3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(11):2081-2093. doi:10.7150/ijms.97217 This issue Cite
Review
A Novel “Endocrine Hormone”: The Diverse Role of Extracellular Vesicles in Multiorgan Insulin Resistance
1. The Key Laboratory of Geriatrics, Beijing Institute of Geriatrics, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing Hospital/ National Center of Gerontology of National Health Commission, 100730, Beijing, P.R. China.
2. Department of Dermatology, Beijing hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, 100730, Beijing, P.R. China.
† These authors have contributed equally to this work and share first authorship.
Received 2024-4-11; Accepted 2024-7-24; Published 2024-8-6
Abstract
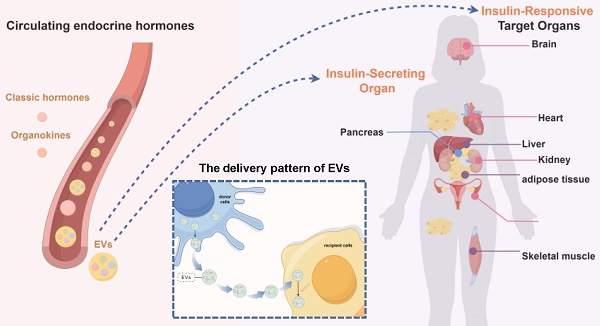
Insulin resistance is the primary contributor to the disruption in glucose homeostasis in the body, playing a significant causative role in many metabolic diseases. Insulin resistance is characterized by compensatory insulin secretion and reduced insulin responsiveness in target organs. Dysregulation of the interaction between insulin-secreting cells and insulin-responsive target organs is an important factor driving the progression of insulin resistance. Circulating endocrine hormones are important mediators mediating the interaction between insulin-secreting cells and insulin-responsive target organs. In addition to the classical hormones secreted by endocrine glands and organ-specific hormones secreted by metabolism-related organs (adipose tissue, muscle, liver, etc.), extracellular vesicles have been recognized as a novel class of endocrine hormones with a complex composition. Extracellular vesicles can transport signaling molecules, such as miRNAs and LncRNAs, to vital organs related to insulin resistance, in a manner akin to conventional hormones. The significant role in regulating the development of insulin resistance underscores the increasing interest in extracellular vesicles as essential contributors to this process. In this review, we summarize the three types of hormones (classical hormones, organokines and extracellular vesicles) that play a regulatory role in insulin resistance, and focus on the novel endocrine hormones, extracellular vesicles, to elaborate the mechanism of extracellular vesicles' regulation of insulin resistance progress from two aspects: the impact on insulin-secreting cells and the influence on insulin-responsive target organs. In addition, this paper outlines the clinical applications of extracellular vesicles in insulin resistance. A comprehensive understanding of the regulatory mechanisms and diagnostic status of the inter-organ network in insulin resistance has great potential to advance targeted therapeutic interventions and diagnostic markers, thereby benefiting both the prevention and treatment of insulin resistance.
Keywords: extracellular vesicles, insulin resistance, intercellular communication, metabolic diseases, endocrine hormones, organokines
Introduction
Insulin resistance (IR) is an important cause of imbalance in the body's glucose homeostasis. It is prevalent in various metabolic diseases, and is considered to be the driving factor of metabolic syndrome [1], obesity, type 2 diabetes mellitus (T2DM) [2], cardiovascular disease [3], metabolic dysfunction related fatty liver disease (MAFLD) [4], polycystic ovary syndrome (PCOS) [5], Alzheimer's disease (AD) [6]and some other tumors [7], all of which affect global public health [8].
IR, which is characterized by compensatory insulin secretion mediated by pancreatic β-cells and a decreased response of multiple insulin-responsive target organs, is a metabolic disease involving multiple organs including insulin-secreting organs and insulin-responsive target organs [7]. Under physiological conditions, insulin-secreting cells and insulin-responsive target organs work together to coordinate interactions among insulin-related organs through metabolic regulatory signals, to maintain blood glucose levels in the body. Under pathological conditions, changes in the cargo and level of these regulatory signals occur, causing dysregulation of insulin-related organ interactions, which is an important factor driving the progression of IR [4].
Circulating endocrine hormones are significant classes that make up the metabolic regulatory network. These hormones include classical hormones secreted by specialized endocrine glands, such as insulin [9], growth hormone [10], and thyroxine [11]; organokines secreted by important metabolic organs, such as adipokines, myokines, and hepatokines [12]; and a novel endocrine hormone, which has recently received a great deal of attention ‒ extracellular vesicles (EVs) [13-15]. Together, these hormone signals play a role in the interactions among organs related to IR [16].
EVs are vesicles secreted by a variety of cells that contain complex contents and are the new mediators that regulate organ-organ and cell‒cell interactions [17]. Similar to classical hormones and organokines, EVs can transport specific cargos to target cells via blood or body fluids to perform physiological or pathological functions. Therefore, they are referred to as a new type of endocrine hormone [18-21].
This review focuses on the important role of EVs in disease pathogenesis, diagnosis and treatment, with a particular emphasis on EV-mediated interactions among vital organs related to insulin resistance.
Insulin resistance is a metabolic disease involving multiple organs
IR refers to a disorderly biological response in which the insulin signaling pathway in target tissues is disrupted, resulting in a significant reduction in sensitivity to insulin [7]. It is closely related to the dysregulation of insulin secretion and impaired insulin utilization. As a result, IR-related organs mainly include insulin-secreting organs and insulin-responsive target organs.
Insulin-secreting organs/cells
The pancreas is an endocrine organ that plays a key role in the regulation of glucose homeostasis. Endocrine cells in the pancreas aggregate to form islets of Langerhans, which maintain glucose homeostasis by releasing a variety of hormones. The core hormone molecule insulin, which is secreted by pancreatic islet β-cells, is present throughout the pathological process of IR.
When IR occurs, the sensitivity of target tissues to insulin decreases, leading to a decrease in the efficiency of insulin in promoting glucose uptake and utilization, and an increase in body glucose levels. To maintain blood glucose homeostasis, pancreatic β-cells compensate for IR by either increasing the cell numbers or increasing the amount and/or frequency of insulin secretion. However, prolonged compensatory effects lead to a decrease in the number of pancreatic β-cells and/or gradual impairment of function [22].
Insulin-responsive target organs
Insulin-responsive organs are distributed throughout the body. Among them, the liver, muscle, and adipose tissue are considered to be important target organs for IR. In addition, other organs such as the kidney, heart, ovary, and brain, also undergo IR and are involved in several pathophysiological processes through the target organ IR [2, 23].
After insulin is transported through the bloodstream to target organs, these organs respond to insulin's actions, contributing to the maintenance of the body's glucose homeostasis. Under normal physiological conditions, the precise regulation of target cell functions within target organs by insulin involves four sequential steps: insulin binding to its specific receptor, receptor substrate activation, signal transmission, and the generation of physiological effects [23].
When IR occurs, the insulin signaling pathway and its mediating effects on organs are disrupted. For example, in the case of liver IR, glycogen synthesis in the liver is reduced, gluconeogenesis is inhibited, and liver fat production is reduced. In skeletal muscle IR, glucose uptake by skeletal muscle decreases, and muscle glycogen synthesis is reduced. In adipose tissue IR, adipogenesis decreases and lipolysis increases. When these target organs exhibit reduced insulin sensitivity, they do not respond effectively to insulin, which is an important contributor to the sustained elevation of blood glucose level in the body [23].
