3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(12):2390-2401. doi:10.7150/ijms.101114 This issue Cite
Research Paper
Using oral molecular hydrogen supplements to combat microinflammation in humans: a pilot observational study
1. Division of Nephrology, Department of Internal Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
2. Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
3. Division of Nephrology, Department of Internal Medicine, Fu-Jen Catholic University Hospital, School of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan.
4. Rheumatology/Immunology and Allergy, Department of Medicine, Armed Forces Taoyuan General Hospital, Taoyuan, Taiwan.
5. Division of Medicine, Armed Forces Taoyuan General Hospital, Taoyuan, Taiwan.
6. Nursing Department, Min-Sheng General Hospital, Taoyuan, Taiwan.
7. Division of Endocrinology and Metabolism, Department of Medicine, Armed Forces Taoyuan General Hospital, Taoyuan, Taiwan.
8. Department of Life Sciences, National Central University, Taoyuan, Taiwan.
9. Rheumatology/Immunology and Allergy, Department of Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan.
10. Division of Nephrology, Department of Internal Medicine, Taoyuan Armed Forces General Hospital, Taoyuan, Taiwan.
§Equal contribution.
Received 2024-7-18; Accepted 2024-9-3; Published 2024-9-9
Abstract
Background: Persistent inflammation over time can cause gradual harm to the body. Molecular hydrogen has the potential to specifically counteract reactive oxygen species (ROS), reduce disease severity, and enhance overall health. Investigations of the anti-inflammatory and antioxidant properties of oral solid hydrogen capsules (OSHCs) are currently limited, prompting our examination of the beneficial effects of OSHCs. Subsequently, we conducted a clinical study to assess the impact of OSHCs supplementation on individuals with chronic inflammation.
Materials and methods: Initially, we evaluated the oxidative reduction potential (ORP) properties of the OSHCs solution by comparing it to hydrogen-rich water (HRW) and calcium hydride (CaH2) treated water. In our outpatient department, stable patients with chronic illnesses who were treated with varying doses of OSHCs were randomized into low-, medium-, and high-dose groups for 4 weeks. Primary outcomes included changes in the serum erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentrations after four weeks of OSHCs consumption. Secondary outcomes included changes in the Brief Fatigue Inventory-Taiwan (BFI-T) fatigue scale, Control Status Scale for Diabetes (CSSD70) scores, and Disease Activity Score 28 (DAS28).
Results: Compared to HRW and CaH2, OSHCs demonstrated a prolonged reduction in ORP for 60 minutes in vitro and enabled a regulated release of hydrogen over 24 hours. A total of 30 participants, with 10 in each dosage (low/medium/high) group, completed the study. The average ESR120 significantly decreased from the first week to the fourth week, with a noticeable dose effect (low-dose group, p = 0.494; high-dose group, p = 0.016). Overall, the average CRP concentration showed a distinct decreasing trend after four weeks of OSHCs administration (w0 vs. w4, p = 0.077). The average DAS28 score demonstrated a significant decrease following OSHCs treatment. Furthermore, there were improvements in the BFI-T and CSSD70 scores.
Conclusion: OSHCs supplementation may exert anti-inflammatory and antioxidant effects on individuals with chronic inflammation. However, further clinical studies could be investigated to explore the potential therapeutic effects of OSHCs.
Keywords: antioxidant, anti-inflammatory effects, chronic diseases, chronic inflammation, molecular hydrogen, oral solid hydrogen capsules (OSHCs)
Introduction
Inflammation is the natural immune defense of the body against foreign pathogens. Prolonged immune system activation leads to chronic inflammation and a persistent systemic response involving continuous cytokine release. Persistent chronic inflammation can result in cumulative damage to the human body, contributing to the development of various chronic diseases, including cardiovascular disease, brain damage, cancer, rheumatoid arthritis, asthma, diabetes, metabolic disorders, and dementia, as indicated by recent studies [1-6]. The persistent release of proinflammatory cytokines from immune-related cells and activation of the innate immune system can lead to chronic systemic inflammation, thereby playing a role in the development or progression of chronic medical conditions. C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) serve as clinical markers to aid physicians in the accurate diagnosis and monitoring of various acute and chronic conditions [4-6]. Treating the intricate clinical manifestations of chronic inflammation remains challenging, and no effective treatments are currently available.
Recent research highlights that employing molecular hydrogen as an antioxidant effectively improves symptoms associated with chronic inflammation-related diseases [7]. Molecular hydrogen has notable effects on cellular oxygen radicals. It selectively reduces highly toxic oxygen radicals such as hydroxyl radicals and peroxynitrite, demonstrating a protective role against oxidative stress [8]. In addition, the present data suggest that the cellular protective effects facilitated by molecular hydrogen can be attributed to the adjustment of cellular antioxidant defenses, including antioxidant and cytoprotective genes, along with intracellular and extracellular redox signaling [9]. Furthermore, hydrogen interferes with reactive oxygen species (ROS) in living systems and serves as an antioxidant by selectively reducing cytotoxic oxygen radicals [10]. While the antioxidant hypothesis posits that the scavenging of hydroxyl radicals can partially explain certain biological effects of H2, the long debate surrounding H2 status stems from the significantly lower reaction rate of H2 with hydroxyl radicals than with other cellular antioxidants. In addition to its antioxidant function, H2 has been shown to have various effects, including anti-inflammatory, antiapoptotic, antishock, antidysmetabolic, and autophagy-activating effects and the ability to preserve mitochondrial function [8, 11]. In summary, supplementation with molecular hydrogen eliminates the damage caused by malignant free radicals while also eliminating chronic inflammation. For example, in patients with Parkinson's disease [9], cardiovascular disease [9], rheumatoid arthritis [12], allergic rhinitis [13, 14], atopic dermatitis [15, 16], asthma [17, 18], chronic obstructive pulmonary disease [19], and other diseases, hydrogen supplementation has been shown to reduce inflammation and improve symptoms.
