3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(14):2640-2654. doi:10.7150/ijms.99724 This issue Cite
Research Paper
The Gustave Roussy Immune score (GRIm score) as a novel prognostic score for early breast cancer patients: A real-world retrospective study
1. Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150081, P.R. China.
2. Endoscope Department, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150081, P.R. China.
Received 2024-6-17; Accepted 2024-10-2; Published 2024-10-14
Abstract
Background and objective: The aim of this research is to investigate whether the GRIm score serves as a novel prognostic tool for predicting the survival rates among early breast cancer patients undergoing surgical treatment.
Methods: This retrospective study included 313 cases of breast cancer patients hospitalized in our hospital from January 2015 to November 2015. All enrolled patients received surgery and had no metastasis. The GRIm score was based on five objective markers: (1) albumin level (<3.5 g/L = 1 point), (2) LDH level (≥245 U/L = 1 point); (3) AST-to-ALT ratio (≥1.44 = 1 point); (4) total bilirubin level (≥21 μmol/ml = 1 point); (5) NLR (≥1.51 = 1 point). The best critical value was 1.51 for NLR by ROC. Patients were categorized into two groups based on GRIm scores: low-score group (0 point) and high-score group (1 to 5 points). Kaplan-Meier method and log rank test were utilized to estimate disease free survival (DFS) and overall survival (OS). Both univariate analysis and multivariate Cox analysis were used to analyze the relationship among the enrolled parameters. Nomograms were formulated reliant on the outcomes of multivariate Cox analysis.
Results: Based on the GRIm score, the cohort was divided into two groups: a low-score group with 81 cases and a high-score group with 232 cases. The mean DFS and OS were significantly prolonged in low-score group compared to high-score group (DFS: 74.39 vs. 66.20 months, χ2=8.729, P=0.0031; OS: 83.71 vs. 76.40 months, χ2=8.729, P=0.0031). According to multivariable analysis, GRIm score was notably correlated with DFS (HR: 2.789, 95% CI: 1.304-5.965, P= 0.004) and OS (HR: 3.015, 95% CI: 1.409-10.087, P=0.004). Nomograms exhibited excellent predictive performance for DFS (C-index: 0.823) and OS (C-index: 0.807).
Conclusions: GRIm score serves as a predictive tool for assessing the prognosis of early breast cancer patients. Nomograms based on GRIm score show good prediction ability.
Keywords: breast cancer, Gustave Roussy immune score, NLR, lactate dehydrogenase, prognosis
Introduction
Traditionally, the main treatments for breast cancer patients include surgery and chemotherapy based on anthracyclines and taxanes, which results in the recovery rates varying by clinical stage and subtype [1]. Although the prognosis of breast cancer patients has been gradually improved by adding endocrine therapy or radiotherapy on the basis of cytotoxic chemotherapy, breast cancer remains the leading cause of cancer-related deaths among women globally [2]. In the past 10 years, the new combination treatments for breast cancer, such as the combination of cyclin-dependent kinase (CDK) 4/6 inhibition and endocrine therapy [3], standard chemotherapy in conjunction with immunotherapy [4], have significantly improved the prognosis of breast cancer patients.
Recently, many studies have reported the relationship between nutrition, inflammation, immunity, and tumor development [5-8]. At present, the hematological examinations commonly used in clinic include blood routine, biochemical tests, coagulation function tests, and tumor marker tests. A variety of scoring systems used to guide the prognosis of clinical trials and patient selection divide patients into different prognostic risk groups by using the combination of clinical and laboratory parameters based on blood routine or/and biochemical tests [7,9-11]. In recent years, with the increasing application of immunotherapy in the field of oncology, new scoring systems (such as MD Anderson immune checkpoint inhibitor score and Gustave Roussy immune score) have been developed and constructed to evaluate patient selection in clinical trials of immune checkpoint inhibitors [12,13]. Gustave Roussy Immune score (GRIm score) was firstly reported by Bigot et al., based on lactate dehydrogenase (LDH) level, albumin level, total bilirubin level, AST-to-ALT ratio, and neutrophil to lymphocyte ratio (NLR). This scoring system was developed in some malignant tumors, such as advanced non-small cell lung cancer (aNSCLC), metastatic colorectal cancer (mCRC), advanced pancreatic adenocarcinoma (PDAC), undergoing immune checkpoint inhibitors to ascertain patients who may respond favorably to the current treatment [14]. These findings indicate that the GRIm score may be a valuable prognostic marker for cancer patients, which clinical doctors can use to stratify patients and develop personalized treatment plans. However, the representativeness of breast cancer patients is insufficient in the discovery and validation cohorts by this scoring system. The role of this prognostic score to forecast the survival outcome among breast cancer patients is still uncertain. Hence, the aim of our study was to investigate whether the GRIm score determines the prognosis, and to provide practical guidance for breast cancer patients who have undergone surgery.
Methods
Patients
The 313 cases of breast cancer patients who received operations in our Hospital from January 2015 to November 2015 were enrolled in this retrospective cohort study. The demographic, clinical, and pathological data were gathered retrospectively from the electronic medical records of each enrolled patient. This research received approval from the Ethics Committee of Harbin Medical University Cancer Hospital (Grant Number: KY2023-38). In this study, all participants were thoroughly informed about the objectives, procedures, potential risks, and their rights related to the research. Specifically, written informed consent was obtained from each participant, documenting their agreement to participate in the research and authorizing the use of their data for scientific analysis.
Based on the histological examination, a histological diagnosis of breast cancer was confirmed for all enrolled patients. The inclusion criteria were: 1) Blood routine, biochemical examination, coagulation function, and tumor marker examination one week before surgery; 2) Complete medical records, and follow-up information; 3) Without distant organ metastasis. Exclusion criteria were: 1) Suffering from metastatic tumor or other malignant tumors; 2) Preoperative chemotherapy or radiotherapy; 3) Accompanied by underlying diseases that were difficult to control, and could not be treated surgically.
Definition of GRIm score
For the first time, the GRIm score was calculated as outlined in Bigot's study [14]. In the current study, the GRIm score was based on five objective markers:(1) albumin level (<3.5 g/L = 1 point, ≥3.5 g/L = 0 point); (2) LDH level (<245 U/L = 0 point, ≥245 U/L = 1 point); (3) AST-to-ALT ratio (<1.44 = 0 point, ≥1.44 = 1 point); (4) total bilirubin level (≥21μmol/ml = 1 point, <21μmol/ml = 0 point); (5) NLR (≥1.51 = 1 point, <1.51 = 0 point). The calculation of the AST-to-ALT ratio involved dividing the serum level of Aspartate aminotransferase (AST) by the serum level of Alanine aminotransferase (ALT). The baseline peripheral neutrophil count was divided by the lymphocyte count prior to surgery to calculate the NLR. In our cohort, the best critical value for NLR by ROC with the highest sensitivity and specificity to predict OS. And the best critical value for NLR was 1.51 in this study. Patients were categorized into two groups based on their GRIm scores: the low-score group (0 point) and the high-score group (ranging from 1 to 5 points).
Followed-up and statistical methods
The disease-free survival (DFS) referred to the duration between the date following curative resection and the occurrence of either local or distant metastasis. The overall survival (OS) was defined as the duration that begins on the date following curative resection and ends either with the death of the patient for any reason or at the date of the last follow-up, depending on the context. All statistical analyses were conducted using the SPSS Statistics software version 22.0, provided by IBM Corp., as well as the R statistical computing language, version 4.2.2, originating from Vienna, Austria. The URL for R is: http://www.R-project.org/. Numerical variables were presented using the median and interquartile range, while categorical variables were expressed as percentages with their corresponding numbers in parentheses. Statistical analysis was made by Fisher exact test and chi-square test. The Kaplan-Meier method was utilized for computing the survival curves of both DFS and OS, followed by a comparison using the log-rank test. The Cox proportional hazards regression model was employed to identify the underlying independent variables that were associated with DFS and OS. In the multivariate analyses, the hazard ratio (HR) along with the corresponding 95% confidence interval (CI) were calculated for each factor involved. The Nomogram models were additionally developed to assess the DFS and OS rates. The clinical utility of the prediction models was analyzed by using calibration curve and decision curve analysis.