Previous studies have explored the specific signaling pathway of IR in different target organs, which starts from the activation of insulin receptor in target cells to the final stages of glycolipid synthesis and decomposition. However, these studies are mostly focused on the specific role of a single target organ, and few addressing how cells/organs regulate and influence each other. In the early stage, the inter-organ communication was limited to classical hormone molecules. From the perspective of macro endocrine, the synergistic or antagonistic effects of different hormone molecules on insulin were discussed. [10, 11, 24-27]. With the continuous updating of experimental methodology, researchers linked organokines and EVs with IR [13, 16]. These molecules established connections between donor and recipient cells through "hormone" like effects, refining the way some signal molecules in the microenvironment communicate with different cells [28].
Hormone mediators of cell/organ interactions related to insulin resistance
Intercellular/interorgan communication is the means by which an organism regulates its physiological functions and serves as a regulatory mechanism for many pathological processes. Mediators of cell/organ communication play a crucial role in the regulation of both physiological and pathological processes. In the physiological state, interorgan/intercell coordination and cooperation between cells are realized through signaling and interaction to maintain the homeostasis, development and functional performance of the organism. Dysregulated intercellular/organ communication is an important factor driving disease progression. Cells under stress or injury contribute to the disease process by releasing stress and danger signals that affect target cells, causing changes in the proliferation, metabolism or function of the cells involved [29, 30].
A complex network of interactions exists between insulin-secreting organs and insulin-responsive target organs, in which hormones play a major role. Classical hormones are chemical substances produced by specialized endocrine glands (e.g. pancreatic islets, adrenal glands, and thyroid glands) that are delivered via the bloodstream and initiate signaling either through cell surface receptors or through the cell membrane into the cell and bind to intracellular receptors to initiate signaling, thus playing a regulatory role in the organism [31]. Classical hormonal mediators, involving insulin, glucagon [24], epinephrine [25], norepinephrine [26], cortisol [27], growth hormone [10] and thyroxine [11], play a central regulatory role in coordinating interactions between insulin-related organs and maintaining the body's blood glucose levels (Table 1).
With the deepening understanding of the endocrine system, the concept of hormones has expanded from a narrow definition of hormones secreted by endocrine glands to encompass a broader category of molecules or substances that exhibit characteristics similar to traditional hormones. This category includes organokines secreted by metabolism-related organs and a novel class of endocrine hormone secreted by various cells, known as EVs [13, 16].
Organokines, also known as organ hormones, refers to a complex array of cytokines secreted by organs such as adipose tissue, skeletal muscle, intestine and bone, which function as endocrine organs [12]. Organokines share similarity with classical hormones in that they mediate interorgan crosstalk and participate in the regulation of organismal homeostasis through autocrine, paracrine, or endocrine actions [32-35]. IR-related organ hormones primarily include Adipokines, Myokines, Hepatokines, Gut cytokines, and Osteokines [16, 36, 37], and certain non-tissue-specific inflammatory factors such as tumor necrosis factor (TNF)-α [38], interleukin (IL)-6[39], and connexin (Cx)43 [40] are also included. These factors are involved in IR processes by modulating insulin signaling pathways in insulin target organs (Table 1).
EVs are a collective term for lipid bilayer-enclosed particles released by cells [17], which are important mediators of cell/organ communication. There are several ways to classify EVs, and the most widely recognized classification in the academic community is to classify EVs into three categories based on their size, biogenesis, and synthesis process: exosomes, microparticles, and apoptotic vesicles. Exosomes are vesicular bodies with a diameter of 30-150 nm and a double-layer membrane structure, that are generated through the endocytosis-endosomal pathway and are released into the extracellular space by exocytosis. Microparticles, also known as microvesicles, which are 100-1000 nm in size, differ from exosomes in that they are formed by separation of cell membranes after direct outward budding. In addition, apoptotic bodies are membrane encapsulated vesicular bodies containing cytoplasm and organelles formed by cell shrinkage and fragmentation during the process of cell apoptosis, with a diameter of approximately 100-5000nm, which are released by the contraction of apoptotic cell membrane by budding [18]. Due to their unique structure and formation mechanism, EVs can transport various contents, including proteins, nucleic acids and lipids, from donor cells to be released into the body fluids, which are ingested by recipient cells, so as to play the role of cell communication, information transmission and regulation of cell functions, and participate in a number of physiological and pathological processes [19]. The uptake of EVs is divided into two steps. In the first step, EVs first connect with the receptor cell membrane through classical adhesion molecules (integrin, tetra transmembrane protein, lactoferrin, etc.) to bind to the surface receptor. In the second step, the receptor cells internalized EVs through macropinocytosis, phagocytosis, and clathrin/caveolin mediated endocytosis; or directly fuse with the cell membrane to release the contents into the target cells [41].
As a new type of endocrine hormone, EVs act in a classical endocrine hormone-like manner. They transmit signals through signaling molecules in the organism to regulate the function of target cells and maintain homeostasis [28]. However, unlike classical hormones and organokines, which are typically single molecules, EVs carry complex cargos like proteins, nucleic acids, and lipids. This allows EVs to have a more detailed regulatory function in cell and organ interactions.[42]. In the physiological state, the cargos transported by EVs play a role in regulating the physiological functions of insulin-secreting organs and insulin-responsive target organs. However, in the pathological state, cells/organs influence the disease process by altering the composition of cargos in the EVs, either by decreasing the beneficial cargos or increasing the pathogenic cargos.
