3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(10):1945-1963. doi:10.7150/ijms.94335 This issue Cite
Research Paper
Exploring Causal Links Between Gut Microbiota and Geriatric Syndromes: A Two-Sample Mendelian Randomization Analysis
1. Department of Rehabilitation Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, China.
2. School of Nursing, Southern Medical University, Guangzhou, China.
3. School of Rehabilitation Sciences, Southern Medical University, Guangzhou, China.
4. Department of Cardiology, Zhujiang Hospital, Southern Medical University, Guangzhou, China.
5. Dongguan Key Laboratory of Stem Cell and Regenerative Tissue Engineering, Guangdong Medical University, Dongguan, Guangdong, China.
6. Faculty of Health and Social Sciences, The Hong Kong Polytechnic University, Hong Kong, China.
†Their contributions to this work are equal, and they share first authorship.
Received 2024-1-16; Accepted 2024-5-21; Published 2024-7-22
Abstract
Background: Both observational studies and clinical trials have demonstrated a link between the gut microbiota and the geriatric syndrome. Nevertheless, the exact nature of this relationship, particularly concerning causality, remains elusive. Mendelian randomization (MR) is a method of inference based on genetic variation to assess the causal relationship between an exposure and an outcome. In this study, we conducted a two-sample Mendelian randomization (TSMR) study to fully reveal the potential genetic causal effects of gut microbiota on geriatric syndromes.
Methods: This study used data from genome wide association studies (GWAS) to investigate causal relationships between the gut microbiota and geriatric syndromes, including frailty, Parkinson's disease (PD), delirium, insomnia, and depression. The primary causal relationships were evaluated using the inverse-variance weighted method, MR Egger, simple mode, weighted mode and weighted median. To assess the robustness of the results, horizontal pleiotropy was examined through MR-Egger intercept and MR-presso methods. Heterogeneity was assessed using Cochran's Q test, and sensitivity was evaluated via the leave-one-out method.
Results: We identified 41 probable causal relationships between gut microbiota and five geriatric syndrome-associated illnesses using the inverse-variance weighted method. Frailty showed five positive and two negative causal relationships, while PD revealed three positive and four negative causal connections. Delirium showed three positive and two negative causal relationships. Similarly, insomnia demonstrated nine positive and two negative causal connections, while depression presented nine positive and two negative causal relationships.
Conclusions: Using the TSMR method and data from the public GWAS database and, we observed associations between specific microbiota groups and geriatric syndromes. These findings suggest a potential role of gut microbiota in the development of geriatric syndromes, providing valuable insights for further research into the causal relationship between gut microbiota and these syndromes.
Keywords: Geriatric syndrome, Gut microbiota, Mendelian randomization, SNPs
Background
Geriatric syndromes refer to typical age-related symptoms that gradually affect individuals' social and daily living abilities including difficulties in mobility, balance disorders, cognitive impairment, and incontinence, among others [1]. Common geriatric syndromes include frailty, Parkinson's disease, delirium, insomnia, and depression. Frailty is a multi-system physiological state characterized by an individual's increased vulnerability, decreased physiological reserve capacity, and increased susceptibility to stressful events [2]. The most widely used tools for assessing frailty in current clinical practice are the physical frailty phenotype proposed by Fried and the Frailty Index (FI) of accumulative deficits proposed by Rookwood et al. The Fried phenotype scale ranges from slowing of gait speed, loss of grip strength, weight loss (unexplained), perceived fatigue, and low physical activity to rate five dimensions of frailty [3, 4]. Rookwood et al. [5, 6] developed the Frailty Index (FI) based on the theory of accumulative deficits, which is essentially a multidimensional approach to assessing frailty, covering physical functioning, multiple comorbidities, cognition, and psychosocial aspects. Parkinson's disease (PD) is the second most prevalent neurodegenerative illness worldwide, following Alzheimer's disease. There is a strong association between Parkinson's disease and geriatric syndromes. Parkinson's disease has a high prevalence in the elderly population, and its symptoms, such as movement disorders and cognitive decline, often overlap with the debilitating, cognitively impaired manifestations of geriatric syndromes. Therefore, although Parkinson's disease is a neurodegenerative disease in terms of medical classification, it is closely related to geriatric syndromes from the point of view of clinical practice, and together they affect the quality of life of the elderly. Depression is an affective disorder characterized by persistent low mood. Severe cases may include slowness of thought and behavior, as well as various somatization symptoms [7]. The WHO predicts that by 2030, depression will become the leading cause of disease burden [8]. Given the significant health, economic, and social burdens imposed by these conditions, there is an urgent need for a comprehensive understanding of their underlying mechanisms and appropriate treatment.
The gut microbiota constitutes a vital component of the human microecosystem. Microbiome alterations are acknowledged among the hallmarks of aging [9]. Complex interactions between microbes and hosts during aging have been suggested to either accelerate or delay the onset of aging [10], providing a biomedical basis for preventing and treating age-related syndromes. However, the causal relationship between senescence and commensal microbes remains unclear.
Recently, there has been increased investigation into the relationship between gut microbiota and the health of older adults. Research has shown that changes in the microbiota are influenced by factors such as age, polypharmacy, lifestyle, and diet. Older adults have altered gut microbiota structure and diversity compared to younger individuals, which can lead to various disorders [11]. However, the specific mechanisms underlying the link between gut microbiota and frailty remain unknown and require further investigation. Frail older adults often experience significant dietary changes due to declines in hearing, vision, mobility, and chewing ability. Additionally, reduced physical activity and gut motility, as well as changes in the living environment due to decreased self-care ability, can all impact the composition of gut microorganisms. Many bioactive metabolites produced by the microbiome are known to accumulate with aging have been implicated in various aspects of frailty [9]. Moreover, alterations in the microbiome may lead to a loss of control over the rate of accumulation of senescent cells, which could have a significant impact on frailty [12].
PD is significantly influenced by the gut-brain axis, which links gastrointestinal microbiota, neural development, and neurological diseases [13]. Short-chain fatty acids (SCFAs) such as butyrate, propionate, isobutyrate, and valerate are reduced in the feces of PD patients, suggesting that early pathogenic pathways in the gut may play a role in PD development. These SCFAs can trigger immune responses in the brain, leading to inflammation, which may contribute to cell damage and the various symptoms of PD [14]. Changes in the microbiota may lead to metabolic alterations in patients with PD, with SCFA being the most examined gut microbial metabolite. A potential hypothesis is that elevated SCFA levels triggered by PD pathogenesis may be a secondary trigger for the development of PD [15]. Ecological dysregulation of the gut microbiota may be further exacerbated by secondary changes in SCFAs levels. Studies have shown that SCFAs have certain beneficial functions, such as protecting the integrity of the intestinal barrier [16] and reducing the permeability of the blood-brain barrier [17]. Contrary views also exist based on in vivo and in vitro evidence [18, 19]. Various studies have attempted to elucidate the mechanism of action of SCFAs in PD; however, there are still many unanswered questions, and the results of different studies may differ or even contradict each other [17, 20, 21], and further studies are needed to characterize the role of SCFAs in the pathogenesis of PD and the exact mechanism.
Insomnia is the most prevalent sleep condition, and mounting research indicates that gut bacteria may play a role in its etiology [22, 23]. Previous research has demonstrated links between biological cycles, immunological response, and nutrient metabolism, all of which may contribute to the prevalence of insomnia [24-26]. Furthermore, substantial evidence suggests that gut microbiota not only affects host digestion, metabolism, and immune function but can also regulate host sleep through the microbiota-gut-brain axis [25, 27]. The exact etiology and pathogenesis of depression have remained elusive. A study led by Guillaume Méric, a Finnish microbial bioinformatician, analyzed the genetic makeup and gut microbiome status of over 6,000 subjects and concluded that certain gut microbes, such as Morganella and Klebsiella, are associated with depression, with the underlying transmission mechanism related to genes [28].
Older adults often present with multiple coexisting diseases, complex etiologies, and undergo polypharmacy interventions, making it challenging to conduct single-disease gut microbiota studies on this population. Consequently, establishing causal analyses becomes essential for a better understanding of the mechanisms originating from the gut microbiota and for providing new perspectives for microbiome-focused therapeutic approaches. Traditional observational epidemiological studies face limitations due to the potential for confounding and reverse causality. To address these limitations, Mendelian randomization (MR) is a valuable technique used to discern causal relationships between exposures and outcomes [29, 30]. By utilizing genetic variants closely related to the exposure as instrumental variables (IV), MR serves as a robust method for determining causal links between exposures and outcomes. MR can be thought of as a natural randomized controlled trial (RCT), offering strong evidence and being less susceptible to confounding variables. In contrast to single-sample MR methods, two-sample MR (TSMR) is particularly effective and powerful for establishing associations between “genetic risk factors” and “genetic outcomes.” However, this approach has not been previously used to explore a causal relationship between gut microbiota and geriatric syndromes. To assess the relationship between gut microbiota composition and geriatric syndromes, we conducted comprehensive two-sample MR analyses for five disorders using data from the IEU Open GWAS project including frailty, PD, psychosis, insomnia, and depression.
Methods
Study design
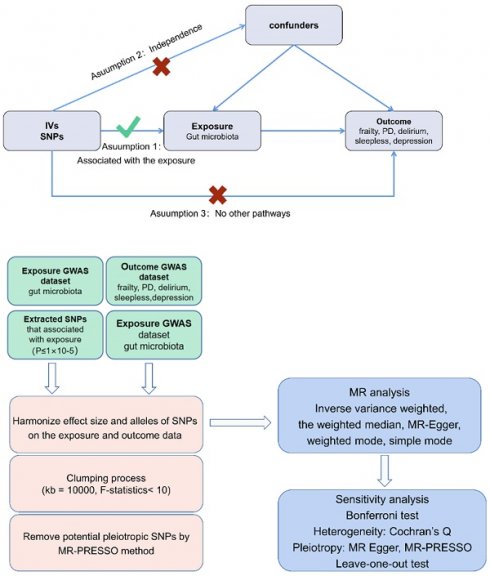
Figure 1A presents a flowchart illustrating the MR analysis. Through the TSMR analysis, we identified a connection between several gut bacterial families and five prevalent geriatric syndrome disorders: frailty, PD, delirium, sleeplessness, and depression. In the initial step, where we considered the gut microbiome as the exposure and the five diseases as the outcomes, we aimed to determine whether the gut microbiota plays a role in either promoting or preventing these disorders.
To perform a two-sample MR, Bowden et al. outline three essential assumptions [31]: (1) a strong correlation exists between SNPs and exposure factors; (2) confounding factors do not influence SNPs; (3) SNPs solely impact the outcomes through exposure factors (Figure 1B).