There have been an increasing number of clinical studies on molecular hydrogen, such as nasal inhalation of hydrogen, injection of hydrogen saline, and drinking of hydrogen-rich water (HRW). A recent human study revealed that gaseous hydrogen reduces lung damage from cigarette poisoning [20]. Our recent article also demonstrates that molecular hydrogen not only scavenges harmful oxygen radicals but also promotes the production of antioxidant enzymes through various redox signaling pathways [21]. However, studies on solid dosage forms of molecular hydrogen have been limited to animal experiments [22]. We performed an observational study focusing on the use of oral molecular hydrogen supplements in humans. This study aimed to evaluate the effect of oral solid hydrogen capsules (OSHCs) supplementation on the inflammatory status of patients with chronic inflammation through scientific assessments and objective clinical indicators.
Materials and methods
Study design
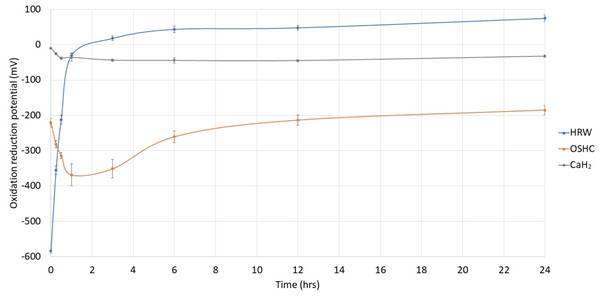
In a fundamental investigation, we explored the antioxidant effects of OSHCs by contrasting them with those of HRW and calcium hydride (CaH2). To investigate the antioxidant capacity of OSHCs, calcium hydride, and HRW, we measured the reduction potential of these different solutions using a reduction potential detector (Pen type oxidative reduction potential (ORP) Meter, ORP5041). The greater the negative value of the reduction potential, the stronger the antioxidant capacity. The longer the negative value of reduction potential lasts, the longer the anti-oxidation time. For the OSHCs and CaH2, 1 g of each was added to 100 ml of pure water and stirred with a glass rod for 3 minutes. The hydrogen-rich water (HRW) was obtained through electrolysis, resulting in a hydrogen concentration of 1,200 ppb.
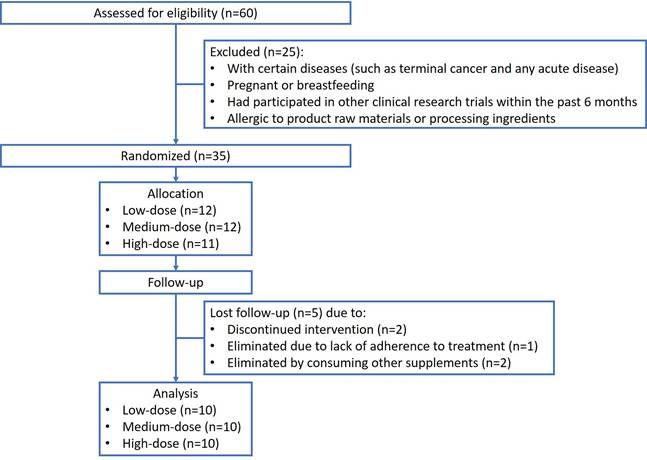
In the clinical study, participants were selected from the hospital's general medicine outpatient department. The screening criteria included adults aged 20 years or older with chronic stable conditions, such as diabetes, hypertension, and metabolic syndrome. Regarding recruitment, researchers followed up with chronic patients with recurring outpatient visits for more than three months before enrolment. During outpatient visits, the researchers explained the content and procedure of the study, including patient cooperation, to the patients. The researchers ensured that each patient understood the entire study process and signed an informed consent form. This clinical study was performed in July 2022 (Figure 1).
Patients who had unstable or advanced medical diseases (such as acute illness or terminal cancer), who were pregnant or breastfeeding, who had participated in other clinical research trials within the past 6 months, or who were allergic to the raw materials or processing ingredients in the supplements were excluded. None of these stable patients had any adjustment to their medications in the outpatient setting. The main outcomes assessed were changes in the blood ESR (120) and serum CRP concentrations. In addition, the Disease Activity Score 28 (DAS28) is a metric used to assess disease activity in rheumatoid arthritis (RA). "DAS" stands for "disease activity score," and the number 28 refers to the 28 joints evaluated in this measurement.
Secondary outcomes included changes in scores on the Brief Fatigue Inventory-Taiwan (BFI-T) fatigue scale, the Control Status Scale for Diabetes (CSSD70) questionnaire, and the DAS28.
Research method: Antioxidant hydrogen supplements
The OSHCs were integrated into the existing treatment regimen without altering other prescriptions and while maintaining the same dosage and frequency of clinical treatment. The original methods for coral calcium-carrying hydrogen have been described [23, 24]. The OSHCs were provided in solid dosage form and used as adjuvants every day.
- Low-dose: 1 capsule per day (in the morning on an empty stomach; 1 capsule × 28 days = 28 capsules)
- Medium dose: 3 capsules per day (in the morning on an empty stomach; 3 capsules × 28 days = 84 capsules)
- High-dose: 6 capsules per day (3 capsules in the morning and 3 capsules in the evening; 6 capsules × 28 days = 168 capsules)
The patients were instructed to take the capsules for 4 weeks and were informed that they could leave the study at any time.
Sample size. The anti-inflammatory effects of OSHCs supplementation in patients with chronic diseases were the primary outcome. In this study, it was assumed that based on a 10% improvement in anti-inflammatory effects with a power of 90% and an alpha value of 0.05, a sample size of 30 patients was needed.
Study flowchart.
Demonstration of OSHCs, purchased from HOHO Biotech Co., Ltd. (Taipei, Taiwan).

Materials
The OSHCs (hydrogen-rich coral calcium) were purchased from HOHO Biotech Co., Ltd. (Taipei, Taiwan; Figure 2). Each capsule contained 0.17 mg of hydrogen-rich coral calcium, and each gram of capsule contained 4.4 × 10^20 molecules of hydrogen, equivalent to 7.5 × 10^16 molecules of hydrogen per capsule. Coral calcium infused with hydrogen is a safe and convenient source of hydrogen. The original method for coral calcium-carrying hydrogen has been described previously. The coral powder with calcium carbonate was treated by circulating 100% H2 gas at 800°C for 1 h (high temperature) and at 300°C for 4 h (low temperature) to obtain hydrogen powder with a 10 μm particle size through grinding.