The role of classical hormones and organokines in insulin resistance
| Classification | Scientific Name | Molecular Mechanism | Effect in insulin resistance (+ : promote; - : inhibit) | |
|---|---|---|---|---|
| Classical hormone | Glucagon | Promote fat decomposition and oxidation; Promote insulin secretion in the body. | + | |
| Epinephrine, Norepinephrine | Bind to insulin receptor and inhibit the biological activity of insulin; Promote fat decomposition and increase the level of free fatty acids, thus inhibiting insulin stimulated glucose utilization. | + | ||
| Cortisol | Inhibit insulin secretion in pancreas β cells; Promote gluconeogenesis in liver and muscle; Promote fat decomposition; Promote the decomposition of muscle protein, increase the level of amino acids, thereby stimulating insulin secretion. | + | ||
| Growth hormone | Promote the uptake and utilization of glucose in muscle and adipose tissue; Inhibit insulin secretion in pancreas β cells. | - | ||
| Promote the decomposition of adipose tissue; Promote gluconeogenesis in liver and muscle. | + | |||
| Thyroxine | Promote protein synthesis; Increase glucose utilization in muscle and adipose tissue; Inhibit insulin secretion in pancreas β cells; Promote the decomposition of adipose tissue. | - | ||
| Promote protein metabolism; Promote gluconeogenesis in liver and muscle. | + | |||
| Organokines | Adipokines | Leptin | Enhance glucose uptake in brown adipose tissue and skeletal muscle. | - |
| Inhibit hepatic glucose output; Inhibit the secretion of corticosterone and glucagon. | + | |||
| Adiponectin | Improve inflammatory response and oxidative stress; Enhance the utilization of glucose and fatty acids in skeletal muscle; Inhibit gluconeogenesis and glycogen decomposition in the liver. | - | ||
| ZAG | Restore the damaged hepatic IRS/Akt signal transduction induced by HFD or palmitic acid. | - | ||
| CCN4 | Weaken the effect of insulin on the phosphorylation of insulin receptor B, Akt and GSK3β | + | ||
| Myokines | Irisin | Improve the lipid and glucose metabolism of skeletal muscle and liver through PI3K/Akt and AMPK pathways; Increase β Cell viability through Akt/Bcl2 signaling pathway. | - | |
| MSTN | Degrade IRS1 protein by CBLB in a Smad3 dependent manner. | + | ||
| BABA | Reduce gluconeogenesis through IRS1/Akt and AMPK pathways. | - | ||
| Hepatokines | Fetuin-A | Increase the production of non-esterified fatty acids, inflammatory precursors of IL-6, IL1β and TNF-α in macrophages and adipocytes, and decrease the production of anti-inflammatory cytokines such as lipocalin; Inhibit the tyrosine kinase of insulin receptor and destroy the downstream phosphorylation pathway of insulin receptor; As an endogenous ligand of Toll like receptor 4, saturated fatty acids induce pro-inflammatory signals and IR through this ligand. | + | |
| FGF21 | Inhibit SREBP1c-mediated lipogenesis and hepatic gluconeogenesis through AMPK - SIRT1 pathway. | - | ||
| Seleno protein P | unclear | - | ||
| Gut cytokines | FGF15/19 | Stimulate glycogen synthesis. | - | |
| Reduce glycogen production by inactivating GSK3 and CREB | + | |||
| GLP-1 | Enhance insulin sensitivity through PKC/PPARγ signaling. | |||
| Osteokines | Osteopontin | Inhibit STAT3 and increase expression of the PEPCK and glucose-6-phosphatase. | + | |
| Osteocalcin | Inhibit NF-KB signaling pathway to attenuate endoplasmic reticulum stress. | - | ||
| inflammatory cytokines | TNF-α | Weaken insulin signaling through phosphorylation of serine to induces IR in adipocytes and peripheral tissues. | + | |
| IL-6 | Mediate GLUT4 translocation and fatty acid oxidation via the AMPK pathway in muscle and fat; Increase hepatic glucose output through increased hepatic glycogenolysis, gluconeogenesis, and glucose release; Stimulate insulin secretion in β-cells by increasing glucagon-like peptide-1 in intestinal L-cells. | - | ||
| Cx43 | mediate an increase in intercellular coupling to synergize endoplasmic reticulum stress to promote hepatocyte IR. | + | ||
In the following sections, we will explore how EVs contribute to the regulation of disease progression and outline the clinical applications of EVs in IR-related diseases.
The role of extracellular vesicles in the pathogenesis of insulin resistance
In recent years, the regulatory role of EV-mediated cell/organ communication in IR has attracted much attention. As mentioned earlier, the pathological process of IR can be divided into two aspects: the compensatory secretion of insulin by pancreatic islet cells into the circulatory system, and the subsequent impact of insulin on various target organs, resulting in the biological effects of IR. In this section, we will delve into the regulatory mechanism of IR involving EVs, specifically exploring the contributions of EVs derived from different organ/tissue sources in the regulation of insulin secretion and their effects on insulin-responsive target organ (Figure 1).
Extracellular vesicles are involved in the regulation of insulin secretion of pancreatic islets
In recent years, insulin secretion has been shown to be regulated not only by blood glucose levels and inflammatory factors, but also by EVs. EVs can target pancreatic islet cells via their cargos, directly affecting the secretion level of pancreatic islet cells or indirectly affecting the proliferation or apoptosis of pancreatic islet cells, thus participating in the regulation of insulin secretion [43].
In several animal models of IR-related diseases, researchers have found that EVs of well-defined sources enhance insulin secretion by transporting noncoding RNAs targeted to islet cells. For instance, In the T2DM mouse model, the content of lncRNA-p3134 in EVs released by mouse islet cells increased. These EVs act on adjacent islet β cells through the cell microenvironment, and promote compensatory insulin secretion by promoting the expression of PDX-1, MafA, GLUT2 and TCF7L2 [44]. In the high-fat fed (HFD) mouse model, EVs derived from skeletal muscle can enter the islet of Langerhans and enhance insulin secretion by carrying miR-16 to promote the proliferation of islet β cells [45]. In the ovariectomized rat model simulating postmenopausal women, after treatment with liraglutide, the EVS derived from bone marrow are enriched with miR-322-3p and miR-335. These EVs enter the pancreas with blood and are internalized by islet cells to enhance insulin secretion by inhibiting target genes adcy5, CBP and Fra-1 [46].
In contrast, some studies have identified circulating EVs of unknown origin in the circulatory system that are capable of triggering impaired insulin secretion. In obese mice, the expression of miR-26 in serum exosomes decreased. These circulating exosomes can target pancreatic islet cells and inhibit insulin secretion by inhibiting ten regulatory factors including mtpn, onecut2, cacna1c, plcb1, pja2, ESR1, EXT1, PAK2, GSK3 β and itga5 in pancreatic islet cells [47]. However, in T2DM mice, the expression of miR-223 in serum exosomes is reduced, and it enters pancreatic islet cells with circulation, and insulin secretion is impaired by inhibiting FoxO1 and Sox6 pathways [48].
Extracellular vesicles are involved in the regulation of insulin resistance in various insulin-responsive target organs
EVs carrying specific cargos to act on target cells are considered to be key factors in the development of IR in target organs. Currently, a large number of studies have focused on exploring the mechanisms by which EVs are involved in the regulation of IR in the three primary target organs-the liver, adipose tissue, and skeletal muscle. Whereas the mechanisms by which EVs are involved in IR in other target organs (e.g., heart, kidney, ovary, and brain) have not yet been elucidated [49, 50].
The role of extracellular vesicles in regulating hepatic insulin resistance
The liver, as an important site of venous return, receives an enriched supply of insulin through the portal vein, making the liver as one of the pivotal target organs affected by IR. When IR occurs in the liver, the inhibitory effect of insulin on hepatic glycogenolysis and glucose gluconeogenesis is weakened, which causes a rise in blood glucose.
EVs play a crucial role in the development of hepatic IR. Several studies have shown that EVs from various sources contribute to hepatic IR by delivering microRNAs (miRNAs) to hepatocytes. In the HFD mouse model, elevated free fatty acids (FFAs) increase miR-155 in the exosomes secreted by M1 macrophages in adipose tissue, and act on PPAR after being ingested by hepatocytes, thus blocking the expression of GLUT2, thereby aggravating hepatic IR [51]. Elevated FFAs can also increase miR-222 in exosomes derived from white adipose tissue of mouse gonad, and then enter the liver, thereby down regulating the levels of IRS-1 and phosphorylated Akt in hepatocytes and promoting hepatic IR [52]. Moreover, FFAs stimulation makes mouse islet cells secrete exosomes, carrying a significantly increased miR-29 family, targeting the liver with blood circulation, downregulating the phosphorylation of PI3K Akt GSK pathway in hepatocytes, thereby inducing hepatic IR [53].
EVs with specific cell source regulate IR in different organs by distant secretion or paracrine. (1) Distant secretion of extracellular vesicles: extracellular vesicles with specific cargos secreted by different cells reach the target organ with blood circulation, and are taken up into the cell by the target cell, and then participate in the regulation of insulin secretion or insulin resistance of the target organ by acting on specific molecules. (2) Paracrine of extracellular vesicles: in the pancreas or insulin target organs (liver, adipose tissue, kidney), extracellular vesicles with specific cargos secreted by different cells act on adjacent cells through diffusion, and participate in regulating insulin secretion or insulin resistance of target organs.
