexual function37, which aligns with the findings of the present study. The present study also revealed a potential positive correlation between the thickness of the obturator internus muscle and the width of the urethra with respect to sexual function scores. However, this may be attributed to chance, and no relevant literature has been identified to date.
The present study has several notable strengths. This study integrated preoperative clinical baseline characteristics and MRI characteristics of patients undergoing RARP to predict postoperative urinary continence and sexual function, explored relevant predictors, and successfully constructed a predictive model with relatively superior predictive ability. This model enables the anticipation of postoperative urinary control and sexual function prior to RARP, thereby guiding postoperative recovery. However, there is a paucity of research in this specific domain. Furthermore, the prediction models constructed in this study integrated the variables selected through univariate/multivariate analysis and LASSO regression analysis, thereby enhancing their predictive ability. Therefore, this study has significant clinical implications.
This study has several limitations that should be taken into account. First, we excluded patients who underwent surgical procedures performed by a single surgeon. Despite the surgeon's extensive experience, variations in surgical practices inevitably arise. Second, pertinent data on radiotherapy and endocrine therapy were not included, which may have potentially affected the investigation of QoL in this study. The validation of the findings in this study calls for an increased sample size and more comprehensive data in the future.
Conclusion
Our study suggests that the predictive model incorporating preoperative clinical baseline characteristics and MRI characteristics enables the assessment of postoperative urinary control and sexual function in patients after RARP. These findings could offer valuable insights and clinical guidance for healthcare professionals and patients in the future.
Abbreviations
UI: Urinary incontinence; ED: Erectile dysfunction; RP: Radical prostatectomy; RARP: Robotic-assisted radical prostatectomy; PCa: prostate cancer; QoL: Quality of life; MRI: Magnetic resonance imaging; TURP: Transurethral resection of the prostate; cT: Clinical T-stage; GS: Gleason score; MUL: Membranous urethral length; PAL: Pubic symphysis-prostate apex length; BMI: Body mass index; PSA: Prostate-specific antigen; SD: Standard deviation; OR: Odds ratio; CI: Confidence interval; Lasso: Least absolute shrinkage and selection operator; AUC: Area under the ROC curve; EGA: Error grid analysis; ASA: American Society of Anesthesiologists.
Supplementary Material
Supplementary tables.
Acknowledgements
Funding
This work was supported by the National Key Research and Development Program of China (2022YFC3602902, 2022YFC3602901, and 2020YFC2008601), the National Natural Science Foundation of China (Grant No. 82160379), Programs from the Science and Technology Department of Sichuan Province (2023NSFSC1906 and 2021YJ0462), the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023LC007 and Z2024LC003), and the Postdoctoral Fellowship Program of CPSF (GZC20241158).
Ethics approval and consent to participate
This study was approved by the Ethics Committee of West China Hospital of Sichuan University (2021YS-1142). Written consent was obtained from each patient or the next of kin.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Author contributions
QW, SQ, ZHL, YGB, and LY participated in the design of the study. WYW, JBW, WJZ, XYS, BZ, SYZ, XYL and YMJ carried out the data analysis and acquisition. WYW, JBW, HXZ, ZLZ, JKL, LHD, and YMS were involved in data interpretation. WYW, JBW and XHZ wrote the original draft. All the authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249 doi:10.3322/caac.21660
2. Zhai Z, Zheng Y, Li N. et al. Incidence and disease burden of prostate cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer. 2020;126(9):1969-1978 doi:10.1002/cncr.32733
3. Wang F, Wang C, Xia H. et al. Burden of Prostate Cancer in China, 1990-2019: Findings From the 2019 Global Burden of Disease Study. Front Endocrinol (Lausanne). 2022;13:853623 doi:10.3389/fendo.2022.853623
