3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(14):2824-2836. doi:10.7150/ijms.101865 This issue Cite
Research Paper
Abnormal miR-122-5p expression in decidual NK cells and its impact on trophoblast behavior: insights into unexplained recurrent pregnancy loss
1. Center of Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children of Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, 610041, China.
2. Department of Andrology/Sichuan Human Sperm Bank, West China Second University Hospital, Sichuan University, Chengdu, 610041, China.
Received 2024-8-3; Accepted 2024-10-10; Published 2024-10-28
Abstract
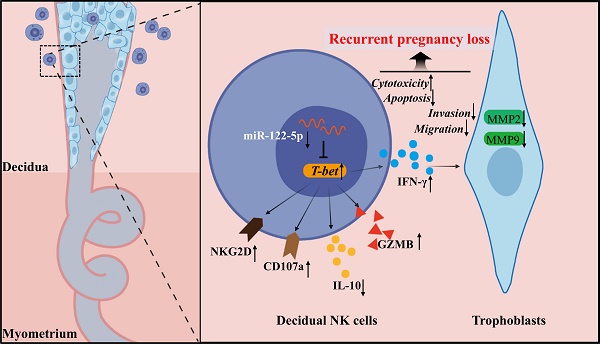
In the early stages of pregnancy, the maternal-fetal interface is enriched with natural killer (NK) cells that release growth factors to support fetal development and promote the remodeling of uterine spiral arteries. Previous studies have shown that the aberrant frequency and activity of decidual natural killer (dNK) cells are associated with recurrent pregnancy loss (RPL). Various factors regulate the roles of dNK cells and their interactions with trophoblasts to facilitate the colonization and maturation of semiallogeneic embryos. However, knowing precise molecular mechanisms involved in this requires further investigation. Earlier studies revealed that microRNAs (miRNAs) play a significant role in regulating the functions of decidual stromal and trophoblast cells. Although there are few studies on the intervention of malfunctioning dNK cells, this strategy shows promise in regulating abnormal miRNA production in NK cells. This study confirmed miR-122-5p downregulation in dNK cells from patients experiencing unexplained RPL. miR-122-5p regulates apoptosis, inflammatory factor secretion, and cytotoxicity of NK cells. miR-122-5p may contribute to immune tolerance at the maternal-fetal interface by targeting transcription factor T-bet. This study provides a deeper understanding of the mechanisms by which miR-122-5p regulates the function of dNK cells and trophoblasts at the maternal-fetal interface to ensure successful pregnancy.
Keywords: recurrent pregnancy loss, maternal-fetal interface, decidual natural killer cells, miR-122-5p, trophoblast
Introduction
Recurrent pregnancy loss (RPL) is defined as the occurrence of two or more pregnancy losses before 24 weeks of pregnancy, causing significant sorrow to many families globally. Fifty percent of RPL instances lacking a definitive reason were categorized as unexplained RPL (URPL) [1]. The prevention and treatment of RPL pose a pressing challenge for medical professionals specializing in reproductive medicine. Pregnancy is a unique immune phenomenon wherein the mother coexists harmoniously with the semiallogenic fetus [2]. Decidual immune cells (DICs), predominantly decidual natural killer (dNK) cells (approximately 70%), T cells, macrophages, and dendritic cells, are abundant at the maternal-fetal interface. Their interactions with trophoblast cells produced from the conceptus efficiently inhibit harmful immune responses to paternally transmitted alloantigens and confer unique features that differ from their counterparts in peripheral blood [3-5]. These cells exhibit intense residency, secrete numerous cytokines, angiogenic factors, and growth factors, and play crucial roles in decidual transformation, uterine vascular adaptation, and process of placentation [6-9]. DICs play a vital role in establishing the ability to receive, initiating pregnancy at the embryonic stage, and producing growth factors necessary for maintaining the fetus. Multiple studies have suggested that deficits or abnormal activation states in dNK cells contribute to pathophysiological processes leading to unfavorable prenatal outcomes and pregnancy complications [9-11]. The potential of diagnosing and treating aberrant dNK cells shows promise despite the lack of research in this field.
MicroRNAs (miRNAs) are tiny noncoding RNAs with a negative regulatory role in gene expression and have been implicated in the development and advancement of many diseases [12]. More than 400 miRNAs have been identified in NK cells, indicating their involvement in regulating cellular function [13]. Cichocki et al. revealed that miR-181 expression significantly affects the development of human NK cells from CD34+ hematopoietic progenitor cells and the production of primary CD56+ NK cell-derived interferon-γ (IFN-γ) [14]. Increased miR-146a expression has been observed in NK cells of patients with chronic hepatitis B and hepatocellular carcinoma (HCC) [15]. miR-362-5p also improves the performance of NK cells by specifically targeting cylindromatosis, which negatively regulates the nuclear factor-κB signaling pathway. Therefore, manipulating NK cell function by modulating miRNA expression represents a potential strategy for modulating the local immune microenvironment.
This study revealed a substantial decrease in miR-122-5p expression in dNK cells from patients with URPL. The regulatory impact of miR-122-5p on NK cells was determined via in vitro experiments, finally revealing the existence of the miR-122-5p/T-bet/IFN-γ pathway in NK cells, which might have a crucial function at the interface between the mother and the fetus.
Materials and methods
Specimen collection and ethical permission
Decidua samples from the healthy control (n = 13, HC) group and the unexplained pregnancy loss (n = 15, URPL) group were collected from the West China Second Hospital of Sichuan University from November 2021 to May 2022 (baseline characteristics of the population are listed in Supplementary Table S1). The exclusion criteria included pregnant women with (a) autoimmune disease; (b) chromosomal diseases such as gene-linked defection and abnormal embryo chromosomes; (c) bacterial, fungal, or viral infection such as hepatitis B or syphilis; (d) abnormalities of physiological structures such as genital tract malformations; (e) abnormalities in endocrine hormone levels; and (f) organ transplantation or blood transfusion. Fresh tissue samples were collected under aseptic conditions and transferred to the laboratory for processing as soon as possible. Peripheral blood mononuclear cells (PBMCs) for NK cell expansion were collected from 10 healthy women of childbearing age; the exclusion criteria for volunteers were consistent with those described above. The Ethics Committee of West China Second Hospital of Sichuan University approved the study protocol [Approval number: Medical Research 2020 (029)]. All human specimens were processed in a biosafety cabinet following the guidelines of West China Second Hospital of Sichuan University.
Isolation of immunocytes from decidua and peripheral blood
Human decidual tissue was collected from the operating room, and the appropriate phosphate buffer solution (PBS) containing penicillin-streptomycin (PS, Gibco, USA) was added. After cleaning with PBS, surgical scissors were cut into tissue blocks of 1-3 mm3. Then, 1 mg/ mL collagenase IV (Sigma, United States) and 150 U/mL DNase I (Applichem, Germany) were added to a total volume of 10 mL for 40 min at 37 ℃ with gentle agitation. The 2 mL fetal bovine serum (FBS, Hyclone, USA) was used to terminate the digestion, and the suspension was filtered successively through 100 μm and 70 μm filters. The filtered suspension was centrifuged at 500×g for 10 min, the upper liquid was discarded, and the lower cells were resuspended in a complete medium. Percoll solution (Pharmacia, Sweden) with a concentration gradient of 20%, 40%, and 60% were prepared and successively added to the centrifuge tube. The suspension was added to the upper layer and centrifuged at 500×g for 20 min to draw 40-60% white membrane layer cells.