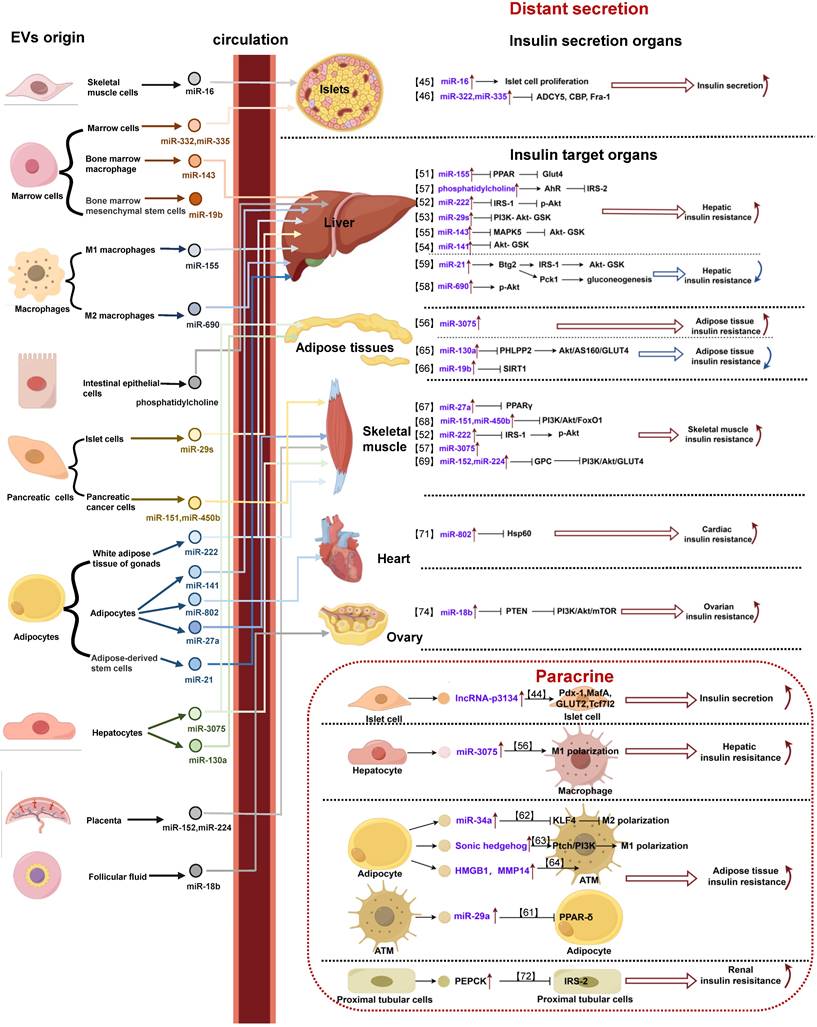
In addition, in HFD mice, the expression of miR-141-3p in exosomes released from adipose tissue decreased significantly. When hepatocytes ingested these exosomes, their insulin sensitivity and glucose uptake ability decreased, promoting IR [54]. However, in obese mice, exosomes derived from bone marrow macrophages carry elevated miR-143-5p into hepatocytes and induce liver IR by inhibiting the expression of mitogen activated protein kinase 5 (MPK5) [55]; Liver parenchymal cells can secrete exosomes and carry elevated miR-3075, which acts on liver macrophages through the cell microenvironment, promotes macrophage infiltration and inflammatory reaction, thus inhibiting liver insulin sensitivity and participating in IR [56]. In addition, EVs can also induce liver IR by delivering lipids to hepatocytes. In the HFD mouse model, exosomes derived from intestinal epithelial cells, containing phosphatidylcholine, were found to activate the hepatocyte AhR signaling pathway in an HFD mouse model. This activation downregulates IRS-2 expression and contributes to hepatic insulin signaling deficiency upon uptake by hepatocytes [57].
In contrast, several other studies have shown that EV-derived miRNAs can ameliorate hepatic IR once they enter hepatocytes. In HFD mouse model, the exocrine secretion secreted by M2 macrophages can act on hepatocytes with miR-690, upregulate Akt phosphorylation level and improve hepatic IR [58]. In addition, in a PCOS rat model, exosomes derived from adipose stem cells carried miR-21-5p into hepatocytes, targeted the BTG antiproliferation factor 2 (Btg2), and induced the cAMP-response element binding protein (CREB) in hepatocytes to bind to the CREB cis element on the target gene promoter. This, in turn, increased the transcription of phosphoenolpyruvate carboxykinase (PEPCK) 1, promoting liver gluconeogenesis. Moreover, it activated the IRS1 and Akt pathways, thereby improving liver insulin sensitivity [59].
Extracellular vesicles are involved in the regulation of insulin resistance in adipose tissue
Adipose tissue serves as an important immune and endocrine organ, where inflammation and increased lipocalin secretion can lead to IR. When IR occurs in adipose tissue, fat synthesis decreases while catabolism is enhanced, leading to elevated blood lipids. Then, a large amount of free fatty acid is absorbed by the liver and converted to glucose, which subsequently leads to elevated blood glucose.
In recent years, several studies have reported that EVs, as a regulatory mediator, are also involved in the regulation of IR in adipose tissue [60]. Among them, some studies have revealed that different kinds of cells in adipose tissue carried cargos, such as miRNA, via EVs to promote IR. In obese mice model, miR-29a was significantly elevated in exosomes secreted by adipose tissue macrophages (ATMs), which acted on adipocytes and induced adipose tissue IR by significantly inhibiting PPAR-δ [61]. In addition, exosomes secreted by mature adipocytes promoted adipose tissue IR by translocating miR-34a into ATMs via paracrine secretion and inhibiting macrophage M2 polarization by suppressing the expression of Krüppel-like factor 4(KLF4) [62]. As mentioned above, hepatocyte-derived exosomes carrying miR-3075 can also act on adipocytes via a compatible mechanism to inhibit adipose tissue insulin sensitivity to promote IR [56]. In addition to miRNAs, EVs also carry DNA and proteins to induce adipose tissue IR. The exosomes secreted by adipocytes can carry the enhanced sonic hedgehog gene to target ATMs, and promote adipose tissue IR by mediating M1 macrophage polarization through the PTCH/PI3K signaling pathway [63]. Adipocyte-derived exosomes carrying elevated high mobility group protein (HMG) B1 and matrix metalloproteinase (MMP) 14 acted on ATMs, leading to the induction of adipose tissue IR [64].
On the other hand, researchers have demonstrated that EVs derived from other tissues ameliorate adipose tissue IR by delivering miRNAs and targeting adipocytes. In HFD mouse model, exosomes secreted from hepatocytes could carry miR-130a-3p into adipocytes. The process subsequently inhibited the PH domain and leucine rich repeat protein phosphatase 2 (PHLPP2) gene, leading to the activation of the Akt/AS160/GLUT4 signaling pathway in adipocytes. This activation resulted in improved glucose tolerance and the subsequent restoration of adipose tissue IR [65]. In addition, in an aging mouse model, miR-19b-3p released from mouse bone marrow mesenchymal stem cell-derived exosomes was taken up by adipocytes to ameliorate adipose tissue IR through the inhibition of silencing information regulatory factor 2-related enzyme 1(SIRT1) [66].
Extracellular vesicles are involved in the regulation of skeletal muscle insulin resistance
As the largest glucose storage and consumption organ in the body, skeletal muscle is the main target tissue for insulin. When IR occurs in skeletal muscle, the uptake and utilization of glucose by skeletal muscle cells are impaired, which in turn leads to elevated blood glucose. When IR occurs, the contractile and diastolic functions of skeletal muscle are impaired, which prevents the effective regulation of blood glucose levels and further leads to elevated blood glucose.