4. Schaeffer EM, Srinivas S, Adra N. et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298 doi:10.6004/jnccn.2022.0063
5. Stolzenburg J-U, Holze S, Neuhaus P. et al. Robotic-assisted Versus Laparoscopic Surgery: Outcomes from the First Multicentre, Randomized, Patient-blinded Controlled Trial in Radical Prostatectomy (LAP-01). Eur Urol. 2021;79(6):750-759 doi:10.1016/j.eururo.2021.01.030
6. Hoffman KE, Penson DF, Zhao Z. et al. Patient-Reported Outcomes Through 5 Years for Active Surveillance, Surgery, Brachytherapy, or External Beam Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA. 2020;323(2):149-163 doi:10.1001/jama.2019.20675
7. Briganti A, Karnes RJ, Gandaglia G. et al. Natural history of surgically treated high-risk prostate cancer. Urol Oncol. 2015;33(4):163.e7-163.13 doi:10.1016/j.urolonc.2014.11.018
8. Muldoon MF, Barger SD, Flory JD, Manuck SB. What are quality of life measurements measuring? BMJ. 1998;316(7130):542-545
9. Schmidt S, Garin O, Pardo Y. et al. Assessing quality of life in patients with prostate cancer: a systematic and standardized comparison of available instruments. Qual Life Res. 2014;23(8):2169-2181 doi:10.1007/s11136-014-0678-8
10. Lam WWT, Tse MA, Ng CNL, Chung EKM, Fielding R. Psychometric Assessment of the Chinese Version of the Abbreviated Expanded Prostate Cancer Index Composite (EPIC-26) and the Clinical Practice Version (EPIC-CP) in Chinese Men With Prostate Cancer. J Pain Symptom Manage. 2017;53(6):1085-1090 doi:10.1016/j.jpainsymman.2017.02.010
11. O'Callaghan ME, Raymond E, Campbell J. et al. Tools for predicting patient-reported outcomes in prostate cancer patients undergoing radical prostatectomy: a systematic review of prognostic accuracy and validity. Prostate Cancer Prostatic Dis. 2017;20(4):378-388 doi:10.1038/pcan.2017.28
12. Mitsui Y, Sadahira T, Maruyama Y. et al. Impact of Sarcopenia on Erectile Function after Nerve-Sparing Robot-Assisted Radical Prostatectomy. World J Mens Health. 2021;39(4):673-682 doi:10.5534/wjmh.200036
13. Jeong SJ, Kim HJ, Kim JH. et al. Urinary continence after radical prostatectomy: predictive factors of recovery after 1 year of surgery. International Journal of Urology: Official Journal of the Japanese Urological Association. 2012;19(12):1091-1098 doi:10.1111/j.1442-2042.2012.03106.x
14. Jin D, Jin K, Chen B. et al. Complete androgen blockade vs. medical castration alone as adjuvant androgen deprivation therapy for prostate cancer patients following radical prostatectomy: a retrospective cohort study. Chinese Medical Journal. 2022;135(7):820-827 doi:10.1097/cm9.0000000000002021
15. Mathew G, Agha R, Albrecht J. et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. 2021;96:106165 doi:10.1016/j.ijsu.2021.106165
16. Bannay A, Chaignot C, Blotière P-O. et al. The Best Use of the Charlson Comorbidity Index With Electronic Health Care Database to Predict Mortality. Med Care. 2016;54(2):188-194 doi:10.1097/MLR.0000000000000471
17. Daniels J, Herrero P, Georgiou P. A Multitask Learning Approach to Personalized Blood Glucose Prediction. IEEE J Biomed Health Inform. 2022;26(1):436-445 doi:10.1109/JBHI.2021.3100558
18. Boettcher C, Dost A, Wudy SA. et al. Accuracy of blood glucose meters for self-monitoring affects glucose control and hypoglycemia rate in children and adolescents with type 1 diabetes. Diabetes Technol Ther. 2015;17(4):275-282 doi:10.1089/dia.2014.0262
19. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148
20. Howard LA, Lidbury JA, Jeffery N, Washburn SE, Patterson CA. Evaluation of a flash glucose monitoring system in nondiabetic dogs with rapidly changing blood glucose concentrations. J Vet Intern Med. 2021;35(6):2628-2635 doi:10.1111/jvim.16273
21. Stanford JL, Feng Z, Hamilton AS. et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354-360
22. Jeong SJ, Yeon JS, Lee JK. et al. Development and validation of nomograms to predict the recovery of urinary continence after radical prostatectomy: comparisons between immediate, early, and late continence. World J Urol. 2014;32(2):437-444 doi:10.1007/s00345-013-1127-y
23. Matsushita K, Kent MT, Vickers AJ. et al. Preoperative predictive model of recovery of urinary continence after radical prostatectomy. BJU Int. 2015;116(4):577-583 doi:10.1111/bju.13087