Data from genome-wide association studies
We utilized SNPs linked to the human gut microbiota as instrumental variables (IVs) obtained from the MiBioGen Genome-Wide Association Study (GWAS) dataset, provided by the International Consortium (https://mibiogen.gcc.rug.nl/). MiBioGen collected samples from 18 populations and 19,000 individuals worldwide, including 16S rRNA sequencing data of the gut microbiota and genome-wide SNP data. They conducted a large-scale meta-GWAS analysis and have established a complete, open-source, and standardized analysis process. This process effectively eliminates technical errors caused by 16S rRNA amplification intervals [32].
For this study, we focused on five prevalent disorders in geriatric syndromes and summarized findings from publicly available GWAS analyses. The Frailty Index (FI) is a quantitative measure comprising more than 40 components and is reported as a ratio of the total number of age-related health deficiencies, serving as a continuous measure for assessing frailty. FI data were obtained from the IEU Open GWAS database (https://gwas.mrcieu.ac.uk), which includes data from 42,351 GWAS datasets.
Summary statistics for PD were sourced from the IEU Open GWAS database, which contains data from 449,056 European-ancestry controls and 33,674 cases [33]. These statistics were based on the seventh iteration of the Finngen Biobank, a prospective cohort study with 342,499 participants as of December 2022 [34]. Summary statistics for insomnia and depression were obtained from the UK Biobank study. The UK Biobank produced GWAS summary statistics on insomnia and depression based on data from 501,500 and 122,938 United Kingdom residents, respectively (https://www.ukbiobank.ac.uk/).
Choosing genetic manipulative variables
In this study, the gut microbiome served as the exposure, and we investigated its potential causal relationship with five common disorders in geriatric syndromes. To ensure the accuracy and reliability of the results, we implemented several control procedures. Firstly, we selected SNPs that showed significant associations with the gut microbiota as the IVs. To ensure the truth and accuracy of the causal relationship between gut microbes and diseases, we identified SNPs with p-values below the significance threshold of 1105 for further analysis [35].
In addition, we set the linkage disequilibrium coefficient R2<0.01 and the region width was set as 10,000 kb to exclude the effect of gene pleiotropy. F-statistics were used to estimate the strength of instrumental variables. Among them F<10, assuming a weak instrumental variable bias. SNPs with palindromic structures were automatically excluded during the analysis. SNPs with palindromic structures were automatically excluded. Thirdly, we applied a minor allele frequency (MAF) threshold of 0.01 to the variant of interest. This ensured that rare alleles were considered in our analysis [36].
To evaluate the potential effects of horizontal pleiotropy, we used two regression tests: namely MR-PRESSO and MR-Egger. MR-PRESSO helps exclude specific SNPs, eliminating outliers to obtain estimates closer to the true values. MR-Egger did not constrain the regression lines to pass through the origin, allowing for the presence of directed genetic pleiotropy among the instrumental variables. When the regression intercept is nonzero and p < 0.05, it indicates the presence of genetic pleiotropy.
Flowchart describing the Mendelian randomization investigation in this study. MR, Mendelian randomization; PD, Parkinson's Disease (A) and three assumptions of Mendelian randomization (B).
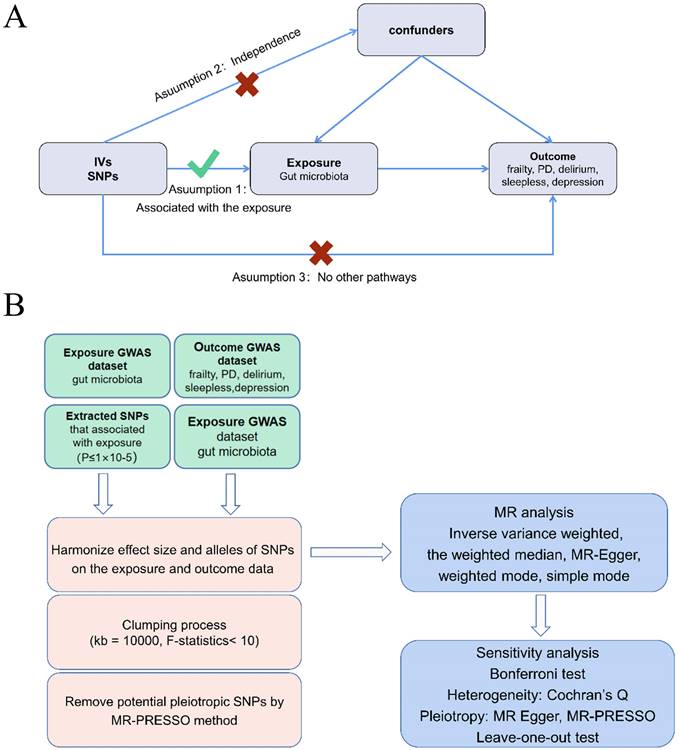
To address potential pleiotropy, we sequentially removed each SNP from the list and retested the remaining SNPs globally using the MR-PRESSO test. The global test's p-value was iterated upon until it reached statistical significance (p > 0.05), at which point the process was repeated. The list of SNPs that remained after accounting for pleiotropic effects was used for the MR analysis to ensure the accuracy of our findings.
Statistical analysis
We used a two-sample MR method to investigate the potential relationship between the composition of the gut microbiota and the presence of frailty. To explore potential causal connections between the gut microbiota and frailty, we used five distinct MR techniques, including the inverse variance weighted (IVW) method [37], the weighted median [38], MR-Egger [31], the weighted mode method [39], and the simple mode. The IVW approach served as the primary analysis method to provide precise estimations, with the other four methods used as supplementary analyses [40]. Moreover, we assessed potential horizontal pleiotropy effects using MR-PRESSO and MR-Egger.
For sensitivity analysis, we employed the “leave-one-out” approach from the R package. This involved systematically reanalyzing the results by sequentially removing each instrumental variable (IV) to evaluate the influence of each SNP on the outcome. The results of this analysis were presented in a forest plot. To minimize the impact of measurement errors in the included IVs, we conducted a heterogeneity test. Cochran's Q test was used to evaluate potential bias in the causal effect estimates due to measurement errors stemming from diverse analysis platforms, experimental setups, and study populations. This test was calculated using the “mr_heterogeneity” function from the “TwoSampleMR” package. We considered heterogeneity not to affect the study results when the test result indicated P > 0.05 [41]. The results were presented in a table. Additionally, we used the Bonferroni correction to assess the significance of multiple testing at each feature level (P < 0.05/n, where n is the number of bacterial taxa included in each feature level) to more precisely identify causal associations [35].
Results
Instrumental variable selection
First, we identified 14,587 SNPs associated with the gut microbiota from the MiBioGen Consortium at a stringent significance level (P<1×10-5). Furthermore, none of the IV's had an F-statistic lower than 10, mitigating the potential for weak instrument bias. Specifically, 101 independent SNPs were associated with 7 microbiomes in frailty, 80 SNPs with 7 microbiomes in PD, 70 SNPs with 5 microbiomes in delirium, 143 SNPs with 11 microbiomes in insomnia, and 137 SNPs with 11 microbiomes in depression (Supplementary Tables).
Causal effects of gut microbiota on frailty
Figure 2A provided evidence of the causal effects of 196 gut microbiomes on the occurrence of frailty. According to Table 1, a lower risk of frailty was associated with a higher genetically predicted abundance of class Bacteroidia (OR: 0.958, 95% CI: 0.924-0.993, p = 0.020) and genus Eubacterium ruminantium group (OR: 0.973, 95% CI: 0.950-0.997, p = 0.028). In contrast, class Betaproteobacteria (OR: 1.050, 95% CI: 1.002-1.101, p = 0.042), genus Clostridium innocuum group (OR: 1.023, 95% CI: 1.001-1.045, p = 0.036), genus Eubacterium coprostanoligenes (OR: 1.056, 95% CI: 1.019-1.094, p = 0.003), genus Allisonella (OR: 1.033, 95% CI: 1.007-1.059, p = 0.012) and genus Bifidobacterium (OR: 1.041, 95% CI: 1.007-1.076, p = 0.016) showed a positive genetic relationship with frailty risk (Figure 2B).
Causal effects of gut microbiota on PD
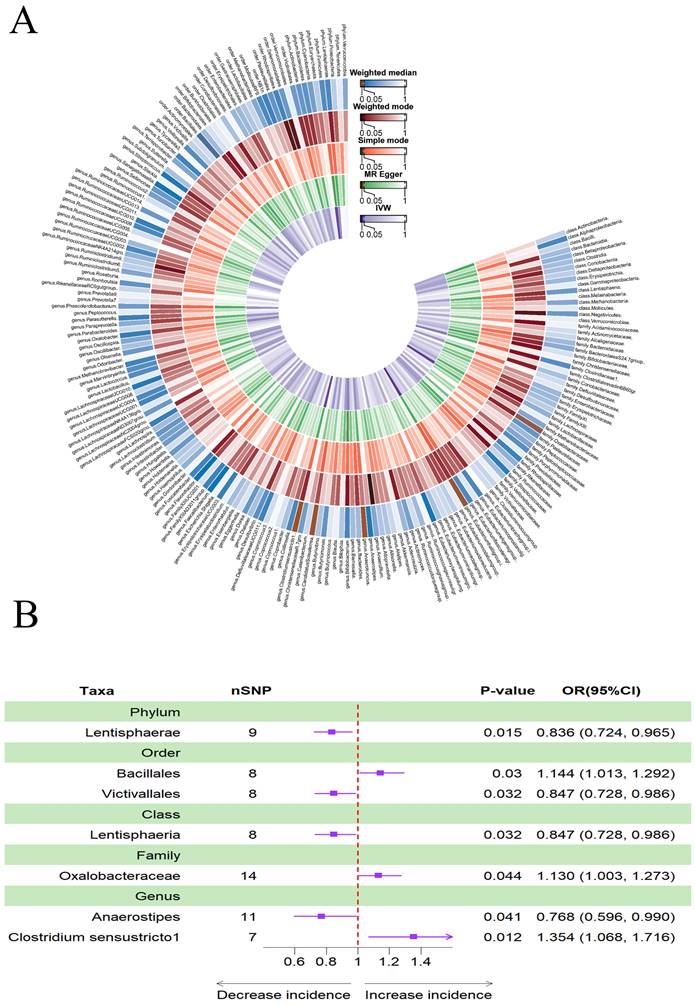
In addition, Figure 3A provided evidence of the causal effects of 196 gut microbiomes on the occurrence of PD. The results obtained using the IVW method revealed that a lower risk of PD was associated with a higher genetically predicted abundance of phylum Lentisphaerae (OR: 0.836, 95% CI: 0.724-0.965, p = 0.015), order Victivallales (OR: 0.847, 95% CI: 0.728-0.986), class Lentisphaeria (OR: 0.847, 95% CI: 0.728-0.986), and genus Anaerostipes (OR: 0.768, 95% CI: 0.596-0.990, p = 0.041). Conversely, the genetically predicted abundance of the family Oxalobacteraceae (OR: 1.130, 95% CI: 1.003-1.273, p = 0.044), genus Clostridium sensu stricto1 (OR: 1.354, 95% CI: 1.068-1.716, p = 0.012), and order Bacillales (OR: 1.144, 95% CI: 1.013-1.292, p = 0.030) showed a positive correlation with the risk of PD (Table 2, Figure 3B).