Measurements of adverse effects/symptoms
During the four weeks, each participant consumed the prescribed capsules. Upon their subsequent visit to the hospital, 20 ml of blood and 10 ml of urine were obtained. Additionally, body weight, blood pressure, and blood oxygen levels were evaluated. Adverse reactions were meticulously documented, and the participants completed a health assessment questionnaire.
Statistical analysis
Statistical analyses were conducted using R statistical software (version 4.0.4; R Core Team 2021) on a Windows platform. The results are presented as the mean or interquartile range (IQR). To compare the values before and after the use of OSHCs for each group, a paired t-test was used. To compare age, the ESR120, CRP concentrations, and the BFI-T-and CSSD70 scores among the three groups, one-way ANOVA was employed, and the chi-square test was used to compare the sex distribution of patients in different groups. The data are expressed as the means ± standard deviations (means ± SDs) along with 95% confidence intervals (CIs). A p-value <0.05 was considered to indicate statistical significance.
Results
Comparison of the reduction potential of OSHCs, HRW, and CaH2
In contrast to the limited 60-minute decrease reduction potential (antioxidative effect) of the HRW solution, the OSHCs solution enabled controlled hydrogen release over 24 hours with a sustained strong reduction potential. The higher the reduction potential value, the OSHCs solution demonstrated superior persistent pharmacological effects and greater stability in terms of reduction potential compared to the CaH2 and HRW solutions. In vitro experiments confirmed a 24-hour sustained antioxidant effect, as the reduction potential of the OSHCs persisted for the same duration (Figure 3).
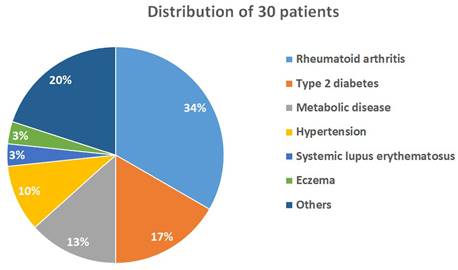
Demographics. A total of 30 patients were randomly assigned to 3 10-person groups (low-, medium-, and high-dose groups) and completed the study. The participants included 10 males and 20 females, with a mean age of 64.86 years (range: 32-82 years; Table 1). Notably, none of the patients who underwent OSHCs supplementation reported adverse effects or toxicity. All participants had preexisting chronic illnesses and were in a state of chronic inflammation. Among the cohort, approximately one-third of the participants had rheumatoid arthritis (n=10), five had type 2 diabetes mellitus, four had metabolic diseases, and three had hypertension (Figure 4).
The demographic and baseline characteristics of the patients (n=30).
| Characteristics | Dose: 1 capsule | Dose: 3 capsules | Dose: 6 capsules | P-value | Total |
|---|---|---|---|---|---|
| Patients (n) | 10 | 10 | 10 | -- | 30 |
| Age, mean ± SD (years) | 67.80±12.44 | 69.67±8.31 | 57.60±14.13 | 0.052 | 64.86±12.77 |
| Gender | M: n=4 F: n=6 | M: n=4 F: n=6 | M: n=2 F: n=8 | 0.549 | M: n=10 F: n= 20 |
| ESR120 | 76.6±48.5 | 59.3±35.4 | 87.8±41.7 | 0.465 | 74.6±42.4 |
| CRP (mg/dl) | 2.7±5.2 | 0.2±0.1 | 1.3±2.5 | 0.463 | 1.5±3.4 31.1±20.1 |
| BFI-T | 35.6±17.2 | 34.5±22.4 | 23.2±20.0 | 0.152 | |
| CSSD70 | 14.2±6.5 | 16.3±3.8 | 15.7±4.4 | 0.562 | 15.4±4.9 |
*: p-value<0.05; One-way ANOVA test was employed to compare values of the three groups for Age, ESR120, CRP, BFI-T, and CSSD70, and Chi-square test was used to compare the distribution of patients in different groups for Gender.
Abbreviations: BFI-T, Brief Fatigue Inventory Taiwan; CRP, C-reactive protein; CSSD70, Control status scale for diabetes; ESR, Erythrocyte sedimentation rate; F, Female; M, Male.
The primary outcome measures focused on alterations in inflammatory markers (the ESR120 and CRP concentrations)
This study aimed to assess the antioxidative effects of OSHCs supplementation as an adjuvant treatment. Thirty participants were involved in the study over 4 weeks. Blood and urine test results, along with diagnostic scale scores, were evaluated at three time points: before capsule intake (w0), one-week post-capsule intake (w1), and four weeks post-capsule intake (w4). The participants were randomly allocated into three groups—low-, medium-, and high-dose—to investigate dose-related effects.
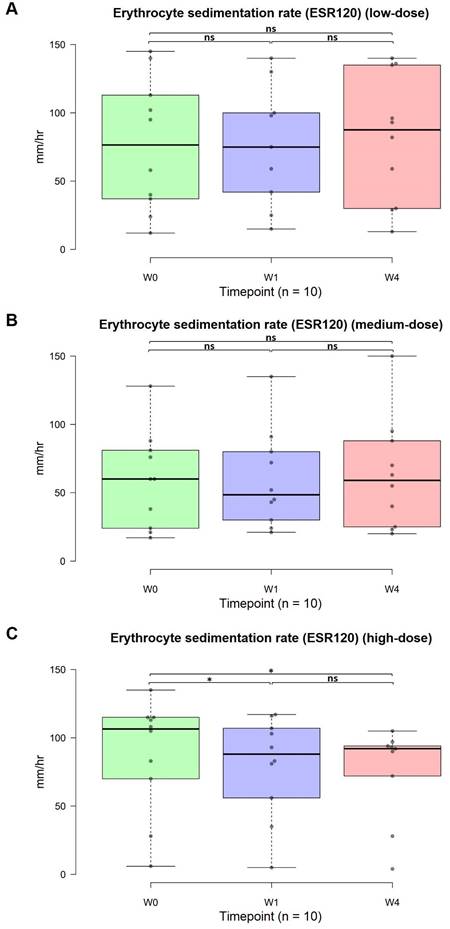
Changes in the ESR120
The standard value for the ESR120 is 0-20 mm/h. Notably, in the high-dose group, the anti-inflammatory response exhibited a rapid onset, achieving significant effects in the first week and further improvement in the fourth week. Specifically, the mean ESR120 in the high-dose group decreased from an average of 87.8 to 79.6 after the first week (w0 vs. w1, p = 0.034, 95% CI: 56.66, 102.54) to 75 after the fourth week (w0 vs. w4, p = 0.016, 95% CI: 53.26, 96.74). Although no significant differences were detected between the low-dose (p = 0.494, 95% CI: 51.93, 110.67; Figure 5A) and medium-dose (p = 0.195, 95% CI: 37.81, 87.99; Figure 5B) groups, a significant dose-dependent difference was detected in the high-dose group (p = 0.016, 95% CI: 53.26, 96.74; Figure 5C). Further clarification is warranted to determine whether extending the administration of OSHCs from four weeks to three or six months at low and medium doses could lead to improved anti-inflammatory effects.