Results
Construction and evaluation of GRIm score with survival
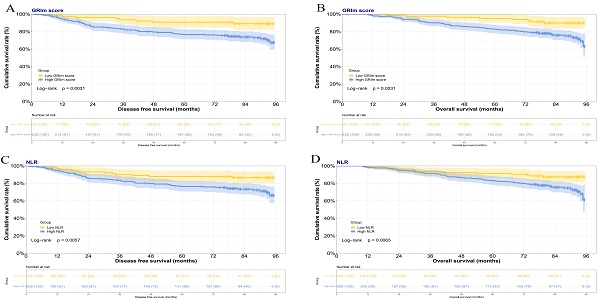
In this study, the GRIm score was constructed by the albumin level, LDH level, total bilirubin level, AST-to-ALT ratio, and NLR. Based on GRIm scores, there were 81 cases for GRIm score 0 divided into the low-score group, and 232 cases for GRIm score from 1 to 5 points divided into the high-score group. In the low GRIm score group, the mean duration of DFS was 74.39 months, while the mean OS was 83.71 months. Conversely, in the high GRIm score group, the mean DFS was 66.20 months, and the mean OS was 76.40 months. The Kaplan-Meier estimations of both DFS and OS, categorized according to prognostic risk for each GRIm score, are displayed in Figures 1A and 1B, respectively. Significant variations in DFS and OS were observed among the different prognostic risk groups for the GRIm score. (DFS: χ2=8.729, P=0.0031; OS: χ2=8.729, P=0.0031). Moreover, according to the NLR, 105 cases were in the low NLR group, 208 cases were in the high NLR group. In the low NLR group, the mean duration of DFS was 72.05 months, while the mean OS was 81.07 months. By contrast, in the high NLR group, the mean DFS was 66.44 months, and the mean OS was 76.88 months. The Kaplan-Meier estimations of both DFS and OS, categorized according to prognostic risk for NLR, are displayed in Figures 1C and 1D, respectively. Significant variations in DFS and OS were observed among the different prognostic risk groups for the NLR (DFS: χ2=7.628, P=0.0057; OS: χ2=7.416, P=0.0065).
Baseline characteristics according to the GRIm score
The 313 cases of breast cancer patients were consecutively enrolled in this discovery cohort between January 2015 and November 2015. All enrolled patients were females, the median age was 51 years, with a range of 25 to 78 years. According to the TNM stage, 85 (27.2%) cases were the stage I, 138 (44.1%) cases were the stage II, 90 (28.8%) cases were the stage III. Breast cancer was divided into four distinct molecular subtypes, dependent on the expression profiles of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67. In the study, the distribution of breast cancer molecular subtypes was as follows: 58 cases (18.5%) belonged to the Luminal A subtype, 65 cases (20.8%) were identified as the HER2-enriched subtype, 63 cases (20.1%) were categorized as the Luminal B HER2-negative subtype, another 63 cases (20.1%) fell into the Luminal B HER2-positive subtype, and finally, 64 cases (20.4%) were designated as the Triple-negative subtype. The GRIm score exhibited a statistically significant association with menarche age (P=0.004). Detailed information is shown in Table 1.
Comparison of performance of GRIm score according to common hematological parameters
In this study, we enrolled the common hematological parameters, including AST, ALT, LDH, γ-glutamyl transpeptidase (GGT), albumin (ALB), direct bilirubin (DBIL), indirect bilirubin (IBIL), total bilirubin (TBIL), total protein (TP), globularproteins (G), albumin/globularproteins (A/G), prealbumin (PAB), carcinoembryonic antigen (CEA), cancer antigen 153 (CA153), fibrinogen (FBG), international normalized ratio (INR), D-Dimer (D-D), white blood cell (W), lymphocyte (L), monocyte (M), neutrophil (N), platelet (P). The median values of these enrolled hematological parameters served as the basis for grouping. The GRIm score exhibited a statistically significant association with AST/ALT (P=0.002), FBG (P=0.041), N (P<0.001), L (P<0.001), W (P=0.002), M (P=0.03), and NLR (P<0.001). Detailed information is presented in Table 2.
Comparison of performance of GRIm score according to pathological features
All patients included in this study underwent surgical treatment. Of all enrolled patients, the maximum short diameter of the tumor in 154 (49.2%) patients was less than 2 cm, in 149 (47.6%) patients was between 2 cm and 5cm, in 10 (3.2%) patients was more than 5 cm. The GRIm score exhibited a statistically significant association with positive axillary lymph nodes (PALN) (P=0.042). Details are shown in Table 3.
Correlation between GRIm score and organ metastasis
All patients were followed up after surgery. In the current study, 32 (10.2%) patients had lung metastasis of breast cancer after operation, 40 (12.8%) patients had bone metastasis of breast cancer after operation, 29 (9.3%) patients had liver metastasis of breast cancer after operation, 12 (3.8%) patients had chest wall metastasis of breast cancer after operation, 14 (4.5%) patients had mediastinal metastasis of breast cancer after operation, 16 (5.1%) patients had brain metastasis of breast cancer after operation, 11 (3.5%) patients had pleural metastasis of breast cancer after operation, 159 (50.8%) patients had axillary metastasis of breast cancer after operation, 49 (15.7%) patients had clavicle metastasis of breast cancer after operation, respectively. The GRIm score exhibited a statistically significant association with axillary metastasis (P=0.048). Detailed information is shown in Table S1.
The Kaplan-Meier survival curves illustrating the DFS and the OS among patients with breast cancer, stratified based on the GRIm score or NLR. A) Kaplan-Meier survival curves illustrating the DFS among patients with breast cancer stratified based on the GRIm score; B) Kaplan-Meier survival curves illustrating the OS among patients with breast cancer stratified based on the GRIm score; C) Kaplan-Meier survival curves illustrating the DFS among patients with breast cancer stratified based on the NLR; D) Kaplan-Meier survival curves illustrating the OS among patients with breast cancer stratified based on the NLR.
The univariate analysis and multivariate analysis
In the current study, we enrolled the GRIm score, NLR, age, BMI, family history, basic disease, menarche age, menopause, ALT, AST, AST/ALT, LDH, ALB, TBIL, CA153, CEA, D-D, FBG, white blood cell, neutrophil, lymphocyte, monocyte, platelet, tumor size, pathological TNM stage, TALN, PALN, molecular subtype, chemotherapy, radiotherapy, endocrine therapy, targeted therapy to construct the univariate and multivariate COX analysis. For these enrolled variables, we adjusted for the confounding factors, including age, BMI, menarche age. In the univariate analysis, GRIm score, NLR, family history, basic disease, menopause, pathological TNM stage, PALN, chemotherapy, and endocrine therapy were identified as meaningful factors. The multivariate analysis identified the GRIm score, NLR, family history, menopause status, pathological TNM stage, chemotherapy, and endocrine therapy as potential prognostic factors for DFS in Table 4. Furthermore, the univariate analysis identified the GRIm score, NLR, family history, ALT, pathological TNM stage, chemotherapy, and endocrine therapy as significant factors. Additionally, the multivariate analysis revealed that the GRIm score, NLR, family history, ALT, pathological TNM stage, chemotherapy, and endocrine therapy were potential predictors of outcome for OS in Table 5. For these results, the multivariate analyses demonstrated that the GRIm score significantly and adversely impacted both DFS and OS. (DFS, HR: 2.789, 95% CI: 1.304-5.965, P=0.008; OS: HR: 3.015, 95% CI: 1.409-10.087, P=0.004). After adjusting for variables such as age, BMI, menarche age, the GRIm score was significantly associated with chemotherapy (DFS, HR: 0.214, 95% CI: 0.107-0.427, P<0.001; OS: HR: 0.141, 95% CI: 0.069-0.289, P<0.001) and endocrine therapy (DFS, HR: 0.241, 95% CI: 0.137-0.423, P<0.001; OS: HR: 0.264, 95% CI: 0.148-0.468, P<0.001).