Causal effects of gut microbiota on delirium
Figure 4A provided evidence of the causal effects of 196 gut microbiomes on the occurrence of delirium. The IVW method revealed that a lower risk of delirium was associated with a higher genetically predicted abundance of the genus Ruminococcus gnavus group (OR: 0.731, 95% CI: 0.557-0.960, p = 0.024) and class Holdemania (OR: 0.737, 95% CI: 0.737-0.545, 0.997, p = 0.048) (Table 3). Conversely, phylum Verrucomicrobia (OR: 1.444, 95% CI: 1.009-2.065, p = 0.044), family Desulfovibrionaceae (OR: 1.926, 95% CI: 1.259-2.946, p = 0.003) and class Candidatus Soleaferrea (OR: 1.381, 95% CI: 1.027-1.857, p = 0.033) showed a positive genetic relationship with delirium risk (Figure 4B).
(A)Causal effects of the gut microbiome on frailty based on MR analyses. From inside to outside, the P values of IVW, MR Egger, SM, Wmode and WM represented, respectively. IVW, inverse variance weighted; SM, simple mode; Wmode weighted mode; WM, weighted median. (B)Forest plot showing Mendelian randomization results for causal effects of gut microbiota on frailty risk. CI: confidence interval; OR: odds ratio.
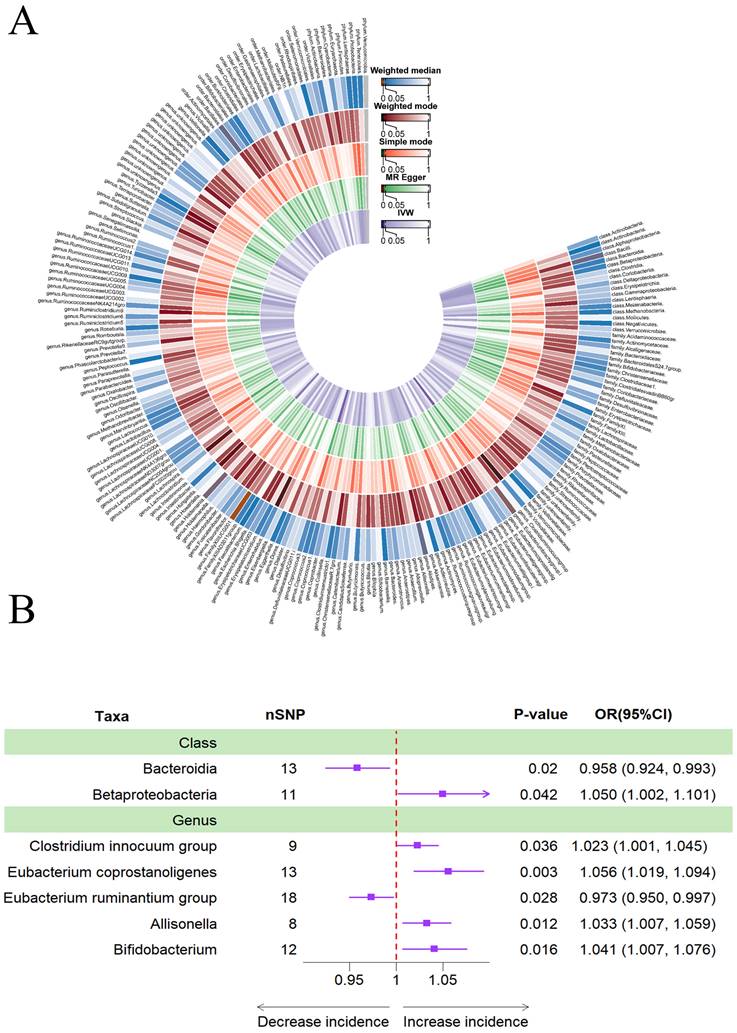
Mendelian randomization result of casual effects between gut microbiome and the risk of frailty.
| Group | Bacterial | Nsnp | Methods | SE | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Class | Bacteroidia | 13 | Inverse variance weighted | 0.018 | 0.958 (0.924, 0.993) | 0.020 |
| MR Egger | 0.042 | 0.944 (0.870, 1.026) | 0.202 | |||
| Simple mode | 0.039 | 0.954 (0.885, 1.030) | 0.257 | |||
| Weighted median | 0.026 | 0.960 (0.913, 1.009) | 0.107 | |||
| Weighted mode | 0.031 | 0.957 (0.901, 1.016) | 0.174 | |||
| Betaproteobacteria | 11 | Inverse variance weighted | 0.024 | 1.050 (1.002, 1.101) | 0.042 | |
| MR Egger | 0.087 | 1.021 (0.861, 1.210) | 0.820 | |||
| Simple mode | 0.048 | 1.092 (0.993, 1.200) | 0.100 | |||
| Weighted median | 0.028 | 1.079 (1.022, 1.139) | 0.006 | |||
| Weighted mode | 0.048 | 1.087 (0.990, 1.194) | 0.112 | |||
| Genus | Clostridiuminnocuum group | 9 | Inverse variance weighted | 0.011 | 1.023 (1.001, 1.045) | 0.036 |
| MR Egger | 0.055 | 1.133 (1.017, 1.263) | 0.059 | |||
| Simple mode | 0.023 | 1.017 (0.972, 1.065) | 0.490 | |||
| Weighted median | 0.015 | 1.022 (0.993, 1.052) | 0.137 | |||
| Weighted mode | 0.022 | 1.017 (0.974, 1.062) | 0.465 | |||
| Eubacterium coprostanoligenes | 13 | Inverse variance weighted | 0.018 | 1.056 (1.019, 1.094) | 0.003 | |
| MR Egger | 0.073 | 1.072 (0.929, 1.237) | 0.363 | |||
| Simple mode | 0.037 | 1.084 (1.008, 1.166) | 0.051 | |||
| Weighted median | 0.024 | 1.070 (1.021, 1.122) | 0.005 | |||
| Weighted mode | 0.037 | 1.085 (1.008, 1.168) | 0.050 | |||
| Eubacteriumruminantiumgroup | 18 | Inverse variance weighted | 0.012 | 0.973 (0.950, 0.997) | 0.028 | |
| MR Egger | 0.043 | 1.042 (0.958, 1.133) | 0.350 | |||
| Simple mode | 0.025 | 1.003 (0.954, 1.054) | 0.921 | |||
| Weighted median | 0.015 | 0.997 (0.969, 1.027) | 0.860 | |||
| Weighted mode | 0.023 | 1.003 (0.959, 1.050) | 0.891 | |||
| Allisonella | 8 | Inverse variance weighted | 0.013 | 1.033 (1.007, 1.059) | 0.012 | |
| MR Egger | 0.072 | 0.898 (0.779, 1.034) | 0.186 | |||
| Simple mode | 0.029 | 1.061 (1.003, 1.122) | 0.078 | |||
| Weighted median | 0.015 | 1.017 (0.988, 1.047) | 0.255 | |||
| Weighted mode | 0.022 | 1.003 (0.961, 1.046) | 0.904 | |||
| Bifidobacterium | 12 | Inverse variance weighted | 0.017 | 1.041 (1.007, 1.076) | 0.016 | |
| MR Egger | 0.045 | 1.089 (0.997, 1.189) | 0.089 | |||
| Simple mode | 0.041 | 1.015 (0.935, 1.101) | 0.732 | |||
| Weighted median | 0.023 | 1.032 (0.987, 1.079) | 0.166 | |||
| Weighted mode | 0.042 | 1.014 (0.933, 1.101) | 0.757 |
MR result of casual effects between gut microbiome and the risk of PD.
| Group | Bacterial | Nsnp | Methods | SE | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Phylum | Lentisphaerae | 9 | Inverse variance weighted | 0.074 | 0.836 (0.724, 0.965) | 0.015 |
| MR Egger | 0.258 | 0.715 (0.431, 1.186) | 0.235 | |||
| Simple mode | 0.162 | 0.743 (0.542, 1.020) | 0.104 | |||
| Weighted median | 0.099 | 0.762 (0.628, 0.924) | 0.006 | |||
| Weighted mode | 0.149 | 0.745 (0.556, 0.998) | 0.084 | |||
| Order | Bacillales | 8 | Inverse variance weighted | 0.077 | 1,144 (1.013, 1.292) | 0.030 |
| MR Egger | 0.255 | 0.743 (0.450, 1.225) | 0.288 | |||
| Simple mode | 0.166 | 0.747 (0.540, 1.035) | 0.123 | |||
| Weighted median | 0.105 | 0.783 (0.638, 0.962) | 0.020 | |||
| Weighted mode | 0.155 | 0.751 (0.555, 1.017) | 0.107 | |||
| Victivallales | 8 | Inverse variance weighted | 0.077 | 0.847 (0.728, 0.986) | 0.032 | |
| MR Egger | 0.255 | 0.743 (0.450, 1.225) | 0.288 | |||
| Simple mode | 0.169 | 0.747 (0.537, 1.040) | 0.128 | |||
| Weighted median | 0.108 | 0.783 (0.634, 0.969) | 0.024 | |||
| Weighted mode | 0.163 | 0.751 (0.546, 1.033) | 0.122 | |||
| Class | Lentisphaeria | 8 | Inverse variance weighted | 0.077 | 0.847 (0.728, 0.986) | 0.032 |
| MR Egger | 0.255 | 0.743 (0.450, 1.225) | 0.288 | |||
| Simple mode | 0.177 | 0.747 (0.528, 1.058) | 0.145 | |||
| Weighted median | 0.107 | 0.783 (0.635, 0.966) | 0.022 | |||
| Weighted mode | 0.160 | 0.751 (0.549, 1.027) | 0.116 | |||
| Family | Oxalobacteraceae | 14 | Inverse variance weighted | 0.061 | 1.130 (1.003, 1.273) | 0.044 |
| MR Egger | 0.259 | 1.422 (0.856, 2.362) | 0.198 | |||
| Simple mode | 0.135 | 1.194 (0.915, 1.557) | 0.213 | |||
| Weighted median | 0.079 | 1.177 (1.008, 1.375) | 0.040 | |||
| Weighted mode | 0.137 | 1.202 (0.919, 1.571) | 0.202 | |||
| Genus | Anaerostipes | 11 | Inverse variance weighted | 0.129 | 0.768 (0.596, 0.990) | 0.041 |
| MR Egger | 0.411 | 0.568 (0.254, 1.270) | 0.201 | |||
| Simple mode | 0.291 | 0.976 (0.552, 1.726) | 0.935 | |||
| Weighted median | 0.169 | 0.792 (0.569, 1.103) | 0.168 | |||
| Weighted mode | 0.304 | 1.024 (0.565, 1.858) | 0.938 | |||
| Clostridium sensustricto1 | 7 | Inverse variance weighted | 0.121 | 1.354 (1.068, 1.716) | 0.012 | |
| MR Egger | 0.275 | 1.728 (1.009, 2.959) | 0.103 | |||
| Simple mode | 0.220 | 1.404 (0.912, 2.161) | 0.174 | |||
| Weighted median | 0.160 | 1.413 (1.034, 1.933) | 0.030 | |||
| Weighted mode | 0.195 | 1.416 (0.966, 2.075) | 0.125 |
(A)Causal effects of the gut microbiome on Parkinson's disease (PD) based on MR analyses. From inside to outside, the P values of IVW, MR Egger, SM, Wmode and WM represented, respectively. IVW, inverse variance weighted; SM, simple mode; Wmode weighted mode; WM, weighted median. (B)Forest plot of Mendelian randomization results for causal effects of gut microbiota on PD risk.