In various studies, researchers have independently reported that EVs secreted by different cells, carrying their specific miRNAs and targeting skeletal muscle, play a role in the promotion of its IR. In a T2DM mouse model, exosomes secreted by adipose tissue carrying elevated miR-27a into skeletal muscle cells induced skeletal muscle IR by inhibiting the target gene PPAR-γ [67]. In a mouse model of pancreatic cancer, increasing exosomal miR-151-3p and miR-450b-3p derived from pancreatic cancer cell were absorbed by C2C12 cells in skeletal muscle myotubes. These miRNAs promoted skeletal muscle IR by inhibiting PI3K/Akt/FoxO1 signaling pathway [68]. As mentioned above, exosomes from gonadal white adipose tissue originating from HFD-fed mice carry miR-222 into skeletal muscle cells, where it induces skeletal muscle IR by a similar molecular mechanism [52]. Hepatocyte-derived exosomal miR-3075 can also promote skeletal muscle IR [56]. In human studies, placenta-derived exosomes from patients with diabetic pregnancies were found to transport miR-152-3p and miR-224-5p into human skeletal muscle cells. These miRNAs targeted the regulation of glypican (GPC) genes, resulting in the inhibition of the insulin signaling PI3K/Akt/GLUT4 pathway and a reduction in skeletal muscle glucose uptake, thereby inducing IR [69].
In addition, some unknown EVs carrying miRNAs were also found in the circulatory system to participate in the induction of skeletal muscle IR. For instance, the circulating exosomes of T2DM patients highly express miR-20b-5p. After entering human skeletal muscle cells, these exosomes downregulate the activity of glycogen synthase by inhibiting the expression of Akt interacting proteins and signal transduction and transcription activators, and subsequently participate in the occurrence of skeletal muscle IR [70]. However, how EVs ameliorate skeletal muscle IR remains to be further investigated.
Extracellular vesicles are involved in the regulation of insulin resistance in other target organs
Extracellular vesicles are involved in the regulation of cardiac insulin resistance
Insulin regulates the uptake and utilization of glucose by cardiomyocytes, as well as the recycling of calcium ions by the sarcoplasmic reticulum of cardiomyocytes. When IR occurs in the heart, the uptake and utilization of glucose by the heart are reduced, leading to elevated blood glucose and impaired diastolic and systolic function. However, there is only one study on the mechanism which EVs are involved in regulating cardiac IR. In this article, Wen et al. found in a neonatal rat model that hypertrophic adipocyte-derived exosomes highly expressed miR-802-5p, and that these exosomes entered ventricular myocytes and targeted silencing of heat shock protein (HSP) 60, thereby inducing cardiomyocyte IR and attenuating the insulin-sensitizing effects of lipocalin [71].
Extracellular vesicles are involved in the regulation of renal insulin resistance
The kidney is an important component in the regulation of glucose metabolism, and insulin promotes renal glucose uptake and utilization. When IR occurs in the kidney, in addition to impaired renal glucose utilization, renal function significantly changes, and even progresses to renal insufficiency. Up to date, no study has clearly reported the role that EVs play in the mechanism of renal IR, but some studies have shown that exosomes secreted by human proximal tubule cells, which carry large amounts of PEPCK, enter surrounding proximal tubule cells, inhibit IRS-2, and impair renal gluconeogenesis [72].
Extracellular vesicles are involved in the regulation of ovarian insulin resistance
Insulin is involved mainly in glucose metabolism and hormone synthesis in the ovary. When IR occurs, in addition to the decrease in glucose uptake and utilization by ovarian cells, hyperinsulinemia also promotes the production of excessive androgens in the adrenal gland and ovary, and leads to a decrease in sex hormone globulin levels and an increase in free testosterone by inhibiting liver synthesis, thus contributing to the occurrence of PCOS [73].
Due to the lack of relevant studies, the role of EVs in ovarian IR needs to be further clarified. In PCOS rats, miR-18b-5p is enriched in exosomes in follicular fluid. After these exosomes are ingested by ovarian granulosa cells, they target PTEN and PI3K/Akt/mTOR signaling pathway mediated by PTEN, thereby inhibiting ovarian IR [74]. However, there is an unknown source of exosomes in the plasma of PCOS mice. The expression of miR-126-3p in these exosomes is increased and acts on ovarian granulosa cells to participate in ovarian IR by inhibiting platelet-derived growth factor receptor β (PDGFR β) and its downstream PI3K/Akt pathway [75].
Extracellular vesicles are involved in the regulation of insulin resistance in the brain
Insulin regulates cerebral vascular function and is also involved in maintaining brain proteostasis, influencing amyloid β-peptide clearance and tau protein phosphorylation. When brain IR occurs, brain's bioenergetics are severely affected. Specifically, when brain tissues have difficulty utilizing glucose, the brain adopts an energy-saving mechanism to survive by inhibiting functions such as learning and memory, which can cause cerebral atrophy or long-term AD [76]. In addition, brain IR is involved in the onset and development of Parkinson's disease by causing mitochondrial dysfunction, oxidative stress, and neuroinflammation, and is closely associated with cognitive dysfunction in Parkinson's disease [77]. However, no study has reported the specific role of EVs in cerebral IR until now, but some clinical studies have shown that neurogenic exosomes can be detected in the blood, which suggests that EVs can be involved in the pathological process of IR in the brain [78].
In conclusion, EVs from different cell sources act on different target cells in a similar "hormone like" manner. Under the pathological condition of IR, high glucose, high fat and other stimulating factors act on the donor cells to change the expression levels of EVs contents. These EVs reach the specific recipient cells through remote secretion of blood, paracrine secretion of tissue fluid or microenvironment autocrine secretion, and are taken up by the recipient cells. Once inside, they release the effective content in the recipient cells to participate in the regulation of IR. However, because EVs carry different effectors, their mechanisms of action in target cells are completely different. For example, non-coding RNAs participate in IR signal pathway by inhibiting downstream target genes, while proteins or lipids directly interact with signal molecules to affect IR signal pathway. It is worth noting that current determinations of effector content are mostly based on detecting expression differences in donor cells, recipient cells and EVs. These contents are highly correlated with donor cells and participate in the regulation of the proliferation, secretion and other functions of donor cells. However, whether these effector content determines the targeting of EVs to some extent is worth further exploration. In addition, the interaction between adhesion molecules on EVs and specific receptors on the target cytoplasmic membrane makes the secretion of EVs have a certain targeting. However, due to the limitations of experimental technology, current research on the targeted uptake of EVs by recipient cells is limited, and clear conclusions are still lacking.
The role of extracellular vesicles in the diagnosis and treatment of insulin resistance
Diagnostic potential of extracellular vesicles in insulin resistance related diseases
EVs are widely present in various biological fluids, including blood and urine, and the contents of these vesicles are closely correlated with disease states. Due to their unique structure, EVs exhibit remarkable stability in body fluids, enduring long-term storage, repeated freeze‒thaw cycles, and extreme pH conditions. Therefore, EVs are expected to play an important role as non-invasive markers for the diagnosis and early screening of IR in the body (Figure 2).