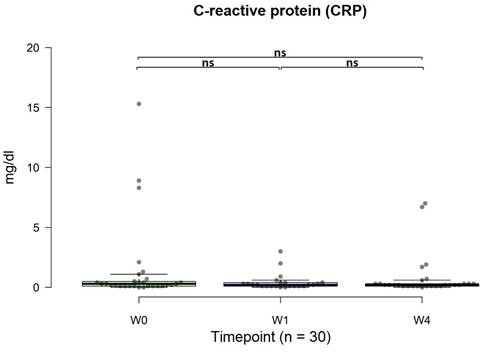
Changes in CRP concentrations
The standard CRP concentration was <1 mg/dl. In general, there was an anti-inflammatory effect on CRP concentrations, irrespective of dosage. The average CRP level before OSHCs usage was 1.46 mg/dl, and after four weeks of use, the average CRP level was 0.80 mg/dl, meeting the criterion of <1 mg/dl, with no distinction based on dosage. Although not statistically significant, there was an observable anti-inflammatory trend (w0 vs. w4, p = 0.077, 95% CI: -0.29, 1.90) (Figure 6).
The use of oral OSHCs provides advantages over hydrogen-rich water (HRW) and calcium hydride (CaH2) due to their stable and sustained strong reduction potential.
Chronic illness and distribution of all patients.
Changes in the erythrocyte sedimentation rate (ESR)-120 from 0 to 4 weeks (n=30). Normal range: males, <10; females, <15 mm/hr. (A) Low-dose group. (B) Medium-dose group. (C) High-dose group. The data are represented by boxplots (ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001) and were analyzed using paired t-tests.
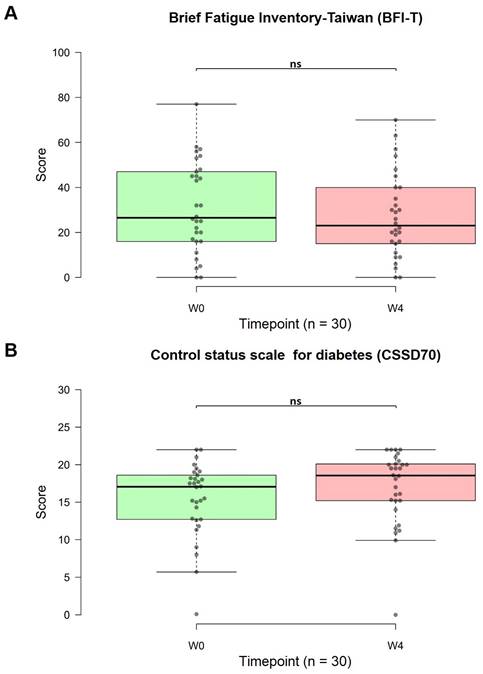
The secondary outcome measures were the changes in the BFI-T, CSSD70, and DAS28 scores
Eight out of 30 patients experienced improved sleep quality, while seven out of 30 patients reported increased energy levels. Lower BFI-T scores indicated reduced fatigue. The use of OSHCs for one month improved chronic fatigue. The average score before capsule consumption was 31.10, and after four weeks of use, the average score decreased to 27.03. Although the difference was not statistically significant, a noticeable downward trend was observed (p = 0.125, 95% CI: 15.56, 38.51; Figure 7A).
Changes in C-reactive protein (CRP) from 0 to 4 weeks (n=30). The normal range was < 1 mg/dl. The data are represented by boxplots (ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001) and were analyzed using paired t-tests.
Higher CSSD70 scores indicate improved diabetes control. After one month of OSHC use, the CSSD70 scores increased. The average CSSD70 score before OSHCs consumption was 15.38, which increased to 16.97 after four weeks of use. Although the difference was not statistically significant, there was an observable upward trend (p = 0.063, 95% CI: 13.93, 20.00; Figure 7B). Extending the duration of use or expanding the sample size may have resulted in statistically significant differences.
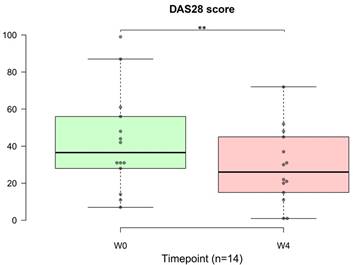
Among the entire cohort of 30 patients, 14 (47%) were diagnosed with autoimmune disease. In the context of the DAS28, a decrease in the score signifies an improvement in disease activity in patients with autoimmune conditions. Notably, one month of OSHCs supplementation resulted in a significant improvement in DAS28 score in 14 patients with autoimmune diseases (p = 0.002, 95% CI: 18.38, 39.62; Figure 8). The average DAS28 score recorded before OSHC consumption was 42.14, and after four weeks of use, it decreased to 29.00.
Discussion
Chronic inflammation poses a substantial and escalating burden to the global healthcare system. The prevailing consensus is that a high intake of dietary bioactive compounds, particularly nutritional supplements, may be linked to a reduced risk of chronic illnesses. Nutritional supplements, including oral molecular hydrogen, have the potential to aid in the management of chronic diseases by diminishing oxidative stress and the associated inflammatory pathways. Our findings revealed that OSHCs supplementation exerts a significant dose-dependent anti-inflammatory effect on chronic inflammation in the human body. Importantly, none of the patients exhibited adverse reactions, and no patients withdrew from the study. These results effectively underscore the potential of OSHCs supplementation to mitigate chronic inflammation in humans, providing optimism for its future application as a new adjunct in clinical treatments.