(A) Causal effects of the gut microbiome on delirium based on MR analyses. From inside to outside, the P values of IVW, MR Egger, SM, Wmode and WM represented, respectively. IVW, inverse variance weighted; SM, simple mode; Wmode weighted mode; WM, weighted median. (B) Forest plot of Mendelian randomization results for causal effects of gut microbiota on delirium risk.
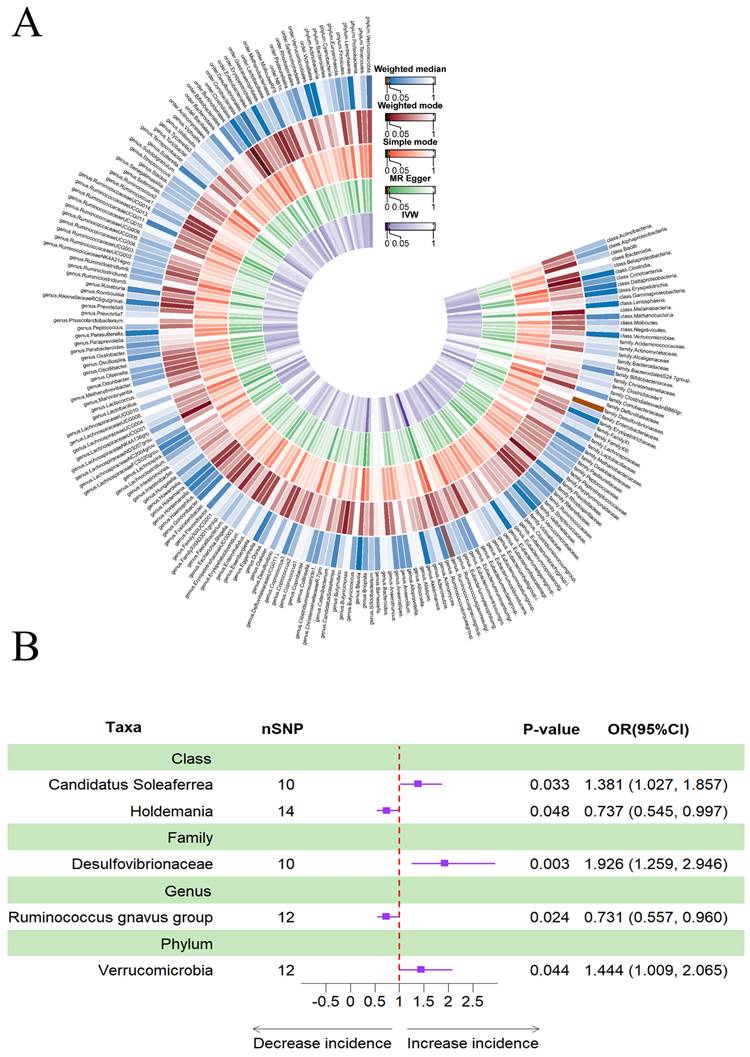
MR result of casual effects between gut microbiome and the risk of delirium.
| Group | Bacterial | Nsnp | Methods | SE | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Phylum | Verrucomicrobia | 12 | Inverse variance weighted | 0.183 | 1.444 (1.009, 2.065) | 0.044 |
| MR Egger | 0.478 | 1.740 (0.682, 4.438) | 0.273 | |||
| Simple mode | 0.411 | 2.053 (0.917, 4.596) | 0.108 | |||
| Weighted median | 0.259 | 1.505 (0.906, 2.500) | 0.114 | |||
| Weighted mode | 0.347 | 1.473 (0.746, 2.906) | 0.288 | |||
| Family | Desulfovibrionaceae | 10 | Inverse variance weighted | 0.217 | 1.926 (1.259, 2.946) | 0.003 |
| MR Egger | 0.507 | 0.975 (0.361, 2.631) | 0.961 | |||
| Simple mode | 0.536 | 1.540 (0.538, 4.404) | 0.441 | |||
| Weighted median | 0.311 | 1.492 (0.811, 2.745) | 0.198 | |||
| Weighted mode | 0.351 | 1.252 (0.630, 2.490) | 0.537 | |||
| Genus | Ruminococcus gnavus group | 12 | Inverse variance weighted | 0.139 | 0.731 (0.557, 0.960) | 0.024 |
| MR Egger | 0.656 | 0.538 (0.149, 1.943) | 0.366 | |||
| Simple mode | 0.269 | 0.608 (0.359, 1.030) | 0.091 | |||
| Weighted median | 0.187 | 0.648 (0.449, 0.933) | 0.020 | |||
| Weighted mode | 0.268 | 0.616 (0.365, 1.043) | 0.099 | |||
| Class | Candidatus Soleaferrea | 10 | Inverse variance weighted | 0.151 | 1.381 (1.027, 1.857) | 0.033 |
| MR Egger | 1.621 | 1.344 (0.056, 32.211) | 0.860 | |||
| Simple mode | 0.343 | 1.132 (0.577, 2.219) | 0.726 | |||
| Weighted median | 0.201 | 1.240 (0.835, 1.840) | 0.286 | |||
| Weighted mode | 0.323 | 1.114 (0.592, 2.096) | 0.747 | |||
| Holdemania | 14 | Inverse variance weighted | 0.154 | 0.737 (0.545, 0.997) | 0.048 | |
| MR Egger | 0.453 | 0.401 (0.165, 0.973) | 0.066 | |||
| Simple mode | 0.315 | 0.764 (0.412, 1.415) | 0.407 | |||
| Weighted median | 0.202 | 0.749 (0.504, 1.113) | 0.153 | |||
| Weighted mode | 0.319 | 0.738 (0.394, 1.380) | 0.358 |
Causal effects of gut microbiota on insomnia
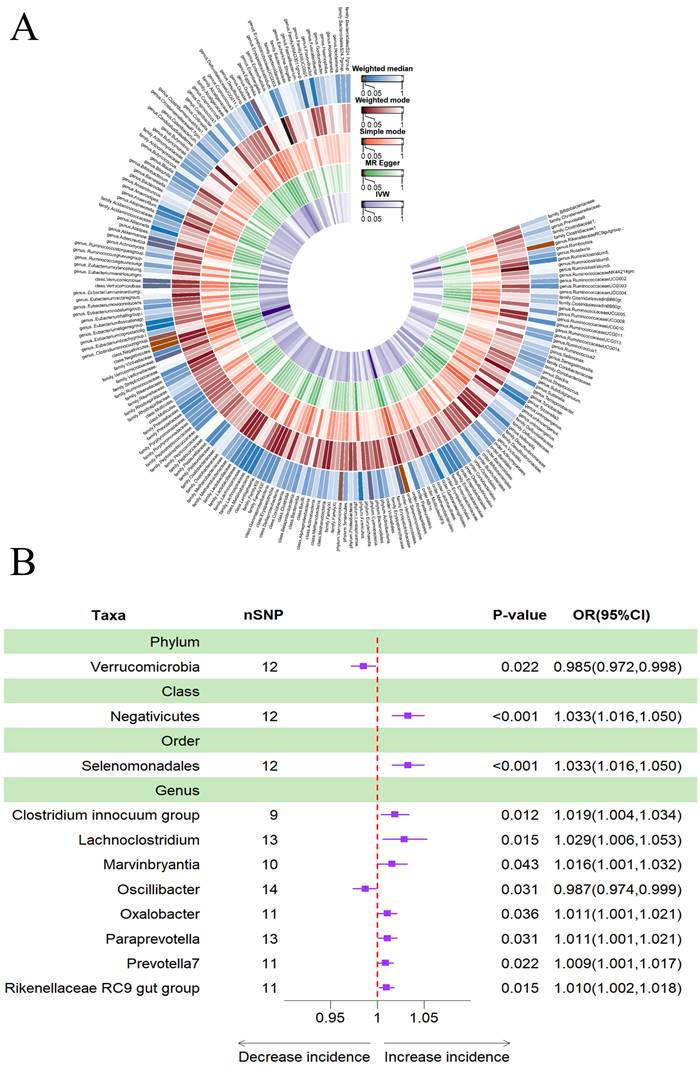
The results obtained using the IVW method indicated that a higher genetically predicted abundance of phylum Verrucomicrobia (OR: 0.985, 95% CI: 0.972-0.998, p = 0.022) and genus Oscillibacter (OR: 0.987, 95% CI: 0.974-0.999, p = 0.031) was associated with a reduced risk of sleeplessness (Table 4). In contrast, class Negativicutes (OR: 1.033, 95% CI: 1.016-1.050, p = 0.000), order Selenomonadales (OR: 1.033, 95% CI: 1.016-1.050, p = 0.000), genus Clostridium innocuum group (OR: 1.019, 95% CI: 1.004-1.034, p = 0.012), genus Lachnoclostridium (OR: 1.029, 95% CI: 1.006-1.053, p = 0.015), genus Marvinbryantia (OR: 1.016, 95% CI: 1.001-1.032, p = 0.043), genus Oxalobacter (OR: 1.011, 95% CI: 1.001-1.021, p = 0.036), genus Paraprevotella (OR: 1.011, 95% CI: 1.001-1.021, p = 0.031), genus Prevotella7 (OR: 1.009, 95% CI: 1.001-1.017, p = 0.022) and genus Rikenellaceae RC9 gut group (OR: 1.010, 95% CI: 1.002-1.018, p = 0.015) showed a positive genetic relationship with the risk of insomnia (Figure 5B). Additionally, Figure 5A provided evidence of the causal effects of 196 gut microbiomes on the occurrence of insomnia.