Serum EVs play an important role in the diagnosis of T2DM. Several studies have reported that the serum exosomal miR-26a is significantly lower in overweight individuals than in healthy individuals and negatively correlates with the clinical features of T2DM [47]. Additionally, miR-223 expression in the serum EVs is reduced in T2DM patients compared to healthy individuals [48]. Conversely, levels of lncRNA-p3134, miR-20b-5p, and miR-222 were elevated in the serum EVs of patients with T2DM [44, 54, 70], and the population of sonic hedgehog positive exosomes in the serum of T2DM patients increased significantly [63]. Urinary exosomes are also valuable in the diagnosis of pre-T2DM, where PEPCK is significantly elevated and positively correlates with systemic insulin sensitivity [72].
In terms of PCOS diagnosis, miR-18b-5p levels are elevated in follicular fluid exosomes from female patients [74]. In terms of AD diagnosis, AD patients have higher expression of pSer312-IRS-1 and lower expression of p-panTyr-IRS-1 in peripheral blood exosomes, and brain volume in AD patients is positively correlated with P-panTyr-IRS-1 and negatively correlated with pSer312-IRS-1[77, 78].
The mechanism by which these EV-derived small molecules target individual cells and participate in the IR of each target organ has been described in detail in the previous section, and will not be repeated here. However, their sensitivity and specificity need to be further verified in clinical tests, but provide a new direction for the diagnosis and screening of IR-related diseases.
Therapeutic perspectives of extracellular vesicles in insulin resistance-related diseases
With the deepening of our understanding of EVs, EVs have the following advantages in disease therapy. Firstly, EVs possess tough lipid bilayer vesicles to maintain bioactivity while being repeatedly manipulated. Secondly, they are immune-tolerant, easy to manipulate, and highly permeable to biological barriers. Therefore, EVs hold great promise for the treatment of a wide range of diseases involving IR (Figure 2).
In clinical therapeutic applications, one study revealed that using EVs secreted by human endothelial progenitor cells to treat human pancreatic islet cells in vitro can improve glucose stimulated insulin secretion and reduce the β apoptosis rate [79]. Another study reported the inhibition of skeletal muscle IR in patients with diabetic gestation by administering placental exosomes secreted from the body fluids of healthy pregnant women [69].
The application of EVs in the diagnosis and treatment of IR related diseases. The application of extracellular vesicles in insulin resistance related diseases is mainly divided into two types: as a biomarker for diagnosis and participation in treatment. Starting from the diagnostic biomarkers, the extracellular vesicles are isolated and purified from body fluid, and the content of specific cargos was detected. The increase or decrease of these cargos suggested the related progress of type 2 diabetes, polycystic ovary syndrome and Alzheimer's disease. From the perspective of treatment, after the isolation and purification of extracellular vesicles in body fluid or cell culture medium, using these extracellular vesicles to treat patients or animals with insulin resistance related diseases can significantly relieve the symptoms of insulin resistance in their insulin target organs (liver, skeletal muscle and heart) or improve insulin secretion.
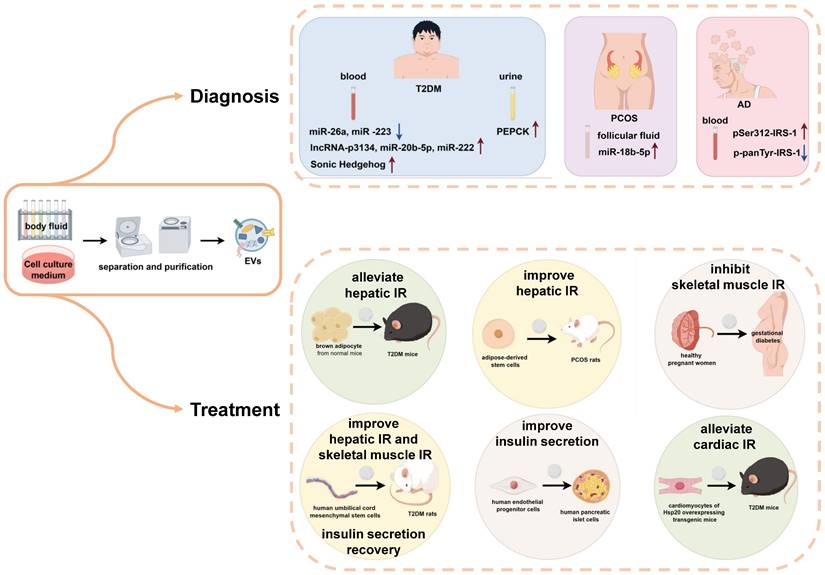
On the other hand, in animal studies, injection of brown adipocyte-derived exosomes from normal mice into T2DM mice was found to alleviate hepatic IR and impaired glucose tolerance [80]. After injecting EVs derived from human umbilical cord mesenchymal stem cells into T2DM rats, the phosphorylation of IRS-1 and Akt in skeletal muscle and liver was restored, which promoted the expression and membrane translocation of GLUT4 in muscle, and the synthesis of glycogen in the liver. Moreover, these exosomes could inhibit β-cell apoptosis and promote the recovery of insulin secretion function [81-83]. Exosomes secreted by cardiomyocytes from Hsp20 overexpressing transgenic mice were collected and injected into mice with diabetic cardiomyopathy. These exosomes carry a large number of phosphorylated Akt molecules, which can alleviate IR in cardiomyocytes [84]. The application of exosomes derived from adipose derived stem cells in the treatment of rats with PCOS can improve liver glucose metabolism in rats, thereby alleviating the symptoms of PCOS, through a therapeutic mechanism as described previously [59]. Although, there are few reports on the use of EVs for the treatment of IR-associated diseases, they also provide new ideas for future disease treatment.
Conclusion
As our understanding of EVs deepens, it becomes increasingly clear that EVs carry specific small molecules and play important roles in the development and progression of IR in various organs of the body, essentially acting as a novel endocrine hormone in intercellular communication. Although research on the use of EVs in the diagnosis and treatment of IR-related diseases is still in the early stage, their great potential should not be underestimated. Therefore, further investigation into the biological source, release, targeted regulation of EVs, their underlying regulatory mechanisms, and the exploration of new molecular markers associated with EVs in the diagnosis and treatment of IR-related diseases will undoubtedly have far-reaching significance for the prevention and treatment of IR-related diseases.