Bioengineering and clinical application of molecular hydrogen
As the smallest molecule in nature, hydrogen can readily diffuse into organs and tissues of the human body. In 2007, Professor Ohsawa recommended the use of hydrogen gas as a therapeutic antioxidant, emphasizing its ability to selectively reduce cytotoxic oxygen radicals [7]. Initially, molecular hydrogen functions as a mild antioxidant, eliminating toxic free radicals without disrupting the body's redox balance. After 16 years of extensive animal and human experiments, the antioxidant and anti-inflammatory effects, along with the mechanism of action of molecular hydrogen, have been elucidated in numerous supporting studies. Despite repeated validation of its beneficial effects and the absence of biological toxicity in thousands of studies and dozens of clinical trials over 16 years, molecular hydrogen has not been widely applied as a medical treatment in hospitals. The primary obstacle is the low clinical availability of this technology. For instance, HRW has a low hydrogen content, and molecular hydrogen volatilizes quickly, resulting in a lower effective concentration in HRW. In addition, hydrogen inhalation and HRW machines are expensive, bulky, and complex to operate, making it challenging to determine the appropriate dosage. Consequently, large-scale human trials are challenging to conduct. To address these clinical usability issues, there is a need for a new technology that delivers an adequate dose of hydrogen, is portable and user-friendly, and allows precise dosage control.
Changes in the Brief Fatigue Inventory-Taiwan (BFI-T) and Control Status Scale for Diabetes (CSSD70) scores from before to after treatment (n=30). (A) The BFI-T consists of 10 questions, each scored from 0 to 10, with 0 = most healthy and 10 = most severe (total range: 0 to 100). (B) The CSSD70 questionnaire consists of 11 questions, each scored from 0 to 2 (total range: 0 to 22). The data are represented by boxplots (ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001) and were analyzed using paired t-tests.
Distribution of patients and changes in the Disease Activity Score in 28 joints (DAS28) from before to after treatment (n=14). (A) Distribution of all 30 patients. There were 14 patients with autoimmune diseases, accounting for nearly half of all patients (47%). (B) The DAS28 is a measure of disease activity in autoimmune diseases. The data are represented by boxplots (ns: not significant; *p < 0.05; **p < 0.01; ***p < 0.001) and were analyzed using paired t-tests.
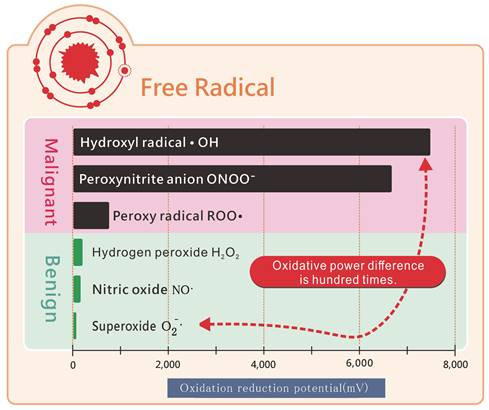
Molecular hydrogen eliminates toxic free radicals but not benign free radicals.
OSHCs offer a solution to the limitations associated with hydrogen inhalation and HRW. In terms of pharmacology, dose adjustments, and clinical availability, OSHCs have advantages over earlier technologies, such as HRW and CaH2, as they provide controlled release and improved stability (Figure 3).
1. The stability of the reduction potential is notable, featuring 24-hour controlled release characteristics. In contrast to HRW, which has an antioxidative effect lasting only 60 min, OSHCs ensure the controlled release of hydrogen over 24 hours. This resulted in favorable pharmacokinetic effects, offering improved stability of the reduction potential. In vitro experiments confirmed the 24-hour antioxidant effects, confirming that the anti-inflammatory effects of OSHCs also extended for 24 h.
2. Convenient dosage adjustment is facilitated, allowing for precise customization based on individual patient needs and conditions. Patients with varying indications and weights can take one, three, or six tablets per day. The established safe dose is up to 13 tablets daily, exceeding which is not toxic; however, surpassing this limit would result in calcium intake surpassing the recommended daily limit set by the Taiwan Food and Drug Administration (TFDA) (https://consumer.fda.gov.tw/Food/CapsuleAuditQueryDetail.aspx?nodeID=165&id=1096000144).
3. Optimal clinical usability is achieved by avoiding bulky high-pressure steel cylinders or machines, which are typically necessary for producing HRW. Such equipment is expensive, intricate, and inconvenient to operate.
To measure molecular hydrogen concentration in an aqueous solution, methods differ in terms of sensitivity, accuracy, and available equipment. Gas chromatography is one such method, which analyzes gas samples from the solution using either a thermal conductivity detector or a flame ionization detector after conversion to a detectable form [25]. Molecular hydrogen influences the ORP of a solution by acting as a reducing agent. Dissolved hydrogen lowers the ORP, indicating a more reductive environment. The Nernst equation captures this relationship, demonstrating that increased hydrogen concentrations result in a more negative ORP, reflecting greater reduction [26]. While hydrogen activity can be equated to concentration in very dilute solutions, this relationship diverges at higher concentrations. To accurately apply the Nernst equation in such cases, the true activity of molecular hydrogen must be determined experimentally. ORP and hydrogen concentration may not always correlate, we present the ORP data, which may better represent the physicochemical effects of molecular hydrogen, as illustrated in Figure 3.
Molecular hydrogen can selectively target toxic free radicals. Functional benign free radicals, which are crucial for immunity against viruses and pathogens or for promoting blood vessel elasticity, do not require complete neutralization. While these benign free radicals have low oxidative capacity, malignant free radicals exhibit 100 times greater oxidative capacity (Figure 9). Molecular hydrogen, which is relatively mild, does not neutralize benign free radicals but focuses on toxic free radicals with super-high oxidizing power. Among the most toxic free radicals, ●OH- and molecular hydrogen promptly combine and react with it, generating water that can be easily excreted from the body [9].