Causal effects of gut microbiota on depression
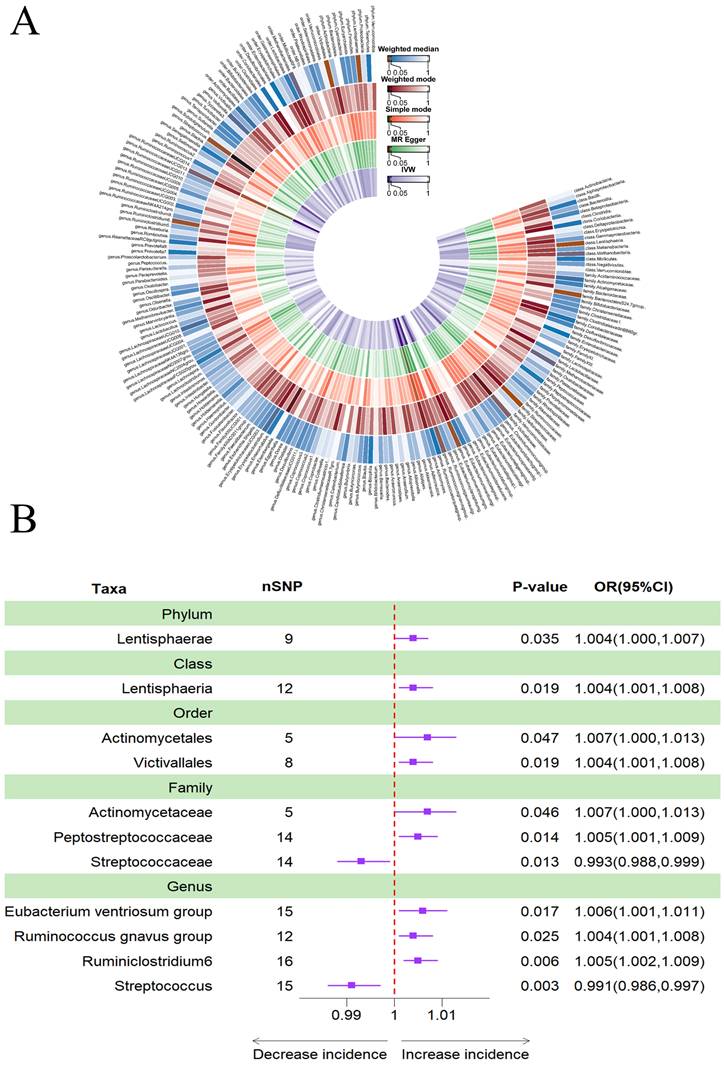
According to Table 5, a higher genetically predicted abundance of phylum Lentisphaerae (OR: 1.004, 95% CI: 1.000-1.007, p = 0.035), class Lentisphaeria (OR: 1.004, 95% CI: 1.001-1.008, p = 0.019), order Actinomycetales (OR: 1.007, 95% CI: 1.000-1.013, p = 0.047), order Victivallales (OR: 1.004, 95% CI: 1.001-1.008, p = 0.019), family Actinomycetaceae (OR: 1.007, 95% CI: 1.000-1.013, p = 0.046), family Peptostreptococcaceae (OR: 1.005, 95% CI: 1.001-1.009, p = 0.014), genus Eubacterium ventriosum group(OR: 1.006, 95% CI: 1.001-1.011, p = 0.017), genus Ruminococcus gnavus group (OR: 1.004, 95% CI: 1.001-1.008, p = 0.025), and genus Ruminiclostridium6 (OR: 1.005, 95% CI: 1.002-1.009, p = 0.006) were associated with a reduced risk of depression. Conversely, family Streptococcaceae (OR: 0.993, 95% CI: 0.988-0.999, p = 0.013) and genus Streptococcus (OR: 0.991, 95% CI: 0.986-0.997, p = 0.003) showed a positive genetic relationship with the risk of depression (Figure 6B). Figure 6A provided evidence of the causal effects of 196 gut microbiomes on the occurrence of depression.
Discussion
As far as we are aware, our two-sample MR study is the first attempt to use a publicly accessible genetic database to investigate the causal link between the gut microbiota and five geriatric disorders: frailty, PD, delirium, insomnia, and depression. Our research demonstrates that 41 gut microbiota are causally linked to geriatric syndromes and their phenotypes, significantly advancing our understanding of the gut microbiota's role in the pathology of these conditions. These findings provide fresh insights into prevention and diagnosis strategies for these conditions.
(A) Causal effects of the gut microbiome on insomnia based on MR analyses. From inside to outside, the P values of IVW, MR Egger, SM , Wmode and WM represented, respectively. IVW, inverse variance weighted; SM, simple mode; Wmode weighted mode; WM, weighted median. (B) Forest plot of Mendelian randomization results for causal effects of gut microbiota on insomnia risk.
(A) Causal effects of the gut microbiome on depression based on MR analyses. From inside to outside, the P values of IVW, MR Egger, SM, Wmode and WM represented, respectively. IVW, inverse variance weighted; SM, simple mode; Wmode weighted mode; WM, weighted median. (B) Forest plot of Mendelian randomization results for causal effects of gut microbiota on depression risk.
Mendelian randomization result of casual effects between gut microbiome and the risk of insomnia.
| Group | Bacterial | Nsnp | Methods | SE | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Phylum | Verrucomicrobia | 12 | Inverse variance weighted | 0.007 | 0.985(0.972,0.998) | 0.022 |
| MR Egger | 0.019 | 0.983(0.947,1.020) | 0.377 | |||
| Simple mode | 0.015 | 0.990(0.961,1.019) | 0.515 | |||
| Weighted median | 0.009 | 0.986(0.969,1.003) | 0.107 | |||
| Weighted mode | 0.014 | 0.989(0.962,1.017) | 0.450 | |||
| Class | Negativicutes | 12 | Inverse variance weighted | 0.008 | 1.033(1.016,1.050) | 0.000 |
| MR Egger | 0.026 | 1.042(0.990,1.098) | 0.147 | |||
| Simple mode | 0.019 | 1.047(1.008,1.087) | 0.035 | |||
| Weighted median | 0.011 | 1.046(1.023,1.069) | 0.000 | |||
| Weighted mode | 0.020 | 1.047(1.008,1.088) | 0.038 | |||
| Order | Selenomonadales | 12 | Inverse variance weighted | 0.008 | 1.033(1.016,1.050) | 0.000 |
| MR Egger | 0.026 | 1.042(0.990,1.098) | 0.147 | |||
| Simple mode | 0.019 | 1.047(1.008,1.088) | 0.038 | |||
| Weighted median | 0.011 | 1.046(1.023,1.069) | 0.000 | |||
| Weighted mode | 0.020 | 1.047(1.008,1.088) | 0.039 | |||
| Genus | Clostridium innocuum group | 9 | Inverse variance weighted | 0.007 | 1.019(1.004,1.034) | 0.012 |
| MR Egger | 0.040 | 0.998(0.924,1.079) | 0.968 | |||
| Simple mode | 0.009 | 1.008(0.990,1.027) | 0.403 | |||
| Weighted median | 0.007 | 1.012(0.997,1.026) | 0.111 | |||
| Weighted mode | 0.009 | 1.007(0.989,1.026) | 0.448 | |||
| Lachnoclostridium | 13 | Inverse variance weighted | 0.012 | 1.029(1.006,1.053) | 0.015 | |
| MR Egger | 0.043 | 1.009(0.926,1.098) | 0.844 | |||
| Simple mode | 0.022 | 1.027(0.983,1.072) | 0.254 | |||
| Weighted median | 0.013 | 1.028(1.002,1.056) | 0.038 | |||
| Weighted mode | 0.022 | 1.024(0.980,1.070) | 0.305 | |||
| Marvinbryantia | 10 | Inverse variance weighted | 0.008 | 1.016(1.001,1.032) | 0.043 | |
| MR Egger | 0.030 | 0.982(0.926,1.043) | 0.576 | |||
| Simple mode | 0.020 | 1.028(0.988,1.069) | 0.207 | |||
| Weighted median | 0.010 | 1.0182(0.998,1.039) | 0.078 | |||
| Weighted mode | 0.020 | 1.026(0.986,1.067) | 0.233 | |||
| Oscillibacter | 14 | Inverse variance weighted | 0.006 | 0.987(0.974,0.999) | 0.031 | |
| MR Egger | 0.023 | 1.007(0.962,1.053) | 0.779 | |||
| Simple mode | 0.014 | 0.983(0.957,1.011) | 0.250 | |||
| Weighted median | 0.008 | 0.987(0.972,1.003) | 0.109 | |||
| Weighted mode | 0.015 | 0.983(0.955,1.012) | 0.277 | |||
| Oxalobacter | 11 | Inverse variance weighted | 0.005 | 1.011(1.001,1.021) | 0.036 | |
| MR Egger | 0.024 | 1.019(0.971,1.069) | 0.461 | |||
| Simple mode | 0.009 | 1.011(0.994,1.029) | 0.242 | |||
| Weighted median | 0.006 | 1.012(1.000,1.024) | 0.058 | |||
| Weighted mode | 0.008 | 1.010(0.994,1.027) | 0.250 | |||
| Paraprevotella | 13 | Inverse variance weighted | 0.005 | 1.011(1.001,1.021) | 0.031 | |
| MR Egger | 0.016 | 0.980(0.949,1.012) | 0.237 | |||
| Simple mode | 0.011 | 1.014(0.992,1.037) | 0.228 | |||
| Weighted median | 0.007 | 1.009(0.996,1.022) | 0.185 | |||
| Weighted mode | 0.011 | 1.010(0.989,1.031) | 0.368 | |||
| Prevotella7 | 11 | Inverse variance weighted | 0.004 | 1.009(1.001,1.017) | 0.022 | |
| MR Egger | 0.024 | 0.989(0.943,1.037) | 0.665 | |||
| Simple mode | 0.009 | 1.006(0.989,1.024) | 0.505 | |||
| Weighted median | 0.006 | 1.009(0.998,1.020) | 0.129 | |||
| Weighted mode | 0.009 | 1.007(0.989,1.024) | 0.470 | |||
| Rikenellaceae RC9 gut group | 11 | Inverse variance weighted | 0.004 | 1.010(1.002,1.018) | 0.015 | |
| MR Egger | 0.025 | 0.997(0.949,1.046) | 0.898 | |||
| Simple mode | 0.008 | 1.011(0.996,1.027) | 0.186 | |||
| Weighted median | 0.005 | 1.010(1.000,1.020) | 0.040 | |||
| Weighted mode | 0.008 | 1.011(0.995,1.027) | 0.217 |
There is mounting evidence that the so-called “gut-brain axis” influences the risk of several age-related chronic diseases and syndromes, including frailty and neurodegenerative diseases [42]. Age-related frailty is a distinctive geriatric syndrome characterized by lower gut microbial diversity in the older adults compared to younger individuals, with significant interindividual variations.