Baseline characteristics according to the GRIm score.
| Parameters | level | Overall | Low GRIm score | High GRIm score | p |
|---|---|---|---|---|---|
| n | 313 | 81 | 232 | ||
| Age | <51 | 154 (49.2) | 39 (48.1) | 115 (49.6) | 0.927 |
| ≥51 | 159 (50.8) | 42 (51.9) | 117 (50.4) | ||
| Weight | <62 | 149 (47.6) | 37 (45.7) | 112 (48.3) | 0.784 |
| ≥62 | 164 (52.4) | 44 (54.3) | 120 (51.7) | ||
| Height | <1.60 | 105 (33.5) | 22 (27.2) | 83 (35.8) | 0.202 |
| ≥1.60 | 208 (66.5) | 59 (72.8) | 149 (64.2) | ||
| BMI | <23.8 | 156 (49.8) | 37 (45.7) | 119 (51.3) | 0.459 |
| ≥23.8 | 157 (50.2) | 44 (54.3) | 113 (48.7) | ||
| Family history | No | 242 (77.3) | 60 (74.1) | 182 (78.4) | 0.512 |
| Yes | 71 (22.7) | 21 (25.9) | 50 (21.6) | ||
| Basic disease | No | 244 (78.0) | 66 (81.5) | 178 (76.7) | 0.463 |
| Yes | 69 (22.0) | 15 (18.5) | 54 (23.3) | ||
| Hypertension | No | 273 (87.2) | 73 (90.1) | 200 (86.2) | 0.474 |
| Yes | 40 (12.8) | 8 (9.9) | 32 (13.8) | ||
| Diabetes mellitus | No | 297 (94.9) | 77 (95.1) | 220 (94.8) | 1.000 |
| Yes | 16 (5.1) | 4 (4.9) | 12 (5.2) | ||
| Coronary heart disease | No | 300 (95.8) | 77 (95.1) | 223 (96.1) | 0.930 |
| Yes | 13 (4.2) | 4 (4.9) | 9 (3.9) | ||
| Menarche age | <15 | 121 (38.7) | 20 (24.7) | 101 (43.5) | 0.004 |
| ≥15 | 192 (61.3) | 61 (75.3) | 131 (56.5) | ||
| Menopause | No | 152 (48.6) | 33 (40.7) | 119 (51.3) | 0.132 |
| Yes | 161 (51.4) | 48 (59.3) | 113 (48.7) | ||
| Primary tumor site | Upper outer quadrant | 178 (56.9) | 44 (54.3) | 134 (57.8) | 0.359 |
| Lower outer quadrant | 29 (9.3) | 10 (12.3) | 19 (8.2) | ||
| Lower inner quadrant | 23 (7.3) | 3 (3.7) | 20 (8.6) | ||
| Upper inner quadrant | 44 (14.1) | 11 (13.6) | 33 (14.2) | ||
| Central | 39 (12.5) | 13 (16.0) | 26 (11.2) | ||
| Operative time | <75 | 143 (45.7) | 30 (37.0) | 113 (48.7) | 0.092 |
| ≥75 | 170 (54.3) | 51 (63.0) | 119 (51.3) | ||
| Type of surgery | Mastectomy | 293 (93.6) | 74 (91.4) | 219 (94.4) | 0.485 |
| Breast-conserving surgery | 20 (6.4) | 7 (8.6) | 13 (5.6) | ||
| Pathological T Stage | T1 | 167 (53.4) | 45 (55.6) | 122 (52.6) | 0.802 |
| T2 | 134 (42.8) | 33 (40.7) | 101 (43.5) | ||
| T3 | 10 (3.2) | 2 (2.5) | 8 (3.4) | ||
| T4 | 2 (0.6) | 1 (1.2) | 1 (0.4) | ||
| Pathological N Stage | N0 | 129 (41.2) | 38 (46.9) | 91 (39.2) | 0.377 |
| N1 | 97 (31.0) | 26 (32.1) | 71 (30.6) | ||
| N2 | 50 (16.0) | 11 (13.6) | 39 (16.8) | ||
| N3 | 37 (11.8) | 6 (7.4) | 31 (13.4) | ||
| Pathological TNM Stage | I | 85 (27.2) | 25 (30.9) | 60 (25.9) | 0.305 |
| II | 138 (44.1) | 38 (46.9) | 100 (43.1) | ||
| III | 90 (28.8) | 18 (22.2) | 72 (31.0) | ||
| Molecular subtype | Luminal A | 58 (18.5) | 18 (22.2) | 40 (17.2) | 0.712 |
| Luminal B HER2+ | 63 (20.1) | 17 (21.0) | 46 (19.8) | ||
| Luminal B HER2- | 63 (20.1) | 15 (18.5) | 48 (20.7) | ||
| HER2 enriched | 65 (20.8) | 18 (22.2) | 47 (20.3) | ||
| Triple negative | 64 (20.4) | 13 (16.0) | 51 (22.0) | ||
| Chemotherapy | No | 23 (7.3) | 10 (12.3) | 13 (5.6) | 0.079 |
| Yes | 290 (92.7) | 71 (87.7) | 219 (94.4) | ||
| Radiotherapy | No | 220 (70.3) | 53 (65.4) | 167 (72.0) | 0.332 |
| Yes | 93 (29.7) | 28 (34.6) | 65 (28.0) | ||
| Endocrine therapy | No | 150 (47.9) | 35 (43.2) | 115 (49.6) | 0.391 |
| Yes | 163 (52.1) | 46 (56.8) | 117 (50.4) | ||
| Targeted therapy | No | 279 (89.1) | 69 (85.2) | 210 (90.5) | 0.263 |
| Yes | 34 (10.9) | 12 (14.8) | 22 (9.5) |
Abbreviation: GRIm score: Gustave Roussy Immune score; BMI: body mass index.
A performance comparison between GRIm score and common hematological parameters.
| Parameters | level | Overall | Low GRIm score | High GRIm score | p |
|---|---|---|---|---|---|
| n | 313 | 81 | 232 | ||
| ALT | <21 | 142 (45.4) | 33 (40.7) | 109 (47.0) | 0.400 |
| ≥21 | 171 (54.6) | 48 (59.3) | 123 (53.0) | ||
| AST | <23 | 147 (47.0) | 36 (44.4) | 111 (47.8) | 0.690 |
| ≥23 | 166 (53.0) | 45 (55.6) | 121 (52.2) | ||
| AST/ALT | <1.44 | 267 (85.3) | 78 (96.3) | 189 (81.5) | 0.002 |
| ≥1.44 | 46 (14.7) | 3 (3.7) | 43 (18.5) | ||
| LDH | <170 | 156 (49.8) | 38 (46.9) | 118 (50.9) | 0.629 |
| ≥170 | 157 (50.2) | 43 (53.1) | 114 (49.1) | ||
| GGT | <14 | 142 (45.4) | 41 (50.6) | 101 (43.5) | 0.331 |
| ≥14 | 171 (54.6) | 40 (49.4) | 131 (56.5) | ||
| ALB | <45 | 145 (46.3) | 43 (53.1) | 102 (44.0) | 0.198 |
| ≥45 | 168 (53.7) | 38 (46.9) | 130 (56.0) | ||
| TBIL | <12.45 | 156 (49.8) | 44 (54.3) | 112 (48.3) | 0.419 |
| ≥12.45 | 157 (50.2) | 37 (45.7) | 120 (51.7) | ||
| DBIL | <3.9 | 155 (49.5) | 47 (58.0) | 108 (46.6) | 0.099 |
| ≥3.9 | 158 (50.5) | 34 (42.0) | 124 (53.4) | ||
| IBIL | <8.29 | 156 (49.8) | 43 (53.1) | 113 (48.7) | 0.583 |
| ≥8.29 | 157 (50.2) | 38 (46.9) | 119 (51.3) | ||
| TP | <74 | 132 (42.2) | 33 (40.7) | 99 (42.7) | 0.863 |
| ≥74 | 181 (57.8) | 48 (59.3) | 133 (57.3) | ||
| G | <29 | 137 (43.8) | 31 (38.3) | 106 (45.7) | 0.304 |
| ≥29 | 176 (56.2) | 50 (61.7) | 126 (54.3) | ||
| A/G | <1.5 | 104 (33.2) | 34 (42.0) | 70 (30.2) | 0.071 |
| ≥1.5 | 209 (66.8) | 47 (58.0) | 162 (69.8) | ||
| PAB | <267 | 156 (49.8) | 36 (44.4) | 120 (51.7) | 0.318 |
| ≥267 | 157 (50.2) | 45 (55.6) | 112 (48.3) | ||
| CA153 | <9.82 | 156 (49.8) | 44 (54.3) | 112 (48.3) | 0.419 |
| ≥9.82 | 157 (50.2) | 37 (45.7) | 120 (51.7) | ||
| CEA | <1.49 | 156 (49.8) | 35 (43.2) | 121 (52.2) | 0.209 |
| ≥1.49 | 157 (50.2) | 46 (56.8) | 111 (47.8) | ||
| D-D | <0.25 | 151 (48.2) | 41 (50.6) | 110 (47.4) | 0.713 |
| ≥0.25 | 162 (51.8) | 40 (49.4) | 122 (52.6) | ||
| FBG | <2.6 | 153 (48.9) | 48 (59.3) | 105 (45.3) | 0.041 |
| ≥2.6 | 160 (51.1) | 33 (40.7) | 127 (54.7) | ||
| INR | <0.97 | 139 (44.4) | 43 (53.1) | 96 (41.4) | 0.090 |
| ≥0.97 | 174 (55.6) | 38 (46.9) | 136 (58.6) | ||
| White blood cell | <5.45 | 156 (49.8) | 53 (65.4) | 103 (44.4) | 0.002 |
| ≥5.45 | 157 (50.2) | 28 (34.6) | 129 (55.6) | ||
| Neutrophil | <3.23 | 155 (49.5) | 68 (84.0) | 87 (37.5) | <0.001 |
| ≥3.23 | 158 (50.5) | 13 (16.0) | 145 (62.5) | ||
| Lymphocyte | <1.70 | 154 (49.2) | 19 (23.5) | 135 (58.2) | <0.001 |
| ≥1.70 | 159 (50.8) | 62 (76.5) | 97 (41.8) | ||
| NLR | Low | 105 (33.5) | 80 (98.8) | 25 (10.8) | <0.001 |
| High | 208 (66.5) | 1 (1.2) | 207 (89.2) | ||
| Monocyte | <0.35 | 151 (48.2) | 48 (59.3) | 103 (44.4) | 0.030 |
| ≥0.35 | 162 (51.8) | 33 (40.7) | 129 (55.6) | ||
| Platelet | <233 | 153 (48.9) | 37 (45.7) | 116 (50.0) | 0.589 |
| ≥233 | 160 (51.1) | 44 (54.3) | 116 (50.0) |
Abbreviation: GRIm score: Gustave Roussy Immune score; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; GGT: γ-glutamyl transpeptidase; ALB: albumin; TBIL: total bilirubin; DBIL: direct bilirubin; IBIL: indirect bilirubin; TP: total protein; G: globularproteins; PAB: prealbumin; CA153: cancer antigen 153; CEA: carcinoembryonic antigen; D-D: D-Dimer; FBG: fibrinogen; INR: international normalized ratio; NLR: neutrophil to lymphocyte ratio.