Density centrifugation was performed to isolate PBMCs using Ficoll-Hypaque (TBD Science, Tianjin, China) according to the manufacturer's protocol. Peripheral blood supplemented with heparin lithium was diluted with an appropriate volume of PBS. Second, the same volume of Ficoll gradient was used, and the mixture was centrifuged at 500×g for 20 min. A pasteur pipette was used to harvest the PBMCs, after which the PBMCs were washed with PBS.
Flow cytometric analysis
Cells were washed and prepared as a single-cell suspension at a concentration of 2×106 cells/mL. Then, 100 μL aliquots of the samples were added to a round-bottom tube (Becton Dickinson, USA). Next, dead/live cells were stained using the Zombie Aqua fixable viability kit (BioLegend, USA) and then blocked with 5 μL Fc-Block (BioLegend, USA) at room temperature for 30 min. For the intracellular cytokines, 2 μL Cell Activation Cocktail (BioLegend, USA), brefeldin A (BioLegend, USA), and monensin (BioLegend, USA) be added to each round bottom tube for incubation at 37°C for 4 h. Before the staining of intracellular cytokines and lytic granules, the cells had been fixed and permeabilized for 30 min. The 3 μL monoclonal antibody was added and incubated at room temperature for 30 min. The gating strategy is shown in supplementary Figure S1, and all the antibodies used are listed in Table 1.
List of antibodies.
| Antibodies | Fluorochrome | Clone | Dilution | Sources |
|---|---|---|---|---|
| anti-CD3 | FITC | UCHT1 | 1:100 | BioLegend |
| anti-CD45 | APC-cy7 | HI30 | 1:100 | BioLegend |
| anti-CD49a | APC | TS2/7 | 1:100 | BioLegend |
| anti-CD56 | PE-cy7 | HCD56 | 1:100 | B&D |
| anti-CD69 | FITC | FN50 | 1:100 | BioLegend |
| anti-CD16 | BV421 | 3GB | 1:100 | BioLegend |
| anti-NKG2D | BV605 | 1D11 | 1:100 | BioLegend |
| anti-Granzyme B | BV421 | QA18A28 | 1:50 | BioLegend |
| anti-Perforin | FITC | dG9 | 1:50 | BioLegend |
| anti-Granulysin | APC | DH2 | 1:50 | BioLegend |
| anti-TNF-α | APC | MAb11 | 1:50 | BioLegend |
| anti-IFN-γ | FITC | B27 | 1:50 | BioLegend |
| anti-IL17A | BV605 | BL168 | 1:50 | BioLegend |
| anti-IL10 | BV421 | JES3-9D7 | 1:50 | BioLegend |
| anti-CD107a | BV421 | H4A3 | 1:50 | BioLegend |
| anti-Ki67 | BV421 | Ki-67 | 1:50 | BioLegend |
| anti-7AAD | FITC | - | 1:100 | BioLegend |
| Annexin V | PE | - | 1:100 | BioLegend |
| Propidium Iodide | PE | - | 1:100 | Thermo Fisher |
| Dioc | FITC | - | 1:500 | Thermo Fisher |
| Zombie AquaTM | BV510 | - | 1:500 | BioLegend |
Cell culture
PBMCs for NK cell expansion were collected from the peripheral blood of healthy fertile female volunteers as described previously. We utilized a natural killer cell culture kit plus (NK-KITP, DAKEWE) to perform the NK cell culture. The process involved adding a medium and recording the NK cell purity according to the manufacturer's protocol. The cells were cultured in 6-well plates at an initial density of 1.5×106/mL in a humid atmosphere with 5% CO2 at 37°C. To induce dNK-like cells, TGF-β1 (5 ng/mL, 240-B, R&D, USA), hCG (15 IU/mL, Lizhu, Zhuhai, China), and IL-15 (10 ng/mL, AF-200-15, PeproTech, USA) were added to the medium on the 7th, 9th, and 11th days of the process. At the end of day 14, the cells were harvested, and negative selection was performed based on the purity of the cultured NK cells.
The K562 cells and HTR-8/SVneo cell lines (Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences, China) were grown and maintained in RPMI-1640 medium supplemented with 10% FBS and 1% PS (Gibco, USA) in a 5% CO2 humid atmosphere at 37°C. Medium replacement and cell passage were performed according to cell density observed under the microscope.
Purification of NK cells
NK cells were purified via negative selection with a magnetic-activated cell sorter (MACS, Miltenyi Biotec) according to the manufacturer's protocol. The purity of the NK cell population obtained was over 90%, as confirmed by flow cytometry.
Transfection of NK cells
NK cells were transfected with miR-122-5p mimic (double-stranded RNA), inhibitor (single-stranded RNA), and their negative control (Ribo, Guangzhou) using RiboFECT™CP or Celetrix (DAKEWE, 560 V, 10 ms). Then, the electroporated NK cells were subjected to further experimentation after a rest period of 24 hours. A dead cell removal kit (Miltenyi Biotechnology Incorporation, Germany) was used to remove the dead cells according to the manufacturer's instructions.
Proliferation potential assay
The proliferation potential of NK cells was analyzed by Ki-67 staining. 2 mL of pre-cooled eBioscienceTM Fixation Permeabilization (BD Biosciences) was added into NK cells and incubated for 1 h. The cells were washed with PBS and resuspended in 100 μL PBS. Then, 2 μL of anti-human Ki-67 antibody was added and incubated for 1 h. The cells were resuspended in 100 μL of PBS. The percentage of Ki-67+ NK cells was analyzed using flow cytometry.
Apoptosis assay
The apoptosis of the transfected NK cells was evaluated via Annexin V staining. Annexin V (2.5 μL) and 7-AAD (1.5 μL) antibodies were added to the resuspended cells (1×106/mL, 100 μL) for 30 min. Then, 300 μL of binding buffer (BioLegend, USA) was added to the stained NK cells, and the percentage of apoptotic cells (Annexin V+) was determined using flow cytometry.
Cytotoxicity assay
First, transfected NK cells (1×106/mL) were cocultured with targeted tumor cells (K562) at an effector-to-target (E: T) ratio of 10:1 in 200 µL of relevant culture medium in a 5% CO2 at 37°C for 4 h. After centrifuging at 300×g for 10 min, the cells were resuspended in 100 µL PBS. The degranulation of NK cells was assessed by CD107a staining. Dead K562 cells were analyzed by PI staining, while Dioc was used to stain NK cells.
ELISA
Transfected NK cells from different treatment groups were centrifuged at 300×g for 10 min to harvest the supernatant. The secretion of IFN-γ, TNF-α, IL-17A, IL-10, granzyme B, perforin, and granulysin in the medium supernatants was measured with ELISA kits (Ruixinbio Quanzhou, China) following the manufacturer's protocol.
RNA isolation, cDNA synthesis, and RT-qPCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, USA) under RNAase-free conditions as per the manufacturer's protocol. Reverse transcription of the miRNAs was performed using the All-in-One™ miRNA First-Strand cDNA Synthesis Kit (GeneCopoeia, USA). An All-in-One™ miRNA RT-qPCR Detection Kit (GeneCopoeia, USA) was used to quantify the expression of miR-122-5p (HmiRQP0274). Reverse transcription of mRNAs was completed using an Evo M-MLV RT kit with gDNA removed with a qPCR II kit (AG111728, Accurate Biotechnology, Hunan, China). HsnoRNA-U48 (HmiRQP9021) and GAPDH served as controls. RT-qPCR was carried out using 2×SYBR®Green Pro Taq HS Premix (AG11701, Accurate Biotechnology, Hunan, China), and the results were analyzed via the qTOWER3G Analytik system (Jena, Germany). The sequences of primer used are listed in Table 2.