Previous research has suggested that a low distinctness index of the gut microbiome and a high prevalence of Bacteroides are independently associated with mortality in older adults. Conversely, a high abundance of Lactobacillus and Bifidobacterium is indicative of a healthier microbiome, often observed in centenarians [43].
Our findings indicate a correlation between a higher genetic abundance of Bacteroidia and frailty, suggesting an increase in Bacteroides in frail older adults [44]. Bifidobacteria have also been recognized as an important factor in sarcopenia and frailty among older adults [45]. Intestinal microbiota associated with frailty and sarcopenia have been linked to changes in the abundance of Bifidobacterium, which is associated with better health, as suggested by animal studies. Bifidobacterium has shown the potential to significantly reduce the peripheral tiredness index associated with exercise in mice and decrease the damage index associated with oxidative stress, possibly through its role in regulating inflammation [10, 46]. Both the MR Egger and weighted median techniques consistently revealed directional effects across all studies, suggesting that Bacteroidia may be a promising target for frailty prevention.
In frail older adults, Eubacterium is less prevalent [47-49]. Due to its production of short-chain fatty acids (SCFAs), particularly butyrate, and its role in immune system regulation, Eubacterium is considered a protective colonic bacterium [50]. Abundance of Eubacterium is inversely correlated with gut health, and its decline may have systemic consequences. The reduction of Eubacterium in older adults and frail individuals may contribute to the protective impact of SCFAs on the human gut [49].
Mendelian randomization result of casual effects between gut microbiome and the risk of depression.
| Group | Bacterial | Nsnp | Methods | SE | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Phylum | Lentisphaerae | 9 | Inverse variance weighted | 0.002 | 1.004(1.000,1.007) | 0.035 |
| MR Egger | 0.007 | 1.008(0.995,1.021) | 0.261 | |||
| Simple mode | 0.004 | 1.001(0.994,1.009) | 0.725 | |||
| Weighted median | 0.002 | 1.003(0.998,1.007) | 0.266 | |||
| Weighted mode | 0.004 | 1.002(0.995,1.009) | 0.626 | |||
| Class | Lentisphaeria | 12 | Inverse variance weighted | 0.002 | 1.004(1.001,1.008) | 0.019 |
| MR Egger | 0.007 | 1.009(0.996,1.022) | 0.227 | |||
| Simple mode | 0.004 | 1.002(0.995,1.010) | 0.533 | |||
| Weighted median | 0.002 | 1.003(0.998,1.007) | 0.263 | |||
| Weighted mode | 0.004 | 1.003(0.995,1.010) | 0.527 | |||
| Order | Actinomycetales | 5 | Inverse variance weighted | 0.003 | 1.007(1.000,1.013) | 0.047 |
| MR Egger | 0.009 | 1.011(0.993,1.030) | 0.320 | |||
| Simple mode | 0.005 | 1.007(0.997,1.018) | 0.255 | |||
| Weighted median | 0.004 | 1.007(1.000,1.015) | 0.047 | |||
| Weighted mode | 0.005 | 1.007(0.997,1.017) | 0.241 | |||
| Victivallales | 8 | Inverse variance weighted | 0.007 | 1.004(1.001,1.008) | 0.019 | |
| MR Egger | 0.002 | 1.009(0.996,1.022) | 0.227 | |||
| Simple mode | 0.007 | 1.002(0.995,1.010) | 0.563 | |||
| Weighted median | 0.004 | 1.003(0.998,1.007) | 0.265 | |||
| Weighted mode | 0.002 | 1.002(0.995,1.010) | 0.541 | |||
| Family | Actinomycetaceae | 5 | Inverse variance weighted | 0.003 | 1.007(1.000,1.013) | 0.046 |
| MR Egger | 0.009 | 1.011(0.993,1.030) | 0.319 | |||
| Simple mode | 0.005 | 1.007(0.996,1.018) | 0.262 | |||
| Weighted median | 0.004 | 1.007(1.000,1.014) | 0.046 | |||
| Weighted mode | 0.005 | 1.007(0.997,1.017) | 0.244 | |||
| Peptostreptococcaceae | 14 | Inverse variance weighted | 0.002 | 1.005(1.001,1.009) | 0.014 | |
| MR Egger | 0.005 | 1.009(0.992,1.010) | 0.865 | |||
| Simple mode | 0.005 | 1.009(0.100,1.019) | 0.079 | |||
| Weighted median | 0.003 | 1.006(0.100,1.012) | 0.065 | |||
| Weighted mode | 0.004 | 0.100(0.991,1.009) | 0.981 | |||
| Streptococcaceae | 14 | Inverse variance weighted | 0.003 | 0.993(0.988,0.999) | 0.013 | |
| MR Egger | 0.011 | 0.984(0.963,1.007) | 0.194 | |||
| Simple mode | 0.007 | 0.992(0.979,1.005) | 0.251 | |||
| Weighted median | 0.004 | 0.993(0.986,1.001) | 0.069 | |||
| Weighted mode | 0.007 | 0.993(0.980,1.007) | 0.330 | |||
| Genus | Eubacterium ventriosum group | 15 | Inverse variance weighted | 0.002 | 1.006(1.001,1.011) | 0.017 |
| MR Egger | 0.011 | 1.023(1.002,1.045) | 0.052 | |||
| Simple mode | 0.005 | 1.012(1.001,1.023) | 0.049 | |||
| Weighted median | 0.003 | 1.008(1.002,1.015) | 0.010 | |||
| Weighted mode | 0.005 | 1.011(1.000,1.022) | 0.060 | |||
| Ruminococcus gnavus group | 12 | Inverse variance weighted | 0.002 | 1.004(1.001,1.008) | 0.025 | |
| MR Egger | 0.009 | 1.009(0.991,1.027) | 0.332 | |||
| Simple mode | 0.004 | 1.007(0.100,1.015) | 0.084 | |||
| Weighted median | 0.002 | 1.006(1.002,1.011) | 0.008 | |||
| Weighted mode | 0.004 | 1.007(1.000,1.015) | 0.073 | |||
| Ruminiclostridium6 | 16 | Inverse variance weighted | 0.002 | 1.005(1.002,1.009) | 0.006 | |
| MR Egger | 0.005 | 0.999(0.990,1.009) | 0.860 | |||
| Simple mode | 0.006 | 1.012(1.001,1.024) | 0.050 | |||
| Weighted median | 0.003 | 1.005(0.999,1.010) | 0.084 | |||
| Weighted mode | 0.005 | 0.999(0.988,1.010) | 0.856 | |||
| Streptococcus | 15 | Inverse variance weighted | 0.003 | 0.993(0.986,0.997) | 0.003 | |
| MR Egger | 0.009 | 0.969(0.952,0.986) | 0.002 | |||
| Simple mode | 0.006 | 0.989(0.977,1.002) | 0.122 | |||
| Weighted median | 0.003 | 0.992(0.985,0.999) | 0.020 | |||
| Weighted mode | 0.006 | 0.990(0.978,1.001) | 0.095 |
However, further research is needed to elucidate the precise mechanism. It remains unclear how the other bacterial genera in our study, which showed significant alterations, may be associated with the onset of frailty.
Our findings demonstrated a potential causal relationship between the increased diversity of the phylum Lentisphaerae, class Lentisphaeria, and order Victivallales and a potential protective effect against PD. However, as this observation did not survive multiple corrections and has not been reported in previous literature, a definitive conclusion about causality cannot be drawn. It should be interpreted with caution and seen as a potential causal relationship.
The associations between family Oxalobacteraceae, order Bacillales, Anaerostipes, and Clostridium sensu stricto 1 and PD are consistent with previous results [21, 51, 52]. Furthermore, our investigation revealed that several microbial clusters capable of producing SCFAs, including Clostridium sensu stricto 1 and Bacillales, were associated with a higher risk of PD [53]. Increasing SCFA levels are generally considered beneficial for health [54], and PD symptoms can be mitigated or even eliminated by introducing SCFAs or reestablishing gut flora [55, 56].
The exact mechanism by which gut microbial dysbiosis contributes to PD remains unclear. Microbial SCFAs could potentially be one of the primary mediators of the microbiota-gut-brain axis, with a role in the onset and progression of PD. To uncover the function and precise mechanisms of SCFAs in the pathogenesis of PD, further research will be necessary in the future.
Data on gut microbiota dysbiosis in acute neuropsychiatric illnesses are currently lacking. Delirium, characterized by inattention, disorganized thinking, and altered awareness that fluctuates over time, is the most prevalent acute neuropsychiatric problem in hospitalized older adults. Delirium is associated with a range of negative outcomes, including functional and cognitive decline, the need for hospitalization, and increased mortality [57].
One study found that an animal model of postoperative delirium showed a dysbiotic gut microbiome, with decreased levels of Ruminococcus and Roseburia and increased levels of Rikenellaceae in fecal samples of postoperative delirious mice. These findings are similar to our results, although the exact mechanisms remain unclear [58]. Notably, postoperative delirious patients had high levels of Proteobacteria, Enterobacteriaceae, Escherichia shigella, Klebsiella, Ruminococcus, Roseburia, Blautia, Holdemanella, Anaerostipes, Burkholderiaceae, Peptococcus, Lactobacillus, and Dorea, whereas patients without postoperative delirium had high levels of Streptococcus [59]. This research provides new perspectives and approaches for the prevention and treatment of delirium, which is crucial for improving the well-being and quality of life of older adults. However, further research is needed to determine how these bacteria may be related to the potential causes of postoperative delirium.
Currently, there is evidence suggesting a certain association between gut microbiota and insomnia. Moreover, Firmicutes and Proteobacteria were more abundant in healthy individuals compared to insomnia patients, leading to a reduced Firmicutes-to-Bacteroidetes ratio, with Bacteroidetes being the predominant phylum in the insomnia group [60]. Moreover, insomnia patients showed a significant decrease in the genus Bacteroides and a notable increase in the genus Prevotella. They also showed a prevalence of Gemmiger and Fusicatenibacter. In contrast, Peptostreptococcaceae, Coprococcus, Oscillibacter, and the genus Clostridium were dominant in healthy individuals [24]. Moreover, a strong correlation was observed between higher sleep efficiency and cognitive ability and the presence of the genus Lachnoclostridium [61], aligning with our findings. Research by Agrawal et al. [62] indicated that short sleepers had a lower relative abundance of Lactobacillus compared to regular sleepers.