Abbreviations
AD: Alzheimer's disease
Adcy5: adenylate cyclase 5
Akt/PKB: protein kinase B
ATMs: adipose tissue macrophages
Bcl2: B-cell lymphoma-2
Bhlhe22: basic helix-loop-helix family, member e22
Btg2: anti-proliferation factor 2
Cacna1c: calcium channel, voltage-dependent, L type, alpha 1C subunit
CBLB: biquitin-protein ligase B
CBP: sarcoplasmic calcium-binding protein
CREB: cAMP-response element binding protein
Crem: cAMP responsive element modulator;
Cx: connexin
Esr1: estrogen receptor 1
EVs: extracellular vesicles
Ext1: exostosin glycosyltransferase 1
FFAs: free fatty acids
FOXO1: forkhead box O1
FRA1: P-loop containing nucleoside triphosphate hydrolases superfamily protein
GLUT: glucose transporter protein
GPC: glypican
GSK: glycogen synthase kinase
GSV: glucose transporter protein type 4 storage vesicles
Hes1 : hes family bHLH transcription factor 1
HFD: high-fat-diet
HMG: high mobility group protein
HSP: heat shock protein
IL: interleukin
Insm1: INSM transcriptional repressor 1
Insm2: INSM transcriptional repressor 2
IR: insulin resistance
IRS: insulin receptor substrate
Itga5: integrin alpha 5 (fibronectin receptor alpha)
KLF4: Krüppel-like factor 4
MafA: MAF bZIP transcription factor A
MAFLD: metabolic dysfunction related fatty liver disease
miRNA: microRNA
MMP: matrix metalloproteinase
MPK5: mitogen-activated protein kinase 5
mTORC2: mechanistic target of rapamycin complex 2
Mtpn: myotrophin
Neuro D: neurogenic differentiation Factor 1
NF-KB: nuclear factor-k-gene binding
Nkx2-2: NK2 homeobox 2
Nkx6.1: NK6 homeobox 1
Onecut2: one cut domain, family member 2
Pak2: p21 (RAC1) activated kinase 2
Pax6: paired box 6
PCOS: polycystic ovary syndrome
PDE3B: phosphodiesterase 3B
PDGFRβ: growth factor receptor β
PDK1: phosphatidylinositol-3-phosphate-dependent kinase-1
Pdx1: pancreatic and duodenal homeobox 1
PEPCK: phosphoenolpyruvate Carboxykinase
PHLPP2: PH domain and leucine rich repeat protein phosphatase 2
PI3K: phosphatidylinositol-3-OH kinase
PIP2: phosphatidylinositol-4,5-bisphosphate
PIP3: phosphatidylinositol-3,4,5-trisphosphate
Pja2: praja ring finger ubiquitin ligase 2
Plcb1: phospholipase C, beta 1
PP: protein phosphatase
p-panTyr-IRS-1: Phospho- Pan-Tyrrosine-Insulin Receptor Substrate 1
PPAR: peroxisome proliferator-activated receptor
p-Ser312-IRS-1: Phospho- Serine312-Insulin Receptor Substrate 1
Pten: phosphatase and tensin homolog
SIRT1: silencing information regulatory factor 2-related enzyme 1
Smad3: SMAD family member 3
Sox6 SRY: (sex determining region Y)-box 6
SREBP-1c: sterol regulatory element binding protein 1c
STAT3: signal transducer and activator of transcription 3
Stx3: syntaxin 3
T2DM: type 2 diabetes mellitus
Tcf7l2: transcription factor 7 like 2
TNF: tumor necrosis factor
Acknowledgements
Figures and Graphical Abstract were created by Figdraw.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82370584), National High Level Hospital Clinical Research Funding (BJ-2024-219, BJ-2023-236, BJ-2022-167), National Natural Science Foundation of China (81600618), Beijing Natural Science Foundation (7232141) and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-050).
Author contributions
XFZ, and HXQ conceived the idea for this review. XFZ and DL performed the literature review, wrote the manuscript, and generated the figures. YDN, WX, LLT and MY provided feedback, perspective, and expertise. HXQ revised the manuscript. All authors read and approved the final manuscript.
Data availability
Data will be made available on request.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415-28
2. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840-6
3. Hill MA, Yang Y, Zhang L. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766
4. Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156
5. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774-800
6. Sędzikowska A, Szablewski L. Insulin and Insulin Resistance in Alzheimer's Disease. Int J Mol Sci. 2021;22(18):9987
7. Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct Target Ther. 2022;7(1):216
8. Napoleão A, Fernandes L, Miranda C, Marum AP. Effects of Calorie Restriction on Health Span and Insulin Resistance: Classic Calorie Restriction Diet vs. Ketosis-Inducing Diet. Nutrients. 2021;13(4):1302
9. Sims EK, Carr A, Oram RA, DiMeglio LA, Evans-Molina C. 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat Med. 2021;27(7):1154-1164
10. Jeffcoate W. Growth hormone therapy and its relationship to insulin resistance, glucose intolerance and diabetes mellitus: a review of recent evidence. Drug Saf. 2002;25(3):199-212
11. Ferrannini E, Iervasi G, Cobb J, Ndreu R, Nannipieri M. Insulin resistance and normal thyroid hormone levels: prospective study and metabolomic analysis. Am J Physiol Endocrinol Metab. 2017;312(5):E429-E436
12. de Oliveira Dos Santos AR, de Oliveira Zanuso B, Miola V. et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int J Mol Sci. 2021;22(5):2639
13. Wang YY, Wang YD, Qi XY, Liao ZZ, Mai YN, Xiao XH. Organokines and Exosomes: Integrators of Adipose Tissue Macrophage Polarization and Recruitment in Obesity. Front Endocrinol (Lausanne). 2022;13:839849
14. Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab. 2017;28(1):3-18
15. Connolly KD, Wadey RM, Mathew D, Johnson E, Rees DA, James PE. Evidence for Adipocyte-Derived Extracellular Vesicles in the Human Circulation. Endocrinology. 2018;159(9):3259-3267
16. Wang YD, Wu LL, Qi XY. et al. New insight of obesity-associated NAFLD: Dysregulated "crosstalk" between multi-organ and the liver. Genes Dis. 2023;10(3):799-812
17. Elsharkasy OM, Nordin JZ, Hagey DW. et al. Extracellular vesicles as drug delivery systems: Why and how. Adv Drug Deliv Rev. 2020;159:332-343
18. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9-17
19. French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin Cell Dev Biol. 2017;67:48-55
20. Vlachakis D, Mitsis Τ, Nicolaides N. et al. Functions, pathophysiology and current insights of exosomal endocrinology (Review). Mol Med Rep. 2021;23(1):26
21. Pardini B, Calin GA. MicroRNAs and Long Non-Coding RNAs and Their Hormone-Like Activities in Cancer. Cancers (Basel). 2019;11(3):378
22. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116(7):1802-12
23. Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. 2018;98(4):2133-2223
24. Sharma R, Sahoo B, Srivastava A, Tiwari S. Reduced insulin signaling and high glucagon in early insulin resistance impaired fast-fed regulation of renal gluconeogenesis via insulin receptor substrate. J Cell Biochem. 2022;123(8):1327-1339
25. Jensen J, Lai YC. Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Arch Physiol Biochem. 2009;115(1):13-21
26. Guo J, Liu G, Guo G. Association of insulin resistance and autonomic tone in patients with pregnancy-induced hypertension. Clin Exp Hypertens. 2018;40(5):476-480
27. Morais J, Severo JS, Beserra JB. et al. Association Between Cortisol, Insulin Resistance and Zinc in Obesity: a Mini-Review. Biol Trace Elem Res. 2019;191(2):323-330
28. BEHRENS OK, BROMER WW. Biochemistry of the protein hormones. Annu Rev Biochem. 1958;27(3):57-100
29. Zhang X, Ji X, Wang Q, Li JZ. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Protein Cell. 2018;9(2):164-177
30. Fafián-Labora JA, O'Loghlen A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020;30(8):628-639
31. Guarnotta V, Amodei R, Frasca F, Aversa A, Giordano C. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int J Mol Sci. 2022;23(10):5710
32. Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9(3):144-52
33. Priest C, Tontonoz P. Inter-organ cross-talk in metabolic syndrome. Nat Metab. 2019;1(12):1177-1188