Anti-inflammatory effects of molecular hydrogen
CRP concentrations and the ESR are widely used blood test markers to assess inflammation in the body [27-29]. Supplementation with molecular hydrogen has been shown to confer a protective effect by diminishing oxidative stress and suppressing the inflammatory response [30, 31]. Additionally, it can regulate energy metabolism within the mitochondria [32]. The results of a pharmacokinetic study using a swine model showed that in addition to hydrogen diffusing throughout the entire body after inhalation, molecular hydrogen is also transported throughout the body via the circulatory system, resulting in a comprehensive impact [33]. Recent studies on molecular hydrogen have demonstrated noteworthy improvements in both patient and animal models, irrespective of the form of molecular hydrogen supplementation. For instance, obese mice exhibit enhanced fatty liver conditions after inhaling hydrogen gas or consuming HRW [34]. In normal rats, inhaling hydrogen and drinking HRW effectively lowered visceral fat and blood lipid concentrations [10]. Furthermore, in hypertensive rats, hydrogen inhalation resulted in a reduction in oxidative stress and the promotion of an anti-inflammatory response, leading to improvements in pulmonary hypertension [35, 36].
In mice experiencing acute pancreatitis, the inhalation of hydrogen was shown to be beneficial for reducing oxidative stress and inhibiting inflammatory responses [31]. In mouse models, the consumption of HRW is effective at reducing chronic inflammation, improving symptoms of depression and anxiety, and relieving neuropathic pain [37, 38]. The documented effects of molecular hydrogen supplementation in humans include a decrease in plasma low-density lipoprotein cholesterol (LDL-C) and free fatty acids along with an increase in extracellular superoxide dismutase after HRW consumption in patients with type 2 diabetes or impaired glucose tolerance [39]. Moreover, HRW consumption resulted in a reduction in LDL-C and an improvement in high-density lipoprotein cholesterol (HDL-C), in addition to enhancements in cardiovascular and metabolic disease-related indicators, in patients with metabolic syndrome [40, 41]. Molecular hydrogen supplementation alleviates inflammation in individuals with diabetes and heart disease [42-44], as well as in those with chronic obstructive pulmonary disease or asthma [19, 45, 46]. There is a notable trend towards improved blood test indicators in patients with nonalcoholic fatty liver disease after eight weeks of HRW consumption [47]. In addition to its anti-inflammatory effects, molecular hydrogen supplementation, whether through inhalation or ingestion of OSHCs, has also been associated with improved athletic performance and protection against fatigue and sports injuries [48, 49]. Recent human research also highlights the potential of OSHC therapy for managing progressive fibrosing interstitial lung disease complicated by pneumonia. Following OSHCs treatment, a significant increase in the number of resting regulatory T cells was observed, along with a marked reduction in the number of Fas-positive helper T cells and cytotoxic T-cell subsets [50].
In summary, molecular hydrogen consumption has remarkable antioxidant and anti-inflammatory effects through specific neutralization of toxic free radicals. Importantly, these effects are achieved without disrupting the body's redox balance; instead, molecular hydrogen consumption modulates chronic inflammation and apoptosis, including a reduction in mitochondrial oxidative damage. Further evidence supports the ability of hydrogen gas to mitigate the side effects induced by chemotherapy and inhibit the growth of tumor cells, highlighting its potential therapeutic application in clinical practice [51, 52]. Oral molecular hydrogen also serves as an effective antioxidant against exercise-induced ROS and can promote improvements in exercise performance in humans [53, 54]. Consequently, future studies should explore the molecular mechanisms targeting redox-associated signaling pathways in chronic diseases. Additionally, the utilization of solid-form hydrogen compounds may represent a potential therapeutic strategy for the chemoprevention of chronic diseases in the future. A recent clinical study highlights the potential therapeutic effects of oral molecular hydrogen as a promising treatment for immunoglobulin G4-related pleuroparenchymal fibroelastosis (IgG4-PF-ILD) [50]. The observational study result demonstrated that oral molecular hydrogen treatment had a regulatory effect on the immune system, particularly on T cell populations. Regulatory T cells (Tregs), which play a crucial role in aintaining immune tolerance and preventing autoimmunity, increased in number. This could indicate that the treatment is enhancing the body's ability to regulate immune responses and prevent excessive inflammation. The decrease in Fas+ helper T cells and cytotoxic T cells is also significant. In addition, a reduction in Fas+ T cells suggests a decrease in the propensity for these cells to undergo apoptosis or a shift in the balance of T cell subtypes towards those that are less prone to Fas-mediated cell death. These changes could reflect a modulation of the immune response, potentially making it more controlled and less prone to autoimmune reactions [50, 55].
The strength of this research is that it is the first to investigate the antioxidant and anti-inflammatory effects of a novel nutritional molecular hydrogen supplement in patients with chronic diseases. However, there are some limitations to this study. The sample size was small, and the treatment duration was short. Nevertheless, these preliminary data indicate that high-dose molecular hydrogen supplementation can induce antioxidative and anti-inflammatory effects, such as improvements in the ESR120, within just one week, and the effect persists for four weeks. Although trends were observed for the low and medium doses, the results were not statistically significant. Therefore, in future clinical studies, it is recommended to increase the number of patients and/or extend the treatment period. Additionally, the incorporation of a negative (placebo) control group is essential. Proinflammatory cytokines, which are mainly produced by activated macrophages, play a role in upregulating inflammatory reactions. Monitoring changes in interleukin-1, interleukin-6, tumor necrosis factor-α, and other significant oxidative and anti-inflammatory markers could provide valuable insights. Finally, gas chromatography may offer high accuracy and sensitivity, but it necessitates specialized equipment and expertise that are often not available in clinical settings.
The results of this study suggest that oral OSHCs supplementation may exert a notable anti-inflammatory effect on outpatients with chronic illnesses. Furthermore, these findings confirm that oral OSHC supplementation has the potential to alleviate symptoms associated with human diseases. We anticipate exploring additional molecular applications of OSHCs supplementation as an adjunct treatment for various diseases or dysfunctions in the future.
Acknowledgements
We are grateful to Min-Sheng General Hospital, Taoyuan, Taiwan, for their assistance in conducting this clinical study.
Funding
This study was supported by grant TYAFGH-D-113032 from the Taoyuan Armed Forces General Hospital, Taiwan.
Institutional review board statement
This study was conducted following the principles of the Institutional Review Board and Good Clinical Practice (ICH GCP). The research protocol was approved by the Institutional Review Board (IRB) of Min-Sheng General Hospital (MSIRB). The approval number for this study was 2021007-A. Informed consent was obtained from all the subjects involved in the study.