24. Grivas N, van der Roest R, Schouten D. et al. Quantitative assessment of fascia preservation improves the prediction of membranous urethral length and inner levator distance on continence outcome after robot-assisted radical prostatectomy. Neurourol Urodyn. 2018;37(1):417-425 doi:10.1002/nau.23318
25. Fukui S, Kagebayashi Y, Iemura Y, Matsumura Y, Samma S. Preoperative MRI Parameters Predict Urinary Continence after Robot-Assisted Laparoscopic Prostatectomy in Prostatic Cancer Patients. Diagnostics (Basel). 2019;9(3):102 doi:10.3390/diagnostics9030102
26. Tienza A, Robles JE, Hevia M, Algarra R, Diez-Caballero F, Pascual JI. Prevalence analysis of urinary incontinence after radical prostatectomy and influential preoperative factors in a single institution. Aging Male. 2018;21(1):24-30 doi:10.1080/13685538.2017.1369944
27. Gupta NP, Singh P, Nayyar R. Outcomes of robot-assisted radical prostatectomy in men with previous transurethral resection of prostate. BJU Int. 2011;108(9):1501-1505 doi:10.1111/j.1464-410X.2011.10113.x
28. Palisaar JR, Roghmann F, Brock M, Löppenberg B, Noldus J, von Bodman C. Predictors of short-term recovery of urinary continence after radical prostatectomy. World J Urol. 2015;33(6):771-779 doi:10.1007/s00345-014-1340-3
29. Zhu G, Wen Y, Cao K, He S, Wang T. A review of common statistical methods for dealing with multiple pollutant mixtures and multiple exposures. Front Public Health. 2024;12:1377685 doi:10.3389/fpubh.2024.1377685
30. Wei H, Sun J, Shan W. et al. Environmental chemical exposure dynamics and machine learning-based prediction of diabetes mellitus. Sci Total Environ. 2022;806(Pt 2):150674 doi:10.1016/j.scitotenv.2021.150674
31. Collette ERP, Klaver SO, Lissenberg-Witte BI, van den Ouden D, van Moorselaar RJA, Vis AN. Patient reported outcome measures concerning urinary incontinence after robot assisted radical prostatectomy: development and validation of an online prediction model using clinical parameters, lower urinary tract symptoms and surgical experience. J Robot Surg. 2021;15(4):593-602 doi:10.1007/s11701-020-01145-9
32. Pinkhasov RM, Lee T, Huang R. et al. Prediction of Incontinence after Robot-Assisted Radical Prostatectomy: Development and Validation of a 24-Month Incontinence Nomogram. Cancers (Basel). 2022;14(7):1644 doi:10.3390/cancers14071644
33. Honda M, Kawamoto B, Morizane S. et al. A prognostic model for predicting urinary incontinence after robot-assisted radical prostatectomy. Int J Med Robot. 2017 13(3). doi:10.1002/rcs.1780
34. Ito T, Watanabe K, Matsushita Y. et al. Impact of Bladder Neck Angle Measured by Postoperative Magnetic Resonance Imaging on Midterm Recovery of Urinary Continence in Prostate Cancer Patients Undergoing Robot-Assisted Radical Prostatectomy. J Endourol. 2021;35(11):1610-1615 doi:10.1089/end.2021.0071
35. Haskins AE, Han PKJ, Lucas FL, Bristol I, Hansen M. Development of clinical models for predicting erectile function after localized prostate cancer treatment. International Journal of Urology: Official Journal of the Japanese Urological Association. 2014;21(12):1227-1233 doi:10.1111/iju.12566
36. Neumaier MF, Segall CH, Hisano M, Rocha FET, Arap S, Arap MA. Factors affecting urinary continence and sexual potency recovery after robotic-assisted radical prostatectomy. Int Braz J Urol. 2019;45(4):703-712 doi:10.1590/S1677-5538.IBJU.2018.0704
37. Cozzi G, Musi G, Monturano M. et al. Sexual function recovery after robot-assisted radical prostatectomy: Outcomes from an Italian referral centre and predicting nomogram. Andrologia. 2019;51(10):e13385 doi:10.1111/and.13385
Author contact
![]() Corresponding author: Shi Qiu,MD. Department of Urology, Institute of Urology and Center of Biomedical Big Data, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China qiushiedu.cn.; Qiang Wei, MD. Department of Urology, Institute of Urology and Center of Biomedical Big Data, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China wq933com.
Corresponding author: Shi Qiu,MD. Department of Urology, Institute of Urology and Center of Biomedical Big Data, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China qiushiedu.cn.; Qiang Wei, MD. Department of Urology, Institute of Urology and Center of Biomedical Big Data, West China Hospital, Sichuan University, Chengdu, Sichuan 610041, China wq933com.

 Global reach, higher impact
Global reach, higher impact