34. Meex R, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509-520
35. Karsenty G, Olson EN. Bone and Muscle Endocrine Functions: Unexpected Paradigms of Inter-organ Communication. Cell. 2016;164(6):1248-1256
36. Chung HS, Choi KM. Organokines in disease. Adv Clin Chem. 2020;94:261-321
37. Choi KM. The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis. Endocrinol Metab (Seoul). 2016;31(1):1-6
38. Akash M, Rehman K, Liaqat A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J Cell Biochem. 2018;119(1):105-110
39. Qu D, Liu J, Lau CW, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171(15):3595-603
40. Tirosh A, Tuncman G, Calay ES. et al. Intercellular Transmission of Hepatic ER Stress in Obesity Disrupts Systemic Metabolism. Cell Metab. 2021;33(2):319-333.e6
41. Xie Q, Hao Y, Li N. et al. Cellular Uptake of Engineered Extracellular Vesicles: Biomechanisms, Engineered Strategies, and Disease Treatment. Adv Healthc Mater. 2024;13(2):e2302280
42. Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200(4):367-71
43. Wei J, Wang Z, Han T. et al. Extracellular vesicle-mediated intercellular and interorgan crosstalk of pancreatic islet in health and diabetes. Front Endocrinol (Lausanne). 2023;14:1170237
44. Ruan Y, Lin N, Ma Q. et al. Circulating LncRNAs Analysis in Patients with Type 2 Diabetes Reveals Novel Genes Influencing Glucose Metabolism and Islet β-Cell Function. Cell Physiol Biochem. 2018;46(1):335-350
45. Jalabert A, Vial G, Guay C. et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59(5):1049-58
46. Li J, Fu LZ, Liu L, Xie F, Dai RC. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist Liraglutide Alters Bone Marrow Exosome-Mediated miRNA Signal Pathways in Ovariectomized Rats with Type 2 Diabetes. Med Sci Monit. 2017;23:5410-5419
47. Xu H, Du X, Xu J. et al. Pancreatic β cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving β cell function. PLoS Biol. 2020;18(2):e3000603
48. Li Y, Deng S, Peng J. et al. MicroRNA-223 is essential for maintaining functional β-cell mass during diabetes through inhibiting both FOXO1 and SOX6 pathways. J Biol Chem. 2019;294(27):10438-10448
49. Li B, Li W, Liu T, Zha L. Extracellular vesicles regulate the transmission of insulin resistance and redefine noncommunicable diseases. Front Mol Biosci. 2022;9:1024786
50. Sáez T, Toledo F, Sobrevia L. Extracellular Vesicles and Insulin Resistance: A Potential Interaction in Vascular Dysfunction. Curr Vasc Pharmacol. 2019;17(5):491-497
51. Ying W, Riopel M, Bandyopadhyay G. et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In vivo and In vitro Insulin Sensitivity. Cell. 2017;171(2):372-384.e12
52. Li D, Song H, Shuo L. et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging (Albany NY). 2020;12(22):22719-22743
53. Li J, Zhang Y, Ye Y. et al. Pancreatic β cells control glucose homeostasis via the secretion of exosomal miR-29 family. J Extracell Vesicles. 2021;10(3):e12055
54. Dang SY, Leng Y, Wang ZX. et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15(2):351-368
55. Li L, Zuo H, Huang X. et al. Bone marrow macrophage-derived exosomal miR-143-5p contributes to insulin resistance in hepatocytes by repressing MKP5. Cell Prolif. 2021;54(12):e13140
56. Ji Y, Luo Z, Gao H. et al. Hepatocyte-derived exosomes from early onset obese mice promote insulin sensitivity through miR-3075. Nat Metab. 2021;3(9):1163-1174
57. Kumar A, Sundaram K, Mu J. et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12(1):213
58. Ying W, Gao H, Dos Reis F. et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021;33(4):781-790.e5
59. Cao M, Zhao Y, Chen T. et al. Adipose mesenchymal stem cell-derived exosomal microRNAs ameliorate polycystic ovary syndrome by protecting against metabolic disturbances. Biomaterials. 2022;288:121739
60. Kita S, Maeda N, Shimomura I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J Clin Invest. 2019;129(10):4041-4049
61. Liu T, Sun YC, Cheng P, Shao HG. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515(2):352-358
62. Pan Y, Hui X, Hoo R. et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129(2):834-849
63. Song M, Han L, Chen FF. et al. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell Physiol Biochem. 2018;48(4):1416-1432
64. Li CJ, Fang QH, Liu ML, Lin JN. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics. 2020;10(16):7422-7435
65. Wu J, Dong T, Chen T. et al. Hepatic exosome-derived miR-130a-3p attenuates glucose intolerance via suppressing PHLPP2 gene in adipocyte. Metabolism. 2020;103:154006
66. Su T, Xiao Y, Xiao Y. et al. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano. 2019;13(2):2450-2462
67. Yu Y, Du H, Wei S. et al. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics. 2018;8(8):2171-2188
68. Wang L, Zhang B, Zheng W. et al. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 2017;7(1):5384
69. Nair S, Jayabalan N, Guanzon D. et al. Human placental exosomes in gestational diabetes mellitus carry a specific set of miRNAs associated with skeletal muscle insulin sensitivity. Clin Sci (Lond). 2018;132(22):2451-2467
70. Katayama M, Wiklander O, Fritz T. et al. Circulating Exosomal miR-20b-5p Is Elevated in Type 2 Diabetes and Could Impair Insulin Action in Human Skeletal Muscle. Diabetes. 2019;68(3):515-526
71. Wen Z, Li J, Fu Y, Zheng Y, Ma M, Wang C. Hypertrophic Adipocyte-Derived Exosomal miR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obesity (Silver Spring). 2020;28(10):1932-1940
72. Sharma R, Kumari M, Prakash P, Gupta S, Tiwari S. Phosphoenolpyruvate carboxykinase in urine exosomes reflect impairment in renal gluconeogenesis in early insulin resistance and diabetes. Am J Physiol Renal Physiol. 2020;318(3):F720-F731
73. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37(5):467-520
74. Zhou Z, Tu Z, Zhang J. et al. Follicular Fluid-Derived Exosomal MicroRNA-18b-5p Regulates PTEN-Mediated PI3K/Akt/mTOR Signaling Pathway to Inhibit Polycystic Ovary Syndrome Development. Mol Neurobiol. 2022;59(4):2520-2531
75. Jiang X, Zhang Z, Hou M, Yang X, Cui L. Plasma exosomes and contained MiRNAs affect the reproductive phenotype in polycystic ovary syndrome. FASEB J. 2023;37(7):e22960
76. Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758-766
77. Nowell J, Blunt E, Edison P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer's and Parkinson's disease. Mol Psychiatry. 2023;28(1):217-229
78. Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D. Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer's disease. Hum Brain Mapp. 2017;38(4):1933-1940
79. Cantaluppi V, Biancone L, Figliolini F. et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21(6):1305-20
80. Thomou T, Mori MA, Dreyfuss JM. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450-455
81. Sun Y, Shi H, Yin S. et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano. 2018;12(8):7613-7628
82. He Q, Wang L, Zhao R. et al. Mesenchymal stem cell-derived exosomes exert ameliorative effects in type 2 diabetes by improving hepatic glucose and lipid metabolism via enhancing autophagy. Stem Cell Res Ther. 2020;11(1):223
83. Yap SK, Tan KL, Abd Rahaman NY. et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Ameliorated Insulin Resistance in Type 2 Diabetes Mellitus Rats. Pharmaceutics. 2022;14(3):649
84. Wang X, Gu H, Huang W. et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65(10):3111-28
Author contact
![]() Corresponding author: Xiuqing Huang. (Tel.): +86-10-58115048; (Fax): +86-10-65237929; (Email): huang_xq118com.
Corresponding author: Xiuqing Huang. (Tel.): +86-10-58115048; (Fax): +86-10-65237929; (Email): huang_xq118com.

 Global reach, higher impact
Global reach, higher impact