This study identified a strong correlation between a higher abundance of the class Negativicutes and order Selenomonadales and an increased incidence of sleeplessness [63]. Notably, melatonin is a common treatment for improving sleep in individuals with insomnia and can also address gut microbiota issues arising from sleep disturbances. Studies involving melatonin treatment in sucking piglets demonstrated a reduction in the prevalence of order Selenomonadales [64]. These findings suggest that by promoting the operation of gut neural networks and the gut barrier, the order Selenomonadales and class Negativicutes class may considerably increase the efficiency of melatonin in treating the symptoms of insomnia [64].
Moreover, this study found a potential association between the genera of Marvinbryantia, Oxalobacter, Paraprevotella, and Rikenellaceae RC9 gut group, with insomnia. This finding represents a novel discovery as it has not been previously reported in existing studies. However, it is needed to do further study to validate and confirm this association.
In previous studies, many researchers have conducted extensive research linking gut microbiota to depression from various perspectives and through different experimental methods [65-67]. However, the contribution of family Streptococcaceae and genus Streptococcus to the pathophysiology of depression remains unclear, as there are limited experimental reports on this topic, warranting further investigation.
According to a case-control study, pro-inflammatory genera like Streptococcus were enriched, while anti-inflammatory genera like Faecalibacterium were decreased in the depressed group [68]. Additionally, our MR study identified a potential association between depression and a higher prevalence of Streptococcaceae or Streptococcus. Furthermore, the genus Ruminococcus gnavus has been linked to mental and behavioral issues in children, including withdrawal, anxiety, despair, and muscle soreness [69]. Ruminococcus has also been associated with various psychiatric conditions, including schizophrenia, mood disorders, and major depressive disorder [70-72]. Ruminococcus plays a role in metabolic processes involving the breakdown of mucin and complex sugars, both of which are essential for providing additional energy [73].
Moreover, Ruminococcus metabolites, particularly SCFAs, are significant chemicals that influence human behavior and brain function, which may contribute to depression [74]. Ruminococcus has the potential to impact lipids, including phosphoethanolamine and glycerophosphorylcholine as well as inflammatory signaling pathways, including the NLRP3 inflammasome, which may contribute to the etiology of depression [75]. However, further research is needed to validate this hypothesis. In conclusion, the mechanisms through which Streptococcus and Ruminococcus influence depression warrant continued exploration in future studies.
Our study has several advantages. Firstly, this is the first MR investigation that has assessed the causal relationship between geriatric illnesses and gut microbiota. This approach reduces the susceptibility to confounding and reverse causality compared to traditional observational studies when examining the connection between gut microbiota and five geriatric syndromes. However, the potential influence of horizontal pleiotropy cannot be completely eliminated due to the uncertain biological mechanisms of many genetic variants. Therefore, the findings should be interpreted with caution. Secondly, by investigating the causative relationships between diverse gut microbiota, from genus to phylum, and diseases, we gained new insights into how to target specific gut bacteria in clinical practice to prevent and treat geriatric syndromes. Thirdly, the reliability and robustness of the causal links indicated by the MR investigation were improved by rigorous quality control procedures and the use of multiple sensitivity analysis techniques. Nonetheless, this study has certain limitations. First, Mendelian randomization relies on the assumption of exclusivity that genetic variation as an instrumental variable affects the outcome only through the exposure factor of interest. Although confounding factors or multiple effects at the gene level were avoided as much as possible, and MR-Egger regression method and MRPRESSO method were used to further ensure the stability of the study results, unmeasured confounding factors may still exist. For example, medications are a potential confounder affecting the composition of the gut microbiota. Antibiotics lead to the reduction of gut microbial diversity and imbalance of gut microbiota by killing or inhibiting specific bacterial groups, a phenomenon commonly referred to as' dysbiosis' [76]. Previous studies have shown that antibiotic use is associated with multiple long-term health outcomes, including an increased risk of frailty [77]. In addition, dietary habits are another important factor in shaping the structure of the gut microbiota, which has a direct impact on the microbial fermentation process by providing different types and amounts of substrates [78]. Thus, although our study provides valuable insights into the relationship between gut microbiota and frailty, these findings still need to be further validated in future studies with stricter control for confounders and more precise exposure assessment. It is worth mentioning that while the issue of pleiotropy may never be proved, it is generally accepted. However, it can be verified that pleiotropy has no effect on the results by performing sensitivity analyses of various MR Models based on different assumptions and methods, complementing each other and corroborating each other. Second, this study only included European populations, and the generalizability of the results may be limited. Therefore, further research in other populations is required. Third, the GWAS data for gut microbiota used in this study was based on the largest population cohort ever analyzed through metagenomic sequencing. However, to comprehensively assess the causal association between gut microbiota and geriatric disorders, summary data from additional gut microbiota will be necessary in the future. Additionally, this study could not to calculate the overlap between participants in exposure and outcome GWASs, which might lead to an overestimation of the study results. Furthermore, the investigation was unable to establish reverse causation due to limited availability of instrumental variables (IVs).
Conclusions
In conclusion, this study provided a comprehensive assessment of the causal relationship between gut microbiota and geriatric syndromes. Using two-sample MR analyses, we identified associations between 7 gut microbiota and frailty, 7 with PD, 5 with delirium, 11 with insomnia, and 11 with depression. It's important to note that the causal relationships derived from MR analyses represent statistical causal relationships and not exact causation. However, our work does present evidence of potential causal links between specific gut microbiota and five geriatric syndromes. These findings contribute to a better understanding of the potential pathways through which gut microbiota may influence geriatric syndromes. Nevertheless, larger GWAS data and further validation through additional MR studies will be in the future to confirm and expand upon these findings.
Acknowledgements
We would like to express our sincere gratitude to the MiBioGen consortium for generously sharing the GWAS summary data publicly, which greatly facilitated the conduct of our research.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82002380, 82072528), Natural Science Foundation of Guangdong Province (2022A1515012460), Health and Appropriate Technology Promotion Project of Guangdong Province (Grant No. 202206252011533513, 202303070834486751, 202303211529097713), China Disabled Persons' Federation Hearing and Language Disability Prevention and Rehabilitation Special (2023CDPFHS12) and Guangdong Medical University-Southern Medical University twinning research team project (No. 4SG23033G).
Data availability statement
All authors agree to share the research data upon publication of the article, and the data will be provided by the corresponding author upon reasonable request.
Ethics approval and consent to participate
This study used publicly available databases and did not require additional ethical approval.
Author Contributions
QZ, GH, JZ designed the research. CL, SW, CY analyzed and processed the data. QY, LC, YC drafted of the manuscript. QZ, YZ, SZ critically revised the manuscript. All of the co-authors have approved the submitted final version and agreed to the publication.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791
2. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376-1386
3. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365-1375
4. Marcozzi S, Bigossi G, Giuliani ME, Giacconi R, Piacenza F, Cardelli M, Brunetti D, Segala A, Valerio A, Nisoli E, Lattanzio F, Provinciali M, Malavolta M. Cellular senescence and frailty: a comprehensive insight into the causal links. Geroscience. 2023;45:3267-3305
5. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173:489-495
6. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol a Biol Sci Med Sci. 2007;62:722-727
7. D'Souza S, Thompson JM, Slykerman R, Marlow G, Wall C, Murphy R, Ferguson LR, Mitchell EA, Waldie KE. Environmental and genetic determinants of childhood depression: The roles of DAT1 and the antenatal environment. J Affect Disord. 2016;197:151-158
8. Herrman H, Kieling C, McGorry P, Horton R, Sargent J, Patel V. Reducing the global burden of depression: a Lancet-World Psychiatric Association Commission. Lancet. 2019;393:e42-e43
9. Schmauck-Medina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, Cao S, Soendenbroe C, Mansell E, Vestergaard MB, Li Z, Shiloh Y, Opresko PL, Egly JM, Kirkwood T, Verdin E, Bohr VA, Cox LS, Stevnsner T, Rasmussen LJ, Fang EF. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany Ny). 2022;14:6829-6839
10. Siddharth J, Chakrabarti A, Pannérec A, Karaz S, Morin-Rivron D, Masoodi M, Feige JN, Parkinson SJ. Aging and sarcopenia associate with specific interactions between gut microbes, serum biomarkers and host physiology in rats. Aging (Albany Ny). 2017;9:1698-1720
11. Wu L, Zeng T, Zinellu A, Rubino S, Kelvin DJ, Carru C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. Msystems. 2019;4:e00325-19
12. Marcozzi S, Bigossi G, Giuliani ME, Lai G, Giacconi R, Piacenza F, Malavolta M. Spreading Senescent Cells' Burden and Emerging Therapeutic Targets for Frailty. Cells. 2023;12:2287
13. Yemula N, Dietrich C, Dostal V, Hornberger M. Parkinson's Disease and the Gut: Symptoms, Nutrition, and Microbiota. J Parkinsons Dis. 2021;11:1491-1505
14. Aho V, Houser MC, Pereira P, Chang J, Rudi K, Paulin L, Hertzberg V, Auvinen P, Tansey MG, Scheperjans F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener. 2021;16:6