A performance comparison between GRIm score and pathological features.
| Parameters | level | Overall | Low GRIm score | High GRIm score | p |
|---|---|---|---|---|---|
| n | 313 | 81 | 232 | ||
| Tumor size | ≤2 | 154 (49.2) | 43 (53.1) | 111 (47.8) | 0.420 |
| >2 and<5 | 149 (47.6) | 37 (45.7) | 112 (48.3) | ||
| ≥5 | 10 (3.2) | 1 (1.2) | 9 (3.9) | ||
| Total lymph nodes (TLN) | <16 | 149 (47.6) | 40 (49.4) | 109 (47.0) | 0.808 |
| ≥16 | 164 (52.4) | 41 (50.6) | 123 (53.0) | ||
| Positive lymph nodes (PLN) | <1 | 134 (42.8) | 39 (48.1) | 95 (40.9) | 0.319 |
| ≥1 | 179 (57.2) | 42 (51.9) | 137 (59.1) | ||
| Total axillary lymph nodes (TALN) | <14 | 149 (47.6) | 42 (51.9) | 107 (46.1) | 0.447 |
| ≥14 | 164 (52.4) | 39 (48.1) | 125 (53.9) | ||
| Positive axillary lymph nodes (PALN) | <1 | 157 (50.2) | 49 (60.5) | 108 (46.6) | 0.042 |
| ≥1 | 156 (49.8) | 32 (39.5) | 124 (53.4) | ||
| ER | 0-25% | 144 (46.0) | 36 (44.4) | 108 (46.6) | 0.918 |
| 26-50% | 26 (8.3) | 6 (7.4) | 20 (8.6) | ||
| 51-75% | 48 (15.3) | 12 (14.8) | 36 (15.5) | ||
| 76-100% | 95 (30.4) | 27 (33.3) | 68 (29.3) | ||
| PR | 0-25% | 192 (61.3) | 51 (63.0) | 141 (60.8) | 0.709 |
| 26-50% | 35 (11.2) | 7 (8.6) | 28 (12.1) | ||
| 51-75% | 35 (11.2) | 11 (13.6) | 24 (10.3) | ||
| 76-100% | 51 (16.3) | 12 (14.8) | 39 (16.8) | ||
| HER2 | Negative | 185 (59.1) | 46 (56.8) | 139 (59.9) | 0.718 |
| Positive | 128 (40.9) | 35 (43.2) | 93 (40.1) | ||
| Ki67 | 0-25% | 147 (47.0) | 42 (51.9) | 105 (45.3) | 0.568 |
| 26-50% | 105 (33.5) | 25 (30.9) | 80 (34.5) | ||
| 51-75% | 46 (14.7) | 12 (14.8) | 34 (14.7) | ||
| 76-100% | 15 (4.8) | 2 (2.5) | 13 (5.6) | ||
| CK5/6 | Negative | 221 (70.6) | 57 (70.4) | 164 (70.7) | 1.000 |
| Positive | 92 (29.4) | 24 (29.6) | 68 (29.3) | ||
| E-cad | Negative | 11 (3.5) | 5 (6.2) | 6 (2.6) | 0.247 |
| Positive | 302 (96.5) | 76 (93.8) | 226 (97.4) | ||
| P120 | Negative | 293 (93.6) | 76 (93.8) | 217 (93.5) | 1.000 |
| Positive | 20 (6.4) | 5 (6.2) | 15 (6.5) | ||
| P53 | Negative | 170 (54.3) | 43 (53.1) | 127 (54.7) | 0.898 |
| Positive | 143 (45.7) | 38 (46.9) | 105 (45.3) | ||
| Blood vessel invasion | No | 289 (92.3) | 76 (93.8) | 213 (91.8) | 0.730 |
| Yes | 24 (7.7) | 5 (6.2) | 19 (8.2) |
Abbreviation: GRIm score: Gustave Roussy Immune score; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; E-cad: E-cadherin.
The univariate analysis and multivariate analysis for DFS.
| Parameters | Group | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Low | High | Low | High | ||||||
| GRIm score | Low | 0.005 | 1(Ref.) | 0.008 | 1(Ref.) | ||||
| High | 2.891 | 1.382 | 6.047 | 2.789 | 1.304 | 5.965 | |||
| NLR | Low | 0.007 | 1(Ref.) | 0.004 | 1(Ref.) | ||||
| High | 2.288 | 1.250 | 4.188 | 2.500 | 1.349 | 4.636 | |||
| Age* | <51 | 0.743 | 1(Ref.) | ||||||
| ≥51 | 0.860 | 0.350 | 2.113 | ||||||
| BMI* | <23.8 | 0.534 | 1(Ref.) | ||||||
| ≥23.8 | 0.819 | 0.437 | 1.537 | ||||||
| Family history | No | 0.008 | 1(Ref.) | 0.015 | 1(Ref.) | ||||
| Yes | 2.435 | 1.265 | 4.687 | 1.964 | 1.137 | 3.391 | |||
| Basic disease | No | 0.015 | 1(Ref.) | 0.052 | 1(Ref.) | ||||
| Yes | 2.190 | 1.165 | 4.115 | 1.730 | 0.995 | 3.010 | |||
| Menarche age* | <15 | 0.131 | 1(Ref.) | ||||||
| ≥15 | 1.618 | 0.866 | 3.023 | ||||||
| Menopause | No | 0.000 | 1(Ref.) | 0.011 | 1(Ref.) | ||||
| Yes | 2.886 | 1.698 | 4.905 | 2.084 | 1.183 | 3.671 | |||
| ALT | <21 | 0.080 | 1(Ref.) | ||||||
| ≥21 | 2.327 | 0.905 | 5.982 | ||||||
| AST | <23 | 0.531 | 1(Ref.) | ||||||
| ≥23 | 0.805 | 0.408 | 1.588 | ||||||
| AST/ALT | <1.44 | 0.348 | 1(Ref.) | ||||||
| ≥1.44 | 1.445 | 0.669 | 3.121 | ||||||
| LDH | <170 | 0.850 | 1(Ref.) | ||||||
| ≥170 | 1.063 | 0.564 | 2.004 | ||||||
| ALB | <45 | 0.114 | 1(Ref.) | ||||||
| ≥45 | 0.623 | 0.347 | 1.120 | ||||||
| TBIL | <12.45 | 0.678 | 1(Ref.) | ||||||
| ≥12.45 | 1.139 | 0.615 | 2.111 | ||||||
| CA153 | <9.82 | 0.309 | 1(Ref.) | ||||||
| ≥9.82 | 1.373 | 0.746 | 2.526 | ||||||
| CEA | <1.49 | 0.203 | 1(Ref.) | ||||||
| ≥1.49 | 1.514 | 0.800 | 2.868 | ||||||
| D-D | <0.25 | 0.077 | 1(Ref.) | ||||||
| ≥0.25 | 0.594 | 0.333 | 1.058 | ||||||
| FBG | <2.6 | 0.984 | 1(Ref.) | ||||||
| ≥2.6 | 1.007 | 0.538 | 1.884 | ||||||
| White blood cell | <5.45 | 0.836 | 1(Ref.) | ||||||
| ≥5.45 | 0.906 | 0.356 | 2.306 | ||||||
| Neutrophil | <3.23 | 0.970 | 1(Ref.) | ||||||
| ≥3.23 | 0.982 | 0.375 | 2.571 | ||||||
| Lymphocyte | <1.70 | 0.514 | 1(Ref.) | ||||||
| ≥1.70 | 0.853 | 0.530 | 1.374 | ||||||
| Monocyte | <0.35 | 0.266 | 1(Ref.) | ||||||
| ≥0.35 | 0.691 | 0.360 | 1.325 | ||||||
| Platelet | <233 | 0.689 | 1(Ref.) | ||||||
| ≥233 | 0.877 | 0.460 | 1.670 | ||||||
| P Tumor size | ≤2 | 0.893 | 1(Ref.) | ||||||
| >2 and<5 | 0.866 | 1.062 | 0.527 | 2.139 | |||||
| ≥5 | 0.726 | 0.784 | 0.201 | 3.057 | |||||
| Pathological TNM Stage | I | 0.000 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| II | 0.104 | 2.251 | 0.847 | 5.982 | 0.370 | 1.401 | 0.670 | 2.932 | |
| III | 0.000 | 24.936 | 5.617 | 110.705 | 0.000 | 6.100 | 3.005 | 12.383 | |
| TALN | <14 | 0.674 | 1(Ref.) | ||||||
| ≥14 | 1.108 | 0.687 | 1.786 | ||||||
| PALN | <1 | 0.004 | 1(Ref.) | ||||||
| ≥1 | 2.053 | 1.253 | 3.365 | ||||||
| Molecular subtype | Luminal A | 0.345 | 1(Ref.) | ||||||
| Luminal B HER2+ | 0.103 | 3.258 | 0.786 | 13.498 | |||||
| Luminal B HER2- | 0.091 | 3.439 | 0.822 | 14.378 | |||||