RT-qPCR primers
| Primer | Sequence |
|---|---|
| GAPDH-F | 5'-ATGACATCAAGAAGGTGGTG-3' |
| GAPDH-R | 5'-CATACCAGGAAATGAGCTTG-3' |
| TBX21-F | 5'-AACCACCTGTTGTGGTCCAA-3' |
| TBX21-R | 5'-CCCGGCCACAGTAAATGACA-3' |
| IFNG-F | 5'-AGTGATGGCTGAACTGTCGC-3' |
| IFNG-R | 5'-CTGGGATGCTCTTCGACCTC-3' |
| EOMES-F | 5'-TCACCAATAACAAAGGCGCAAAT-3' |
| EOMES-R | 5'-CACGCCATCCTCTGTAACTTCAA-3' |
| Tcf1-F | 5'-CACTCCCATGAAGACGCAGAAG-3' |
| Tcf1-R | 5'-CCTTCTTGGTTGGTAGCTCATCA-3' |
| GATA3-F | 5'-GTGAACTGTGGGGCAACCTC-3' |
| GATA3-R | 5'-GGTCTGACAGTTCGCACAGG-3' |
Dual-luciferase reporter assay
A luciferase reporter assay was used to confirm the binding sites for hsa-miR-122-5p in the 3' UTR of T-bet mRNA. The T-bet 3' UTR of the wildtype and mutant sequence was inserted into the pmiR-RB-REPORTTM (Ruibo, Guangzhou, China), and activated NK cells harboring the mutant or wild-type 3' UTR were cotransfected with the hsa-miR-122-5p or NC mimic by electroporation. Transfected NK cells were collected after a 24-hour rest period, and the supernatant was discarded after centrifugation at 300×g for 10 min. 200 µL of lysate was added and centrifuged at 5000×g for 5 min. 100µL of the collected supernatant was added to the microplate, and then the equal volume of firefly and renilla luciferase detection reagent was added in turn to gently mix. Finally, firefly and renilla luciferase activities were measured using the Dual-Glo Luciferase Assay System (Promega, Italy).
Migration and invasion assays
The trophoblast cell line HTR-8/SVneo was cultured in RPMI medium supplemented with 10% FBS and 1% PS. The cells were grown in a 5% CO2 humidified atmosphere at 37°C. Transwell plates with a filter membrane aperture of 8 μm were used to establish an indirect coculture system for transfected NK cells and HTR-8/SVneo cells. 1×105 HTR-8/SVneo cells were inoculated in a 200 µL of medium free of FBS in the upper compartment, and transfected NK cells (2×105/mL) were inoculated in a 1 mL medium containing 10% FBS in the lower compartment. For invasion experiments, a matrix gel was used (50 µL), but not for migration experiments. After coculturing at 37°C, 5% CO2 overnight, the upper chamber was removed, and the migrated or invasive cells were fixed with formaldehyde, stained with crystal violet, and finally viewed under a microscope. In the rescue experiment, the human IFN-γ blocking antibody (10 μg/mL, 502509, BioLegend, USA) was added to the medium.
Western blot analysis
Proteins were extracted from DICs and NK cells using RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with 1% PMSF (Beyotime, Shanghai, China), after which the protein concentration was detected using a BCA Protein Assay Kit (Beyotime, Shanghai, China). The protein samples were resolved via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS‒PAGE) and electroblotted onto polyvinylidene difluoride membranes (Millipore, USA). The blotted membrane was blocked with blocking buffer (Beyotime Shanghai, China) for 1 h at room temperature, followed by incubation with primary antibodies at 4°C overnight. The primary antibodies used were as follows: MMP2 (A19080; ABclonal, Wuhan, China), MMP9 (ET1704-69; Huabio, Hangzhou, China), T-bet (13700-1-AP; ProteinTech, Wuhan, China), IFN-γ (15365-1-AP; ProteinTech, Wuhan, China), and GAPDH (60004-1-Ig; ProteinTech, Wuhan, China). Later, the blotted membranes were rinsed in TBST buffer and then incubated with the secondary antibody HRP-conjugated anti-rabbit/mouse IgG for 1 h. Finally, the bands were detected by an enhanced ECL chemiluminescent substrate kit (Beyotime Shanghai, China), and the relative fluorescence intensity was quantified using ImageJ software.
Statistical analysis
The statistical analysis was performed with GraphPad Prism version 8.0 (GraphPad Software, USA). First, the normality of the distribution of the data was evaluated. The results are expressed as the mean ± S.E.M. Intergroup differences were examined by either unpaired t-tests or the Mann-Whitney U test based on the normality test, and differences were considered significant when the P value < 0.05.
Results
miR-122-5p is downregulated in dNK cells of patients with URPL
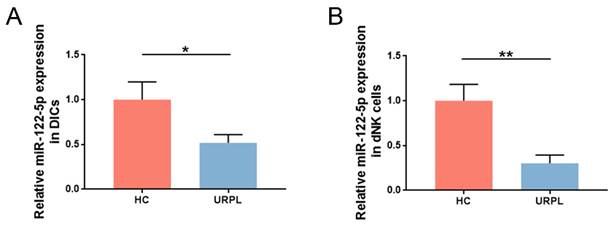
Earlier RNA-sequencing results (NCBI Bioproject ID: PRJNA813430) showed that miR-122-5p expression in DICs was lower in patients with URPL than in those with normal pregnancy [16]. DICs and dNK cells were extracted from the decidua, and miR-122-5p expression was measured using RT-qPCR. Comparative studies were aligned with sequencing findings (P= 0.04; Figure 1A). Considering the high frequency of dNK cells at the maternal-fetal interface in the early stages of pregnancy, miR-122-5p expression in dNK cells from the URPL and HC groups was examined. A significant reduction in miR-122-5p expression was observed in the URPL group compared with the HC group (P= 0.009; Figure 1B), indicating that the miR-122-5p deficit in dNK cells may play a role in the URPL development.
miR-122-5p expression in dNK cells was determined in the HC group and the URPL group. (A) RT-qPCR was performed to determine the difference in miR-122-5p expression in DICs between the HC group (n = 10) and the URPL group (n = 10). *P< 0.05. (B) RT-qPCR was performed to determine the difference in miR-122-5p expression in dNK cells between the HC and URPL groups (n = 5). **P< 0.01. HC, healthy control. URPL, unexplained recurrent pregnancy loss.