Author contributions
The authors declare that they have no conflicts of interest. All the authors contributed to the conception and design of the study. Material preparation was performed by KCL, MCS, PJH, RLW, SHC, YHK, and WWC; data collection was performed by KCL, RLW, MCS, PJH, and WWC; analysis was performed by PJH and FCL; and validation was performed by YHK and PJH. MCS, PJH, and FCL wrote the original drafts. KCL, PJH, MCS, SHC, YHK, and FCL reviewed and edited the manuscript. All authors commented on all versions of the manuscript and have read and approved the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ishida M, Sakai C, Ishida T. Role of DNA damage in the pathogenesis of atherosclerosis. J Cardiol. 2023;81:331-6
2. Duan GY, Silverberg JI. Sleep impairment in patients with chronic inflammatory skin diseases: A review of mechanisms and management. J Am Acad Dermatol. 2023;88:421-7
3. Ke Q, Greenawalt AN, Manukonda V, Ji X, Tisch RM. The regulation of self-tolerance and the role of inflammasome molecules. Front Immunol. 2023;14:1154552
4. Li Q, von Ehrlich-Treuenstatt V, Schardey J, Wirth U, Zimmermann P, Andrassy J. et al. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J Gastrointest Surg. 2023;27:1466-72
5. Quan X, Lim SO, Jung G. Reactive oxygen species downregulate catalase expression via methylation of a CpG island in the Oct-1 promoter. FEBS Lett. 2011;585:3436-41
6. Thapa K, Shivam K, Khan H, Kaur A, Dua K, Singh S. et al. Emerging Targets for Modulation of Immune Response and Inflammation in Stroke. Neurochem Res. 2023;48:1663-90
7. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-94
8. Zhang X, Xie F, Ma S, Ma C, Jiang X, Yi Y. et al. Mitochondria: one of the vital hubs for molecular hydrogen's biological functions. Front Cell Dev Biol. 2023;11:1283820
9. Barancik M, Kura B, LeBaron TW, Bolli R, Buday J, Slezak J. Molecular and Cellular Mechanisms Associated with Effects of Molecular Hydrogen in Cardiovascular and Central Nervous Systems. Antioxidants (Basel). 2020;9:1281
10. Adzavon YM, Xie F, Yi Y, Jiang X, Zhang X, He J. et al. Long-term and daily use of molecular hydrogen induces reprogramming of liver metabolism in rats by modulating NADP/NADPH redox pathways. Sci Rep. 2022;12:3904
11. Slezak J, Kura B, LeBaron TW, Singal PK, Buday J, Barancik M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr Pharm Des. 2021;27:610-25
12. Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y. et al. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res. 2012;2:27
13. Fang S, Li X, Wei X, Zhang Y, Ma Z, Wei Y. et al. Beneficial effects of hydrogen gas inhalation on a murine model of allergic rhinitis. Exp Ther Med. 2018;16:5178-84
14. Yu S, Zhao C, Che N, Jing L, Ge R. Hydrogen-rich saline attenuates eosinophil activation in a guinea pig model of allergic rhinitis via reducing oxidative stress. J Inflamm (Lond). 2017;14:1
15. Kajisa T, Yamaguchi T, Hu A, Suetake N, Kobayashi H. Hydrogen water ameliorates the severity of atopic dermatitis-like lesions and decreases interleukin-1beta, interleukin-33, and mast cell infiltration in NC/Nga mice. Saudi Med J. 2017;38:928-33
16. Yoon YS, Sajo ME, Ignacio RM, Kim SK, Kim CS, Lee KJ. Positive Effects of hydrogen water on 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Biol Pharm Bull. 2014;37:1480-5
17. Huang P, Wei S, Huang W, Wu P, Chen S, Tao A. et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol. 2019;74:105646
18. Zhang N, Deng C, Zhang X, Zhang J, Bai C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract. 2018;4:3
19. Wang ST, Bao C, He Y, Tian X, Yang Y, Zhang T. et al. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM. 2020;113:870-5
20. Yang HJ, Tsou WH, Shen MC, Liu CY, Saunders HM, Wang KY. et al. The effects of hydrogen treatment in a cigarette smoke solution-induced chronic obstructive pulmonary disease-like changes in an animal model. J Thorac Dis. 2022;14:4246-55
21. Zheng CM, Hou YC, Liao MT, Tsai KW, Hu WC, Yeh CC. et al. Potential role of molecular hydrogen therapy on oxidative stress and redox signaling in chronic kidney disease. Biomed Pharmacother. 2024;176:116802
22. Wu HT, Chao TH, Ou HY, Tsai LM. Coral Hydrate, a Novel Antioxidant, Improves Alcohol Intoxication in Mice. Antioxidants (Basel). 2022;11:1290
23. Ma K, Hu X, Nambu K, Ueda D, Ichimaru N, Fujino M. et al. Coral calcium carried hydrogen ameliorates the severity of non-alcoholic steatohepatitis induced by a choline deficient high carbohydrate fat-free diet in elderly rats. Sci Rep. 2023;13:11646
24. Chiu SH, Douglas FL, Chung JR, Wang KY, Chu CF, Chou HY. et al. Evaluation of the safety and potential lipid-lowering effects of oral hydrogen-rich coral calcium (HRCC) capsules in patients with metabolic syndrome: a prospective case series study. Front Nutr. 2023;10:1198524
25. Lin YP, Lee YL, Hung CY, Chang CF, Chen Y. Detection of adulterated drugs in traditional Chinese medicine and dietary supplements using hydrogen as a carrier gas. PLoS One. 2018;13:e0205371
26. Hopper A, Beswick-Jones H, Brown AM. The Nernst equation: using physico-chemical laws to steer novel experimental design. Adv Physiol Educ. 2022;46:206-10
27. Lau DC, Yan H, Dhillon B. Metabolic syndrome: a marker of patients at high cardiovascular risk. Can J Cardiol. 2006 22 Suppl B: 85B-90B
28. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391-7