| HER2 enriched | 0.391 | 2.064 | 0.394 | 10.811 | |||||
| Triple negative | 0.382 | 2.059 | 0.408 | 10.399 | |||||
| Chemotherapy | No | 0.000 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| Yes | 0.166 | 0.065 | 0.423 | 0.214 | 0.107 | 0.427 | |||
| Radiotherapy | No | 0.456 | 1(Ref.) | ||||||
| Yes | 0.736 | 0.328 | 1.650 | ||||||
| Endocrine therapy | No | 0.000 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| Yes | 0.178 | 0.069 | 0.461 | 0.241 | 0.137 | 0.423 | |||
| Targeted therapy | No | 0.814 | 1(Ref.) | ||||||
| Yes | 0.882 | 0.308 | 2.521 |
*Confounding factor. Abbreviation: DFS: disease free survival; GRIm score: Gustave Roussy Immune score; BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; GGT: γ-glutamyl transpeptidase; ALB: albumin; TBIL: total bilirubin; DBIL: direct bilirubin; IBIL: indirect bilirubin; TP: total protein; G: globularproteins; PAB: prealbumin; CA153: cancer antigen 153; CEA: carcinoembryonic antigen; D-D: D-Dimer; FBG: fibrinogen; INR: international normalized ratio; NLR: neutrophil to lymphocyte ratio; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; E-cad: E-cadherin.
The univariate analysis and multivariate analysis for OS.
| Parameters | Group | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | ||||
| Low | High | Low | High | ||||||
| GRIm score | Low | 0.005 | 1(Ref.) | 0.004 | 1(Ref.) | ||||
| High | 3.613 | 1.383 | 13.708 | 3.015 | 1.409 | 10.087 | |||
| NLR | Low | 0.008 | 1(Ref.) | 0.011 | 1(Ref.) | ||||
| High | 2.264 | 1.237 | 4.145 | 2.225 | 1.203 | 4.116 | |||
| Age* | <51 | 0.446 | 1(Ref.) | ||||||
| ≥51 | 0.688 | 0.263 | 1.800 | ||||||
| BMI* | <23.8 | 0.629 | 1(Ref.) | ||||||
| ≥23.8 | 0.858 | 0.460 | 1.600 | ||||||
| Family history | No | 0.027 | 1(Ref.) | 0.011 | 1(Ref.) | ||||
| Yes | 1.772 | 1.066 | 2.946 | 2.020 | 1.174 | 3.473 | |||
| Basic disease | No | 0.115 | 1(Ref.) | ||||||
| Yes | 1.675 | 0.882 | 3.179 | ||||||
| Menarche age* | <15 | 0.401 | 1(Ref.) | ||||||
| ≥15 | 1.296 | 0.708 | 2.370 | ||||||
| Menopause | No | 0.052 | 1(Ref.) | ||||||
| Yes | 2.732 | 0.990 | 7.536 | ||||||
| ALT | <21 | 0.010 | 1(Ref.) | 0.002 | 1(Ref.) | ||||
| ≥21 | 3.545 | 1.350 | 9.306 | 2.301 | 1.357 | 3.901 | |||
| AST | <23 | 0.505 | 1(Ref.) | ||||||
| ≥23 | 0.792 | 0.399 | 1.572 | ||||||
| AST/ALT | <1.44 | 0.453 | 1(Ref.) | ||||||
| ≥1.44 | 1.342 | 0.622 | 2.895 | ||||||
| LDH | <170 | 0.440 | 1(Ref.) | ||||||
| ≥170 | 0.784 | 0.423 | 1.454 | ||||||
| ALB | <45 | 0.478 | 1(Ref.) | ||||||
| ≥45 | 0.842 | 0.523 | 1.355 | ||||||
| TBIL | <12.45 | 0.659 | 1(Ref.) | ||||||
| ≥12.45 | 1.151 | 0.616 | 2.150 | ||||||
| CA153 | <9.82 | 0.913 | 1(Ref.) | ||||||
| ≥9.82 | 1.034 | 0.565 | 1.895 | ||||||
| CEA | <1.49 | 0.067 | 1(Ref.) | ||||||
| ≥1.49 | 1.837 | 0.959 | 3.517 | ||||||
| D-D | <0.25 | 0.125 | 1(Ref.) | ||||||
| ≥0.25 | 0.636 | 0.357 | 1.133 | ||||||
| FBG | <2.6 | 0.938 | 1(Ref.) | ||||||
| ≥2.6 | 0.975 | 0.516 | 1.843 | ||||||
| White blood cell | <5.45 | 0.887 | 1(Ref.) | ||||||
| ≥5.45 | 1.072 | 0.410 | 2.804 | ||||||
| Neutrophil | <3.23 | 0.650 | 1(Ref.) | ||||||
| ≥3.23 | 0.806 | 0.318 | 2.044 | ||||||
| Lymphocyte | <1.70 | 0.462 | 1(Ref.) | ||||||
| ≥1.70 | 0.836 | 0.519 | 1.347 | ||||||
| Monocyte | <0.35 | 0.727 | 1(Ref.) | ||||||
| ≥0.35 | 0.890 | 0.461 | 1.715 | ||||||
| Platelet | <233 | 0.807 | 1(Ref.) | ||||||
| ≥233 | 0.924 | 0.491 | 1.740 | ||||||
| P Tumor size | ≤2 | 0.964 | 1(Ref.) | ||||||
| >2 and<5 | 0.790 | 0.909 | 0.448 | 1.842 | |||||
| ≥5 | 0.960 | 0.967 | 0.259 | 3.606 | |||||
| Pathological TNM Stage | I | 0.000 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| II | 0.085 | 2.482 | 0.883 | 6.980 | 0.393 | 1.383 | 0.657 | 2.907 | |
| III | 0.000 | 24.430 | 5.325 | 112.074 | 0.000 | 9.852 | 4.540 | 21.383 | |
| TALN | <14 | 0.703 | 1(Ref.) | ||||||
| ≥14 | 1.097 | 0.681 | 1.768 | ||||||
| PALN | <1 | 0.284 | 1(Ref.) | ||||||
| ≥1 | 0.554 | 0.188 | 1.632 | ||||||
| Molecular subtype | Luminal A | 0.256 | 1(Ref.) | ||||||
| Luminal B HER2+ | 0.114 | 3.144 | 0.759 | 13.021 | |||||
| Luminal B HER2- | 0.118 | 3.032 | 0.756 | 12.163 | |||||
| HER2 enriched | 0.322 | 2.307 | 0.441 | 12.079 | |||||
| Triple negative | 0.669 | 1.437 | 0.273 | 7.569 | |||||
| Chemotherapy | No | 0.000 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| Yes | 0.147 | 0.057 | 0.378 | 0.141 | 0.069 | 0.289 | |||
| Radiotherapy | No | 0.122 | 1(Ref.) | 0.008 | 1(Ref.) | ||||
| Yes | 0.526 | 0.233 | 1.188 | 0.412 | 0.214 | 0.793 | |||
| Endocrine therapy | No | 0.001 | 1(Ref.) | 0.000 | 1(Ref.) | ||||
| Yes | 0.193 | 0.072 | 0.517 | 0.264 | 0.148 | 0.468 | |||
| Targeted therapy | No | 0.286 | 1(Ref.) | ||||||
| Yes | 0.559 | 0.192 | 1.626 |
*Confounding factor. Abbreviation: OS: overall survival; GRIm score: Gustave Roussy Immune score; BMI: body mass index; ALT: alanine aminotransferase; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; GGT: γ-glutamyl transpeptidase; ALB: albumin; TBIL: total bilirubin; DBIL: direct bilirubin; IBIL: indirect bilirubin; TP: total protein; G: globularproteins; PAB: prealbumin; CA153: cancer antigen 153; CEA: carcinoembryonic antigen; D-D: D-Dimer; FBG: fibrinogen; INR: international normalized ratio; NLR: neutrophil to lymphocyte ratio; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; E-cad: E-cadherin.