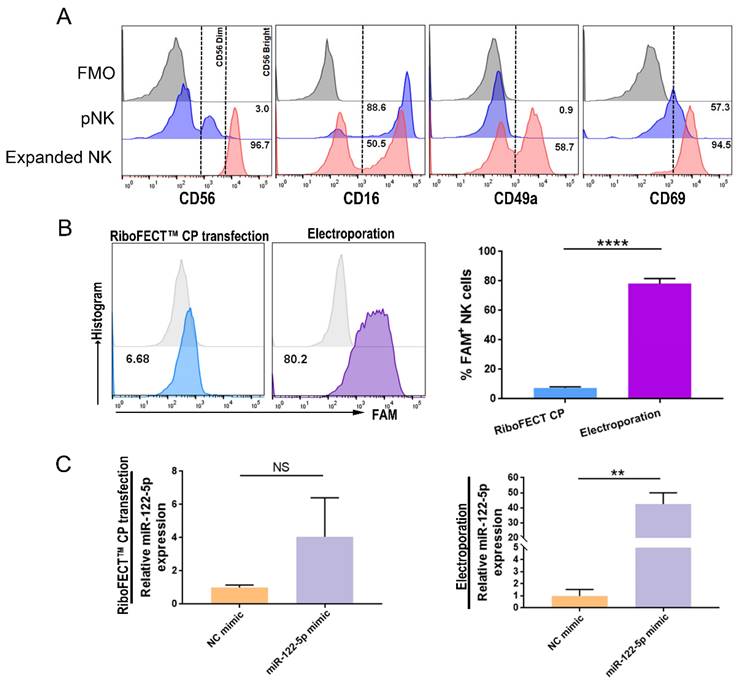
miR-122-5p overexpression or knockdown in NK cells
Grown NK cells had a phenotype similar to that of decidua, which was characterized by higher expression of the activation marker CD69 and the resident marker CD49a (Figure 2A). The transfection efficacy of RiboFECT™ CP reagent-driven transfection and electroporation was assessed using flow cytometry (P< 0.0001; Figure 2B). Results indicated that electroporation of a miR-122-5p mimic resulted in an approximately 48-fold elevation in the miR-122-5p expression in NK cells (P= 0.26; P= 0.005; Figure 2C).
Regulation of activation and apoptosis of NK cells by miR-122-5p
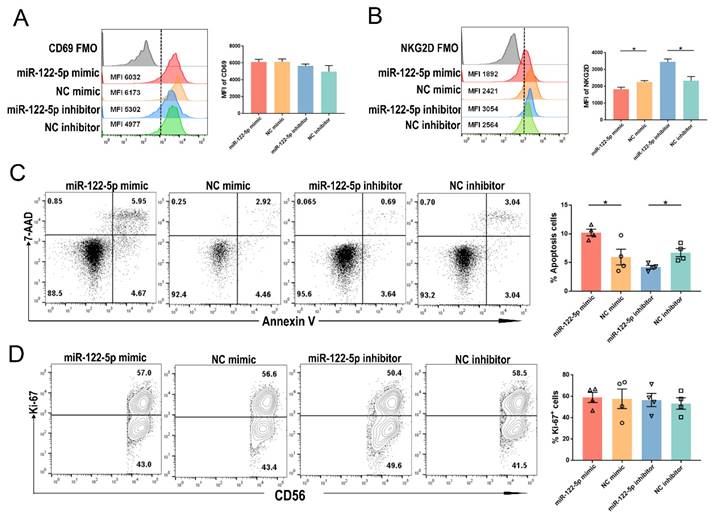
The URPL group showed a higher percentage of CD69+ NK cells than the HC group (P= 0.03; Supplementary Figure S2A). The experiment involved introducing a miR-122-5p mimic (inhibitor) or control into cells to investigate the impact of miR-122-5p on the biological functions of NK cells. NK cells transfected with miR-122-5p inhibitor (mimic) exhibited a higher (lower) mean fluorescence intensity (MFI) of NKG2D compared to the control group (P= 0.02; P= 0.04). However, no significant changes were observed in the MFI of CD69 between the mimic (inhibitor) and control groups (P= 0.39; P= 0.94; Figure 3A and 3B). Flow cytometric analysis revealed an increased (decreased) apoptosis rate in NK cells in the mimic (inhibitor) group compared to the control group (P= 0.02; P= 0.01; Figure 3C). Nevertheless, there was no significant difference in the percentage of Ki-67+ NK cell proliferation potential across the four groups with varying miR-122-5p expression (P= 0.90; P= 0.70; Figure 3D).
miR-122-5p regulates inflammation and cytotoxicity in NK cells
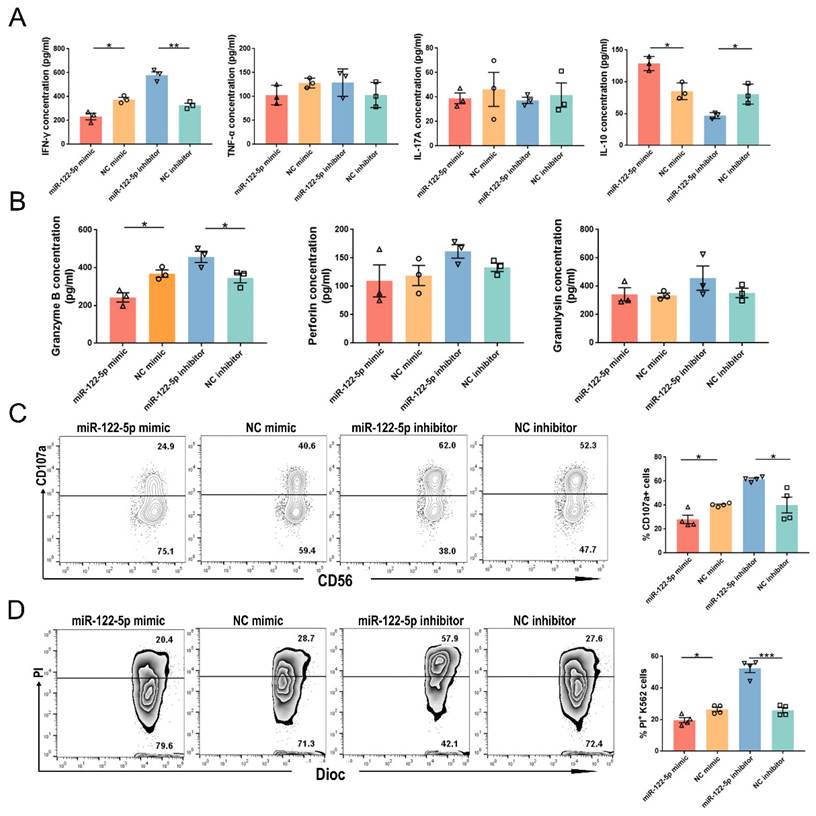
The release of inflammatory factors and lytic granules is essential for the proper functioning of dNK cells. Flow cytometry analysis showed that the URPL group had a higher proportion of IFN-γ+ and interleukin (IL)-17A+ NK cells than the HC group (P= 0.009; P= 0.01; Supplementary Figure S3). The URPL group had a lower frequency of IL-10+ NK cells (P= 0.008). The involvement of miR-122-5p in the regulation of cytokine secretion was confirmed using ELISA. After treatment with the miR-122-5p mimic (inhibitor), along with a reduction (elevation) in IFN-γ secretion (P= 0.01; P= 0.001), there was an elevation (reduction) in IL-10 secretion (P= 0.01; P= 0.02) by NK cells. However, no discernable IL-17A or TNF-α secretion alterations were detected (P> 0.05; Figure 4A).
miR-122-5p overexpression and knockdown in expanded NK cells. (A) The percentage of CD56bright+, CD16+, CD49a+, and CD69+ expanded NK cells were analyzed via flow cytometry. (B) The efficiency of RiboFECTTM and electroporation transfection were determined by flow cytometry (n = 4). ****P< 0.0001. (C) RT-qPCR was performed to determine the miR-122-5p expression in transfected NK cells after treatment with the miR-122-5p mimic or NC mimic (n = 3). **P< 0.01. pNK, peripheral natural killer cell. NC, negative control. NS, no significance. FMO, fluorescence minus one.