29. Alende-Castro V, Alonso-Sampedro M, Fernandez-Merino C, Sanchez-Castro J, Sopena B, Gude F. et al. C-Reactive Protein versus Erythrocyte Sedimentation Rate: Implications Among Patients with No Known Inflammatory Conditions. J Am Board Fam Med. 2021;34:974-83
30. Zhang Y, Tan S, Xu J, Wang T. Hydrogen Therapy in Cardiovascular and Metabolic Diseases: from Bench to Bedside. Cell Physiol Biochem. 2018;47:1-10
31. Li K, Yin H, Duan Y, Lai P, Cai Y, Wei Y. Pre-inhalation of hydrogen-rich gases protect against caerulein-induced mouse acute pancreatitis while enhance the pancreatic Hsp60 protein expression. BMC Gastroenterol. 2021;21:178
32. Tian Y, Zhang Y, Wang Y, Chen Y, Fan W, Zhou J. et al. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front Physiol. 2021;12:789507
33. Cole AR, Sperotto F, DiNardo JA, Carlisle S, Rivkin MJ, Sleeper LA. et al. Safety of Prolonged Inhalation of Hydrogen Gas in Air in Healthy Adults. Crit Care Explor. 2021;3:e543
34. Kamimura N, Nishimaki K, Ohsawa I, Ohta S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring). 2011;19:1396-403
35. Sugai K, Tamura T, Sano M, Uemura S, Fujisawa M, Katsumata Y. et al. Daily inhalation of hydrogen gas has a blood pressure-lowering effect in a rat model of hypertension. Sci Rep. 2020;10:20173
36. Kishimoto Y, Kato T, Ito M, Azuma Y, Fukasawa Y, Ohno K. et al. Hydrogen ameliorates pulmonary hypertension in rats by anti-inflammatory and antioxidant effects. J Thorac Cardiovasc Surg. 2015;150:645-54 e3
37. Coral-Perez S, Martinez-Martel I, Martinez-Serrat M, Batalle G, Bai X, Leite-Panissi CRA. et al. Treatment with Hydrogen-Rich Water Improves the Nociceptive and Anxio-Depressive-like Behaviors Associated with Chronic Inflammatory Pain in Mice. Antioxidants (Basel). 2022;11:2153
38. Martinez-Serrat M, Martinez-Martel I, Coral-Perez S, Bai X, Batalle G, Pol O. Hydrogen-Rich Water as a Novel Therapeutic Strategy for the Affective Disorders Linked with Chronic Neuropathic Pain in Mice. Antioxidants (Basel). 2022;11:1826
39. Kajiyama S, Hasegawa G, Asano M, Hosoda H, Fukui M, Nakamura N. et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr Res. 2008;28:137-43
40. Song G, Li M, Sang H, Zhang L, Li X, Yao S. et al. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res. 2013;54:1884-93
41. LeBaron TW, Singh RB, Fatima G, Kartikey K, Sharma JP, Ostojic SM. et al. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab Syndr Obes. 2020;13:889-96
42. Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013;3:10
43. Ming Y, Ma QH, Han XL, Li HY. Molecular hydrogen improves type 2 diabetes through inhibiting oxidative stress. Exp Ther Med. 2020;20:359-66
44. Tamura T, Suzuki M, Hayashida K, Kobayashi Y, Yoshizawa J, Shibusawa T. et al. Hydrogen gas inhalation alleviates oxidative stress in patients with post-cardiac arrest syndrome. J Clin Biochem Nutr. 2020;67:214-21
45. Zheng ZG, Sun WZ, Hu JY, Jie ZJ, Xu JF, Cao J. et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir Res. 2021;22:149
46. Guan WJ, Chen RC, Zhong NS. Strategies for the prevention and management of coronavirus disease 2019. Eur Respir J. 2020;55:2000597
47. Kura B, Szantova M, LeBaron TW, Mojto V, Barancik M, Szeiffova Bacova B. et al. Biological Effects of Hydrogen Water on Subjects with NAFLD: A Randomized, Placebo-Controlled Trial. Antioxidants (Basel). 2022;11:1935
48. Javorac D, Stajer V, Ratgeber L, Betlehem J, Ostojic S. Short-term H(2) inhalation improves running performance and torso strength in healthy adults. Biol Sport. 2019;36:333-9
49. Ostojic SM, Vukomanovic B, Calleja-Gonzalez J, Hoffman JR. Effectiveness of oral and topical hydrogen for sports-related soft tissue injuries. Postgrad Med. 2014;126:187-95
50. Lui SW, Lu JW, Ho YJ, Tang SE, Ko KH, Hsieh TY. et al. Molecular Hydrogen as a Promising Therapy Could Be Linked With Increased Resting Treg Cells or Decreased Fas+ T Cell Subsets in a IgG4-PF-ILD Patient: A Case Report. In Vivo. 2024;38:1512-8
51. Li S, Liao R, Sheng X, Luo X, Zhang X, Wen X. et al. Hydrogen Gas in Cancer Treatment. Front Oncol. 2019;9:696
52. Li C, Zhao X, Gu X, Chen Y, Yu G. The Preventive Role of Hydrogen-Rich Water in Thioacetamide-Induced Cholangiofibrosis in Rat Assessed by Automated Histological Classification. Front Pharmacol. 2021;12:632045
53. Alharbi AAD, Ebine N, Nakae S, Hojo T, Fukuoka Y. Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans. Nutrients. 2021;13:459
54. Alharbi AAD, Iwamoto N, Ebine N, Nakae S, Hojo T, Fukuoka Y. The Acute Effects of a Single Dose of Molecular Hydrogen Supplements on Responses to Ergogenic Adjustments during High-Intensity Intermittent Exercise in Humans. Nutrients. 2022;14:3974
55. Ishibashi T. Molecular hydrogen: new antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr Pharm Des. 2013;19:6375-81
Author contact
![]() Corresponding author: Po-Jen Hsiao MD, PhD, Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, Number 325, Section 2, Cheng-Kung Road, Neihu 114, National Defense Medical Center, Taipei, Taiwan, Republic of China. Tel.: +886-2-87927213 Fax: +886-2-87927134; Email: doc10510gov.tw; a2005a660820com.tw.
Corresponding author: Po-Jen Hsiao MD, PhD, Division of Nephrology, Department of Internal Medicine, Tri-Service General Hospital, Number 325, Section 2, Cheng-Kung Road, Neihu 114, National Defense Medical Center, Taipei, Taiwan, Republic of China. Tel.: +886-2-87927213 Fax: +886-2-87927134; Email: doc10510gov.tw; a2005a660820com.tw.

 Global reach, higher impact
Global reach, higher impact