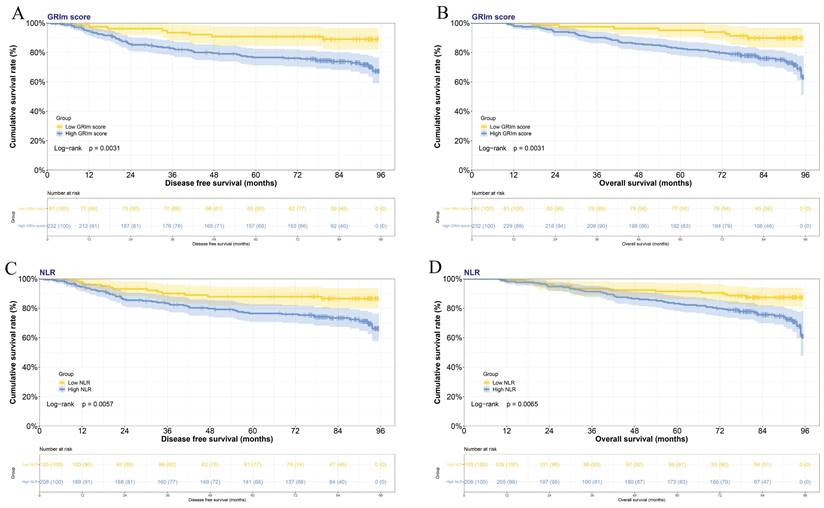
Nomograms constructed and validated
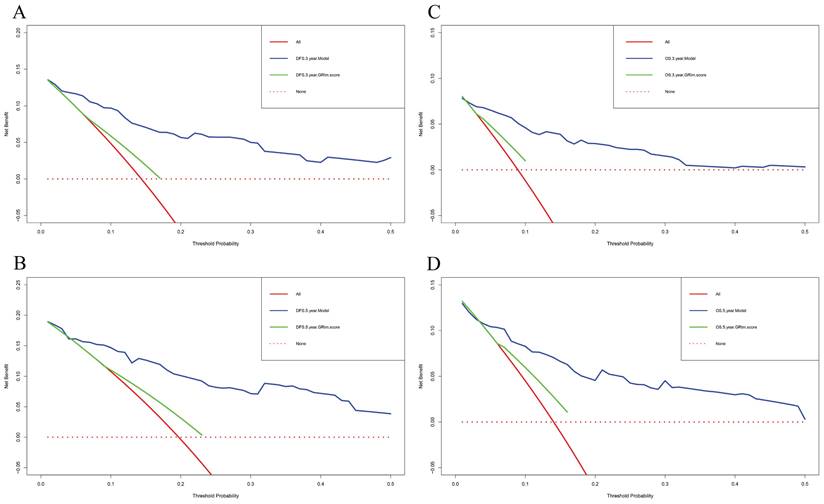
In this study, the parameters with P < 0.05, including GRIm score, NLR, family history, menopause, pathological TNM stage, chemotherapy, and endocrine therapy, were selected based on multivariate analyses to construct a nomogram for the prediction of DFS in Figure 2A. The C-index for this nomogram model predicting DFS was 0.823 (95%CI: 0.699-0.903). Furthermore, the parameters with P < 0.05, including GRIm score, NLR, family history, ALT, pathological TNM stage, chemotherapy, and endocrine therapy by multivariate analyses were chose to comprise nomogram for the prediction of OS in Figure 2B. The C-index for this nomogram model predicting OS was 0.807 (95%CI: 0.684-0.890). The calibration curves used to predict DFS at 1-, 3-, and 5-year intervals exhibited significant correlation between predictions and actual observations, indicating minimal departure from an ideal fit in Figure 3A-C. Also, the calibration curves used to evaluate 1-, 3-, and 5-year OS showed that good correlation between predictions and actual observations, and no significant deviation from perfect fit in Figure 3D-F. Furthermore, the nomograms constructed indicated a superior positive net benefit compared to the GRIm score in predicting 3- and 5-year DFS, as shown in Figure 4A-B, and in predicting 3- and 5-year DFS in Figure 4C-D. In addition, the nomograms constructed indicated a better positive net benefit than NLR in predicting 3- and 5-year DFS, as illustrated in Figure S1A-B, and in predicting 3- and 5-year DFS in Figure S1C-D.
Subgroup analyses
According to multivariate analyses, TNM stage was also a potential factor in this study. Of those patients, 85 (27.2%) cases were stage I breast cancer, 138 (44.1%) cases were stage II breast cancer, 90 (28.8%) cases were stage III breast cancer, respectively. The mean DFS and OS were 75.27 and 83.17 months in stage I breast cancer, 73.58 and 83.05 months in stage II breast cancer, 53.69 and 68.58 months in stage III breast cancer, respectively. Kaplan-Meier estimations of both DFS and OS, categorized according to prognostic risk for each TNM stage, are presented in Figure S2A and S2B. Significant variations in both DFS and OS were observed among the distinct TNM stage groups. (DFS: χ2=30.140, P<0.0001; OS: χ2=260.43, P<0.0001). We analyzed the relationship between TNM stage and the common hematological parameters. Compared to patients with stage I or stage II, patients with stage III were notably linked to TBIL (P=0.002), CA153 (P=0.001), CEA (P=0.004), monocyte (P=0.031). Detailed information is shown in Table S2. Furthermore, we also analyzed the prognostic effect of the GRIm score and its components by different TNM stage. The results indicated that the GRIm score was related to NLR, neutrophil, and lymphocyte. Detailed information is shown in Table S3.
Nomograms to predict the survival time among patients with breast cancer. A) Nomograms to predict the DFS among patients with breast cancer; B) Nomograms to predict the OS among patients with breast cancer.
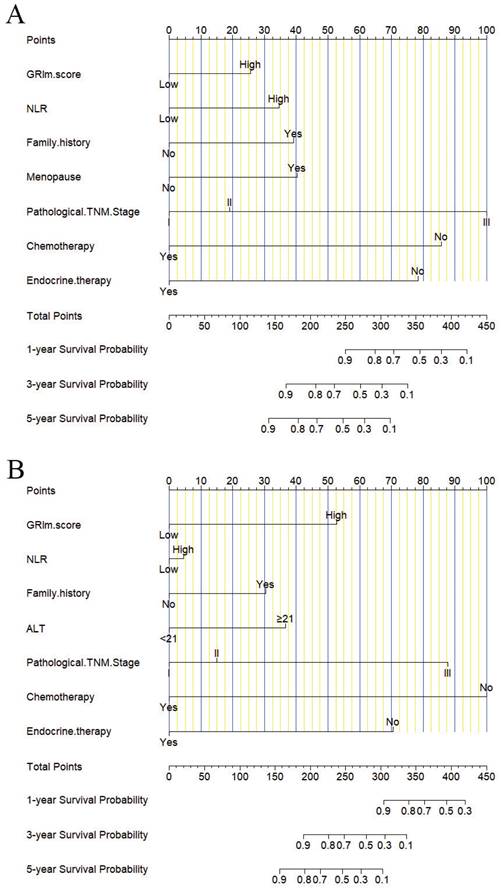
The predicted 1-, 3-, and 5-year DFS rates and OS rates of breast cancer patients using the nomogram closely matched the actual observed values by calibration curves. A) The predicted 1-year DFS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve; B) The predicted 3-year DFS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve; C) The predicted 5-year DFS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve; D) The predicted 1-year OS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve; E) The predicted 3-year OS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve; F) The predicted 5-year OS rate of breast cancer patients using the nomogram closely matched the actual observed values by calibration curve.