NK cells treated with the miR-122-5p mimic (inhibitor) showed a reduction (increase) in the granzyme B secretion (P= 0.01; P= 0.04; Figure 4B). There were no noticeable disparities in the perforin or granulysin production (P> 0.05). Alteration of lytic granules corresponded to the variation in dNK cells of patients with URPL (Supplementary Figure S4). Ultimately, NK cell cytotoxicity was observed after treatment with miR-122-5p mimic or inhibitor. The percentages of CD107a+ NK cells (P= 0.01; P= 0.01) and PI+ K562 cells (P= 0.01; P= 0.0002) declined (increased) in the miR-122-5p mimic (inhibitor) transfection group after effective cell-target cell coculture (Figure 4C and 4D).
miR-122-5p regulates IFN-γ in NK cells by targeting T-bet
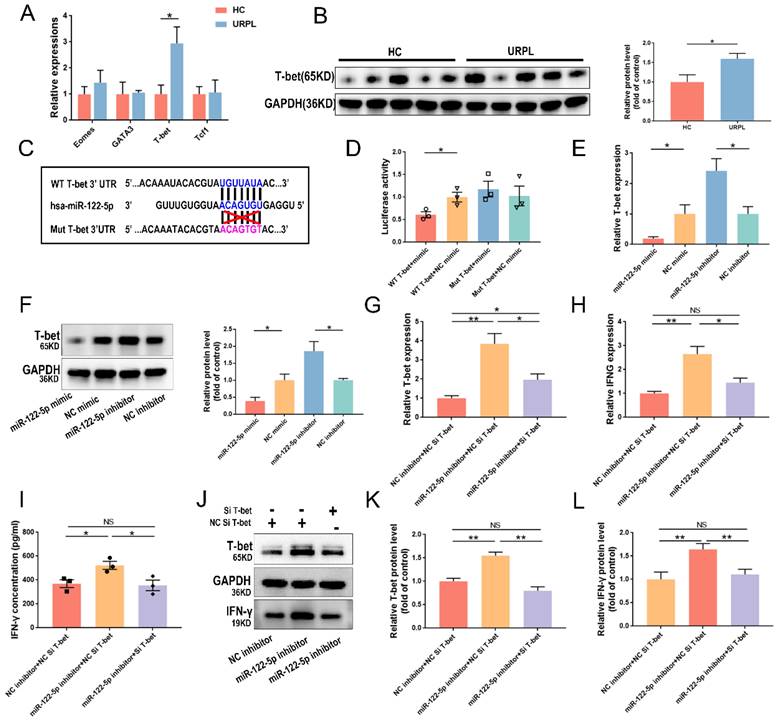
T-bet, Eomes, GATA3, and Tcf1 were selected as target genes as predicted by the RNAhybrid database. The upregulation of T-bet mRNA expression in dNK cells from patients with URPL was verified using RT-qPCR (P= 0.02; Figure 5A). The T-bet protein level increased significantly in patients with URPL, and it was inversely related to miR-122-5p expression in dNK cells (P= 0.03; Figure 5B). To understand this further, wild-type and mutant plasmid vectors for T-bet were created using the sequence predicted by RNAhybrid database (Figure 5C). The dual-luciferase reporter experiment provided evidence of the interaction between miR-122-5p and T-bet (Figure 5D). Results of subsequent experiments showed that miR-122-5p can negatively regulate the mRNA (P= 0.03; P= 0.02) and protein expression of T-bet. (P= 0.04; P= 0.03) (Figure 5E and 5F).
Efficacy of T-bet silencing and its influence on IFN-γ in NK cells following T-bet siRNA treatment was evaluated (P< 0.05). T-bet siRNA-transfected NK cells showed reduction in IFN-γ production (P< 0.01) (Supplementary Figure S5A and S5B). Cotransfection of NK cells with a miR-122-5p inhibitor and T-bet siRNA was performed to investigate potential cascading relationships among miR-122-5p, T-bet, and IFN-γ. Results showed that T-bet siRNA reversed the increase in mRNA expression (Figure 5G and 5H) of T-bet and IFNG in transfected NK cells with miR-122-5p inhibitor and secretion of IFN-γ (Figure 5I). Further rescue experiments confirmed the presence of a miR-122-5p/T-bet/IFN-γ regulatory axis in NK cells (Figure 5J-5L).
Changes in miR-122-5p expression in NK cells affect the biological behavior of trophoblasts
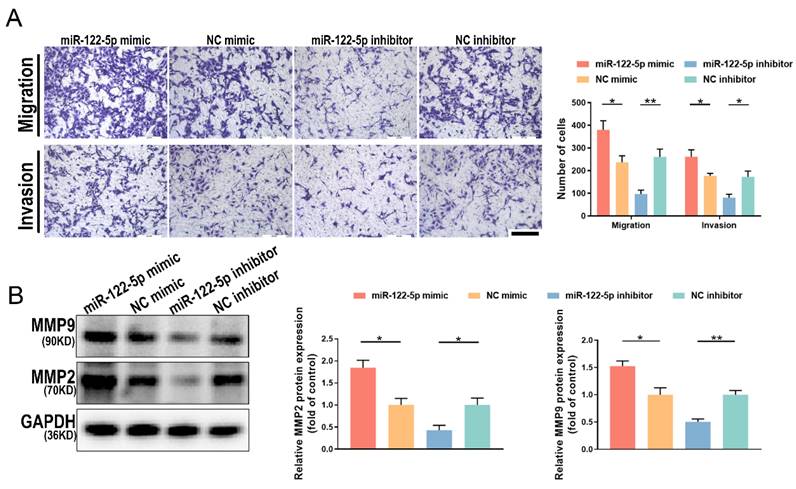
For a successful pregnancy, the appropriate development of the spiral artery and trophoblast cell invasion requires good communication between the fetus and mother. To investigate the impact of NK cell alteration of miR-122-5p expression on embryonic development, NK cells were cultured alongside HTR-8/SVneo cells after exposure to a miR-122-5p mimic or inhibitor. Results demonstrated that the quantity of migrating and invasive HTR-8/SVneo cells in the miR-122-5p mimic group increased (P= 0.02; P= 0.04), whereas the group treated with an inhibitor decreased (P= 0.005; P= 0.01; Figure 6A). Matrix metalloproteinase (MMP) 2 and 9 belong to the MMP family and are essential enzymes that break down the basement membrane. Additional experiments were conducted to quantify the protein expression levels of MMP2 and MMP9 in different treatment groups of HTR-8/SVneo cells (Figure 6B). Results revealed a correlation between fluctuations in the expression levels of MMP2 (P= 0.04; P= 0.04) and MMP9 (P= 0.03; P= 0.005) and the alterations in migration and invasion numbers, strengthening our initial findings.
miR-122-5p is involved in the regulation of NK cell activation and apoptosis. (A) The MFI of CD69 in NK cells transfected with miR-122-5p mimic and inhibitor treatment was determined by flow cytometry (n = 3). (B) The MFI of NKG2D in NK cells treated with miR-122-5p mimic and inhibitor treatment was determined by flow cytometry (n = 3) *P< 0.05. (C) Effect of miR-122-5p mimic and inhibitor on the percentage of apoptotic NK cells detected by flow cytometry. *P< 0.05. (D) Effect of miR-122-5p mimic and inhibitor on the proliferation potential of NK cells, as detected by flow cytometry. NC, negative control. FMO, fluorescence minus one. MFI, mean fluorescence intensity.