15. Jiang L, Li JC, Tang BS, Guo JF. Associations between gut microbiota and Parkinson disease: A bidirectional Mendelian randomization analysis. Eur J Neurol. 2023;30:3471-3477
16. Chen G, Ran X, Li B, Li Y, He D, Huang B, Fu S, Liu J, Wang W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. Ebiomedicine. 2018;30:317-325
17. Shin C, Lim Y, Lim H, Ahn TB. Plasma Short-Chain Fatty Acids in Patients With Parkinson's Disease. Mov Disord. 2020;35:1021-1027
18. Qiao CM, Sun MF, Jia XB, Li Y, Zhang BP, Zhao LP, Shi Y, Zhou ZL, Zhu YL, Cui C, Shen YQ. Sodium Butyrate Exacerbates Parkinson's Disease by Aggravating Neuroinflammation and Colonic Inflammation in MPTP-Induced Mice Model. Neurochem Res. 2020;45:2128-2142
19. Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167:1469-1480
20. Chen SJ, Chen CC, Liao HY, Lin YT, Wu YW, Liou JM, Wu MS, Kuo CH, Lin CH. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids With Gut Microbiota and Clinical Severity in Patients With Parkinson Disease. Neurology. 2022;98:e848-e858
21. Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66-72
22. Han M, Yuan S, Zhang J. The interplay between sleep and gut microbiota. Brain Res Bull. 2022;180:131-146
23. Wagner-Skacel J, Dalkner N, Moerkl S, Kreuzer K, Farzi A, Lackner S, Painold A, Reininghaus EZ, Butler MI, Bengesser S. Sleep and Microbiome in Psychiatric Diseases. Nutrients. 2020;12:2198
24. Zhou J, Wu X, Li Z, Zou Z, Dou S, Li G, Yan F, Chen B, Li Y. Alterations in Gut Microbiota Are Correlated With Serum Metabolites in Patients With Insomnia Disorder. Front Cell Infect Microbiol. 2022;12:722662
25. Li Y, Hao Y, Fan F, Zhang B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front Psychiatry. 2018;9:669
26. Xiang B, Liu K, Yu M, Liang X, Huang C, Zhang J, He W, Lei W, Chen J, Gu X, Gong K. Systematic genetic analyses of GWAS data reveal an association between the immune system and insomnia. Mol Genet Genomic Med. 2019;7:e742
27. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, Zale E, Wang Y, Huang Y, Theriault B, Dinner AR, Musch MW, Kudsk KA, Prendergast BJ, Gilbert JA, Chang EB. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. 2015;17:681-689
28. Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, Valsta L, Brożyńska M, Zhu Q, Tripathi A, Vázquez-Baeza Y, Loomba R, Cheng S, Jain M, Niiranen T, Lahti L, Knight R, Salomaa V, Inouye M, Méric G. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134-142
29. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4:330-345
30. Sekula P, Del GMF, Pattaro C, Köttgen A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol. 2016;27:3253-3265
31. Bowden J, Davey SG, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44:512-525
32. Wang J, Kurilshikov A, Radjabzadeh D, Turpin W, Croitoru K, Bonder MJ, Jackson MA, Medina-Gomez C, Frost F, Homuth G, Rühlemann M, Hughes D, Kim HN, Spector TD, Bell JT, Steves CJ, Timpson N, Franke A, Wijmenga C, Meyer K, Kacprowski T, Franke L, Paterson AD, Raes J, Kraaij R, Zhernakova A. Meta-analysis of human genome-microbiome association studies: the MiBioGen consortium initiative. Microbiome. 2018;6:101
33. Fang S, Hu X, Wang T, Yang Y, Xu R, Zhang X, Luo J, Ma Y, Patel AB, Dmytriw AA, Jiao L. Parkinson's Disease and Ischemic Stroke: a Bidirectional Mendelian Randomization Study. Transl Stroke Res. 2022;13:528-532
34. Yu H, Wan X, Yang M, Xie J, Xu K, Wang J, Wang G, Xu P. A large-scale causal analysis of gut microbiota and delirium: A Mendelian randomization study. J Affect Disord. 2023;329:64-71
35. Chen S, Han H, Sun X, Zhou G, Zhou Q, Li Z. Causal effects of specific gut microbiota on musculoskeletal diseases: a bidirectional two-sample Mendelian randomization study. Front Microbiol. 2023;14:1238800
36. Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. Bmc Med. 2023;21:66
37. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658-665
38. Bowden J, Davey SG, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314
39. Hartwig FP, Davey SG, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985-1998
40. Mao M, Zhai C, Qian G. Gut microbiome relationship with arrhythmias and conduction blocks: A two-sample Mendelian randomization study. J Electrocardiol. 2023;80:155-161
41. Nazarzadeh M, Rahimi K. Heterogeneity Between Genetic Variants as a Proxy for Pleiotropy in Mendelian Randomization-Reply. Jama Cardiol. 2020;5:108
42. Horn J, Mayer DE, Chen S, Mayer EA. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl Psychiatry. 2022;12:164
43. Strasser B, Ticinesi A. Intestinal microbiome in normal ageing, frailty and cognition decline. Curr Opin Clin Nutr Metab Care. 2023;26:8-16
44. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, Fitzgerald GF, Deane J, O'Connor M, Harnedy N, O'Connor K, O'Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O'Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178-184
45. Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, Meschi T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11:1633
46. Huang WC, Hsu YJ, Huang CC, Liu HC, Lee MC. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients. 2020;12:1145
47. Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O'Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8
48. Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Junior HJ, Gervasoni J, Primiano A, Putignani L, Del CF, Reddel S, Gasbarrini A, Landi F, Bernabei R, Marzetti E. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients. 2019;12:65
49. Zhang L, Liao J, Chen Q, Chen M, Kuang Y, Chen L, He W. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res. 2020;32:2001-2011
50. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front Microbiol. 2016;7:979
51. Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson's disease and Parkinson's disease medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32:739-749
52. Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson's disease patients. Genome Med. 2017;9:39
53. Rivera-Chávez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, Olsan EE, Xu G, Velazquez EM, Lebrilla CB, Winter SE, Bäumler AJ. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe. 2016;19:443-454
54. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. Bmj. 2018;361:k2179
55. Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun. 2018;70:48-60
56. Ostendorf F, Metzdorf J, Gold R, Haghikia A, Tönges L. Propionic Acid and Fasudil as Treatment Against Rotenone Toxicity in an In Vitro Model of Parkinson's Disease. Molecules. 2020;25:2502
57. Garcez FB, Garcia DAJ, Fernandez S, Avelino-Silva VI, Sabino EC, Martins R, Franco L, Lima RS, Possolo DSH, Avelino-Silva TJ. Association Between Gut Microbiota and Delirium in Acutely Ill Older Adults. J Gerontol a Biol Sci Med Sci. 2023;78:1320-1327
58. Zhang J, Bi JJ, Guo GJ, Yang L, Zhu B, Zhan GF, Li S, Huang NN, Hashimoto K, Yang C, Luo AL. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. Cns Neurosci Ther. 2019;25:685-696
59. Liu H, Cheng G, Xu YL, Fang Q, Ye L, Wang CH, Liu XS. Preoperative Status of Gut Microbiota Predicts Postoperative Delirium in Patients With Gastric Cancer. Front Psychiatry. 2022;13:852269
60. Jiang Z, Zhuo LB, He Y, Fu Y, Shen L, Xu F, Gou W, Miao Z, Shuai M, Liang Y, Xiao C, Liang X, Tian Y, Wang J, Tang J, Deng K, Zhou H, Chen YM, Zheng JS. The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases. Nat Commun. 2022;13:3002
61. Haimov I, Magzal F, Tamir S, Lalzar M, Asraf K, Milman U, Agmon M, Shochat T. Variation in Gut Microbiota Composition is Associated with Sleep Quality and Cognitive Performance in Older Adults with Insomnia. Nat Sci Sleep. 2022;14:1753-1767
62. Agrawal R, Ajami NJ, Malhotra S, Chen L, White DL, Sharafkhaneh A, Hoffman KL, Graham DY, El-Serag HB, Petrosino JF, Jiao L. Habitual Sleep Duration and the Colonic Mucosa-Associated Gut Microbiota in Humans-A Pilot Study. Clocks Sleep. 2021;3:387-397
63. Yue M, Jin C, Jiang X, Xue X, Wu N, Li Z, Zhang L. Causal Effects of Gut Microbiota on Sleep-Related Phenotypes: A Two-Sample Mendelian Randomization Study. Clocks Sleep. 2023;5:566-580
64. Xia S, Gao W, Li Y, Ma J, Gong S, Gao Z, Tang W, Tian W, Tang S. Effects of melatonin on intestinal function and bacterial compositions in sucking piglets. J Anim Physiol Anim Nutr (Berl). 2022;106:1139-1148
65. Foster JA, McVey NK. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312
66. Bastiaanssen T, Cowan C, Claesson MJ, Dinan TG, Cryan JF. Making Sense of … the Microbiome in Psychiatry. Int J Neuropsychopharmacol. 2019;22:37-52
67. Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics. 2018;15:36-59
68. Ling Z, Cheng Y, Chen F, Yan X, Liu X, Shao L, Jin G, Zhou D, Jiang G, Li H, Zhao L, Song Q. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: A case-control study. Front Immunol. 2022;13:964910
69. Lee MJ, Lai HC, Kuo YL, Chen VC. Association between Gut Microbiota and Emotional-Behavioral Symptoms in Children with Attention-Deficit/Hyperactivity Disorder. J Pers Med. 2022;12:1634
70. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194
71. Painold A, Mörkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, Birner A, Fellendorf F, Platzer M, Queissner R, Schütze G, Schwarz MJ, Moll N, Holzer P, Holl AK, Kapfhammer HP, Gorkiewicz G, Reininghaus EZ. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. 2019;21:40-49
72. Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29
73. Cervera-Tison M, Tailford LE, Fuell C, Bruel L, Sulzenbacher G, Henrissat B, Berrin JG, Fons M, Giardina T, Juge N. Functional analysis of family GH36 α-galactosidases from Ruminococcus gnavus E1: insights into the metabolism of a plant oligosaccharide by a human gut symbiont. Appl Environ Microbiol. 2012;78:7720-7732
74. Yu S, Wang L, Jing X, Wang Y, An C. Features of gut microbiota and short-chain fatty acids in patients with first-episode depression and their relationship with the clinical symptoms. Front Psychol. 2023;14:1088268
75. Zhao H, Jin K, Jiang C, Pan F, Wu J, Luan H, Zhao Z, Chen J, Mou T, Wang Z, Lu J, Lu S, Hu S, Xu Y, Huang M. A pilot exploration of multi-omics research of gut microbiome in major depressive disorders. Transl Psychiatry. 2022;12:8
76. Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Crit Rev Food Sci Nutr. 2022;62:3509-3534
77. Lynn MA, Eden G, Ryan FJ, Bensalem J, Wang X, Blake SJ, Choo JM, Chern YT, Sribnaia A, James J, Benson SC, Sandeman L, Xie J, Hassiotis S, Sun EW, Martin AM, Keller MD, Keating DJ, Sargeant TJ, Proud CG, Wesselingh SL, Rogers GB, Lynn DJ. The composition of the gut microbiota following early-life antibiotic exposure affects host health and longevity in later life. Cell Rep. 2021;36:109564
78. Al BZ, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17:7618
Author contact
![]() Corresponding authors: Jihua Zou (E-mail: zoujihuaedu.cn), Guozhi Huang (E-mail: drhuang66com), and Qing Zeng (E-mail: zengqingyang203com).
Corresponding authors: Jihua Zou (E-mail: zoujihuaedu.cn), Guozhi Huang (E-mail: drhuang66com), and Qing Zeng (E-mail: zengqingyang203com).

 Global reach, higher impact
Global reach, higher impact