Based on multivariate analyses, chemotherapy and endocrine therapy were also a potential factor in this study. We further analyzed the data and found that patients who received chemotherapy had longer survival time than those who did not receive chemotherapy (DFS: P=0.00084; OS: P=0.00034). Moreover, for those received chemotherapy patients (290 cases), patients with low GRIm score group had survived longer than those with high GRIm score group (DFS: P=0.0073; OS: P=0.0075). We also analyzed the effects of endocrine therapy and found that patients who received endocrine therapy had longer survival times than those who did not receive it (DFS: P<0.0001; OS: P<0.0001). Moreover, for those who received endocrine therapy patients (163 cases), patients with low GRIm score group had survived longer than those with high GRIm score group (DFS: P=0.026; OS: P=0.030).
Decision curve analyses (DCA) of nomogram and GRIm score prediction model for predicting the DFS and OS rates in 3- and 5-year. A) DCA of nomogram and GRIm score prediction model for predicting the DFS rates in 3-year; B) DCA of nomogram and GRIm score prediction model for predicting the DFS rates in 5-year; C) DCA of nomogram and GRIm score prediction model for predicting the OS rates in 3-year; D) DCA of nomogram and GRIm score prediction model for predicting the OS rates in 5-year.
Discussion
In this study, the GRIm score was constructed by the albumin level, LDH level, total bilirubin level, AST-to-ALT ratio, and NLR. To our knowledge, the GRIm score serves as a valuable predictor of survival outcomes among patients with diverse malignant tumor types, such as esophageal squamous cell carcinoma [15], non-small cell lung cancer [16], hepatocellular carcinoma [17], operable pancreatic adenocarcinoma [18]. Despite its potential utility, limited studies have explored the predictive value of the GRIm score specifically in the context of breast cancer. Our study concluded that the GRIm score serves as an independent predictor of DFS and OS in patients with breast cancer. Our findings revealed that patients exhibiting a low GRIm score exhibited prolonged DFS and OS.
The blood albumin level, a widely recognized nutritional index, has been established to have a correlation with liver function and prognosis. In Tanriverdi O's study, they demonstrated that patients with low serum albumin levels exhibited shorter PFS and OS. Furthermore, a reduced level of serum albumin was determined as a standalone factor that was significantly linked to a worse outcome in patients with Stage IIIB non-small cell lung cancer [19]. Fujii T's study investigated the relationship between serum levels of albumin and breast cancer, and demonstrated that low serum albumin levels were related to worse prognosis, but not a stand-alone prognostic indicator for the tumor microenvironment in breast cancer [20].
As is known to all, the serum level of total bilirubin reflects the liver function. Serum bilirubin is the final product of blood metabolism and has many protective properties, such as anti-inflammatory, antioxidant, and anticancer activities; and it is negatively correlated with various malignant tumors [21]. In Gao C's study, their results indicated that higher serum direct bilirubin concentrations were related to the increased risk of poor prognosis in rectal cancer [22]. Another study showed that the serum total bilirubin levels prior to treatment may serve as a potential biomarker for anticipating the clinical outcomes among patients diagnosed with primary central nervous system lymphoma undergoing a combination of chemotherapy and immunochemotherapy [23]. According to these studies, the results were summarized as follows those patients with lower albumin levels or higher total bilirubin levels had poor survival.
Lactate dehydrogenase (LDH) serves as a well-recognized biomarker of inflammatory responses, and is detected in many tissues of the human body. LDH not only is a simple indicator of tumor burden but also is a complex biomarker associated with immunogenicity, metabolic activity, and invasiveness of numerous tumors [24]. A meta-analysis examined the association between LDH levels prior to treatment and clinical results in patients with non-small cell lung cancer, indicating that patients with elevated LDH values lead to poorer PFS and OS [25].
The levels of AST and ALT are widely recognized markers for assessing the preservation of liver function. A prior study indicated that the serum AST-to-ALT ratio emerged as a reliable prognostic factor for DFS, but not for OS in patients suffering from colorectal cancer that was non-metastatic and at stages II and III [26]. Wang F's study indicated that the pre-surgical serum AST-to-ALT ratio might serve as a predictive marker for patients with hepatocellular carcinoma undergoing simultaneous thermal ablation and transarterial chemoembolization [27]. Another study also indicated that a higher ratio of AST-to-ALT in the blood serum was associated with a poorer prognosis for patients with HBV-induced hepatocellular carcinoma receiving hepatectomy [28]. Even though the precise mechanism is not yet clear, a speculated mechanism is that as liver function becomes increasingly impaired, invasive tumor progression results in a marked elevation of AST levels and a concomitant reduction in the rate of AST clearance [28]. Therefore, we assume that an elevated AST-to-ALT ratio can be applied to impair liver function and predict the prognosis of tumors.
Inflammation, being a vital part of the tumor microenvironment, is instrumental in driving cancer progression [29]. As a sensitive biomarker of inflammation, NLR has different effects on the development of tumors [30]. Certain studies have shown that tumor-stimulated neutrophils facilitate angiogenesis, immune suppression, and enhance the infiltration, migration, and metastatic abilities of tumor cells [31]. In contrast, the number of tumor-infiltrating lymphocytes can vary among different types of tumors, and its quantitation has been explored as a potential means to enhance clinical response to chemotherapy. Exactly, the increased neutrophil count often indicates a higher risk of inflammation, while a higher lymphocyte count signifies a stronger immune response. Therefore, we hypothesize that an elevated NLR can potentially be used to predict the prognosis of tumors, as it reflects the balance between inflammation and immune response within the tumor microenvironment.
According to the above biological mechanisms, it is entirely plausible to anticipate expected cancer patient survival rates with GRIm score. The relationship between GRIm score and the prognosis of malignant tumors has been determined [18,32,33]. The current study suggests that patients with a high GRIm score exhibited a poorer prognosis and shorter survival time (DFS, HR: 2.789, 95% CI: 1.304-5.965, P=0.008; OS: HR: 3.015, 95% CI: 1.409-10.087, P=0.004) in early breast cancer. The utility of neutrophils and lymphocytes in tumors and immunotherapy is reported. NLR was one of the comprised of GRIm score factors. Then, the patients in high NLR value had poor prognosis and short survival time (DFS, HR: 2.500, 95% CI: 1.349-4.636, P=0.004; OS: HR: 2.225, 95% CI: 1.203-4.116, P=0.011). Moreover, the multivariate analysis identified the GRIm score, NLR, TNM stage, chemotherapy, and endocrine therapy as potential prognostic factors. TNM stage was the common factor for predicting prognosis for tumors. The GRIm score was related to NLR, neutrophil, and lymphocyte by different TNM stage. Moreover, patients, who received chemotherapy or endocrine therapy, with low GRIm score group had survived longer than those with high GRIm score group. Furthermore, nomograms were constructed by these prognostic factors. In addition, the nomograms constructed by GRIm score performed superior predictive capabilities than NLR or only by GRIm score.
It's worth noting that the present research has various constraints that ought to be considered. Firstly, our retrospective analysis was restricted to data from one single institution only, thus our findings necessitate further prospective validation through multicenter studies involving independent patient groups. Secondly, due to the restricted duration of observation in the present study, a more extended period of observation could potentially have led to different findings. Lastly, the nomograms constructed need to be tested and verified by an external validation cohort in the following study.
Conclusion
In conclusion, our pooled results demonstrate that GRIm score, as a novel prognostic score, could be a valuable prognostic tool for breast cancer patients. The nomograms formulated using the GRIm score have demonstrated their capacity to precisely forecast the DFS and OS rates among breast cancer patients. The GRIm score can help oncologists discuss prognosis, treatment decisions, and patient selection for clinical trials.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
The authors thank the patients for their contributions to the investigation of clinical data and sample collection.