miR-122-5p is involved in the immune function regulation of NK cells. (A) Effect of miR-122-5p mimic and inhibitor on the secretion of inflammatory factors by NK cells, as detected by flow cytometry. *P< 0.05, **P< 0.01. (B) Effect of miR-122-5p mimic and inhibitor on the secretion of lytic granules by NK cells, as detected by ELISA. *P< 0.05. (C) Effect of miR-122-5p mimic and inhibitor on the degranulation of NK cells detected by flow cytometry. *P< 0.05. (D) Effect of miR-122-5p mimic and inhibitor on the killing ability of NK cells, as detected by flow cytometry. *P< 0.05, ***P< 0.001.
miR-122-5p in NK cells affects trophoblasts via the miR-122-5p/T-bet/IFN-γ axis
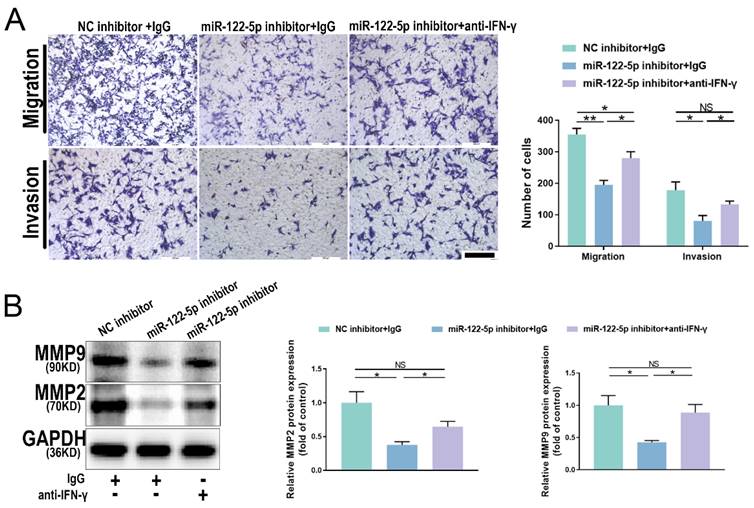
The proportion of IFN-γ+ NK cells was increased in patients with URPL, and NK cells with reduced miR-122-5p expression released more IFN-γ. To understand the molecular mechanisms by which IFN-γ affects trophoblast migration and invasion, NK cells treated with a miR-122-5p inhibitor were cultured with a neutralizing antibody against IFN-γ. Results indicated that the combined use of the miR-122-5p inhibitor and neutralizing antibody effectively restored the migratory and invasive abilities of trophoblast cells, surpassing those of the control group (P= 0.01; P= 0.03; Figure 7A). Similarly, the protein levels for MMP2 and MMP9 noticeably increased (P= 0.04; P= 0.02; Figure 7B). This analysis showed that the miR-122-5p/T-bet/IFN-γ axis plays a crucial role in maternal-fetal interactions by controlling the expression of invasion-related proteins, which affects the biological activity of trophoblast cells.
Verification of the miR-122-5p/T-bet/IFN-γ axis in NK cells. (A) T-bet, Eomes, GATA3, and Tcf1 expression in decidual NK cells was compared between the HCs and URPL patients (n = 5). *P< 0.05. (B) The protein expression of T-bet in decidual NK cells was compared between the HC group and the URPL group (n = 5). *P< 0.05. (C-D) The binding site between T-bet and miR-122-5p, as predicted by the RNAhybrid database was detected via a luciferase reporter assay. *P< 0.05. (E-F) RT-qPCR and Western blot analysis of the effects of miR-122-5p mimic and inhibitor on T-bet mRNA and protein levels in NK cells. *P< 0.05 (G-I) RT-qPCR and ELISA analysis of the effects of cotransfection of miR-122-5p inhibitor and T-bet siRNA on the mRNA expression of T-bet and IFNG and the secretion of IFN-γ in NK cells (n = 4; n = 3). *P< 0.05, **P< 0.01. (J-L) Western blot analysis of the effects of co-transfection of NK cells with miR-122-5p inhibitor and T-bet siRNA on the protein expression of T-bet and IFN-γ (n = 4). *P< 0.05, **P< 0.01. HC, healthy control. URPL, unexplained recurrent pregnancy. NS, no significance.
Discussion
The mechanisms governing the biological and immunological functions of dNK cells, an essential component of the immune microenvironment at the maternal-fetal interface that upholds immune tolerance and balance, are diverse and complex. This study revealed a noteworthy miR-122-5p downregulation in dNK cells, which is implicated in URPL. miR-122-5p regulates NK cell apoptosis and immune activity. This study supported the existence of the miR-122-5p/T-bet/IFN-γ regulatory axis within NK cells, which may affect the biological functions of trophoblast cells (Figure 8).
Studies on noncoding RNAs at the maternal-fetal interface have mostly focused on regulating trophoblasts and decidual stromal cell activities [17, 18]. The immunological mechanisms underlying RPL remain unknown, and it is difficult to transfect primary immune cells from tissue in a durable and successful manner [19]. According to Du et al.'s study [20], the expansion of NK cells with a resident phenotype was induced to partially substitute for dNK cells. Our data suggest that miR-122-5p impairs dNK cell immunological function, compromising immune tolerance at the maternal-fetal interface and hindering embryo colonization.
Altered miR-122-5p expression in NK cells affects the biological function of HTR-8/SVneo cells. (A) Effect of miR-122-5p mimic and inhibitor in NK cells on the migration and invasion of HTR-8/SVneo cells detected by coculture without or with matrigel (n = 4). The scale bar represents 200 μm. *P< 0.05, **P< 0.01. (B) Effect of miR-122-5p mimic and inhibitor in NK cells on the protein levels of MMP2 and MMP9 in HTR-8/SVneo cells detected by coculture without or with matrigel (n = 3). *P< 0.05, **P< 0.01.
IFN-γ alterations affect the biological function of HTR-8/SVneo cells. (A) Effect of miR-122-5p inhibitor in NK cells on the migration and invasion of HTR-8/SVneo cells detected by coculture without or with the addition of anti-IFN-γ (n = 4). The scale bar represents 200 μm. *P< 0.05, **P< 0.01. (B) Effect of miR-122-5p inhibitor in NK cells on the protein levels of MMP2 and MMP9 of HTR-8/SVneo cells detected by coculture without or with the addition of anti-IFN-γ (n = 3). *P< 0.05. NS, no significance.
Schematic diagram of the action of miR-122-5p signaling in dNK cells at the maternal-fetal interface in patients with URPL. The decreased miR-122-5p expression in decidual natural killer cells may lead to reduced apoptosis, heightened activation, and immunological dysregulation. Moreover, it indirectly affects the normal biological behavior of trophoblast cells by targeting T-bet and subsequent IFN-γ secretion, ultimately resulting in the occurrence of recurrent pregnancy loss. GZMB, granzyme B.