Funding
This research was supported by grants from the Practice and Innovation Project for Postgraduates of Harbin Medical University (Grant Number: YJSCX2023-79HYD).
Author contributions
Yuanxi Huang and Chunlei Tan: Conceptualization. Yuanxi Huang and Chunlei Tan: Data curation. Chunlei Tan, Jinling Xu, Danping Wu and Xiaotian Yang: Formal analysis, Visualization, Validation, Software. Chunlei Tan: Funding acquisition. Chunlei Tan and Shiyuan Zhang: Investigation. Jinling Xu and Shuqiang Liu: Methodology. Yuanxi Huang: Project administration. Yuanxi Huang: Resources. Yuanxi Huang and Boqian Yu: Supervision. Chunlei Tan and Jinling Xu: Writing - original draft. Chunlei Tan and Xiaotian Yang: Writing - Review & Editing.
Data availability statement
The material supporting the conclusion of this article has been included within the article.
Ethics approval and consent to participate
This research received approval from the Ethics Committee of Harbin Medical University Cancer Hospital (Grant Number: KY2023-38). In this study, all participants were thoroughly informed about the objectives, procedures, potential risks, and their rights related to the research. Specifically, written informed consent was obtained from each participant, documenting their agreement to participate in the research and authorizing the use of their data for scientific analysis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674
2. Morrison L, Loibl S, Turner NC. The CDK4/6 inhibitor revolution - a game-changing era for breast cancer treatment. Nat Rev Clin Oncol. 2024;21:89-105
3. Loibl S, André F, Bachelot T. et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:159-82
4. Gardner D, Lucas PB, Cowdry RW. Soft sign neurological abnormalities in borderline personality disorder and normal control subjects. J Nerv Ment Dis. 1987;175:177-80
5. Topkan E, Selek U, Pehlivan B. et al. The Prognostic Value of the Novel Global Immune-Nutrition-Inflammation Index (GINI) in Stage IIIC Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy. Cancers (Basel). 2023;15:4512
6. Liu J, Lai S, Wu P. et al. Impact of a novel immune and nutritional score on prognosis in patients with upper urinary tract urothelial carcinoma following radical nephroureterectomy. J Cancer Res Clin Oncol. 2023;149:10893-909
7. Guo J, Yang Q, Jiang Q, Gu L-W, Lin H-X, Guo L. Integrating Baseline Nutritional and Inflammatory Parameters with Post-Treatment EBV DNA Level to Predict Outcomes of Patients with De Novo Metastatic Nasopharyngeal Carcinoma Receiving Chemotherapy Combination PD-1 Inhibitor. Nutrients. 2023;15:4262
8. Chen L, Liu Q, Tan C. et al. The Age-Male-Albumin-Bilirubin-Platelets (aMAP) Risk Score Predicts Liver Metastasis Following Surgery for Breast Cancer in Chinese Population: A Retrospective Study. Immunotargets Ther. 2024;13:75-94
9. Min-Oo HH, Aung TM, Wongwattanakul M, Maraming P, Proungvitaya T, Proungvitaya S. Development of a Prognostic Score for Cholangiocarcinoma Patients Using a Combination of Biochemical Parameters. In Vivo. 2023;37:1145-55
10. Chen L, Tan C, Li Q. et al. Assessment of the albumin-bilirubin score in breast cancer patients with liver metastasis after surgery. Heliyon. 2023;9:e21772
11. Chen L, Chen Y, Zhang L. et al. In Gastric Cancer Patients Receiving Neoadjuvant Chemotherapy Systemic Inflammation Response Index is a Useful Prognostic Indicator. Pathol Oncol Res. 2021;27:1609811
12. Sen S, Hess K, Hong DS. et al. Development of a prognostic scoring system for patients with advanced cancer enrolled in immune checkpoint inhibitor phase 1 clinical trials. Br J Cancer. 2018;118:763-9
13. Ma LX, Espin-Garcia O, Bach Y. et al. Comparison of Four Clinical Prognostic Scores in Patients with Advanced Gastric and Esophageal Cancer. Oncologist. 2023;28:214-9
14. Bigot F, Castanon E, Baldini C. et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur J Cancer. 2017;84:212-8
15. Shi Y, Shen G, Zeng Y. et al. Predictive values of the hemoglobin, albumin, lymphocyte and platelet score (HALP) and the modified -Gustave Roussy immune score for esophageal squamous cell carcinoma patients undergoing concurrent chemoradiotherapy. Int Immunopharmacol. 2023;123:110773
16. Minami S, Ihara S, Ikuta S, Komuta K. Gustave Roussy Immune Score and Royal Marsden Hospital Prognostic Score Are Biomarkers of Immune-Checkpoint Inhibitor for Non-Small Cell Lung Cancer. World J Oncol. 2019;10:90-100
17. Hatanaka T, Naganuma A, Hiraoka A. et al. The hepatocellular carcinoma modified Gustave Roussy Immune score (HCC-GRIm score) as a novel prognostic score for patients treated with atezolizumab and bevacizumab: A multicenter retrospective analysis. Cancer Med. 2023;12:4259-69
18. Basoglu T, Babacan NA, Ozturk FE. et al. Prognostic value of Gustave Roussy immune score in operable pancreatic adenocarcinoma. Indian J Cancer. 2023;60:179-84
19. Tanriverdi O, Avci N, Oktay E. et al. Pretreatment Serum Albumin Level is an Independent Prognostic Factor in Patients with Stage IIIB Non-Small Cell Lung Cancer: A Study of the Turkish Descriptive Oncological Researches Group. Asian Pac J Cancer Prev. 2015;16:5971-6
20. Fujii T, Tokuda S, Nakazawa Y. et al. Implications of Low Serum Albumin as a Prognostic Factor of Long-term Outcomes in Patients With Breast Cancer. In Vivo. 2020;34:2033-6
21. Cao Y, Deng S, Yan L. et al. A nomogram based on pretreatment levels of serum bilirubin and total bile acid levels predicts survival in colorectal cancer patients. BMC Cancer. 2021;21:85
22. Gao C, Fang L, Li J-T, Zhao H-C. Significance and prognostic value of increased serum direct bilirubin level for lymph node metastasis in Chinese rectal cancer patients. World J Gastroenterol. 2016;22:2576-84
23. Cao J, Li S, Li D, Hua W, Guo L, Xia Z. Development and Validation of Pretreatment Serum Total Bilirubin as a Biomarker to Predict the Clinical Outcomes in Primary Central Nervous System Lymphoma: A Multicenter Cohort Study. Cancers (Basel). 2023;15:4584
24. Claps G, Faouzi S, Quidville V. et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19:749-62
25. Zhang Z, Li Y, Yan X. et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. 2019;8:1467-73
26. Scheipner L, Smolle MA, Barth D. et al. The AST/ALT Ratio Is an Independent Prognostic Marker for Disease-free Survival in Stage II and III Colorectal Carcinoma. Anticancer Res. 2021;41:429-36
27. Wang F, Gao S, Wu M. et al. The prognostic role of the AST/ALT ratio in hepatocellular carcinoma patients receiving thermal ablation combined with simultaneous TACE. BMC Gastroenterol. 2023;23:80
28. Mo Q, Liu Y, Zhou Z. et al. Prognostic Value of Aspartate Transaminase/Alanine Transaminase Ratio in Patients With Hepatitis B Virus-Related Hepatocellular Carcinoma Undergoing Hepatectomy. Front Oncol. 2022;12:876900
29. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44
30. Nøst TH, Alcala K, Urbarova I. et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841-8
31. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141-8
32. Lenci E, Cantini L, Pecci F. et al. The Gustave Roussy Immune (GRIm)-Score Variation Is an Early-on-Treatment Biomarker of Outcome in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients Treated with First-Line Pembrolizumab. J Clin Med. 2021;10:1005
33. Li S-J, Zhao L, Wang H-Y. et al. Gustave Roussy Immune Score based on a three-category risk assessment scale serves as a novel and effective prognostic indicator for surgically resectable early-stage non-small-cell lung cancer: A propensity score matching retrospective cohort study. Int J Surg. 2020;84:25-40
Author contact
![]() Corresponding author: Yuanxi Huang, Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150081, P.R. China. (Email: rxwkcom).
Corresponding author: Yuanxi Huang, Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, Heilongjiang 150081, P.R. China. (Email: rxwkcom).

 Global reach, higher impact
Global reach, higher impact