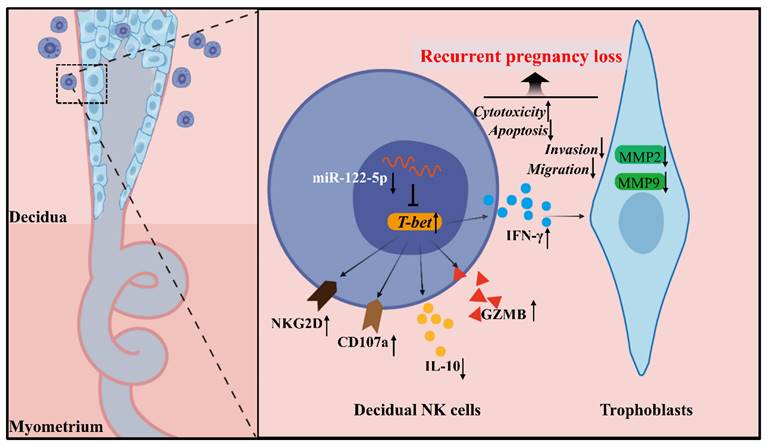
Adverse inflammatory changes in locally infiltrating immune cells will lead to the occurrence of the disease [21, 22]. The balance of inflammatory factors at the maternal-fetal interface may be upset by decreased IL-10 secretion, whereas decreased miR-122-5p expression causes NK cells to secrete more IFN-γ. The cytotoxic properties of NK cells are strictly regulated by hormonal regulation and receptors such as CD49a, Tim-3, and NKG2A [23-25]. Granzyme B release in excess may compromise the immunological tolerance of developing embryos [26]. A previous study demonstrated that miR-30e targets PRF1 to maintain immunological tolerance [27]. miR-122-5p is a significant modulator of lipid metabolism and is widely expressed in the mature liver, where it plays a role in the pathophysiology of nonalcoholic steatohepatitis and HCC [28, 29]. Studies have also shown that miR-122-5p mediates cerebral ischemic injury and acute myocardial infarction [30, 31]. This study reported the function of miR-122-5p at the maternal-fetal interface for the first time and found that it mediates URPL occurrence.
The transcription factors Eomes, KLF2, and Elf1 govern NK cell development and maturation [32, 33]. This study observed an increase in the Th1 transcription factor T-bet in URPL-derived dNK cells; this increase is controlled negatively by miR-122-5p. As demonstrated in earlier studies, T-bet had increased mRNA expression in the decidua of patients with preeclampsia [34]. Normally, CD56bright NK cells show modest levels of T-bet mRNA expression, and a significant increase suggests an aberrant functional difference among NK cells [35]. Based on these supporting investigations, T-bet is a prominent molecule responsible for regulating IFN-γ through miR-122-5p. Therefore, the miR-122-5p/T-bet/IFN-γ regulation pathway in NK cells may significantly enhance maternal-fetal tolerance.
Abnormal invasion of trophoblast cells can lead to complications such as preeclampsia and intrauterine growth restriction, and trophoblast cells employ MMPs and urokinase activator systems to degrade the basement membrane and extracellular matrix [36, 37]. Immune cells and vascular formation, remodeling, and calcification are strongly associated, with the placenta being a highly vascularized tissue [38]. The reciprocal interaction between trophoblasts and dNK cells seems to limit and augment each other, creating the necessary equilibrium for the continuation of the fetus [39, 40]. In this study, we observed a reduced invasive capacity of HTR-8/ SVneo cells due to reduced miR-122-5p expression, leading to excessive IFN-γ secretion. Verma et al. showed that IFN-γ inhibited STAT1 and AKT pathways by upregulating the expression of bone marrow stromal antigen 2 (BST-2) and E-cadherin, thereby curtailing the invasive ability of trophoblast cells [41]. To understand the molecular mechanism responsible for IFN-γ inhibition, an IFN-γ neutralizing antibody was administered to the group exhibiting reduced miR-122-5p expression. There was a partial recovery of the decreased invasion of HTR-8/SVneo cells accompanied by reduced production of MMP2 and MMP9 protein. miR-122-p in dNK cells regulates IFN-γ production, affecting the expression of functional proteins relevant to trophoblasts.
Embryo colonization and development occur in hypoxic conditions [42]. Previous studies have reported that mitochondrial function is closely related to T-bet expression in NK cells and its anti-tumor activity [43]. The anomalous metabolism and function of immune cells in specific environments affect blood perfusion, hyperactivation, and inflammation [44, 45]. Researchers have found that targeted mitochondrial correction improves the ischemic state and stress of myocardial vessels, providing new insights for the future treatment of ischemic placental disease [46, 47]. Recent studies have reported an updated bioswitchable delivery system for achieving controlled delivery based on tetrahedral DNA nanostructures and microRNA loading and release. This suggests the possibility of clinical application of intervention with abnormal miR-122-5p expression [48]. The limitation of this study was that transfection of primary dNK cells was not achieved. Additional in vivo studies are warranted to further corroborate our findings and the impact of miR-122-5p on the entire immune microenvironment at maternal-fetal interface should be explored in the future.
Conclusion
In summary, this study revealed that miR-122-5p plays a crucial role in controlling NK cell activity. Abnormal miR-122-5p expression in dNK cells indirectly affects the biological function of trophoblasts at the maternal-fetal interface, which is implicated in URPL occurrence. Hence, specifically regulating miR-122-5p/T-bet/IFN-γ axis in dNK cells is a prospective therapeutic approach for URPL.
Abbreviations
URPL: Unexplained recurrent pregnancy loss; HC: Healthy control; DICs: Decidual immune cells; dNK: Decidual natural killer cells; GZMB: granzyme B; CD: Cluster of differentiation; hCG: Human chorionic gonadotropin; IL: Interleukin; FMO: Fluorescence minus one; MFI: Mean fluorescence intensity; mRNA: Message RNA; RT-qPCR: Reverse transcription-polymerase chain reaction. ELISA, Enzyme-linked immunosorbent assay.
Supplementary Material
Supplementary figures and table.
Acknowledgements
We would like to thank the Outpatient Operating Room and the Obstetrics Department of West China Second University Hospital for the clinical samples.
Funding
This research was supported by the National Key R&D Program of China (2021YFC2701600) and the Projects of the Sichuan Science and Technology Department (No.2022YFS0235).
Ethics approval and consent to participate
The study was approved by the Ethics Committee of West China Second Hospital of Sichuan University [Approval number: Medical Research 2020 (029)]. All patients signed the informed consent form, and all procedures involving human participants were conducted following the principles of the Declaration of Helsinki.
Author contributions
Y.L., L.L., and H.L. designed the research. Y.L. drafted the manuscript. Y.L., W.Z., Y.L, Y.M., and Z.H. helped collect the clinical samples. Y.L. and T.F. performed the flow cytometry. H.L. was liable for leadership responsibility for research. All authors read and approved the final version of the manuscript.
Availability of data and materials
All data and materials can be obtained from the first author and corresponding author.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bender Atik R, Christiansen OB, Elson J. et al. Eshre guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018:hoy004
2. PrabhuDas M, Bonney E, Caron K. et al. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol. 2015;16:328-34
3. Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387-411
4. Hanna J, Goldman-Wohl D, Hamani Y. et al. Decidual nk cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065-74
5. Tilburgs T, Crespo ÂC, van der Zwan A. et al. Human hla-g+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proceedings of the National Academy of Sciences. 2015;112:7219-24
6. Cartwright JE, James-Allan L, Buckley RJ. et al. The role of decidual nk cells in pregnancies with impaired vascular remodelling. J Reprod Immunol. 2017;119:81-4
7. Fu B, Zhou Y, Ni X. et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity. 2017;47:1100-13
8. Yang F, Zheng Q, Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front Immunol. 2019;10:2317
9. Ortega MA, Fraile-Martínez O, García-Montero C. et al. The pivotal role of the placenta in normal and pathological pregnancies: a focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease. Cells. 2022;11:568
10. Lin F, Yang C, Feng T. et al. The maternal-fetal interface in small-for-gestational-age pregnancies is associated with a reduced quantity of human decidual nk cells with weaker functional ability. Front Cell Dev Biol. 2020;8:633
11. Wei X, Yang X. The central role of natural killer cells in preeclampsia. Front Immunol. 2023;14:1009867
12. Krol J, Loedige I, Filipowicz W. The widespread regulation of microrna biogenesis, function and decay. Nat Rev Genet. 2010;11:597-610
13. Sullivan RP, Leong JW, Fehniger TA. Microrna regulation of natural killer cells. Front Immunol. 2013;4:44
14. Cichocki F, Felices M, McCullar V. et al. Cutting edge: microrna-181 promotes human nk cell development by regulating notch signaling. The Journal of Immunology. 2011;187:6171-5
15. Xu D, Han Q, Hou Z. et al. Mir-146a negatively regulates nk cell functions via stat1 signaling. Cell Mol Immunol. 2017;14:712-20
16. Li L, Liu Y, Feng T. et al. The ahnak induces increased il-6 production in cd4+ t cells and serves as a potential diagnostic biomarker for recurrent pregnancy loss. Clin Exp Immunol. 2022;209:291-304
17. Liu M, Liao L, Gao Y. et al. Bcam deficiency may contribute to preeclampsia by suppressing the pik3r6/p-stat3 signaling. Hypertension. 2022;79:2830-42
18. Soni UK, Chadchan SB, Gupta RK. et al. Mirna-149 targets parp-2 in endometrial epithelial and stromal cells to regulate the trophoblast attachment process. Mol Hum Reprod. 2021;27:gaab039
19. Boissel L, Betancur M, Lu W. et al. Comparison of mrna and lentiviral based transfection of natural killer cells with chimeric antigen receptors recognizing lymphoid antigens. Leuk Lymphoma. 2012;53:958-65
20. Du X, Zhu H, Jiao D. et al. Human-induced cd49a+ nk cells promote fetal growth. Front Immunol. 2022;13:821542
21. Yang X, Tian Y, Zheng L. et al. The update immune-regulatory role of pro- and anti-inflammatory cytokines in recurrent pregnancy losses. International Journal of Molecular Sciences. 2023;24:132
22. Li Y, Yu J, Li R. et al. New insights into the role of mitochondrial metabolic dysregulation and immune infiltration in septic cardiomyopathy by integrated bioinformatics analysis and experimental validation. Cell Mol Biol Lett. 2024;29:21
23. André P, Denis C, Soulas C. et al. Anti-nkg2a mab is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both t and nk cells. Cell. 2018;175:1731-43
24. Li H, Hou Y, Zhang S. et al. Cd49a regulates the function of human decidual natural killer cells. Am J Reprod Immunol. 2019;81:e13101
25. Li Y, Zhou W, Tao Y. et al. The galectin-9/tim-3 pathway is involved in the regulation of nk cell function at the maternal-fetal interface in early pregnancy. Cell Mol Immunol. 2016;13:73-81
26. Zhang X, Wei H. Role of decidual natural killer cells in human pregnancy and related pregnancy complications. Front Immunol. 2021;12:728291
27. Huang Q, Ding J, Gong M. et al. Effect of mir-30e regulating nk cell activities on immune tolerance of maternal-fetal interface by targeting prf1. Biomed Pharmacother. 2019;109:1478-87
28. Bandiera S, Pfeffer S, Baumert TF. et al. Mir-122 -a key factor and therapeutic target in liver disease. J Hepatol. 2015;62:448-57
29. Turato C, Fornari F, Pollutri D. et al. Mir-122 targets serpinb3 and is involved in sorafenib resistance in hepatocellular carcinoma. J Clin Med. 2019;8:171
30. Kong Y, Li S, Cheng X. et al. Brain ischemia significantly alters microrna expression in human peripheral blood natural killer cells. Front Immunol. 2020;11:759
31. Wang Y, Chang W, Zhang Y. et al. Circulating mir-22-5p and mir-122-5p are promising novel biomarkers for diagnosis of acute myocardial infarction. J Cell Physiol. 2019;234:4778-86
32. Choi H, Geng Y, Cho H. et al. Differential requirements for the ets transcription factor elf-1 in the development of nkt cells and nk cells. Blood. 2011;117:1880-7
33. Fang D, Cui K, Cao Y. et al. Differential regulation of transcription factor t-bet induction during nk cell development and t helper-1 cell differentiation. Immunity. 2022;55:639-55
34. Jianjun Z, Yali H, Zhiqun W. et al. Original article: imbalance of t-cell transcription factors contributes to the th1 type immunity predominant in pre-eclampsia. Am J Reprod Immunol. 2010;63:38-45
35. Vazquez J, Chasman DA, Lopez GE. et al. Transcriptional and functional programming of decidual innate lymphoid cells. Front Immunol. 2020;10:3065
36. Espino Y. Sosa S, Flores-Pliego A, Espejel-Nuñez A, et al. New insights into the role of matrix metalloproteinases in preeclampsia. International Journal of Molecular Sciences. 2017;18:1448
37. Huppertz B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. International Journal of Molecular Sciences. 2020;21:289
38. Ortega MA, De Leon-Oliva D, Gimeno-Longas MJ. et al. Vascular calcification: molecular networking, pathological implications and translational opportunities. Biomolecules. 2024;14:275
39. Alexandrova M, Manchorova D, Dimova T. Immunity at maternal-fetal interface: kir/hla (allo) recognition. Immunol Rev. 2022;308:55-76
40. Wallace AE, Fraser R, Cartwright JE. Extravillous trophoblast and decidual natural killer cells: a remodelling partnership. Hum Reprod Update. 2012;18:458-71
41. Verma S, Kang AK, Pal R. et al. Bst2 regulates interferon gamma-dependent decrease in invasion of htr-8/svneo cells via stat1 and akt signaling pathways and expression of e-cadherin. Cell Adh Migr. 2020;14:24-41
42. Zhao H, Wong RJ, Stevenson DK. The impact of hypoxia in early pregnancy on placental cells. International Journal of Molecular Sciences. 2021;22:9675
43. Chamoto K, Chowdhury PS, Kumar A. et al. Mitochondrial activation chemicals synergize with surface receptor pd-1 blockade for t cell-dependent antitumor activity. Proceedings of the National Academy of Sciences. 2017;114:E761-E770
44. Zhang X, Zhou H, Chang X. Involvement of mitochondrial dynamics and mitophagy in diabetic endothelial dysfunction and cardiac microvascular injury. Arch Toxicol. 2023;97:3023-35
45. Park J, Hsueh P, Li Z. et al. Microenvironment-driven metabolic adaptations guiding cd8+ t cell anti-tumor immunity. Immunity. 2023;56:32-42
46. Chang X, Liu R, Li R. et al. Molecular mechanisms of mitochondrial quality control in ischemic cardiomyopathy. Int J Biol Sci. 2023;19:426-48
47. Chang X, Li Y, Liu J. et al. ß-tubulin contributes to tongyang huoxue decoction-induced protection against hypoxia/reoxygenation-induced injury of sinoatrial node cells through sirt1-mediated regulation of mitochondrial quality surveillance. Phytomedicine. 2023;108:154502
48. Li S, Tian T, Zhang T. et al. A bioswitchable delivery system for microrna therapeutics based on a tetrahedral dna nanostructure. Nat Protoc. 2024
Author contact
![]() Corresponding author: lihonghxedu.cn.
Corresponding author: lihonghxedu.cn.

 Global reach, higher impact
Global reach, higher impact